Abstract
Objective
As large single‐surgeon series in the literature are lacking, we sought to review a single‐surgeon's experience with parotidectomy in an academic center, with a focused analysis of pathology, technique, and facial nerve (FN) weakness. Benchmark values for complications and operative times with routine trainee involvement and without continuous FN monitoring are offered.
Materials and Methods
All patients who underwent parotidectomy, performed by D. G. D., for benign and malignant disease between January 2004 and December 2018 at an academic center were reviewed.
Results
A total of 924 parotidectomies, with adequate evaluatable data were identified. The majority of patients had benign tumors (70.9%). Partial/superficial parotidectomy was the most common approach (65.7%). Selective FN branch sacrifice was rare (12.3%), but significantly more common among patients with malignant pathology (33.8% vs 3.5% for benign, P < .0001). Among patients with intact FN, post‐operative short‐ and long‐term FN weaknesses were rare (6.5% and 1.7%, respectively). These rates were lower among patients with benign tumors (5.4% and 1.3%). Partial/superficial parotidectomy for benign tumors was associated with a low rate of short‐ and long‐term FN weaknesses (2.7% and 0.9%). Mean OR time was 185 minutes.
Conclusion
This is the largest single‐surgeon series on parotidectomy, spanning 15 years. We demonstrate excellent long‐ and short‐term FN paresis rates with acceptable operative times without regular use of continuous FN monitoring and with routine trainee involvement. These findings may provide valuable insight into parotid tumor pathology, FN outcomes, and feasibility and expectations of performing parotidectomy in an academic setting.
Level of Evidence
4.
Keywords: academic, epidemiology, extracapsular dissection, facial nerve, facial nerve monitoring, national, parotid, parotid mass, parotid tumor, parotidectomy, single‐surgeon, trend
1. INTRODUCTION
Parotidectomy in the management of salivary gland masses remains one of the central and critical procedures in head and neck surgery, regardless of specific specialty discipline (Otolaryngology‐Head and Neck Surgery, General Surgery, Plastic Surgery, Oral Maxillofacial Surgery). 1 Parotidectomy also represents one of the index cases in Otolaryngology‐Head and Neck Surgery training and is considered fundamental to the development of surgical competency in the specialty. 2 As such, parotidectomy, remains the most common procedure in the management of salivary gland tumors. 3
Consistent with surgery development as a whole, parotidectomy has likewise been marked by a critical evolution from procedures based on facial nerve avoidance, such as enucleation or “shelling out” procedures, to those based on facial nerve identification and preservation. 4 This transition led to the utilization and adoption of the formal superficial parotidectomy with facial nerve identification, dissection and preservation in the management of parotid tumors. This, in turn, led to improved disease control while decreasing associated complications, most specifically those related to the facial nerve. 4
Further evolution and maturation of this approach led to the introduction of less invasive procedures such as partial parotidectomy. 5 , 6 , 7 This approach still utilized formal facial nerve identification, but involved removal of parotid tissue in direct abutment to the mass thus limiting unnecessary dissection and tissue loss. Studies subsequently demonstrated that partial parotidectomy allowed similar disease control while providing fewer associated complications. 5 , 7 , 8 The introduction and increasing utilization of the extracapsular dissection approach to benign parotid tumors are further evidence of this trend. 8 , 9 , 10 , 11 , 12 , 13
Yet, as clinical experience accrued with any of the described techniques, there has been great variance in the published data regarding the features specific to the management of parotid masses such as utilization rationale, specific tumor types, procedure durations and complication rates, specifically those involving the facial nerve. The literature is marked by smaller single institution series limited in number and scope, as well as larger database series lacking in specific detail. 8 , 14 , 15 , 16 , 17 , 18 , 19 Other recent series from single institutions are larger in their scope and consistency, but they do possess the critical limiting variable of combined experiences of multiple surgeons participating over many years. 16 , 20 , 21 , 22 Large single surgeon experiences utilizing a distinct and consistent approach to the management of parotid masses over a multi‐year period in an academic setting are lacking. 23
To address these limitations and to provide insight into the specific issues surrounding parotidectomy surgery, we reviewed the large single surgeon experience of the primary author (D. G. D.) utilizing a consistent operative approach to parotid salivary gland mass management applied in an academic institution with trainee involvement. This 15‐year experience was accrued over a set time period and at a single institution where the practitioner had an established head and neck practice after completion of a head and neck fellowship 8 years prior. Therefore, the initial learning curve of early practice and surgeon development had already been transitioned. Primary attention was placed on issues such as: epidemiology of presenting salivary gland masses, procedures utilized, postoperative complication rates with specific attention to transient and long‐term facial nerve dysfunction, assessment of other complications. Secondarily, benchmark values for complication rates and operative times with routine trainee involvement and without continuous facial nerve monitoring are presented.
