Abstract
In plants, the spatial arrangement of cells within tissues and organs is a direct consequence of the positioning of the new cell walls during cell division. Since the nineteenth century, scientists have proposed rules to explain the orientation of plant cell divisions. Most of these rules predict the new wall will follow the shortest path passing through the cell centroid halving the cell into two equal volumes. However, in some developmental contexts, divisions deviate significantly from this rule. In these situations, mechanical stress, hormonal signalling, or cell polarity have been described to influence the division path. Here we discuss the mechanism and subcellular structure required to define the cell division placement then we provide an overview of the situations where division deviates from the shortest symmetric path.
Keywords: cellular reproduction, developmental biology, plant biology
Introduction
In plants, the presence of cell walls implies that tissue forms a mechanical continuum where cells are attached to their neighbours. The position of new cell walls is thus of importance since it will influence cell shape, tissue topology, patterning, and to some extent the overall organ morphology. The positioning of new cell walls in plants has retained the attention of scientists for more than a century, as their non-random orientation has been noticed quite early, several rules explaining their positions have been proposed. In 1863 Hofmeister proposed that new cell walls formed perpendicular to the main direction of growth of the mother cell [1]. In relation to Hofmeister's rule, Sachs proposed that new cell walls were inserted at a 90° angle from the mother cell wall [2]. Then in 1886, Errera proposed that most planes of plant cell divisions followed the shortest possible path (i.e. minimal surface area), halving the cell into two daughter cells of equal volume in a way similar to soap film formation that tends to optimize the energy by minimising their surface area [3]. More than 100 years later Flanders et al. added another rule on top of these: the new cell wall inserts in the pre-existing one to avoid the formation of four-way junctions [4]. More recently, Besson and Dumais revised Errera's rule by including a probabilistic component: the path followed by the new wall is selected from several alternative paths with an increased probability toward the shortest one [5]. These rules are based on cell geometry or growth but some authors have recently proposed that the positioning of new cell walls maintains the topological properties of the tissue such as each cell having an average of six neighbours [6,7] or homogeneity of the cells network [8]. However, since topology is an emergent property of the division pattern, both geometry-based and topology-based rules predict new cell walls to be (or very close to) the shortest possible one going through the mother cell centroid, halving the cell in two equal volumes. But there are some cases where the selected path deviates significantly from this rule by following a path longer than the shortest one, creating an asymmetry in the volume of the two daughter cells or not passing through the mother cell centroid. Here we present the essential subcellular structures required for the positioning of the new cell wall then we provide an overview of situations where the division path deviates from the shortest one going through the cell centroid.
New cell wall and division orientation establishment
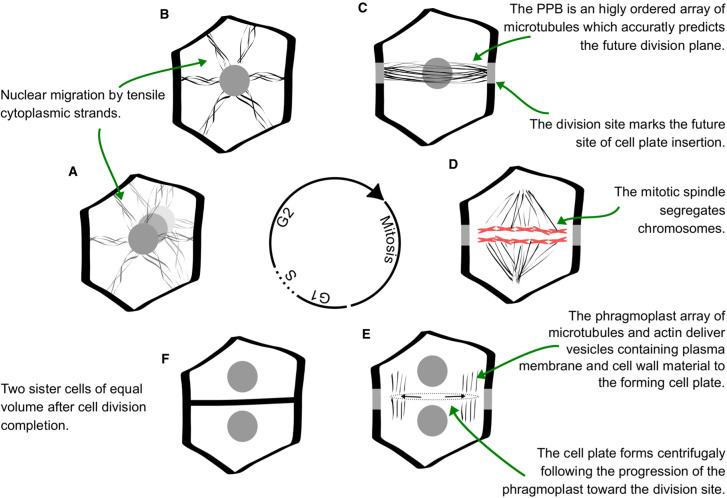
The definition of the division path and the formation of the new cell wall is a multistep process initiated by the end of interphase, here we will briefly present these steps before discussing the potential keystones for the definition of division orientation. The first events relating to the definition of the new cell wall position occur at the end of interphase when the cell nucleus often moves to a new position; central for divisions that pass through the centroid and away from the cell centroid for those that do not [9,10]. The movement of the nucleus is associated with the formation of cytoplasmic strands containing actin and microtubules (MTs) radiating from the nucleus [4,9,11], the nuclear membrane having the ability to nucleate MTs [12] (Figure 1A,B). The Pre Prophase Band (PPB) of microtubules is formed soon after nuclear displacement and consists of highly ordered microtubules [13] (Figure 1C). The cortical division zone (CDZ) that marks the future site