Abstract
Objective
Techniques for reconstruction of skull base defects have advanced greatly since the introduction of the vascular pedicled nasoseptal flap in 2006. The objective of this review is to assess the current state of the field by examining both intranasal and extranasal techniques of vascular pedicled skull base defect repair, their indications and success rates, and novel techniques that are currently under investigation.
Methods
A review of the literature describing the use of vascular pedicled flaps in skull base defect reconstruction was conducted using PubMed and Google Scholar.
Results
The nasoseptal flap remains the most widely used vascular pedicled flap for endoscopic repair of skull base defects. Its ease of harvest, wide arch of rotation, and high success rates make it a popular choice among surgeons. Several variations including a “rescue” nasopseptal flap have been developed. Other less commonly used pedicled intranasal flaps include the middle turbinate flap and the posterior pedicled inferior turbinate flap. Additionally, several novel vascular pedicled flaps have been developed and tested in small cohorts of patients. Extranasal flaps such as the pericranial flap and the temporoparietal fascia flap are used less frequently than intranasal flaps. However, they remain valuable options for reconstruction in certain situations.
Conclusion
Advancements continue to be made in the field of skull base defect reconstruction using vascular pedicled flaps. Though the nasoseptal flap remains the most widely utilized option, additional intranasal techniques continue to be developed and tested to optimize surgical outcomes and patient care.
Level of Evidence
NA
Keywords: endoscopic surgery, skull base reconstruction, surgical outcomes, surgical techniques, vascular pedicled flaps
Endoscopic reconstruction of skull base defects has rapidly advanced since the introduction of the nasoseptal flap in 2006. Here, we review current techniques of vascular pedicled flap reconstruction of skull base defects, their indications and success rates, and novel techniques currently under investigation.
1. INTRODUCTION
Skull base surgery has evolved from its history of primarily open transcranial repairs to increasingly common complex endoscopic approaches. Methods of endoscopic reconstruction of skull base defects are selected based on location, defect size, CSF flow rate, and relevant patient history such as prior surgeries or radiation. 1 The development of the vascular pedicled nasoseptal flap (NSF) in 2006 by Hadad et al was milestone achievement for endoscopic skull base defect repair thanks to its ease of harvest, reliable blood supply, and anatomic reach. 2 As the efficacy of this reconstructive technique became widely known, additional pedicled flaps for skull base reconstruction were developed and described in the literature. Use of vascular pedicled flaps has resulted in the ability to reliably reconstruct larger, more hostile, and more anatomically complex defects than was previously possible. Here, we review current techniques of vascular pedicled flap reconstruction of skull base defects, their indications and success rates, and novel techniques currently under investigation.
2. GENERAL PRINCIPLES OF REPAIRS
The goal of skull base reconstruction is to achieve water‐tight closure of the intracranial space, thereby preventing CSF leakage, pneumocephalus, and infection. A significant factor in successful closure is the presence and flow rate of intraoperative CSF leaks, which can guide the choice of repair technique. Leak‐free sites can be repaired with a single layer free tissue autograft or synthetic biomaterial. 3 , 4 Low‐flow sites can be reconstructed using either free tissue grafts, biomaterials, or vascular pedicled flaps. High‐flow sites (where a direct communication exists between brain cisterns/ventricles and an arachnoid membrane defect) are most successfully reconstructed using vascular pedicled flaps. 1 For both low‐flow and high‐flow sites, use of a multilayer closure has demonstrated higher success rates than single layer reconstructive techniques. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12
Additional, selection criteria for a repair technique include the anterior‐posterior location (Figure 1) and the size of the defect. Several classification systems have been developed to incorporate these variables in an effort to accurately describe skull base defects and facilitate treatment standardization. 13 , 14 , 15 , 16 Recently, Yano et al described a system categorizing skull base defects by the location of the defect center either in the anterior (I) or middle (II) skull base, and by the extension of the defect from its center (a = confined to anatomic boundaries, b = horizontal extension beyond anatomic boundaries, c = vertical extension beyond anatomic boundaries). Involvement of orbit and/or skin is indicated by +O and + S, respectively. Though the system is intuitive, the utility of this or any other location‐based classification systems is limited, as a 2014 systematic review by Soudry et al found that location was not a significant factor in successful defect closure, except for cases of clival defects. Instead, intraoperative CSF flow rate (low vs high) was of greatest utility in predicting closure success of various graft types. Reported estimates of intraoperative CSF leaks occurring in transnasal surgeries such as endoscopic transsphenoidal sellar surgery range from as low as 20% of cases to as high as 89%. 17 , 18 , 19 , 20 , 21 , 22 , 23 Given the frequency of these leaks, there is a need to have multiple versatile repair options available for skull base defects of various sizes, locations, and CSF flow rates. Below we discuss several vascular pedicled flaps which can be employed in the reconstruction of a variety of skull base defects. The technique for harvesting each flap is described and illustrated, along with a brief description of its uses, outcomes, and variants, if applicable.
FIGURE 1.