2. MANAGEMENT APPROACH
A standardized approach was used in the management of all parotid patients. All masses underwent preoperative fine needle aspiration and imaging with either a computed tomography scan with contrast or magnetic resonance imaging with gadolinium contrast. For isolated lesions without evidence of metastatic disease within the lateral lobe of the parotid gland, a partial/superficial parotidectomy approach was chosen. In this approach, facial nerve identification and dissection was completed in the standard anterograde fashion in the vast majority of cases. In selected anterior lesions, a retrograde approach was undertaken with initial identification of the facial nerve branches within the region. For tumors situated within the deep lobe, a standard anterograde approach was utilized to identify the facial nerve in combination with mobilization of the superficial parotid gland. Facial nerve branches were appropriately mobilized and preserved, often with the use of microscopic instrumentation. Such cases were categorized under total parotidectomy.
For cases of malignancy in the context of a functioning facial nerve, all efforts were directed towards facial nerve preservation unless direct involvement of the nerve was noted. In such cases, involved nerves were sacrificed after confirmation by intraoperative frozen section. Sacrificed nerve branches were primarily reconstructed if applicable and functional deficits were addressed with the adjunct use of appropriate rehabilitative procedures. Appropriate selective neck dissection was completed in cases of node positive disease noted preoperatively. Selective neck dissection was also undertaken for node negative high‐grade lesions. In cases of parotid metastases from cutaneous malignancies, superficial parotidectomy approach with selective neck dissection was undertaken.
All cases were done with Loupe magnification. Facial nerve stimulation served as an adjunct in facial nerve identification for all cases. Continuous facial nerve monitoring was rarely used. All cases were completed at an academic medical center with trainee (resident or fellow) involvement appropriate to difficulty of case and level of training.
3. METHODS
A retrospective review of all parotidectomy procedures performed by the primary author (D. G. D.) for benign and malignant diseases between January 2004 and December 2018 was conducted. This investigation received institutional review board approval from the Massachusetts Eye and Ear Institutional Review Board.
Patient demographic and tumor characteristics were extracted from the patient's medical records. Intraoperative details including type of parotidectomy (partial/superficial, total or revision) and selective facial nerve branch sacrifice (yes vs no), if necessary, was recorded. Short‐ and long‐term outcomes including transient or permanent facial weakness were identified. Short‐term weakness was defined as facial nerve weakness resolving after 2 weeks postoperatively, and long‐term weakness defined as weakness persisting at 1‐year follow‐up. All patients had a minimum of 1‐year follow‐up. Tumor pathology was identified based on postoperative pathology reports and characterized as benign vs malignant. Symptomatic Frey's syndrome was defined as postoperative gustatory sweating described by the patient as frequent and affecting their quality of life. Operative time (defined as time from incision till closure) was recorded for patients with available data.
Descriptive analysis was performed to characterize trends in parotidectomy over time, distribution of benign vs malignant disease, and type of parotidectomy performed. Bivariable analysis was performed to identify associations between type of parotidectomy and facial nerve outcomes for both benign and malignant disease cohorts. Operative time was compared for patients who underwent parotidectomy for benign vs malignant disease and tabulated by year. Comparisons in group proportions were performed using Chi Square and Fisher's exact tests and means using t‐tests. Linear regression analysis was performed to identify associations between year of practice and mean operative time. Statistical significance was defined by a type I error threshold of .05, All statistical analyses were conducted using STATA version 15.1 (StataCorp, College Station, TX).
4. RESULTS
A total of 924 patients underwent parotidectomy by the primary author (D. G. D.) between 2004 and 2018. Mean patient age was 57.3 years (standard deviation [SD] 16.5 years). There were an equal proportion of men (49.3%) and women (50.7%). Median follow‐up was 29 months.
The majority of patients underwent parotidectomy for benign (70.9%) vs malignant disease (29.1%) (Table 1). Among patients with benign tumors, the most common etiology was pleomorphic adenoma (58.6%). Metastatic squamous cell carcinoma was the most common malignant parotid tumor (32.7%). Over the study period, the proportion of parotidectomy procedures performed for benign disease increased (Figure 1).
TABLE 1.
Tumor pathology
| Categorization | Pathology | Number | Percentage (%) |
|---|---|---|---|
| Benign | 655 | ||
| Pleomorphic adenoma | 384 | 58.6 | |
| Papillary cystadenoma lymphomatosum | 96 | 14.7 | |
| Oncocytoma | 29 | 4.4 | |
| Basal cell adenoma | 22 | 3.4 | |
| Lymphoepithelial cyst | 20 | 3.1 | |
| Reactive lymphoid hyperplasia | 16 | 2.4 | |
| Lipoma | 16 | 2.4 | |
| Benign cystic lesion | 7 | 1.1 | |
| Myoepithelioma | 6 | 0.9 | |
| Other (frequency < 5) | 59 | 9.0 | |
| Malignant | 269 | ||
| Metastatic squamous cell carcinoma | 88 | 32.7 | |
| Mucoepidermoid carcinoma | 36 | 13.4 | |
| Acinic cell carcinoma | 26 | 9.7 | |
| Metastatic melanoma | 16 | 5.9 | |
| Salivary duct carcinoma | 15 | 5.6 | |
| Basal cell adenocarcinoma | 11 | 4.1 | |
| Lymphoma | 23 | 8.6 | |
| Carcinoma ex‐pleomorphic adenoma | 10 | 3.7 | |
| Adenoid cystic carcinoma | 10 | 3.7 | |
| Other (frequency < 5) | 34 | 12.6 |
FIGURE 1.
