Abstract
Objective: Tinnitus has been shown to be associated with specific cognitive deficits. Contemporary models of tinnitus, based primarily on human behavior, emphasize the influence of the cognitive response to tinnitus in tinnitus manifestation and level of associated annoyance. The models and hypotheses proposed thus far have (a) focused on the cognitive response to the onset of tinnitus, and not necessarily focused on the cognitive consequences of established chronic tinnitus, and (b) failed to dissociate the contributions of cognitive and perceptual load in their theories. Load theory states that we have a limited capacity of neural resources that can be used to process internal and external stimuli. This theory is differentially applied to perceptual load, which refers to the neural resources engaged in the processing of sensory stimuli in our environment, and cognitive load, which refers to the occupation of a more central resource that is involved in higher‐level processing, such as stimulus discrimination, decision making, and working memory processing.
Methods: A focused review was conducted on behavioral and brain‐imaging studies examining cognitive deficits in tinnitus, in an attempt to reexamine the findings in a load theory framework.
Results: Findings of these studies are discussed in the context of load theory, and a novel model for understanding these findings is proposed.
Conclusion: We believe the incorporation of load theory into models of tinnitus may advance understanding of the cognitive impact of tinnitus and lead to better management of tinnitus.
Keywords: cognition, load theory, perception, tinnitus
1. INTRODUCTION AND GOALS
Current models of tinnitus have proposed that failures of cognitive control are critical to the perception of, and emotional response to, tinnitus. 1 , 2 , 3 Such models consider cognitive failures as they specifically relate to the onset of tinnitus; in this review, we consider cognitive failures as seen after continuous tinnitus has been established. We also follow the assumption, as do previous models, that these cognitive failures appear after tinnitus onset, and are likely caused by the percept. We believe that two distinct processes contribute to the observed alterations in cognitive processing—(1) tinnitus onset alters sufferers' cognitive processing (or may indeed be a result of such changes due to other factors such as sensory deprivation), and (2) the process of habituation leads to further changes in this processing. Selective attention and cognitive control studies in this area have greatly advanced our understanding of the relationship between tinnitus and cognition, but we do not yet have a comprehensive model to represent this relationship. Our understanding could be further clarified by considering the roles played by specific perceptual or cognitive processes that may be altered as a result of tinnitus. In this article, we describe load theory and explain its relevance to understanding neural systems of cognition as they relate to the experience of tinnitus. We then review literature linking tinnitus to alterations in cognitive function, highlighting work examining the impact of tinnitus on perceptual and cognitive resources. Adopting a load theory framework, we propose an updated model of chronic tinnitus that builds upon previous models to advance understanding of tinnitus from a cognitive neuroscience perspective.
2. LOAD THEORY AND COGNITIVE CONTROL
Load theory 4 emerged as a mechanism to help us better understand the processes underlying selective attention. It posits that our efficiency in a selective attention paradigm is dependent on the specific perceptual and cognitive demands of that paradigm. Selective attention is an important tool in making sense of the world around us, while the processes of cognitive control help us prioritize responses and execute appropriate responses to specific situations. While some specifics underlying this theory are yet to be uncovered, extensive research has supported the principles of load theory, and it is a prominent theory in the study of selective attention. Load theory is centered around two kinds of neural resources—perceptual resources and cognitive resources.
3. PERCEPTUAL LOAD
3.1. Definition
Perceptual load theory 4 is concerned with the limited capacity of brain regions involved in the sensory processing of attentional selection, such as the auditory and visual processing pathways, and their contribution in selecting target stimuli in crowded or complex perceptual fields. Perceptual load is defined as “the amount of information involved in the processing of the task stimuli.” 5 A stimulus environment would be considered to demand high perceptual load if considerable perceptual resources are required to conduct efficient stimulus selection in said environment, such as a crowded visual display, a noisy auditory environment, or a stimulus setup where objects (visual or auditory) are very similar to each other in terms of their physical or perceptual characteristics.
Perceptual load theory claims that when perceptual load is high, processing capacity is fully engaged in target selection, leaving fewer resources available for the processing of distractors. 4 , 6 , 7 In cases of low perceptual load, perceptual capacity is not exhausted, which leads to a “spill over” of the remaining resources on to the distractors, which in turns leads to increased distractor processing as compared to high load environments.
3.2. Relevant studies
Studies of perceptual load have suggested that processing by perceptual resources may be reflective of the underlying neural mechanisms of bias competition resolution based on the physical characteristics and spacing of objects in the perceptual field. 8 It was hypothesized that the strength of the biasing mechanisms used to resolve competition between stimuli would determine the degree to which distractors are processed, because a visual display that induces stronger interactions between stimuli should require greater top‐down effort to suppress the surrounding distractors and process the target.