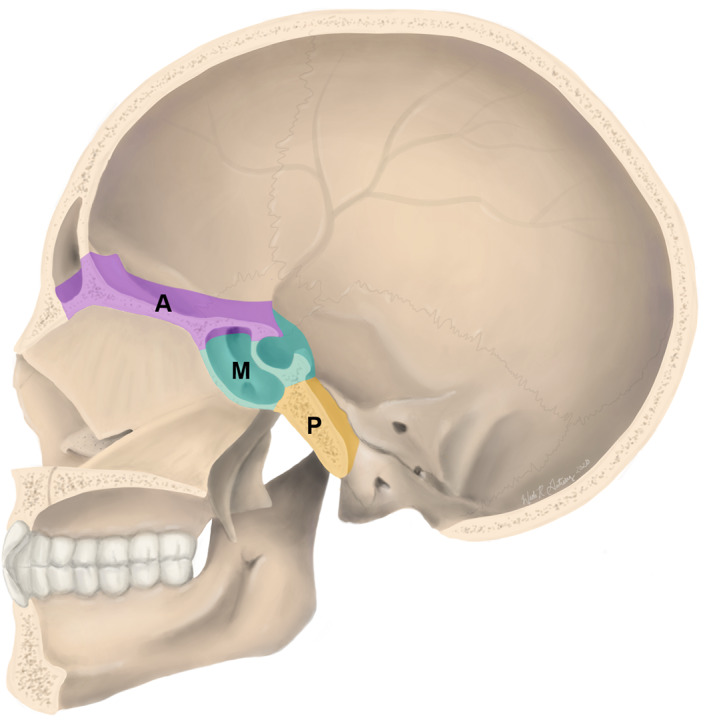
Regions of skull base defects. Vascular pedicled flap reconstruction may be required in the anterior skull base (frontal sinus, cribiform plate, and ethmoid sinus) highlighted in purple (A), the middle skull base (sphenoid sinus and sella turcica) highlighted in teal (M), or the posterior skull base (clivus) highlighted in gold (P). Selection of a specific pedicled flap for reconstruction is based on the anatomy of the defect (location and size), among other factors
3. MULTILAYER CLOSURE FOUNDATION TERMINOLOGY AND PRINCIPLES
Multilayer skull base defect closure consists primarily of a graft with the addition of a vascular pedicle flap. Terminology describing placement of graft layers (underlay, inlay, overlay, and onlay) is largely inconsistent in the body of published literature. Here we define underlay grafts (sometimes referred to as inlay grafts) as a layer that is sized to be slightly larger than the defect and that is placed on the intracranial (proximal) side of the defect between the dura and bone. Common underlay materials include dermal fat grafts, fascia, cartilage, acellular dermal matrix, or biosynthetic dural replacements. 3 , 8 , 17 , 24 , 25 , 26 , 27 True inlay grafts are defined as those sized to fit only within the bony margins of the defect and can include dermal fat or bone. 3 , 28 , 29 Of note, the utility of bone or other nonabsorbable rigid materials as part of a multilayer closure is considered by some to be unnecessary and not recommended due to risk of graft migration and infection. 30 True inlay grafts are not often used in isolation. Onlay grafts (sometimes referred to as overlay grafts) are those that are sized slightly larger than the defect and applied to the nasal, rather than intracranial, side. Materials for onlay grafts include fascia, bone, acellular dermal matrix, free mucosa grafts, and vascular pedicled flaps (intranasal and extranasal). 3 , 4 , 17 , 31 , 32 Pedicled flaps such as NSFs are frequently used as onlay grafts in multilayer repairs to reinforce high‐risk reconstructions. Finally, modified onlay grafts are created by tucking a folded double layer of the graft (often fascia or acellular dermal matrix) between the dura and bone then folding the graft edge onto the outer aspect of the bony margins of the defect (Figure 2). 3 For both conventional onlay and modified onlay grafts, the surgeon must be careful to ensure that all mucosa along the edges of the defect has been removed and that the flap is oriented with the mucosal surface facing outward to minimize risk of mucocele formation. Grafts are most often held in place against the skull base with cellulose strips, tissue glues, or gelfoam, though the use of dural sealant with NSFs is not necessary and may in fact be detrimental. 7 , 33 , 34 , 35 , 36 For cases involving vascular pedicled flaps or tenuous repairs, additional reinforcement may be provided with a nasal tampon, bioresorbable packing (eg, NasoPore), or balloon catheter, which may be removed later. 30 , 34
FIGURE 2.
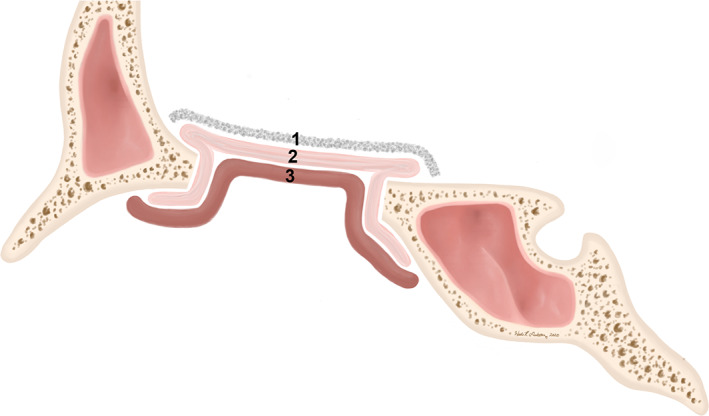
Layers of grafts for reconstruction. Underlay grafts (1) lie entirely on the intracranial (proximal) side of the defect. Onlay grafts (3) lie entirely on the nasal side of the defect. Modified onlay grafts (2) are onlay grafts that are tucked in on the proximal side of the defect around its perimeter
4. INTRANASAL VASCULAR PEDICLED FLAPS
4.1. Nasoseptal flap (NSF)
Due to its large surface area, robust vascular supply, and ease of harvest, the NSF has been the workhorse of intranasal endoscopic reconstruction of the skull base since its first documented use in patients in 2006. 2 The paddle of the graft incorporates the mucoperichondrium and mucoperiostium of the nasal septum, and it receives its vascular supply from the posterior septal branch of the sphenopalatine artery (SPA, Figure 3). The long and robust pedicle allows for a wide arc of rotation, and the large surface area enables ready customization of shape and size for use in various repairs. The NSF can be used in repairs as far posteriorly as the sella turcica. The anterior reach of the flap, however, is limited. Its use in the pediatric population has also been discouraged to prevent disruption of normal septal growth, though successful use of NSFs in patients as young as 1 year old has been reported. 37
FIGURE 3.
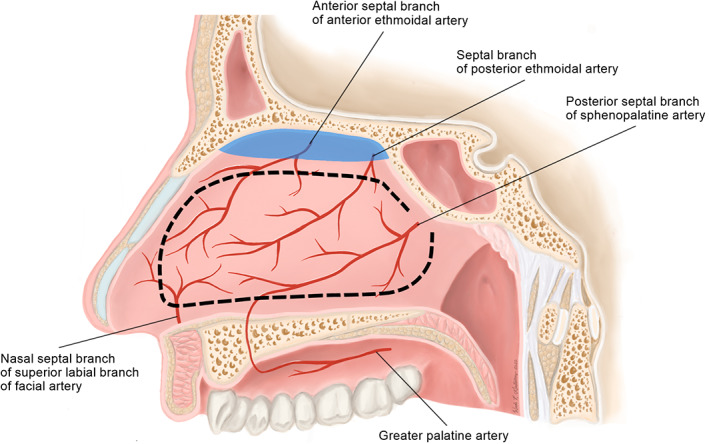
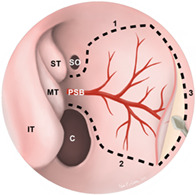
Surgical anatomy of the nasoseptal flap (NSF). The NSF is pedicled on the posterior septal branch of the sphenopalatine artery and can be custom sized and shaped (dotted line) based on reconstructive needs. Olfactory epithelium, highlighted in blue, is preserved during harvesting
Preparation for flap harvest involves mucosal decongestion by placement of intranasal pledgets soaked in 0.5% oxymetazoline and can include optimization of the approach by outfracturing of the inferior turbinate (IT) and removal of the middle turbinate (MT). Selection of flap laterality is influenced by several factors including the laterally of the defect or tumor, presence of existing septal deviation, and preference of the surgeon. Lidocaine 0.5% to 1% with epinephrine 1:100 000 to 1:200 000 is injected into the subperichondrial layer for surgical plane hydrodissection.
Septal mucosal incisions are made using needle‐tip electrocautery to include horizontal superior and inferior incisions, and an anterior vertical incision. The inferior incision begins at the superior margin of the choana below the floor of the sphenoid sinus and proceeds along the posterior free margin of the septum to the nasal floor (Figure 4). The incision continues anteriorly along the maxillary crest and can extend to the junction of the septal mucosa and the vestibular skin. The superior incision starts at the level of the sphenoid ostium and proceeds rostrally and superiorly across the front of the sphenoid sinus toward the skull base and continues anteriorly, leaving an adequate margin superiorly to preserve the olfactory epithelium (blue highlight in Figure 3), which extends approximately 1 cm below the olfactory sulcus. A final vertical incision made anteriorly to connect the inferior and superior incisions in line with the most anterior projection of the inferior turbinate.