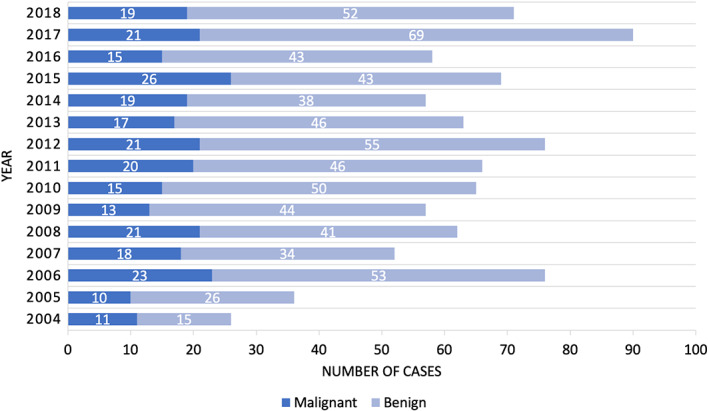
Cases per year (Benign vs Malignant)
For all patients, the most common type of parotidectomy performed was partial or superficial (65.7%) (Table 2). This approach was significantly more common among patient with benign disease as compared to those with malignant disease (68.9% vs 54.7%, P < .0001) (Table 2).
TABLE 2.
Approach to parotidectomy, stratified by tumor pathology
| Tumor pathology | |||
|---|---|---|---|
| Type of operation | Benign (N = 655) | Malignant (N = 269) | Total (N = 924) |
|
Partial/superficial Parotidectomy |
451 (68.9%) | 147 (54.7%) | 598 (65.7%) |
| Total parotidectomy | 160 (24.4%) | 105 (39.0%) | 265 (28.7%) |
| Revision parotidectomy | 34 (5.2%) | 14 (5.2%) | 48 (5.2%) |
| Other | 10 (1.5%) | 3 (1.1%) | 13 (1.4%) |
Complications were overall uncommon. Among patients with complete facial nerve preservation, 6.5% (53/810) had short term nerve weakness and 1.7% (14/810) had long term nerve weakness (Table 3). Short term facial nerve weakness was significantly more common among patients with malignant vs benign tumors (10.7% vs 5.4%, P = .006) in whom facial nerve branches were intact (Table 3). No long‐term facial nerve weakness was greater than House‐Brackmann II‐III.
TABLE 3.
Complications, stratified by tumor type
| Complication | Benign, N (%) | Malignant, N (%) | P value |
|---|---|---|---|
| Selective nerve branch sacrifice | 23 (3.5%) | 91 (33.8%) | <.0001 |
| Short‐term nerve weakness a | 34 (5.4%) | 19 (10.7%) | .006 |
| Long‐term nerve weakness a | 8 (1.3%) | 6 (3.4%) | .167 |
| Frey's syndrome | 12 (1.9%) | 2 (1.1%) | .193 |
Calculated only for patients without intentional selective nerve branch sacrifice intraoperatively and/or without preoperative facial nerve weakness, N = 632 for benign tumors, N = 178 for malignant tumors.
Bold denotes significant variables p < 0.05.
Symptomatic Frey's syndrome, identified at post‐operative visit, was rare (1.5%), with no significant difference between benign and malignant cases (1.9% vs 1.1%, P = .193) (Table 3).
Selective nerve branch sacrifice was required in 114 cases (12.3%) and its incidence was stable over the study period. When stratified by tumor pathology, a significantly higher proportion of malignant tumors required selective nerve branch sacrifice compared to benign tumors (33.8% vs 3.5%, P < .0001). The most common benign tumor associated with selective nerve branch sacrifice was pleomorphic adenoma (16/23). Twenty‐four patients (24/91, 26.4%) with malignant disease had facial nerve dysfunction prior to nerve resection, whereas 1/23 patients (4.3%) with benign disease had dysfunction prior to nerve resection (P = .060). Post‐operatively, 70.3% of nerve resection for malignancy cases had short term weakness, with the majority (61/91) having long‐term weakness. Immediate post‐operative weakness was less in the benign group (7/23), with five patients having long‐term weakness (Table 4).
TABLE 4.