Early research in load theory suggested a similar impact of perceptual load in the auditory domain as described above in the visual domain, 9 while recently an auditory analogue of the famous “gorilla study” of inattentional blindness 10 has been demonstrated, 11 and increased auditory perceptual load has been shown to be associated with diminished performance in an auditory search task. 12 Some of our understanding in this area can be derived from one of the oldest and best‐known studies of auditory selective attention: the “cocktail party phenomenon” seen in dichotic listening paradigms. 13 Dichotic listening demonstrated how we can “ignore” a stream of perceptual information when focused on another one, and while load was not specifically manipulated in this study, the experimental set up could be assumed to be of high perceptual load. These findings align with the claims of perceptual load theory—in a crowded perceptual field, we are able to ignore irrelevant information and focus on relevant stimuli. A detailed review of auditory perceptual load indicated that the majority of results demonstrating an impact of perceptual load within audition are derived from studies that manipulate perceptual demands by varying the complexity of perceptual processes needed to successfully perform the task. 14
There has also mixed evidence on whether the impact of perceptual load on selective attention is modality‐specific 15 , 16 or cross‐modal. 5 , 17 , 18 , 19 , 20 However, cross‐modal studies of selective attention likely induce cognitive load, and so are considered in the next section.
3.3. Brain regions
Perceptual load is primarily processed in regions where the features of perceptual stimuli are processed, such as the auditory cortex (consisting of Heschl's gyrus and the lateral geniculate nucleus 21 ), and visual processing regions (such as the fusiform gyrus, superior occipital gyrus and lateral occipital cortex 22 ). It may also involve regions involved in the processing of other forms of sensory stimuli (such as the somatosensory cortex), but for the purposes of this article, we are primarily interested in visual and auditory processing regions. These regions are highlighted in Figure 1.
FIGURE 1.
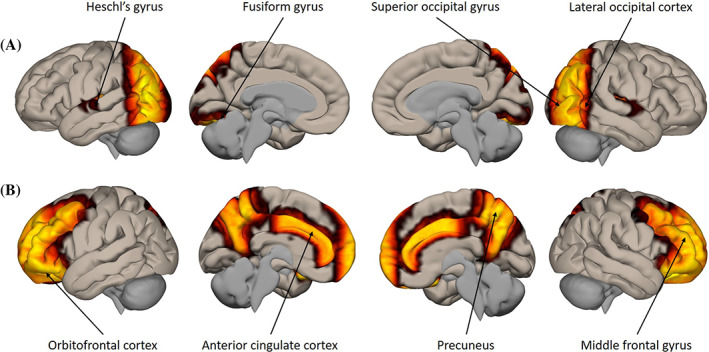
A, Putative brain regions involved in the processing of perceptual load and B, regions involved in the processing of cognitive load
4. COGNITIVE LOAD
4.1. Definition
As load theory was empirically investigated, it became apparent that the efficiency of selective attention may also be impacted by the type of load, and that, in addition to perceptual load, the amount of load placed on cognitive control processes at a given time may also impact the extent to which distractors in an environment are processed. 23 Load theory posits that when perceptual load is low, active cognitive control is exercised to select the target stimulus in the presence of distractors. Active cognitive control engages cognitive resources, leading to increased cognitive load. This higher cognitive load involves executive control functions, which maintain attention to the current task. Scenarios inducing high cognitive load may include multitasking, task‐switching, or conducting any task engaging working memory while completing a selective attention task. If the reader has ever turned down music when nearing their destination while driving, they are familiar with the impact of high cognitive load—we reduce our cognitive load to focus on the task at hand (finding the destination).
4.2. Relevant studies
Foundational work introducing cognitive load demonstrated that performance on selective attention tasks was subject to more distractor interference when working memory was engaged in a secondary task, 23 while increased cognitive load was observed to cause increased distractor interference in a spatial search task. 24 An increase in distractor interference of auditory selective attention when working memory load is highly engaged has also been empirically demonstrated, suggesting that the availability of cognitive resources was important in efficient control of auditory attention, 25 supported by research suggesting a large influence of working memory in control over involuntary switches of auditory attention. 26
Since cognition is a central resource, cross‐modal effects of load become important. In a series of four experiments distractor interference was seen to increase in the nonverbal auditory Stroop task in a high‐load condition as compared to low‐load, but the effect was not seen if the concurrent cognitive load was verbal. 27 The opposite effect was seen for the verbal Stroop task. This demonstrated that the interference effect of cognitive load can be context‐dependent, a finding supporting earlier research in the visual domain. 28 At a physiological level, the auditory‐evoked brainstem response to task‐irrelevant sounds was seen to decrease with an increasing visual‐verbal working memory load, demonstrating the cross‐modal effects of cognitive load, and its impact on selective attention. 29
4.3. Brain regions
Cognitive load is believed to be processed in regions of “higher level” processing, such as the frontal and prefrontal cortices (eg, the precentral gyrus, orbitofrontal cortex and middle frontal gyrus 30 ), posterior parietal cortex (including the precuneus 31 ) and regions of emotional valence processing, such as the anterior cingulate. 31 Figure 1 shows the brain regions believed to be involved in the processing of perceptual and cognitive load.