FIGURE 4.
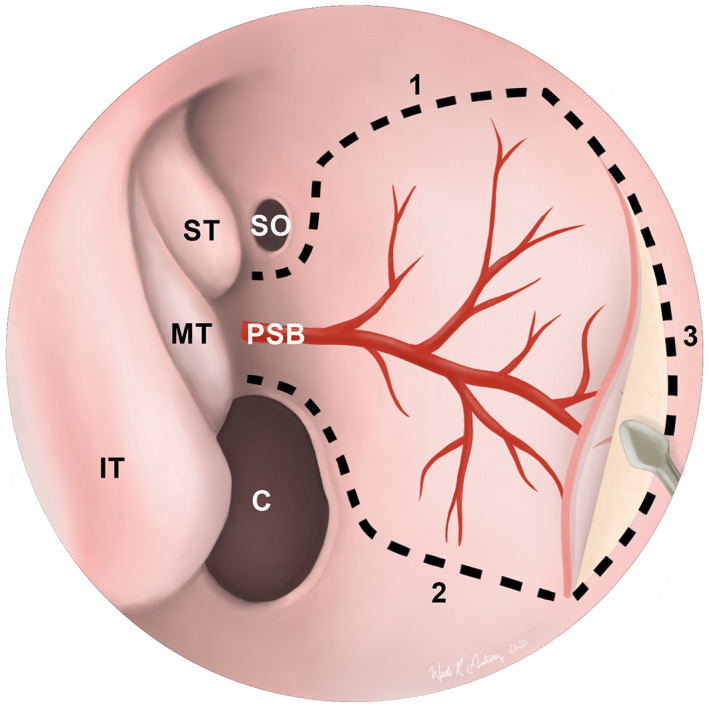
Endoscopic view of a nasoseptal flap (NSF) in the right nasal cavity. Dotted lines depict the superior (1), inferior (2), and anterior (3) incisions of the NSF. The inferior incision can be extended laterally onto the nasal floor to maximize the surface area of the graft. Once elevated, the flap can be tucked into the nasopharynx or maxillary sinus until it is needed for reconstruction. C, choana; IT, inferior turbinate; MT, middle turbinate; PSB, posterior septal branch of the sphenopalatine artery; SO, sphenoid ostium; ST, superior turbinate
Elevation of the flap is performed using a Cottle elevator or suction dissector, beginning anteriorly and proceeding posteriorly in the plane deep to the mucoperichondrial layer anteriorly and mucoperiosteal layer posteriorly. Care is taken to avoid perforations and flap elevation is completed when the vascular pedicle is raised from the sphenoid rostrum. The NSF can be preserved and protected by tucking the flap into the nasopharynx or the maxillary sinus until it is needed for the reconstructive portion of the case.
4.1.1. NSF outcomes
The absence of postoperative CSF leaks is generally considered to be the primary measure of success for reconstruction of skull base defects. 1 , 6 , 17 NSF success rates range from 70% to 100% in single institution studies, with most reporting success rates of 90% or higher. 1 , 21 , 22 , 38 , 39 , 40 Additional NSF complications and their prevalence were assessed in a 2018 systematic review by Lavinge et al: flap necrosis (4 studies; [0%‐1.3%]), mucocele formation (5 studies; [0%‐3.6%]), septal perforation (6 studies; [0%‐14.4%]), and nasal dorsum collapse (2 studies, [0.7%‐5.8%]). Additionally, change in olfaction, crusting, and quality of life outcomes were assessed. Though some studies reported decreases in olfaction at 6 weeks, all studies included in the review reported full recovery of olfaction by 6 months. Similarly, no difference in crusting was reported between NSF and non‐NSF reconstruction groups. Only one study (of eight) reported lower quality of life outcomes for patients with NSFs compared to those with free mucosal graft reconstruction. 41
4.1.2. NSF variants
NSF “rescue” flap
To protect the vascular pedicle of prototypical NSFs, the entire flap must be raised at the beginning of an operation with the anticipation of encountering a CSF leak later. The NSF “rescue” flap is an NSF variant that differs little from conventional NSFs except for its time of creation. Unlike conventional NSFs, only the posterior superior incision is made at the beginning of an operation for NSF “rescue” flaps. This allows for reflection and protection of the vascular supply, should an NSF be needed for the defect repair. However, if an NSF is not required, damage to septal mucosa is limited and the reflected tissue can be easily replaced. 42 , 43
Posterior NSF
A posterior NSF is a smaller variation of the conventional NSF and is used for repair of small posterior skull base defects such as those from a transsphenoidal resection of a pituitary adenoma. The superior and inferior margins of the flap are the same as a full‐sized NSF. However, the anterior margin is made in line with the anterior aspect of the middle turbinate to reduce the length of the paddle. Posterior NSFs utilize septal mucosal tissue that is often discarded during posterior septectomies performed for binostril transsphenoidal approaches. Unlike full‐sized NSFs, it can be raised routinely at the beginning of cases without detriment should it not be later utilized for defect repair. Posterior NSFs provide robust repair, with a 97.7% success rate in preventing postoperative CSF leaks in one retrospective case series of 43 patients. 44
Other NSF variants
Successful use of bilateral NSFs, termed the “Janus flap,” has been reported for defects that may not be adequately repaired by a single NSF. 43 , 45 Single NSFs can also be enlarged by moving the inferior incision laterally to include mucosa from the inferior turbinate or even the entire lateral wall. 46 The structural pedicled mucochondral‐osteal nasoseptal flap, which is reinforced by septal cartilage and bone, can be used to repair defects such as those of the orbital floor that require greater structural support than can be provided by a conventional NSF. 47
4.2. Middle turbinate flap (MTF)
The MTF serves as an option for endonasal reconstruction of the skull base in patients who had previously had a septectomy or NSF. Use of the MTF in patients was first published in 2009. 48 Due to increased technical difficulty from anatomical variations in the MT and the necessity of elevating the flap from the underlying turbinate bone, they are generally of more limited use than NSFs for reconstruction. The MTF can be harvested at the outset and protected within the nasopharynx or maxillary sinus until it is needed, since the MT is otherwise often transected for exposure during the operation. The MTF is pedicled posteriorly posterior lateral branches of the SPA (Figure 5). The posterior location of the pedicle on the superior aspect of the MT makes this flap useful in the reconstruction of the planum, fovea ethmoidalis, and the sella.
FIGURE 5.