Frequency of preoperative facial nerve weakness, postoperative short‐ and long‐term nerve weakness among patients with selective nerve branch sacrifice, stratified by tumor type
| Complication | Benign, N (%) (N = 23) | Malignant, N (%) (N = 91) | P value |
|---|---|---|---|
| Preoperative facial nerve weakness | 1 (4.3%) | 24 (26.4%) | .060 |
| Postoperative short‐term nerve weakness | 7 (30%) | 64 (70.3%) | <.0001 |
| Postoperative long‐term nerve weakness | 5 (21.7%) | 61 (67.9%) | <.0001 |
Bold denotes significant variables p < 0.05.
Complications were compared among all patients when stratified by parotidectomy approach (Table 5). Total parotidectomy was more commonly associated with selective nerve branch sacrifice (26.6%, P < .0001) and was more common in patients with malignancy Revision parotidectomy was most commonly associated with short term nerve weakness (19.0%, P < .0001). These associations persisted after further stratification by tumor pathology (Table 6). Notably, in 440 benign tumors removed with the partial/superficial approach, short‐ and long‐term facial weakness rates were 2.7% and 0.9%, respectively.
TABLE 5.
Complications, stratified by approach to parotidectomy (all tumor pathologies)
| Complication | Partial/superficial parotidectomy | Total parotidectomy | Revision parotidectomy | Other | P value |
|---|---|---|---|---|---|
| Selective nerve branch sacrifice | 35 (5.9%) | 71 (26.6%) | 6 (12.5%) | 2 (15.4%) | <.0001 |
| Short‐term nerve weakness a | 20 (3.6%) | 25 (12.8%) | 8 (19.0%) | 0 (0%) | <.0001 |
| Long‐term nerve weakness a | 7 (1.2%) | 4 (2.0%) | 3 (7.1%) | 0 (0%) | .001 |
Calculated only for patients without intentional selective nerve branch sacrifice intraoperatively, N = 563 for partial parotidectomy, N = 196 for total parotidectomy, N = 42 for revision parotidectomy, N = 11 for other.
Bold denotes significant variables p < 0.05.
TABLE 6.
Complications, stratified by approach to parotidectomy and tumor pathology
| Complication | Partial/superficial parotidectomy | Total parotidectomy | Revision parotidectomy | Other | Total | P value |
|---|---|---|---|---|---|---|
| Benign | ||||||
| Selective nerve branch sacrifice | 11 (2.4%) | 10 (6.3%) | 1 (2.9%) | 1 (10%) | 23 (3.5%) | <.0001 |
| Short‐term nerve weakness a | 12 (2.7%) | 16 (10.7%) | 6 (18.2%) | 0 (0%) | 34 (5.4%) | <.0001 |
| Long‐term nerve weakness a | 4 (0.9%) | 2 (1.3%) | 2 (6.1%) | 0 (0%) | 8 (1.3%) | .005 |
| Malignant | ||||||
| Selective nerve branch sacrifice | 24 (16.3%) | 61 (58.1%) | 5 (35.7%) | 1 (33.3%) | 91 (33.8%) | <.0001 |
| Short‐term nerve weakness b | 8 (6.5%) | 9 (20.5%) | 2 (22.2%) | 0 (0%) | 19 (10.7%) | <.0001 |
| Long‐term nerve weakness b | 3 (2.4%) | 2 (4.5%) | 1 (11.1%) | 0 (0%) | 6 (3.4%) | .521 |
Calculated only for benign tumor patients without intentional selective nerve branch sacrifice intraoperatively, N = 440 for partial parotidectomy, N = 150 for total parotidectomy, N = 33 for revision parotidectomy, N = 9 for other.
Calculated only for malignant tumor patients without intentional selective nerve branch sacrifice intraoperatively, N = 123 for partial parotidectomy, N = 44 for total parotidectomy, N = 9 for revision parotidectomy, N = 2 for other.
Bold denotes significant variables p < 0.05.
Mean operative (OR) time for all cases was 185 minutes (SD 88.7). Overall, OR time was significantly longer among patients with malignant tumors (238.9 minutes, SD 127.0) vs benign tumors (169.4 minutes, SD 66.3) (P < .0001). Mean OR time decreased over the study period, from 210.6 minutes in 2004 to 130.2 minutes in 2018 (beta coefficient [BC]: −6.81, P < .0001) (Figure 2). This trend was similarly reflected among patients with benign (190.9‐100.8 minutes, BC: −7.88, P < .0001) and malignant tumors (279.5‐210.9 minutes, BC: −3.75, P = .087) (Figure 2).
FIGURE 2.