5. LOAD THEORY AND TINNITUS
Cognitive and perceptual load likely have significant influence over both the perception of tinnitus and its impact on emotional well‐being. Some models of tinnitus detail the role played by attention and cognitive control in tinnitus perception and annoyance, 1 , 2 , 3 but they do not consider the role of cognitive or perceptual load. We believe that the role of cognitive control in tinnitus may be better modeled by including load theory.
Research investigating cognitive deficits in tinnitus has been broadly guided by one of two hypotheses. The “general depletion of resources” hypothesis states that the perception of tinnitus causes constant orienting to the sound, thereby reducing overall processing capacity. The “controlled processing” hypothesis claims that tinnitus has a more specific impact—patients should be able to complete simple tasks at a similar level to non‐tinnitus controls, but performance should suffer in more difficult tasks and multitasking paradigms. Considering these hypotheses in terms of load theory, the controlled processing hypothesis of tinnitus implies an overload of cognitive resources, leading to reduced capacity and thus to worse performance on tasks which engage higher‐order cognitive functions, such as working memory. The general depletion hypothesis suggests that tinnitus consumes both perceptual and cognitive resources—if performance suffers across all tests of attention and cognitive control, it is possible that perceptual resources are being occupied sufficiently to lead to worse performance in detection tasks, and cognitive resources are also being overloaded, leading to diminished performance in discrimination tasks.
Typically, two types of cognitive tasks are used in selective attention paradigms—detection tasks and discrimination tasks. Detection tasks prompt participants to indicate when they identify a target stimulus, while discrimination tasks ask participants to make judgments about competing stimuli. These types of tasks involve inherently different mechanisms; detection tasks demand greater perceptual resources, as the task is relatively simple and does not require higher‐level processing, while discrimination tasks demand greater engagement of cognitive resources, as decisions must be made when attempting to differentiate between stimuli. In the context of research on tinnitus, it is important to keep these distinctions in mind when evaluating various tasks incorporated by different studies. If the tinnitus percept, which can be considered the “distractor,” engages primarily perceptual resources, we would expect group differences between tinnitus and non‐tinnitus subjects in detection tasks, while there may not be significant group differences in performance on discrimination tasks. According to load theory, since more perceptual resources would be engaged by tinnitus, fewer are available for distractor processing as the available capacity is applied towards detection of the stimulus, which may lead to different levels of performance in these tasks. Conversely, if tinnitus engages mostly cognitive resources, we would expect diminished performance in discrimination tasks and tasks that involve working memory in tinnitus subjects compared to non‐tinnitus controls.