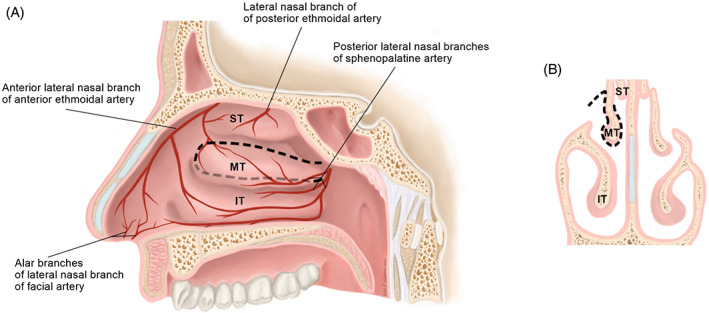
Sagittal, A and coronal, B, views of the middle turbinate flap (MTF) surgical anatomy. The MTF is pedicled on the posterior lateral nasal branches of the sphenopalatine artery. Borders of the flap are depicted by with dotted lines, A. The flap is raised from both the medial and lateral aspects of the turbinate, B. IT, inferior turbinate; MT, middle turbinate; ST: superior turbinate
The mucosa is prepared as described previously for other flaps. A vertical incision is made at the anterior aspect of the head of the MT followed by horizontal incisions along the superior and inferior aspects of the MT that continue posteriorly parallel to the skull base (Figure 5). Next, the subperiosteum of the MT is elevated from superior to inferior, and the exposed MT bone is carefully removed. The vertical attachment of the MT is sharply incised away from the skull base and the lateral mucoperichondrial flap is sharply incised parallel to the skull base, thereby freeing the flap from the underlying bone. Elevation of the flap continues posteriorly until the pedicle provides optimum flap length and rotation.
4.2.1. MTF outcomes
To date, use of MTFs for the reconstruction of skull base defects in patients has been reported in seven studies with a combined total of 51 patients. 48 , 49 , 50 , 51 , 52 , 53 , 54 All but one reported case were considered successful and were without long‐term CSF leaks, infection, pneumocephalus, or significant crusting. The one incidence of MTF failure was due to a necrotic MTF following a failed NSF. 51 Other reported complications include one patient who had transient CSF leakage during the immediate postoperative period that subsided fully, a patient in which an MTF was insufficient to covered the defect, and another in whom an MTF was irreparably torn during the operation. 48 , 54
4.3. Posterior pedicled inferior turbinate flap (PPITF)
The PPITF is of more limited use than the NSF, and is best for small, posterior skull base defects or, more commonly, as an adjunct to the NSF for reconstruction for larger defects. 55 Use of the PPITF for skull base reconstruction in patients was first described in 2007. 56 The PPITF is substantially smaller than the NSF and is limited in its arc of rotation, but can serve as an NSF‐alterative for patients with prior posterior septectomies or prior wide sphenoidotomies. It is pedicled posteriorly on posterior lateral nasal branches of the SPA (Figure 6). Harvest typically occurs after the endoscopic procedure allowing the flap to be fashioned to meet specific criteria for shape and size.
FIGURE 6.
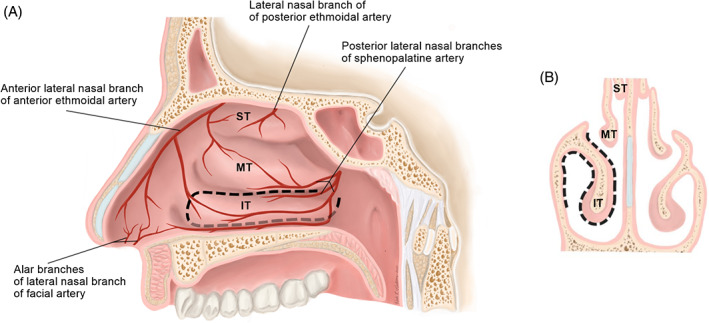
Sagittal, A and coronal, B, views of the posterior pedicled inferior turbinate flap (PPITF) surgical anatomy. The PPITF is pedicled on the posterior lateral nasal branches of the sphenopalatine artery. Borders of the flap are depicted by with dotted lines, A. The flap is raised from both the medial and lateral aspects of the turbinate, B. IT, inferior turbinate; MT, middle turbinate; ST, superior turbinate
The nasal mucosa is prepared for flap harvest with the placement of 0.5% oxymetazoline‐soaked pledgets and infiltration of lidocaine with epinephrine. Visualization of the IT and inferior meatus is aided by medialization of the turbinate. The SPA is first dissected as it exits from the sphenopalatine foramen to where the posterior lateral nasal branches can be identified. Next, parallel incisions are made. The superior incision proceeds above the IT in the middle meatus and the inferior incision runs inferior to the turbinate in the inferior meatus. These are joined by a vertical incision anterior to the head of the turbinate (Figure 6). Elevation of the flap is made from anterior to posterior with care to avoid damaging the pedicle at the superior aspect of the lateral IT attachment.
4.3.1. PPITF outcomes
There is a paucity of reported cases and outcomes for the PPITF in the literature. All published reports of PPITF use contained between two and five patients for a total of 14 patients. 51 , 56 , 57 , 58 The first two patients at a single institution experienced flap necrosis, whereas the remaining three at that institution and the other nine published cases experienced no reported complications.
4.3.2. PPITF variants
Use of an “extended” PPITF has been reported in five patients. 59 To extend the flap, the inferior incision is moved medially to the septum, thereby more than doubling the surface area of the flap. Four of the five cases were successful without complications. The remaining patient had postoperative CSF leakage.
4.4. Emerging techniques
Several novel intranasal vascular pedicled flaps have been described in cadaveric studies with or without testing in a small cohort of clinical patients. Though these techniques are not yet widely used, reports of techniques such as these have historically represented future directions of the field. 59 , 60 , 61 The posterior pedicle lateral nasal wall flap, also known as the Carrau‐Hadad or C‐H flap, is designed for the reconstruction of large cranial base defects in patients whose presentation prohibits the use of an NSF. The C‐H flap is based posteriorly on branches of the sphenopalatine artery. In one case series, four patients underwent reconstruction with the C‐H flap successfully and without complications. 62 In a recently published study using vascular latex‐injected specimens, the blood supply of this flap was characterized as including a smaller‐caliber superficial branch of the inferior turbinate artery as well as a more robust and dominant inferior meatus branch. This report also included data from a larger case series of 24 patients with sellar or posterior cranial fossa defects, wherein reconstruction with this lateral nasal wall flap resulted in recovery without CSF leak in 75% of cases. 63 The anteriorly based inferior turbinate flap (AITF) is a counterpart to the PPITF and is pedicled on the anterior ethmoidal artery rather than the IT artery. 64 In the seven patients who underwent reconstruction with the AITF, no complications were reported. The AITF can be used as an adjunct to the NSF for repairs of the cribiform plate, posterior table of the frontal sinus, and ethmoid roof. In the study, the AITF was used as a singular flap in two patients, in combination with a PPITF in one patient, and in combination with NSFs in the remaining four patients. The anterior pedicle lateral nasal wall flap, also known as the Hadad‐Bassagaisteguy 2 or HB2 flap, is another anteriorly pedicled flap of recent interest. 65 The HB2 flap is pedicled on branches of the facial and anterior ethmoidal arteries, and can be used in reconstruction of large anterior skull base defects in patients whose presentation prohibits the use of an NSF. In two published cases of HB2 flap use in patients, reconstructions were successful with no postoperative CSF leaks or flap necrosis. 51 The nasal floor pedicled flap is another vascular pedicled alterative flap for cases in which the septum cannot be used. 66 The flap is pedicled on the sphenopalatine artery and has been used in 10 patients. All cases were successful and without intraoperative or postoperative complications. The turbinal flap (TF) utilized mucosa from the middle and superior turbinates and is designed for the repair of large defects in the ethmoid roof. 67 The TF is pedicled on both the anterior and posterior ethmoidal arteries. The TF has been used in one clinical patient and was successful and without complications. Finally, the bipedicled anterior septal flap is designed to repair the frontal nasal beak and portions of the posterior frontal table. 68 The flap is pedicled on the superior labial and nasopalatine arteries (Figure 2). The bipedicled anterior septal flap has not yet been tested in published reports but is designed for use in revision cases where the posterior septal artery has been compromised.