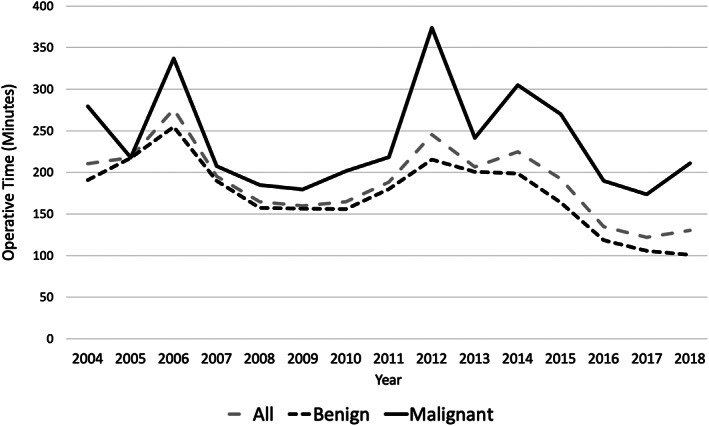
Operative time per year, stratified by tumor pathology (2004‐2018)
5. DISCUSSION
Although parotidectomy remains a relatively common procedure within the specialty of head and neck surgery, the number of procedures performed by a specific practitioner can vary widely from a few per year to dozens or more. Review of institutional experience may garner greater overall numbers, but analysis of these experiences is often divided among numerous providers varying in experience and with inherent technical variations. These limitations can lead to wide variation in the literature regarding the evaluation of key features relating to the efficacy of parotidectomy such as procedure duration, procedure type, facial nerve management and function, and other complications. 15 , 16
The current study reviews the largest published single surgeon experience with parotidectomy for the management of the breadth of parotid tumors. Analysis of this select experience offers meaningful insight into parotidectomy surgery. Such analysis may assist in establishing benchmarks for realistic expectations for experienced head and neck surgeons surrounding parotidectomy, and similarly facilitate pre‐ and post‐operative counseling and decision making with patients.
This 15‐year experience was accrued over a set time period and at a single institution where the practitioner had an established head and neck practice after completion of a head and neck fellowship 8 years prior. Therefore, the initial learning curve of early practice and surgeon development had already been transitioned. A consistent established approach, as described, was utilized in the management of 924 tumors. This served to limit the inherent variation in skill level and technique noted in most series. As demonstrated by the significant decrease in operative time, skill progression occurred in this series. This was further highlighted by the evolving referral nature of the practice in which more challenging cases, such as tumors with deep lobe involvement or large size, increased in number from outside referral in addition to the presentation of more straightforward masses.
As noted, 924 cases reviewed were managed in an established and consistent manner for the time period studied, with commensurate close follow‐up. This represents the largest single surgeon series and compares favorably in size to single institutional series over a similar time period reported in the literature. 8 , 14 , 22 , 23 Table 1 demonstrates the wide and well‐discussed variation in the pathology of the parotid gland leading to parotid surgery. Although malignancy is standardly shown to involve 15% to 20% of the salivary gland parotid masses, malignancy was a diagnosis in 29% of the cases. These malignancies were equally divided between those of primary salivary gland origin and those representing metastatic disease. If metastatic disease is removed from the series and only tumors of salivary gland origin are reviewed, then the incidence of salivary gland malignancy, 20% falls within the expected epidemiologic range. 24 The prevalence of metastatic disease in this series, highlights the importance of consideration of this process in the evaluation of parotid mass. Additional intervention such as primary site identification and treatment, concomitant neck dissection and incorporation of adjuvant treatment may be required.
This series focused on the primary outcomes of short‐ and long‐term facial nerve functions after surgery, which varies significantly in the literature. Some series reporting temporary weakness rates as high as 77%. 25 While others report rates of over 50% independent of specialty types. 26 Yet most large, contemporary series will usually cite temporary paresis rates in the 15% to 20% range and permanent paresis rates in the low to mid‐single digits for parotidectomy for benign disease. 7 , 16 , 27 , 28 , 29 Increase rates are noted in management of malignant lesions.
The current series demonstrated overall temporary and permanent facial nerve paresis rates of 6.5% and 1.7% respectively, for all lesions, which was stable across the study period. Consistent with previous studies, surgery for malignant disease had a statistically significantly higher rate of temporary paresis when compared to benign disease (10.7% vs 5.4%). Although higher, the difference in long term paresis did not reach statistical significance (3.4% vs 1.3%) A similar trend was also noted by Jin et al, in reviewing a series of 794 parotidectomy procedures for benign and malignant pathology with overall temporary and long‐term paresis rates of 9.2% and 5.4%. 16 Rates for malignant disease were 21.7% and 14.4%. 16
Extent of procedure also significantly affected post‐operative facial nerve function. Short‐term weakness rates for total parotidectomy (12.8%) and revision parotidectomy (19.0%) were significantly greater than the partial/superficial parotidectomy group (3.6%) for all lesions. A similar significant difference was noted when benign and malignant lesions were assessed independently. This is an expected finding in the context of the degree of facial nerve dissection and manipulation required in more extensive procedure and is supported by numerous previous studies. In response to this, a trend toward less invasive procedures specifically to address benign parotid tumors, such as extracapsular dissection (ED), have been advocated. These procedures are conceptually based on identification and meticulous removal of the mass while avoiding direct facial nerve identification and dissection if possible. Iro demonstrated decreases in both temporary (22.8%‐9.8%) and permanent (9%‐5.9%) facial paresis with the transition to less radical procedures, primarily extracapsular dissection. 8 Further review supported this finding 12 and a recent study from this group noted temporary and permanent facial paresis rates of 5.9% and 2.0%, respectively, in ED procedures done specifically for pleomorphic adenoma. 30 The least invasive subgroup in the current series was the partial/superficial parotidectomy procedure. When assessing the application of this technique for all benign tumors, temporary (2.7%) and permanent paresis rates (0.9%) were also low indicating the efficacy and safety of procedures based upon facial nerve identification and dissection.