5.1. Cognitive studies in tinnitus
In reevaluating studies conducted on cognition in tinnitus in the context of load theory, it becomes apparent that the focus has primarily been on cognitive resources and their involvement in tinnitus, at the exclusion of perceptual resources. Studies have shown that cognitive deficits in tinnitus manifest in a more profound way in more demanding and complex tasks than single‐task designs, 32 , 33 and tinnitus subjects have been reported to be significantly slower than non‐tinnitus controls in both auditory and visual domains in the Stroop and Go/No‐go tasks (both discrimination tasks). 34 , 35 , 36 , 37 This apparent support for the controlled processing hypothesis aligns with studies demonstrating that tinnitus patients self‐report a greater number of cognitive failures in daily activities than controls, 32 as well as the finding of tinnitus‐specific failures in the executive control tasks of the Attention Network Test. 38 , 39
Neuroimaging studies also play an important role in furthering our understanding of tinnitus through load theory. Tinnitus subjects have shown increased BOLD activity in the ventromedial prefrontal cortex (vmPFC) when presented with a sound, as compared to matched controls, 40 providing a functional analogue for reported decreases in grey‐matter volume in the subcallosal area and vmPFC of tinnitus subjects. 41 , 42 , 43 The functional differences observed were further seen to be correlated with the loudness and duration of perception of the tinnitus signal. 40 Taken together, these results suggest an overloading of cognitive resources in chronic tinnitus. In a tinnitus‐specific emotional Stroop paradigm, increased activation of the orbitofrontal cortex was seen in high distress tinnitus patients as compared to low‐distress tinnitus patients, and tinnitus distress was seen to be positively correlated with activity in the orbitofrontal cortex. 44 Combined with similar behavioral performance between groups, the neuroimaging findings suggest an increased loading of cognitive resources in tinnitus patients when completing this cognitive task. The level of engagement of cognitive resources seems to be related to tinnitus annoyance, as low‐distress tinnitus patients, who have habituated to their tinnitus, do not demonstrate the increased frontal activation when compared to non‐tinnitus controls. In contrast, Stevens et al 37 found similar performance between tinnitus sufferers and controls on an “easy” and a “difficult” task, suggesting support for the general depletion hypothesis. However, the gap between the performance of the two groups increased as task difficulty increased. Further, tinnitus severity may also impact performance—we would expect those with more severe tinnitus to perform worse in a difficult cognitive task than those with milder tinnitus, 44 but since individual tinnitus severity was not reported, it is difficult to parse out specific cognitive mechanisms that may be responsible for this finding in a relatively small sample.
There have been a series of studies investigating alterations in resting state functional connectivity in tinnitus as compared to controls. 45 Differences have been reported between tinnitus and control subjects in the ventral attention network (VAN) 46 and dorsal attention network (DAN). 47 , 48 , 49 , 50 The VAN is believed to be involved in involuntary reorienting of attention to salient stimuli, while the DAN is believed to be involved in voluntary switches of attention. Coupled with the findings that the default mode network may show decreased connectivity in tinnitus patients compared to controls, 49 , 51 it appears that tinnitus sufferers are engaged in increased cognitive processing at rest compared to non‐tinnitus, hearing matched controls. It is plausible that as sufferers habituate to the tinnitus, they are able to “free up” some of these resources, reducing the cognitive load, and are then able to better direct and focus their attention.
Whereas the studies discussed above suggest overwhelming evidence for an overloading of cognitive resources in tinnitus, it is worth noting that none of the studies approached tinnitus from the concept of load. As such, any suggestions about their findings in terms of load theory are speculative. It is also worth noting that few studies have incorporated a detection task aimed specifically at examining engagement of perceptual resources. One of the most pertinent findings in this area might be the observation that those with chronic unilateral tinnitus demonstrated more accurate responses when stimuli were presented in the tinnitus ear compared to the non‐tinnitus ear, demonstrating a “pre‐attentive” effect. 52 Load theory claims that when perceptual resources are overloaded, stimulus processing increases while distractor processing decreases—this pre‐attentive effect may reflect an engagement of perceptual resources by the tinnitus signal.
There is also evidence from neuroimaging studies to suggest an overloading of perceptual resources in the presence of tinnitus. Positron Emission Tomography (PET) studies have shown increased metabolic activity in auditory processing regions of tinnitus sufferers compared to healthy controls, 53 , 54 , 55 while resting state fMRI studies have demonstrated increased neural activity 2 , 56 , 57 and altered spontaneous neuronal activity 3 , 47 in the central auditory pathways. This increase in resting activity likely reflects processing of the tinnitus percept, which indicates an increased perceptual load in tinnitus subjects at rest, as compared to healthy controls. In a PET study, prefrontal and auditory regions associated with the tinnitus percept were identified, 55 which may reflect engagement of both cognitive resources, processed in frontal areas, as well as perceptual resources, processed in the auditory cortex. This suggests that both types of resources are being employed by the tinnitus signal in different ways.
Some work has found that behavioral performance across varying levels of cognitive load is similar between tinnitus sufferers and controls, 58 , 59 but research using fMRI revealed reduced fronto‐parietal activation in the tinnitus group for the task>rest condition in one study. 58 Additionally, reduced activity in the attention network in the auditory domain was observed, with the opposite effect (ie, increased activity) observed in the visual domain. As the authors of this study suggest, the observed patterns of neural activity may underlie group differences in task processing, but it is worth considering that this reduced activation may alternatively stem from increased baseline activity in the tinnitus group, which would explain the reduced activation seen in the tinnitus group. These findings highlight the differential activity in the attention networks of tinnitus sufferers relative to controls, while underscoring the need to better understand the impact of cross‐modal perceptual processing the presence of a high cognitive load.