5. EXTRANASAL VASCULAR PEDICLED FLAPS
A limited number of options are available for vascularized tissue coverage of large skull base defects, especially those created during the resection of large nasal and perinasal sinus malignancies. A variety of extranasal pedicled flaps have been theorized and explored in cadaveric models, including the palatal flap supplied by the greater palatine artery and passed into the nasal cavity through an opening above the greater palatine foramen, the facial buccinator flap pedicled on the facial artery gaining intranasal access via a maxillary window, and the occipital galeopericranial flap based on the occipital artery. 69 However, using currently described approaches, these flaps carry significant risk for morbidity, pedicle compromise, and technical infeasibility and are therefore not further discussed here. Extranasal pedicled flaps that have demonstrated clinical viability include the pericranial flap, for which a minimally‐invasive approach has been described for addressing large anterior defects, 70 and the temporoparietal flap for posterior and lateral defects.
5.1. Pericranial flap (PCF)
The PCF is a versatile and robust pedicled graft for skull base reconstruction that can be used in open and endoscopic procedures when an NSF is unavailable due to prior endonasal surgery or sinonasal malignancy. This large flap is pedicled on the supraorbital and supratrochlear arteries. As such, it is a good option for repair of anterior ethmoid and cribiform defects, though its reach does not extend past the sella turcica to cover more posterior defects.
A bicoronal approach is performed and elevation proceeds in a subgaleal plane. The periosteum is transected on its lateral edges at the superior attachment of the temporalis muscle bilaterally. The posterior incision can be made at the same point as the coronal incision or extended posteriorly if greater length is desired. To improve rotation, two flaps can be created. To narrow the overall size, the flap can be pedicled off a single side. To introduce this pedicled flap into the nasal cavity, a nasionectomy is performed. An incision is made in the glabella and dissection is performed within the subperiosteal plane up to meet the subperiosteal plane of the flap. The nasionectomy is then drilled, allowing for introduction of the PCF into the nasal cavity for use in reconstruction.
5.1.1. PCF outcomes
PCF outcomes are generally good. One report of 25 patients reported complications in 4 patients: one partial flap necrosis, two total flap necroses, and one minor CSF leak. Complications were associated with preoperative radiation therapy. 71 An endoscopic approach to reconstruction with the PCF has been elegantly demonstrated in a patient with a large anterior skull base defect secondary to resection of esthesioneuroblastoma. The defect was successfully repaired endoscopically without the need of raising a bicoronal flap. 70
5.2. Temporopariatel fascia flap (TPFF)
The TPFF is a versatile option for skull base reconstruction when the flaps described above are not available, typically in head and neck cancer cases. Its advantages include its large size that can be readily customized to match defect shapes, the availability of tissue for bulk replacement, and a predictable and reliable blood supply. When combined with a craniotomy, such as in the case of middle cranial fossa approach, this flap has diverse utility in providing coverage for defects of the anterior, middle, and even posterior cranial fossa. The TPFF is pedicled on the superficial temporal artery. It is less suited for far anterior repairs because of the orientation of its pedicle, and there are significant risks of compromising important structures during flap harvest, such as the frontal branch of the facial nerve or the internal maxillary artery. Compared to the pericranial flap, the TPFF carries slightly higher risk of morbidity, including facial nerve damage, alopecia, and scarring. 72
To harvest the TPFF, a hemicoronal incision made, taking care not to injure the superficial temporal artery. The temperoparietal fascia is then elevated off the temporalis muscle. The flap is narrowed at its base, with care taken to protect the superficial temporal artery and vein. The flap can be rotated as an island flap into defects by creating a tunnel through the infratemporal fossa. The TPFF can be transposed into the nasal cavity by way of a guide wire passed through the tunnel.
5.2.1. TPFF outcomes
A small series of five patients treated with TPFF reconstruction after extended endoscopic resection of skull base malignancy reported no major or minor postoperative complications. 73 A retrospective case series compared the rate of CSF leak control among patients who had undergone repair of lateral skull base defects after tumor resection using either at TPFF or adipose packing alone. There were no CSF leaks following vascularized flap reconstruction among 16 patients vs CSF leak in 6 patients out of 20 who had repair with adipose only. 74 Interestingly, an alternative pathway for transposition of the flap was recently proposed in which a supraorbital epidural corridor is accessed via a pterional craniotomy. This expands the access of the flap anteriorly and laterally while obviating the need for dissection through the infratemporal fossa, thereby preserving the internal maxillary artery from harm. 72
6. CONCLUSION
Rapid advancements continue to be made in the field of skull base defect reconstruction. The increasingly common use of vascular pedicled flaps, both intranasal and extranasal, is contributing to meaningful reductions in surgical complication rates and the need for reoperation. The principles for harvesting robust vascular pedicled flaps serve as the basic framework for the successful implementation of novel techniques. As these approaches are refined and technology continues to advance, we will likely continue to see the expansion and evolution of endoscopic skull base defect repair using vascular pedicled flaps for years to come.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Gutierrez WR, Bennion DM, Walsh JE, Owen SR. Vascular pedicled flaps for skull base defect reconstruction. Laryngoscope Investigative Otolaryngology. 2020;5:1029–1038. 10.1002/lio2.471
Wade R. Gutierrez and Douglas M. Bennion contributed equally to this work.