Selective facial nerve sacrifice was expectedly more common and extensive in management of malignant lesions with 26%, having facial nerve dysfunction pre‐operatively. Facial nerve branch sacrifice was rare in benign cases (3.5%) and occurred primarily when small branches could not successfully be separated from the primary tumor. The majority (16/23) had no dysfunction noted post‐operatively most likely due to redundancy within the facial nerve system.
Although evaluation of the impact of the use of intraoperative continuous facial nerve monitoring on the facial nerve function was not a primary objective, this study sheds light regarding potential necessity of this technology. Continuous monitoring was rarely used in this series and only for cases of disrupted anatomy such as recurrences, congenital abnormalities or exceedingly large masses. As such, the rates of temporary and permanent facial nerve paralysis with no continuous monitoring reported here are quite favorable compared to published series and systematic reviews evaluating such monitoring. 31 , 32 , 33 , 34 , 35 Therefore, monitoring may be considered a useful, but not necessary adjunct to successful parotid surgery.
Notable in this series is that all procedures were performed in an academic setting with resident or fellow involvement. Primary surgeon supervisory involvement was maintained in all cases and the exact level of trainee involvement was determined by the level of trainee, individual technical ability of the trainee and overall case complexity. Although the literature is conflicted as to whether trainee involvement may increase overall complications in surgery, the low rate of complications in this series, especially facial nerve weakness both permanent and temporary, would indicate otherwise. These low complication rates compared quite favorably to the series of 963 benign parotid procedures at a university teaching hospital over an 18‐year period reported by Guntinas‐Lichius et al. 27 The transient and long‐term facial paresis rates were 25% and 6%, respectively, average procedure time was 15 minutes greater than noted in this series. Pollei et al noted that resident participation added an average of 27 minutes to the parotidectomy case length, but these results are limited by overall small sample size of 42 parotid procedures. 36 In the latter portion of the current series, operative times with trainee involvement averaged 130 minutes. Ultimately, the current series demonstrates that parotidectomy completed in an academic center with trainee involvement can be undertaken in a timely fashion with excellent success and low complication rates.
The rates of gustatory sweating following parotidectomy vary considerably in the literature, with high rates noted with the utilization of formal starch testing. 37 , 38 , 39 Symptomatic Frey's syndrome is a much rarer occurrence with studies demonstrating rates from 1% to 9%. 23 , 40 The low rate of symptomatic Frey's Syndrome noted at follow‐up visits (1.5%) in this series is consistent with other large series demonstrating this infrequent event.
Limitations of the study include the retrospective design and data acquisition obtained from this large series over a 15‐year period. This is tempered by the consistency and fidelity of the evaluative, surgical and treatment approach afforded in this single‐surgeon series. Similarly, more exhaustive objective analysis could have been utilized in the assessment of facial nerve function, but as with many series the practicality of such analysis is challenging from the standpoint of both access and affordability. Finally, the series does not include the evolving approach of extracapsular dissection as practiced with excellent results around the world. Yet, it does offer benchmark data demonstrating results using a nerve identification and dissection approach which rival the best results in extracapsular dissection series.