6. PROPOSED MODEL AND FUTURE DIRECTIONS
Tinnitus has been proposed as a disorder that has a reciprocal relationship with cognitive control—an individual's cognitive control may predict how quickly and efficiently that individual habituates to their tinnitus. 2 As we further refine our understanding of tinnitus, it is worth taking a higher‐order view of the relationship between tinnitus and cognitive control. Cognitive control is greatly impacted by the level of perceptual and cognitive load at any given time, which makes load an important factor to consider. Rather than considering tinnitus through the general depletion or controlled processing hypotheses, we might think of tinnitus from the perspective of perceptual and cognitive resources, and by extension, load. No study has yet directly investigated the influence of tinnitus on either perceptual or cognitive resources, although as discussed previously, evidence suggests a key role for load theory‐informed designs in advancing knowledge of the mechanisms of tinnitus.
The proposed model in Figure 2 is a synthesis of these theories and the associated studies. It updates previous models of tinnitus, 1 , 2 , 3 incorporating the hypothesized regions involved in the processing of perceptual and cognitive load. Husain 3 listed a variety of regions believed to be implicated in various neural networks and various tinnitus subgroups—here we emphasize brain regions engaged in processing the load tinnitus induces. Neuroimaging studies at rest and during task performance point to an overloading of both perceptual and cognitive resources, so it may be beneficial to approach the relationship between cognition and tinnitus from two perspectives. The first is through perceptual load—tinnitus is an undesired stimulation of the auditory processing pathway, meaning sufferers' perceptual resources are constantly engaged in the processing of the sound itself. While few studies have directly manipulated perceptual load, a possible pre‐attentive effect in the tinnitus ear 52 suggests that baseline perceptual load may be higher in the tinnitus ear of those with unilateral tinnitus. Studies have also demonstrated increased activity in auditory processing regions at rest in tinnitus subjects compared to both hearing loss and normal hearing controls, further supporting this hypothesis. The observed “pre‐attentive” effect may be indicative of perceptual resources processing the tinnitus “signal,” but this may or may not manifest as behavioral differences in detection tasks relative to controls. Further study is needed to truly elucidate this effect.
FIGURE 2.
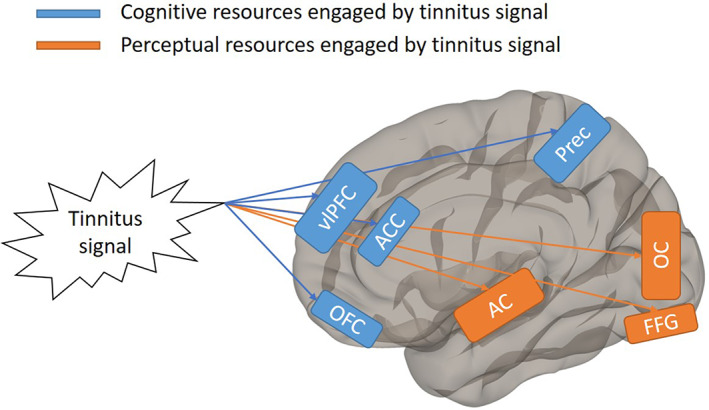
Proposed model of cognitive deficits associated with tinnitus. Blue represents brain regions occupied by cognitive processing of tinnitus signal. Orange represents brain regions occupied by perceptual processing of tinnitus signal. Model is applicable specifically to established chronic tinnitus. AC, auditory cortex; ACC, anterior cingulate cortex; FFG, fusiform gyrus; OC, occipital cortex; OFC, orbitofrontal cortex; Prec, precuneus; vlPFC, ventrolateral prefrontal cortex
A second consideration is that the constant perception of tinnitus affects the cognitive load of a sufferer. Sufferers could be ruminating on the presence of the sound, attempting strategies to mitigate the loudness of the sound, or attempting to minimize the emotional distress caused by the sound, all of which would engage working memory and cognitive resources, leaving fewer resources available for the processing of tasks. This is evidenced in studies where performance is consistently seen to decrease in the presence of tasks of high cognitive load when compared to controls. Additionally, most of the behavioral studies discussed here incorporated visual stimuli in their tasks, and performance was observed to diminish cross‐modally, strengthening the claim for engagement of a central cognitive resource. Importantly, these two hypotheses would give us a better understanding of tinnitus as it relates to cognition, allowing us to design studies to more precisely measure the intricacies of cognition in relation to load, which may lead to the development of more targeted therapy for established, chronic tinnitus.