BIBLIOGRAPHY
- 1. Soudry E, Turner JH, Nayak JV, Hwang PH. Endoscopic reconstruction of surgically created skull base defects: a systematic review. Otolaryngol Head Neck Surg. 2014;150(5):730‐738. 10.1177/0194599814520685. [DOI] [PubMed] [Google Scholar]
- 2. Hadad G, Bassagasteguy L, Carrau RL, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116(10):1882‐1886. 10.1097/01.mlg.0000234933.37779.e4. [DOI] [PubMed] [Google Scholar]
- 3. Germani RM, Vivero R, Herzallah IR, Casiano RR. Endoscopic reconstruction of large anterior skull base defects using acellular dermal allograft. Am J Rhinol. 2007;21(5):615‐618. 10.2500/ajr.2007.21.3080. [DOI] [PubMed] [Google Scholar]
- 4. Ting JY, Metson R. Free graft techniques in skull base reconstruction. Adv Otorhinolaryngol. 2013;74:33‐41. 10.1159/000342266. [DOI] [PubMed] [Google Scholar]
- 5. Kassam A, Carrau RL, Snyderman CH, Gardner P, Mintz A. Evolution of reconstructive techniques following endoscopic expanded endonasal approaches. Neurosurg Focus. 2005;19(1):1‐7. 10.3171/foc.2005.19.1.9. [DOI] [PubMed] [Google Scholar]
- 6. Harvey RJ, Nogueira JF, Schlosser RJ, Patel SJ, Vellutini E, Stamm AC. Closure of large skull base defects after endoscopic transnasal craniotomy: clinical article. J Neurosurg. 2009;111(2):371‐379. 10.3171/2008.8.JNS08236. [DOI] [PubMed] [Google Scholar]
- 7. Liu JK, Schmidt RF, Choudhry OJ, Shukla PA, Eloy JA. Surgical nuances for nasoseptal flap reconstruction of cranial base defects with high‐flow cerebrospinal fluid leaks after endoscopic skull base surgery. Neurosurg Focus. 2012;32(6):E7 10.3171/2012.5.FOCUS1255. [DOI] [PubMed] [Google Scholar]
- 8. Bernal‐Sprekelsen M, Rioja E, Enseñat J, et al. Management of anterior skull base defect depending on its size and location. Biomed Res Int. 2014;2014:1‐7. 10.1155/2014/346873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ibrahim AA, Okasha M, Elwany S. Endoscopic endonasal multilayer repair of traumatic CSF rhinorrhea. Eur Arch Otorhinolaryngol. 2016;273(4):921‐926. 10.1007/s00405-015-3681-y. [DOI] [PubMed] [Google Scholar]
- 10. Ramakrishnan VR, Terella AM, Poonia S, Chiu AG, Palmer JN. Osseous repair in minimally‐invasive reconstruction of anterior skull base defects. J Craniofac Surg. 2017;28(1):36‐39. 10.1097/SCS.0000000000003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oakley GM, Christensen JM, Winder M, et al. Collagen matrix as an inlay in endoscopic skull base reconstruction. J Laryngol Otol. 2018;132(3):214‐223. 10.1017/S0022215117001499. [DOI] [PubMed] [Google Scholar]
- 12. Kim‐Orden N, Shen J, Or M, Hur K, Zada G, Wrobel B. Endoscopic endonasal repair of spontaneous cerebrospinal fluid leaks using multilayer composite graft and vascularized pedicled nasoseptal flap technique. Allergy Rhinol (Providence). 2019;10:215265671988862 10.1177/2152656719888622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Urken ML, Catalano PJ, Sen C, Post K, Futran N, Biller HF. Free tissue transfer for skull base reconstruction analysis of complications and a classification scheme for defining skull base defects. Arch Otolaryngol Head Neck Surg. 1993;119(12):1318‐1325. 10.1001/archotol.1993.01880240054007. [DOI] [PubMed] [Google Scholar]
- 14. Yamamoto Y, Minakawa H, Kawashima K, et al. Experience with 24 cases of reconstructive anterior skull base surgery: classification and evaluation of postoperative facial appearance. Skull Base Surg. 2000;10(2):65‐70. 10.1055/s-2000-7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pusic AL, Chen CM, Patel S, Cordeiro PG, Shah JP. Microvascular reconstruction of the skull base: a clinical approach to surgical defect classification and flap selection. Skull Base. 2007;17(1):5‐15. 10.1055/s-2006-959331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yano T, Okazaki M, Tanaka K, et al. A new concept for classifying skull base defects for reconstructive surgery. J Neurol Surg B Skull Base. 2012;73(2):125‐131. 10.1055/s-0032-1301402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tabaee A, Anand VK, Brown SM, Lin JW, Schwartz TH. Algorithm for reconstruction after endoscopic pituitary and skull base surgery. Laryngoscope. 2007;117(7):1133‐1137. 10.1097/MLG.0b013e31805c08c5. [DOI] [PubMed] [Google Scholar]
- 18. Fujimoto Y, Balsalobre L, Santos FP, Vellutini E, Stamm AC. Endoscopic combined “transseptal/transnasal” approach for pituitary adenoma: reconstruction of skull base using pedicled nasoseptal flap in 91 consecutive cases. Arq Neuropsiquiatr. 2015;73(7):611‐615. 10.1590/0004-282X20150070. [DOI] [PubMed] [Google Scholar]
- 19. Karnezis TT, Baker AB, Soler ZM, et al. Factors impacting cerebrospinal fluid leak rates in endoscopic sellar surgery. Int Forum Allergy Rhinol. 2016;6(11):1117‐1125. 10.1002/alr.21783. [DOI] [PubMed] [Google Scholar]
- 20. Patel PN, Stafford AM, Patrinely JR, et al. Risk factors for intraoperative and postoperative cerebrospinal fluid leaks in endoscopic transsphenoidal sellar surgery. Otolaryngol Head Neck Surg. 2018;158(5):952‐960. 10.1177/0194599818756272. [DOI] [PubMed] [Google Scholar]
- 21. Canfarotta MW, Farzal Z, Sreenath S, et al. Pedicled nasoseptal flap outcomes: an update. J Neurol Surg B: Skull Base. 2019;80:P207 10.1055/s-0039-1679846. [DOI] [Google Scholar]
- 22. Reilly EK, Fastenberg J, Nyquist G, et al. Indications and outcomes of the nasoseptal flap for pituitary surgery. J Neurol Surg B: Skull Base. 2019;80:A118 10.1055/s-0039-1679534. [DOI] [Google Scholar]
- 23. Centeno TR, Villalonga JF, Saenz A, Del Pont FM, Cervio A, Campero A. The sellar barrier and intraoperative CSF leak in elderly patients. J Clin Neurosci. 2020;73:48‐50. 10.1016/j.jocn.2020.01.078. [DOI] [PubMed] [Google Scholar]
- 24. Das PT, Balasubramanian D. Extradural cartilage inlay graft in cerebrospinal fluid fistula repair. J Laryngol Otol. 2010;124(12):1294‐1297. 10.1017/S0022215110001295. [DOI] [PubMed] [Google Scholar]
- 25. Ozturk O, Polat S, Uneri C. Endoscopic endonasal management of cerebrospinal fluid rhinorrhea. J Craniofac Surg. 2012;23(4):1087‐1092. 10.1097/SCS.0b013e31824e6a44. [DOI] [PubMed] [Google Scholar]