of cell plate insertion is formed concomitantly to the PPB and is characterised by a local change in plasma membrane composition and enrichment in a specific type of kinase and microtubule-associated proteins. Changes in plasma membrane composition and associated proteins during cell division are reviewed in detail in [14]. The PPB narrows then coalesce during prophase [15], the mitotic spindle forms during prophase, and metaphase segregate chromosomes in anaphase (Figure 1D). In telophase, vesicles from golgi and trans-golgi networks fuse at the cell equator to form the cell plate. These vesicles are transported by a dense network of microtubules and actin: the phragmoplast, they carry both plasma membrane material, cell wall material, and proteins responsible for cell wall material biosynthesis [16]. The new cell plate is formed centrifugally following the movement of the phragmoplast [17,18], it then fuses with the existing cell wall and the plasma membrane at the division site [19,20] (Figure 1E,F). We will now discuss how some of these events are instrumental in division plane orientation determination.
Figure 1. Schematic view of plant cell division.
(A) Nuclear migration at the end of interphase. (B) Cytoplasmic strands containing actin and microtubules radiates from the nucleus and participate in anchoring it to its news position. (C) Cortical microtubules accumulate in a dense band, the PPB that will mark the future site of cell plate insertion (the division site). (D) The spindle segregates chromosomes between the two daughter cells in anaphase. (E) During cytokinesis, a network of microtubules and actin, the phragmoplast, deliver vesicles containing cell wall material to the forming cell plate. The phragmoplast and cell plate progress centrifugally towards the division site (gray bands). (F) Two sister cells after division is completed.
All division rules imply a mechanism to identify the shortest path, measure the potential angle of cell plate insertion, or evaluate the direction of tensile stress. This mechanism could rely on the physical properties of the structures involved in cell division or on the modulation of these properties by additional molecular actors. One important property of plant structures is the level of tension or compression they support. Tension and compression are forces bearing directional information. The main direction of tensile stress within single cells can align with the shortest path across the cell. For instance, the shortest path predictions made by Besson and Dumais can be recapitulated by a simple inflated elastic membrane model predicting the direction of tensile stress [5,21]. At the subcellular level, MTs have been shown to align with the maximal direction of tensile stress [22] and could be tension sensors per se [23]. During interphase, a dense network of intertwined MT is present at the cell cortex often referenced as Cortical Microtubules array (CMT), its principal direction is influenced by the tensile and compressive force [22,24,25]. Several structures involved in cell division are made of MTs and as we will now discuss, some of them are under tension or align with the principal direction of tensile stress. For instance, cytoplasmic strands associated with nuclear displacement are under tension [26,27], and like a soap film in a rigid frame, they tend to reach the cell edge at 90° following the shortest path [4]. Another structure aligning with tension is the PPB whose orientation often follows the main orientation of cortical microtubules in interphase. PPB position is accurately predicted by the soap-film area minimization model run on real 3D segmented cell templates, in turn, the PPB position predicts the position of the future division plane [13,28]. However, PPB is not present in all plant species [29] and its function has recently been re-evaluated. PPB formation is regulated by the TONNEAU1 (TON1)-TON1 RECRUITING MOTIF (TRM)-PROTEIN PHOSPHATASE 2A (PP2A) (TTP) protein complex. Several mutants affected in this complex do not form a PPB and have cell division orientation defects. Defective expression of the regulatory subunit of PP2A (fass mutant) severely disrupts division plane placement in Arabidopsis embryo [30]. TON1 defectives alleles also exhibit strong cell division orientation defects [31]. However, both fass and ton1 have defects in other microtubules structures such as the interphasic cortical microtubules array, and have strong developmental defects (ton1 mutant is named after ‘tonneau’ the French word for a barrel, as the plantlet looks like a tiny barrel), making the relationships between lack of PPB and cell division defects complex to interpret. Recently, a trm678 triple mutant has been isolated, this line does not form PPB and does not seems to have other microtubules defects [32]. On the contrary of other PPB defectives lines, the mean orientation of division in trm678 roots is similar to wild type although more variable, indicating that, at least in roots, the PPB would ensure the robustness of cell division orientation rather than determining the orientation [32]. Another main MT-based structure involved in cell division is the spindle. It is formed perpendicular to the axis of the PPB during prophase [33,34] but its position does not seem to be the determining factor of division plane position since centrifugation experiments have shown that displaced spindles can trackback to the initial position of the PPB [35–37]. In addition, rotations ending in the alignment of the spindle or even developing cell plates with the PPB position are frequent during guard mother cell division in Allium [38]. All these studies indicate that the division orientation and thus the location of cell plate insertion are defined prior to spindle formation by structures under tension or aligned with the principal direction of tension.
After its specification, the division site has to be maintained to ensure the proper guidance of the phragmoplast, here we provide a brief description of the few known actors involved in this process, a more detailed review of these actors is provided in [39]. Most of the actors identified to have a role in phragmoplast guidance are located at the CDZ underlying the PPB and remain there after PPB disassembly. For instance, the presence of PHRAGMOPLAST ORIENTING KINESIN (POK)1 and POK2 at the CDZ are essential for phragmoplast guidance, their absence in a pok1pok2 mutant causes a mismatch between phragmoplast and PPB orientation leading to severe division orientation defects [40] as the cell plate fuses outside of the CDZ. The positioning of POK1 at the CDZ partially depends on PLECKSTRIN HOMOLOGY GAPs (PHGAPS) proteins, mispositioning of POK1 in phgaps1phgaps2 double mutant also give rise to division orientation defects [41]. The presence of POK1 at the CDZ is required to maintain other important actors at the CDZ after the PPB disassembly. This is the case for TANGLED1 (TAN1) whose location at the CDZ is not maintained after PPB disassembly in pok1pok2 [42]. TAN1 is a microtubule-associated protein whose loss of function in the maize line tan1 results in strong division orientation defects due to a mismatch between phragmoplast and PPB orientation [43]. It has been proposed that the interaction between TAN1 at the division site and microtubules at the leading edge of the phragmoplast would maintain its trajectory [43]. In addition to the CDZ, POK2 is also present in the midzone of the expanding phragmoplast and interacts with microtubule cross-linker MAP65-3/PLEIADE, this interaction would stabilize the phragmoplast and favour its rapid expansion toward the division site [44]. The microtubule severing activity of KATANIN is essential for proper orientation of the spindle and guidance of the phragmoplast to the division site. Strong katanin mutant alleles show defects in these processes resulting in cell division plane defects [45,46]. In addition, a line with a mutation in CORD4, a protein recruiting katanin to the phragmoplast, shows a delayed progression of the phragmoplast to the division site [47]. After the initial definition of the future division plane by the PPB orientation, the spatial information is retained by some of the above-mentioned proteins, but the precise mechanism by which the leading edge of the phragmoplast grows in the precise direction of the division site remains unclear. Although the guidance of the phragmoplast to the division site is an important part of the division orientation process, the actors modulating the division path are likely to act on the early steps of the cell division process prior to spindle formation. Indeed, in a wild type context, no new division has been reported not to match the position of the division site. In the next section, we will describe the situations where the selected division path is not the shortest one going through the cell centre halving the cell in two equal volumes as well as the factors associated with the definition of these alternative division paths.