6. CONCLUSION
In summary, this represents the largest single surgeon experience with parotid surgery in an academic center over a confined time period without the routine use of continuous facial nerve monitoring. Long term and short facial paralysis rates were acceptably low, 6.5% and 1.7% respectively, for all pathologies and lower for benign tumors, 5.4% and 1.3%. Notably, benign tumors treated with partial/superficial parotidectomy had short‐ and long‐term weakness rates of 2.3% and 0.9%, respectively, although such rates did increase with extent of procedure for all pathologies. Overall, this series demonstrates acceptably low rates of facial nerve paralysis for parotid surgery without the routine use of continuous facial nerve monitoring and in an academic setting with trainee involvement while utilizing a surgical approach based on facial nerve identification and preservation.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Deschler DG, Kozin ED, Kanumuri V, et al. Single‐surgeon parotidectomy outcomes in an academic center experience during a 15‐year period. Laryngoscope Investigative Otolaryngology. 2020;5:1096–1103. 10.1002/lio2.480
REFERENCES
- 1. Dykun RJ, Deitel M, Borowy ZJ, Jackson S. Treatment of parotid neoplasms. Can J Surg. 1980;23:14‐19. [PubMed] [Google Scholar]
- 2. (ACGME) ACfGME . Required minimum number of key indicator procedures for graduating residents Surgery RCfO‐HaN. Chicago, IL: American Council for Graduate Medical Education; 2018. [Google Scholar]
- 3. Sethi RKV, Deschler DG. National trends in inpatient parotidectomy: a fourteen‐year retrospective analysis. Am J Otolaryngol. 2018;39:553‐557. [DOI] [PubMed] [Google Scholar]
- 4. Donovan DT, Conley JJ. Capsular significance in parotid tumor surgery: reality and myths of lateral lobectomy. Laryngoscope. 1984;94:324‐329. [DOI] [PubMed] [Google Scholar]
- 5. Mehta V, Nathan CA. Extracapsular dissection versus superficial parotidectomy for benign parotid tumors. Laryngoscope. 2015;125:1039‐1040. [DOI] [PubMed] [Google Scholar]
- 6. Larian B. Parotidectomy for benign parotid tumors. Otolaryngol Clin North Am. 2016;49:395‐413. [DOI] [PubMed] [Google Scholar]
- 7. Witt RL. Facial nerve function after partial superficial parotidectomy: an 11‐year review (1987‐1997). Otolaryngol Head Neck Surg. 1999;121:210‐213. [DOI] [PubMed] [Google Scholar]
- 8. Mantsopoulos K, Koch M, Klintworth N, Zenk J, Iro H. Evolution and changing trends in surgery for benign parotid tumors. Laryngoscope. 2015;125:122‐127. [DOI] [PubMed] [Google Scholar]
- 9. Deschler DG. Extracapsular dissection of benign parotid tumors. JAMA Otolaryngol Head Neck Surg. 2014;140:770‐771. [DOI] [PubMed] [Google Scholar]
- 10. Brennan PA, Ammar M, Matharu J. Contemporary management of benign parotid tumours ‐ the increasing evidence for extracapsular dissection. Oral Dis. 2017;23:18‐21. [DOI] [PubMed] [Google Scholar]
- 11. Sood S, McGurk M, Vaz F. Management of salivary gland tumours: United Kingdom national multidisciplinary guidelines. J Laryngol Otol. 2016;130:S142‐S149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bar B, Mantsopoulos K, Iro H. Paradigm shift in surgery for benign parotid tumors: 19 years of experience with almost 3000 cases. Laryngoscope. 2020;130:1941‐1946. [DOI] [PubMed] [Google Scholar]
- 13. Iro H, Zenk J. Role of extracapsular dissection in surgical management of benign parotid tumors. JAMA Otolaryngol Head Neck Surg. 2014;140:768‐769. [DOI] [PubMed] [Google Scholar]
- 14. Parikh AS, Khawaja A, Puram SV, et al. Outcomes and prognostic factors in parotid gland malignancies: a 10‐year single center experience. Laryngoscope Investig Otolaryngol. 2019;4:632‐639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maithani T, Pandey AK, Agrahari AK. An overview of Parotidectomy for benign parotid lesions with special reference to perioperative techniques to avoid complications: our experience. Indian J Otolaryngol Head Neck Surg. 2019;71:258‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jin H, Kim BY, Kim H, et al. Incidence of postoperative facial weakness in parotid tumor surgery: a tumor subsite analysis of 794 parotidectomies. BMC Surg. 2019;19:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ruohoalho J, Makitie AA, Aro K, et al. Complications after surgery for benign parotid gland neoplasms: a prospective cohort study. Head Neck. 2017;39:170‐176. [DOI] [PubMed] [Google Scholar]
- 18. Al‐Naqeeb NI, Dashti H, Al‐Muhanna AH, Behbehani A. Parotid gland tumours: a 15‐year experience. J R Coll Surg Edinb. 1992;37:89‐93. [PubMed] [Google Scholar]