The understanding of tinnitus through a load theory framework needs to be directly tested to help us better understand the neural bases of tinnitus related to cognition and perception. It is plausible that while the engagement of perceptual resources by the tinnitus signal remains constant throughout the duration of a sufferer's perception of the signal, cognitive resources are likely most heavily engaged immediately following the onset of tinnitus because attention is directed to the signal, attempts to subdue the signal and control the emotional response to it. As participants habituate to the signal, the process of coping with the tinnitus becomes more automated, thus fewer cognitive resources are occupied in the response to tinnitus, freeing up these resources to be used in other tasks. This implies an effect of tinnitus duration (with the accompanying change in annoyance) on performance in cognitive tasks, which have not been traditionally accounted for in this literature.
Modeling tinnitus in a cognitive control framework has advanced the field's understanding of tinnitus and its underlying mechanisms, yet some gaps in understanding exist. Existing models of tinnitus can be further refined through the addition of load theory, giving us a framework to understand the relationship between tinnitus and cognition. The model proposed here extends prior work by considering chronic tinnitus after it has been established, and further accounting for the involvement of perceptual and cognitive resources, proposing that the competition seen between the two types of resources as integral to the cognitive impact of tinnitus.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Khan RA, Husain FT. Tinnitus and cognition: Can load theory help us refine our understanding? Laryngoscope Investigative Otolaryngology. 2020;5:1197–1204. 10.1002/lio2.501
REFERENCES
- 1. Andersson G, McKenna L. The role of cognition in tinnitus. Acta Otolaryngol. 2006;126(sup556):39‐43. [DOI] [PubMed] [Google Scholar]
- 2. Rauschecker JP, Leaver AM, Mühlau M. Tuning out the noise: limbic‐auditory interactions in tinnitus. Neuron. 2010;66(6):819‐826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Husain FT. Neural networks of tinnitus in humans: elucidating severity and habituation. Hear Res. 2016;334:37‐48. [DOI] [PubMed] [Google Scholar]
- 4. Lavie N. Perceptual load as a necessary condition for selective attention. J Exp Psychol Hum Percept Perform. 1995;21(3):451‐468. [DOI] [PubMed] [Google Scholar]
- 5. Macdonald JS, Lavie N. Visual perceptual load induces inattentional deafness. Atten Percept Psychophys. 2011;73(6):1780‐1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lavie N. Distracted and confused?: selective attention under load. Trends Cogn Sci. 2005;9(2):75‐82. [DOI] [PubMed] [Google Scholar]
- 7. Lavie N. Attention, distraction, and cognitive control under load. Curr Dir Psychol Sci. 2010;19(3):143‐148. [Google Scholar]
- 8. Torralbo A, Beck DM. Perceptual‐load‐induced selection as a result of local competitive interactions in visual cortex. Psychol Sci. 2008;19(10):1045‐1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lavie N, Tsal Y. Perceptual load as a major determinant of the locus of selection in visual attention. Percept Psychophys. 1994;56(2):183‐197. [DOI] [PubMed] [Google Scholar]
- 10. Simons DJ, Chabris CF. Gorillas in our midst: sustained inattentional blindness for dynamic events. Perception. 1999;28(9):1059‐1074. [DOI] [PubMed] [Google Scholar]
- 11. Dalton P, Fraenkel N. Gorillas we have missed: sustained inattentional deafness for dynamic events. Cognition. 2012;124(3):367‐372. [DOI] [PubMed] [Google Scholar]
- 12. Fairnie J, Moore BC, Remington A. Missing a trick: auditory load modulates conscious awareness in audition. J Exp Psychol Hum Percept Perform. 2016;42(7):930‐938. [DOI] [PubMed] [Google Scholar]
- 13. Cherry EC. Some experiments on the recognition of speech, with one and with two ears. J Acoust Soc Am. 1953;25(5):975‐979. [Google Scholar]
- 14. Murphy S, Spence C, Dalton P. Auditory perceptual load: a review. Hear Res. 2017;352:40‐48. [DOI] [PubMed] [Google Scholar]
- 15. Parks NA, Hilimire MR, Corballis PM. Steady‐state signatures of visual perceptual load, multimodal distractor filtering, and neural competition. J Cogn Neurosci. 2011;23(5):1113‐1124. [DOI] [PubMed] [Google Scholar]
- 16. Treisman A, Davies A. Divided attention to eye and ear In: Komblum S, ed. Attention and Performance IV. New York: Academic Press; 1973. [Google Scholar]