- 26. Al‐Asousi F, Okpaleke C, Dadgostar A, Javer A. The use of polydioxanone plates for endoscopic skull base repair. Am J Rhinol Allergy. 2017;31(2):122‐126. 10.2500/ajra.2017.31.4411. [DOI] [PubMed] [Google Scholar]
- 27. Dadgostar A, Okpaleke C, Al‐Asousi F, Javer A. The application of a free nasal floor mucoperiosteal graft in endoscopic sinus surgery. Am J Rhinol Allergy. 2017;31(3):196‐199. 10.2500/ajra.2017.31.4430. [DOI] [PubMed] [Google Scholar]
- 28. Geyik M, Erkutlu I, Alptekin M, et al. Anterior skull base defects reconstructed using three‐layer method: 78 consecutive cases with long‐term follow‐up. J Neurol Surg B Skull Base. 2016;77(6):499‐502. 10.1055/s-0036-1583310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Indorewala S, Nemade G, Indorewala A, Mahajan G. Repair of bony lateral skull base defects equal to or larger than 10 mm by extracorporeally sewed unit‐sandwich graft. Eur Arch Otorhinolaryngol. 2018;275(8):2177‐2186. 10.1007/s00405-018-5039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Snyderman CH, Wang EW, Zenonos GA, Gardner PA. Reconstruction after endoscopic surgery for skull base malignancies. J Neurooncol. 2020;27 10.1007/s11060-020-03465-0. [DOI] [PubMed] [Google Scholar]
- 31. Hegazy HM, Carrau RL, Snyderman CH, Kassam A, Zweig J. Transnasal endoscopic repair of cerebrospinal fluid rhinorrhea: a meta‐analysis. Laryngoscope. 2000;110(7):1166‐1172. 10.1097/00005537-200007000-00019. [DOI] [PubMed] [Google Scholar]
- 32. Hoffmann TK, El Hindy N, Müller OM, et al. Vascularised local and free flaps in anterior skull base reconstruction. Eur Arch Otorhinolaryngol. 2013;270(3):899‐907. 10.1007/s00405-012-2109-1. [DOI] [PubMed] [Google Scholar]
- 33. Dehdashti AR, Stofko D, Okun J, Obourn C, Kennedy T. Endoscopic endonasal reconstruction of skull base: repair protocol. J Neurol Surg B Skull Base. 2016;77(3):271‐278. 10.1055/s-0035-1568871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Snyderman CH, Kassam AB, Carrau R, Mintz A. Endoscopic reconstruction of cranial base defects following endonasal skull base surgery. Skull Base. 2007;17(1):73‐78. 10.1055/s-2006-959337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Prickett KK, Wise SK. Grafting materials in skull base reconstruction. Adv Otorhinolaryngol. 2013;74:24‐32. 10.1159/000342265. [DOI] [PubMed] [Google Scholar]
- 36. Eloy JA, Choudhry OJ, Friedel ME, Kuperan AB, Liu JK. Endoscopic nasoseptal flap repair of skull base defects: is addition of a dural sealant necessary? Otolaryngol Head Neck Surg. 2012;147(1):161‐166. 10.1177/0194599812437530. [DOI] [PubMed] [Google Scholar]
- 37. Ben‐Ari O, Wengier A, Ringel B, et al. Nasoseptal flap for skull base reconstruction in children. J Neurol Surg B Skull Base. 2018;79(1):37‐41. 10.1055/s-0037-1617435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zanation AM, Carrau RL, Snyderman CH, et al. Nasoseptal flap reconstruction of high flow intraoperative cerebral spinal fluid leaks during endoscopic skull base surgery. Am J Rhinol Allergy. 2009;23(5):518‐521. 10.2500/ajra.2009.23.3378. [DOI] [PubMed] [Google Scholar]
- 39. Patel MR, Stadler ME, Snyderman CH, et al. How to choose? Endoscopic skull base reconstructive options and limitations. Skull Base. 2010;20(6):397‐404. 10.1055/s-0030-1253573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Riley CA, Tabaee A, Conley L, et al. Long‐term sinonasal outcomes after endoscopic skull base surgery with nasoseptal flap reconstruction. Laryngoscope. 2019;129(5):1035‐1040. 10.1002/lary.27637. [DOI] [PubMed] [Google Scholar]
- 41. Lavigne P, Faden DL, Wang EW, Snyderman CH. Complications of nasoseptal flap reconstruction: a systematic review. J Neurol Surg B. 2018;79(suppl 4):S291‐S299. 10.1055/s-0038-1668158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rawal RB, Kimple AJ, Dugar DR, Zanation AM. Minimizing morbidity in endoscopic pituitary surgery: outcomes of the novel nasoseptal rescue flap technique. Otolaryngol Head Neck Surg. 2012;147(3):434‐437. 10.1177/0194599812443042. [DOI] [PubMed] [Google Scholar]
- 43. Kim BY, Shin J‐H, Kang S‐G, et al. Bilateral modified nasoseptal “rescue” flaps in the endoscopic endonasal transsphenoidal approach. Laryngoscope. 2013;123(11):2605‐2609. 10.1002/lary.24098. [DOI] [PubMed] [Google Scholar]
- 44. Barger J, Siow M, Kader M, et al. The posterior nasoseptal flap: a novel technique for closure after endoscopic transsphenoidal resection of pituitary adenomas. Surg Neurol Int. 2018;9:32 10.4103/sni.sni_192_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nyquist GG, Anand VK, Singh A, Schwartz TH. Janus flap: bilateral nasoseptal flaps for anterior skull base reconstruction. Otolaryngol Head Neck Surg. 2010;142(3):327‐331. 10.1016/j.otohns.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 46. Moon JH, Kim EH, Kim SH. Various modifications of a vascularized nasoseptal flap for repair of extensive skull base dural defects. J Neurosurg. 2019;132(2):371‐379. 10.3171/2018.10.JNS181556. [DOI] [PubMed] [Google Scholar]
- 47. Kalyoussef E, Schmidt RF, Liu JK, Eloy JA. Structural pedicled mucochondral‐osteal nasoseptal flap: a novel method for orbital floor reconstruction after sinonasal and skull base tumor resection. Int Forum Allergy Rhinol. 2014;4(7):577‐582. 10.1002/alr.21306. [DOI] [PubMed] [Google Scholar]
- 48. Bhatki AM, Carrau RL, Prevedello DM, et al. The posteriorly pedicled middle turbinate flap for dural reconstruction after skull base surgery. Skull Base. 2009;19(3):A053 10.1055/s-2009-1242331. [DOI] [Google Scholar]
- 49. Simal Julián JA, Miranda Lloret P, Cárdenas Ruiz‐Valdepeñas E, Barges Coll J, Beltrán Giner A, Botella Asunción C. Middle turbinate vascularized flap for skull base reconstruction after an expanded endonasal approach. Acta Neurochir. 2011;153(9):1827‐1832. 10.1007/s00701-011-1064-8. [DOI] [PubMed] [Google Scholar]
- 50. Otto BA, Carrau RL, Prevedello DM, et al. Middle turbinate vascularized flap: applications in skull base reconstruction. J Neurol Surg B. 2012;73(S 1):A152 10.1055/s-0032-1312200. [DOI] [Google Scholar]