- 19. Laccourreye H, Laccourreye O, Cauchois R, Jouffre V, Menard M, Brasnu D. Total conservative parotidectomy for primary benign pleomorphic adenoma of the parotid gland: a 25‐year experience with 229 patients. Laryngoscope. 1994;104:1487‐1494. [DOI] [PubMed] [Google Scholar]
- 20. Luksic I, Virag M, Manojlovic S, Macan D. Salivary gland tumours: 25 years of experience from a single institution in Croatia. J Craniomaxillofac Surg. 2012;40:e75‐e81. [DOI] [PubMed] [Google Scholar]
- 21. Knopf A, Szyper M, Mansour N, Sonnenberg J, Hofauer B, Niedermeyer H. A critical review of 20 years of parotid gland surgery. Acta Otolaryngol. 2016;136:711‐716. [DOI] [PubMed] [Google Scholar]
- 22. Bussu F, Parrilla C, Rizzo D, Almadori G, Paludetti G, Galli J. Clinical approach and treatment of benign and malignant parotid masses, personal experience. Acta Otorhinolaryngol Ital. 2011;31:135‐143. [PMC free article] [PubMed] [Google Scholar]
- 23. Upton DC, McNamar JP, Connor NP, Harari PM, Hartig GK. Parotidectomy: ten‐year review of 237 cases at a single institution. Otolaryngol Head Neck Surg. 2007;136:788‐792. [DOI] [PubMed] [Google Scholar]
- 24. Subramaniam N, Gao K, Gupta R, Clark JR, Low TH. Trends in parotidectomy over 30 years in an Australian tertiary care center. Head Neck. 2020;42:2905‐2911. [DOI] [PubMed] [Google Scholar]
- 25. Lacosta JL, Zabaleta M, Infante JC. Surgical pathology of parotid gland tumors. Acta Otorrinolaringol Esp. 1997;48:653‐657. [PubMed] [Google Scholar]
- 26. Infante‐Cossio P, Gonzalez‐Cardero E, Garcia‐Perla‐Garcia A, Montes‐Latorre E, Gutierrez‐Perez JL, Prats‐Golczer VE. Complications after superficial parotidectomy for pleomorphic adenoma. Med Oral Patol Oral Cir Bucal. 2018;23:e485‐e492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guntinas‐Lichius O, Klussmann JP, Wittekindt C, Stennert E. Parotidectomy for benign parotid disease at a university teaching hospital: outcome of 963 operations. Laryngoscope. 2006;116:534‐540. [DOI] [PubMed] [Google Scholar]
- 28. Mehle ME, Kraus DH, Wood BG, et al. Facial nerve morbidity following parotid surgery for benign disease: the Cleveland Clinic Foundation experience. Laryngoscope. 1993;103:386‐388. [DOI] [PubMed] [Google Scholar]
- 29. O'Brien CJ. Current management of benign parotid tumors—the role of limited superficial parotidectomy. Head Neck. 2003;25:946‐952. [DOI] [PubMed] [Google Scholar]
- 30. Schapher M, Koch M, Goncalves M, Mantsopoulos K, Iro H. Extracapsular dissection in pleomorphic adenomas of the parotid gland: results after 13 years of follow‐up. Laryngoscope. 2020. [DOI] [PubMed] [Google Scholar]
- 31. Chiesa‐Estomba CM, Larruscain‐Sarasola E, Lechien JR, et al. Facial nerve monitoring during parotid gland surgery: a systematic review and meta‐analysis. Eur Arch Otorhinolaryngol. 2020. [DOI] [PubMed] [Google Scholar]
- 32. Terrell JE, Kileny PR, Yian C, et al. Clinical outcome of continuous facial nerve monitoring during primary parotidectomy. Arch Otolaryngol Head Neck Surg. 1997;123:1081‐1087. [DOI] [PubMed] [Google Scholar]
- 33. Sood AJ, Houlton JJ, Nguyen SA, Gillespie MB. Facial nerve monitoring during parotidectomy: a systematic review and meta‐analysis. Otolaryngol Head Neck Surg. 2015;152:631‐637. [DOI] [PubMed] [Google Scholar]
- 34. Savvas E, Hillmann S, Weiss D, Koopmann M, Rudack C, Alberty J. Association between facial nerve monitoring with postoperative facial paralysis in parotidectomy. JAMA Otolaryngol Head Neck Surg. 2016;142:828‐833. [DOI] [PubMed] [Google Scholar]
- 35. Grosheva M, Klussmann JP, Grimminger C, et al. Electromyographic facial nerve monitoring during parotidectomy for benign lesions does not improve the outcome of postoperative facial nerve function: a prospective two‐center trial. Laryngoscope. 2009;119:2299‐2305. [DOI] [PubMed] [Google Scholar]
- 36. Pollei TR, Barrs DM, Hinni ML, Bansberg SF, Walter LC. Operative time and cost of resident surgical experience: effect of instituting an otolaryngology residency program. Otolaryngol Head Neck Surg. 2013;148:912‐918. [DOI] [PubMed] [Google Scholar]
- 37. Motz KM, Kim YJ. Auriculotemporal syndrome (Frey syndrome). Otolaryngol Clin North Am. 2016;49:501‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Singleton GT, Cassisi NJ. Frey's syndrome: incidence related to skin flap thickness in parotidectomy. Laryngoscope. 1980;90:1636‐1639. [PubMed] [Google Scholar]
- 39. Kadletz L, Taucher K, Janik S, et al. Cross‐sectional study on the occurrence of Frey's syndrome following superficial parotidectomy or extracapsular dissection. J Craniomaxillofac Surg. 2020;48:199‐202. [DOI] [PubMed] [Google Scholar]
- 40. Rustemeyer J, Eufinger H, Bremerich A. The incidence of Frey's syndrome. J Craniomaxillofac Surg. 2008;36:34‐37. [DOI] [PubMed] [Google Scholar]