- 17. Dalton P, Spence C. Attentional capture in serial audiovisual search tasks. Percept Psychophys. 2007;69(3):422‐438. [DOI] [PubMed] [Google Scholar]
- 18. Molloy K, Griffiths TD, Chait M, Lavie N. Inattentional deafness: visual load leads to time‐specific suppression of auditory evoked responses. J Neurosci. 2015;35(49):16046‐16054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raveh D, Lavie N. Load‐induced inattentional deafness. Atten Percept Psychophys. 2015;77(2):483‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klemen J, Büchel C, Rose M. Perceptual load interacts with stimulus processing across sensory modalities. Eur J Neurosci. 2009;29(12):2426‐2434. [DOI] [PubMed] [Google Scholar]
- 21. Xu J, Monterosso J, Kober H, Balodis IM, Potenza MN. Perceptual load‐dependent neural correlates of distractor interference inhibition. PLoS One. 2011;6(1):e14552 10.1371/journal.pone.0014552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lavie N. The role of perceptual load in visual awareness. Brain Res. 2006;1080(1):91‐100. [DOI] [PubMed] [Google Scholar]
- 23. Lavie N, Hirst A, De Fockert JW, Viding E. Load theory of selective attention and cognitive control. J Exp Psychol Gen. 2004;133(3):339‐354. [DOI] [PubMed] [Google Scholar]
- 24. Longstaffe KA, Hood BM, Gilchrist ID. The influence of cognitive load on spatial search performance. Atten Percept Psychophys. 2014;76(1):49‐63. [DOI] [PubMed] [Google Scholar]
- 25. Dalton P, Santangelo V, Spence C. The role of working memory in auditory selective attention. Q J Exp Psychol. 2009;62(11):2126‐2132. [DOI] [PubMed] [Google Scholar]
- 26. Berti S, Schröger E. Working memory controls involuntary attention switching: evidence from an auditory distraction paradigm. Eur J Neurosci. 2003;17(5):1119‐1122. [DOI] [PubMed] [Google Scholar]
- 27. Dittrich K, Stahl C. Selective impairment of auditory selective attention under concurrent cognitive load. J Exp Psychol Hum Percept Perform. 2012;38(3):618‐627. [DOI] [PubMed] [Google Scholar]
- 28. Kim SY, Kim MS, Chun MM. Concurrent working memory load can reduce distraction. Proc Natl Acad Sci. 2005;102(45):16524‐16529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sörqvist P, Stenfelt S, Rönnberg J. Working memory capacity and visual–verbal cognitive load modulate auditory–sensory gating in the brainstem: toward a unified view of attention. J Cogn Neurosci. 2012;24(11):2147‐2154. [DOI] [PubMed] [Google Scholar]
- 30. Tomasi D, Chang L, Caparelli EC, Ernst T. Different activation patterns for working memory load and visual attention load. Brain Res. 2007;1132:158‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yan C, Liu D, He Y, et al. Spontaneous brain activity in the default mode network is sensitive to different resting‐state conditions with limited cognitive load. PLoS One. 2009;4(5):e5743 10.1371/journal.pone.0005743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hallam RS, McKenna L, Shurlock L. Tinnitus impairs cognitive efficiency. Int J Audiol. 2004;43(4):218‐226. [DOI] [PubMed] [Google Scholar]
- 33. Rossiter S, Stevens C, Walker G. Tinnitus and its effect on working memory and attention. J Speech Lang Hear Res. 2006;49(1):150‐160. [DOI] [PubMed] [Google Scholar]
- 34. Andersson G, Eriksson J, Lundh LG, Lyttkens L. Tinnitus and cognitive interference: a Stroop paradigm study. J Speech Lang Hear Res. 2000;43(5):1168‐1173. [DOI] [PubMed] [Google Scholar]
- 35. Araneda R, De Volder AG, Deggouj N, et al. Altered top‐down cognitive control and auditory processing in tinnitus: evidences from auditory and visual spatial stroop. Restor Neurol Neurosci. 2015;33(1):67‐80. [DOI] [PubMed] [Google Scholar]
- 36. Araneda R, De Volder AG, Deggouj N, Renier L. Altered inhibitory control and increased sensitivity to cross‐modal interference in tinnitus during auditory and visual tasks. PLoS One. 2015;10(3):e0120387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stevens C, Walker G, Boyer M, Gallagher M. Severe tinnitus and its effect on selective and divided attention: acufeno severo y sus efectos sobre la atención selectiva y dividida. Int J Audiol. 2007;46(5):208‐216. [DOI] [PubMed] [Google Scholar]
- 38. Heeren A, Maurage P, Perrot H, et al. Tinnitus specifically alters the top‐down executive control sub‐component of attention: evidence from the attention network task. Behav Brain Res. 2014;269:147‐154. [DOI] [PubMed] [Google Scholar]