- 51. Patel MR, Taylor RJ, Hackman TG, et al. Beyond the nasoseptal flap: outcomes and pearls with secondary flaps in endoscopic endonasal skull base reconstruction. Laryngoscope. 2014;124(4):846‐852. 10.1002/lary.24319. [DOI] [PubMed] [Google Scholar]
- 52. Wang X, Zhang X, Hu F, et al. Middle turbinate mucosal flap in endoscopic skull base reconstruction. Turk Neurosurg. 2016;26(2):200‐204. 10.5137/1019-5149.JTN.6250-12.0. [DOI] [PubMed] [Google Scholar]
- 53. Tamura R, Toda M, Kohno M, et al. Vascularized middle turbinate flap for the endoscopic endonasal reconstruction of the anterior olfactory groove. Neurosurg Rev 2016;39(2):297–302; discussion 302. doi: 10.1007/s10143-015-0688-1 [DOI] [PubMed] [Google Scholar]
- 54. George S, Suresh S. Vascularized middle turbinate mucoperiosteal flap in skull base defects: follow‐up analysis of 20 cases. Int J Otorhinolaryngol Head Neck Surg. 2016;3(1):71‐76. 10.18203/issn.2454-5929.ijohns20164786. [DOI] [Google Scholar]
- 55. Boetto J, Labidi M, Watanabe K, et al. Combined nasoseptal and inferior turbinate flap for reconstruction of large skull base defect after expanded endonasal approach: operative technique. Oper Neurosurg (Hagerstown). 2019;16(1):45‐52. 10.1093/ons/opy046. [DOI] [PubMed] [Google Scholar]
- 56. Fortes FSG, Carrau RL, Snyderman CH, et al. The posterior pedicle inferior turbinate flap: a new vascularized flap for skull base reconstruction. Laryngoscope. 2007;117(8):1329‐1332. 10.1097/mlg.0b013e318062111f. [DOI] [PubMed] [Google Scholar]
- 57. Lee DH, Yoon TM, Lee JK, et al. Clinical utility of the inferior turbinate flaps in the reconstruction of the nasal septum and skull base. J Craniofac Surg. 2012;23(4):e322‐e326. 10.1097/SCS.0b013e3182543410. [DOI] [PubMed] [Google Scholar]
- 58. Yip J, Macdonald KI, Lee J, et al. The inferior turbinate flap in skull base reconstruction. J Otolaryngol – Head Neck Surg. 2013;42(1):6 10.1186/1916-0216-42-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Choby GW, Pinheiro‐Neto CD, de Almeida JR, et al. Extended inferior turbinate flap for endoscopic reconstruction of skull base defects. J Neurol Surg B Skull Base. 2014;75(4):225‐230. 10.1055/s-0033-1358791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Prevedello DM, Barges‐Coll J, Fernandez‐Miranda JC, et al. Middle turbinate flap for skull base reconstruction: cadaveric feasibility study. Laryngoscope. 2009;119(11):2094‐2098. 10.1002/lary.20226. [DOI] [PubMed] [Google Scholar]
- 61. Rivera‐Serrano CM, Snyderman CH, Gardner P, et al. Nasoseptal “rescue” flap: a novel modification of the nasoseptal flap technique for pituitary surgery. Laryngoscope. 2011;121(5):990‐993. 10.1002/lary.21419. [DOI] [PubMed] [Google Scholar]
- 62. Rivera‐Serrano CM, Bassagaisteguy LH, Hadad G, et al. Posterior pedicle lateral nasal wall flap: new reconstructive technique for large defects of the skull base. Am J Rhinol Allergy. 2011;25(6):e212‐e216. 10.2500/ajra.2011.25.3693. [DOI] [PubMed] [Google Scholar]
- 63. Lavigne P, Vega MB, Ahmed OH, Gardner PA, Snyderman CH, Wang EW. Lateral nasal wall flap for endoscopic reconstruction of the skull base: anatomical study and clinical series. Int Forum Allergy Rhinol. 2020;10(5):673‐678. 10.1002/alr.22534. [DOI] [PubMed] [Google Scholar]
- 64. Gil Z, Margalit N. Anteriorly based inferior turbinate flap for endoscopic skull base reconstruction. Otolaryngol Head Neck Surg. 2012;146(5):842‐847. 10.1177/0194599811434516. [DOI] [PubMed] [Google Scholar]
- 65. Hadad G, Rivera‐Serrano CM, Bassagaisteguy LH, et al. Anterior pedicle lateral nasal wall flap: a novel technique for the reconstruction of anterior skull base defects. Laryngoscope. 2011;121(8):1606‐1610. 10.1002/lary.21889. [DOI] [PubMed] [Google Scholar]
- 66. Daraei P, Oyesiku NM, Patel ZM. The nasal floor pedicled flap: a novel technique for use in skull base reconstruction. Int Forum Allergy Rhinol. 2014;4(11):937‐943. 10.1002/alr.21369. [DOI] [PubMed] [Google Scholar]
- 67. Schreiber A, Mattavelli D, Ferrari M, et al. The turbinal flap: an additional option for anterior skull base reconstruction. Cadaveric feasibility study and case report. Int Forum Allergy Rhinol. 2017;7(2):199‐204. 10.1002/alr.21857. [DOI] [PubMed] [Google Scholar]
- 68. Bleier BS, Curry WT, Wang EW, Schlosser RJ. The bipedicled anterior septal flap: a radioanatomic and cadaveric study. Laryngoscope. 2011;121(7):1367‐1371. 10.1002/lary.21824. [DOI] [PubMed] [Google Scholar]
- 69. Clavenna MJ, Turner JH, Chandra RK. Pedicled flaps in endoscopic skull base reconstruction: review of current techniques. Curr Opin Otolaryngol Head Neck Surg. 2015;23(1):71‐77. 10.1097/MOO.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 70. Zanation AM, Snyderman CH, Carrau RL, Kassam AB, Gardner PA, Prevedello DM. Minimally invasive endoscopic pericranial flap: a new method for endonasal skull base reconstruction. Laryngoscope. 2009;119(1):13‐18. 10.1002/lary.20022. [DOI] [PubMed] [Google Scholar]
- 71. Yano T, Tanaka K, Kishimoto S, Iida H, Okazaki M. Reliability of and indications for pericranial flaps in anterior skull base reconstruction. J Craniofac Surg. 2011;22(2):482‐485. 10.1097/SCS.0b013e318207b714. [DOI] [PubMed] [Google Scholar]
- 72. Ferrari M, Vural A, Schreiber A, et al. Side‐door temporoparietal fascia flap: a novel strategy for anterior skull base reconstruction. World Neurosurg. 2019;126:e360‐e370. 10.1016/j.wneu.2019.02.056. [DOI] [PubMed] [Google Scholar]
- 73. Bolzoni Villaret A, Nicolai P, Schreiber A, Bizzoni A, Farina D, Tschabitscher M. The temporo‐parietal fascial flap in extended transnasal endoscopic procedures: cadaver dissection and personal clinical experience. Eur Arch Otorhinolaryngol. 2013;270(4):1473‐1479. 10.1007/s00405-012-2187-0. [DOI] [PubMed] [Google Scholar]
- 74. Patel R, Buchmann LO, Hunt J. The use of the temporoparietal fascial flap in preventing CSF leak after lateral skull base surgery. J Neurol Surg B Skull Base. 2013;74(5):311‐316. 10.1055/s-0033-1349059. [DOI] [PMC free article] [PubMed] [Google Scholar]