- 39. Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14(3):340‐347. [DOI] [PubMed] [Google Scholar]
- 40. Seydell‐Greenwald A, Leaver AM, Turesky TK, Morgan S, Kim HJ, Rauschecker JP. Functional MRI evidence for a role of ventral prefrontal cortex in tinnitus. Brain Res. 2012;1485:22‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mühlau M, Rauschecker JP, Oestreicher E, et al. Structural brain changes in tinnitus. Cereb Cortex. 2006;16(9):1283‐1288. [DOI] [PubMed] [Google Scholar]
- 42. Leaver AM, Renier L, Chevillet MA, Morgan S, Kim HJ, Rauschecker JP. Dysregulation of limbic and auditory networks in tinnitus. Neuron. 2011;69(1):33‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Leaver AM, Seydell‐Greenwald A, Turesky T, Morgan S, Kim HJ, Rauschecker JP. Cortico‐limbic morphology separates tinnitus from tinnitus distress. Front Syst Neurosci. 2012;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Golm D, Schmidt‐Samoa C, Dechent P, Kröner‐Herwig B. Tinnitus‐related distress: evidence from fMRI of an emotional stroop task. BMC Ear Nose Throat Disord. 2016;16(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shahsavarani S, Khan RA, Husain FT. Tinnitus and the brain: a review of functional and anatomical magnetic resonance imaging studies. Perspect ASHA Special Interest Groups. 2019;4(5):896‐909. [Google Scholar]
- 46. Burton H, Wineland A, Bhattacharya M, Nicklaus J, Garcia KS, Piccirillo JF. Altered networks in bothersome tinnitus: a functional connectivity study. BMC Neurosci. 2012;13(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Husain FT, Schmidt SA. Using resting state functional connectivity to unravel networks of tinnitus. Hear Res. 2014;307:153‐162. [DOI] [PubMed] [Google Scholar]
- 48. Maudoux A, Lefebvre P, Cabay JE, et al. Auditory resting‐state network connectivity in tinnitus: a functional MRI study. PLoS One. 2012;7(5):e36222 10.1371/journal.pone.0036222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schmidt SA, Akrofi K, Carpenter‐Thompson JR, Husain FT. Default mode, dorsal attention and auditory resting state networks exhibit differential functional connectivity in tinnitus and hearing loss. PLoS One. 2013;8(10):e76488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wineland AM, Burton H, Piccirillo J. Functional connectivity networks in nonbothersome tinnitus. Otolaryngol Head Neck Surg. 2012;147(5):900‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Carpenter‐Thompson JR, Schmidt SA, Husain FT. Neural plasticity of mild tinnitus: an fMRI investigation comparing those recently diagnosed with tinnitus to those that had tinnitus for a long period of time. Neural Plast. 2015;2015:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cuny C, Norena A, El Massioui F, Chery‐Croze S. Reduced attention shift in response to auditory changes in subjects with tinnitus. Audiol Neurootol. 2004;9:294‐302. [DOI] [PubMed] [Google Scholar]
- 53. Arnold W, Bartenstein P, Oestreicher E, Römer W, Schwaiger M. Focal metabolic activation in the predominant left auditory cortex in patients suffering from tinnitus: a PET study with [18F] deoxyglucose. Orl. 1996;58(4):195‐199. [DOI] [PubMed] [Google Scholar]
- 54. Langguth B, Eichhammer P, Kreutzer A, et al. The impact of auditory cortex activity on characterizing and treating patients with chronic tinnitus–first results from a PET study. Acta Otolaryngol. 2006;126(sup556):84‐88. [DOI] [PubMed] [Google Scholar]
- 55. Mirz F. Cortical networks subserving the perception of tinnitus‐a PET study. Acta Otolaryngol. 2000;120(543):241‐243. [DOI] [PubMed] [Google Scholar]
- 56. Roberts LE, Husain FT, Eggermont JJ. Role of attention in the generation and modulation of tinnitus. Neurosci Biobehav Rev. 2013;37(8):1754‐1773. [DOI] [PubMed] [Google Scholar]
- 57. Sedley W, Friston KJ, Gander PE, Kumar S, Griffiths TD. An integrative tinnitus model based on sensory precision. Trends Neurosci. 2016;39(12):799‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Husain FT, Pajor NM, Smith JF, et al. Discrimination task reveals differences in neural bases of tinnitus and hearing impairment. PLoS One. 2011;6(10):e26639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Husain FT, Akrofi K, Carpenter‐Thompson JR, Schmidt SA. Alterations to the attention system in adults with tinnitus are modality specific. Brain Res. 2015;1620:81‐97. [DOI] [PubMed] [Google Scholar]