Variations from the shortest symmetric path
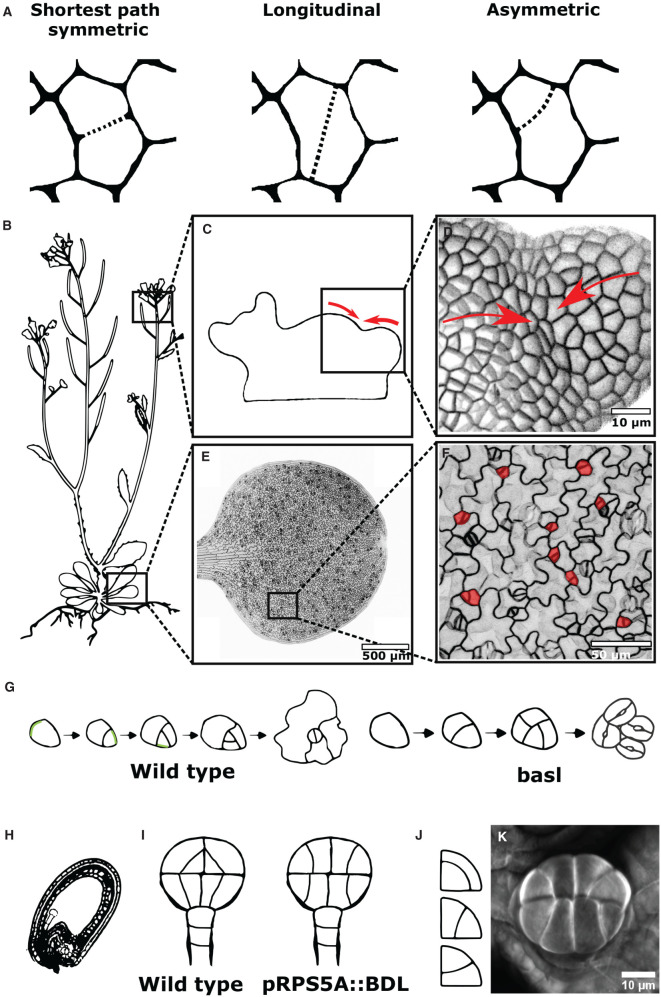
Although most divisions seem to follow the shortest path, there are a number of examples where this is not the case (Figure 2A). Divisions parallel to the longest axis of the cell, referred to as longitudinal divisions hereafter, are frequent during leaf development in maize [48]. However, they tend to occur in nearly isotropic cells, making the selected path not so distant from the shortest path [28]. Besides this situation that can be interpreted as an extreme case of the Besson and Dumais rule, some deviations from the shortest path cannot be explained by cell geometry-based rules alone. For instance in Shoot Apical Meristems (SAM), cells in the boundary region that separates organ primordia from the rest of the meristem almost systematically divide longitudinally [49–53]. These divisions occur perpendicular to the main direction of compression generated by the differential growth between organ primordia and SAM (Figure 2B–D). This is in accordance with the classical experiments using compression or wounding on dividing plant tissues that have repeatedly reported divisions to occur perpendicular to the direction of compression or parallel to the main direction of tensile stress [54–56]. More recently, cell laser ablation experiments further confirmed the influence of mechanical stress on the orientation of cell division. In Arabidopsis SAM, cell ablation leads to the formation of a circumferential pattern of mechanical stress as visualized by the alignment of cortical microtubules [22] and cell division occurring parallel to the main direction of tensile stress [49]. The local or global mechanical stress pattern influences the orientation of cell division but they are not the only factor affecting cell divisions.
Figure 2. Deviations from the shortest symmetric path.
(A) Schematic representations of different cell divisions: shortest path symmetric, longitudinal symmetric, and shortest path asymmetric. (B) Drawing of Arabidopsis thaliana with highlights of some locations where deviations from the shortest path are found. (C) Illustration of the Shoot Apical Meristem (SAM) where differential growth associated with organogenesis generate compressive forces (red arrows) (based on results from [22,86]). (D) Confocal image of the region of the SAM under compression exhibiting longitudinal divisions (as in (c), red arrows represents compressive forces). (E) Confocal image of a cotyledon. (F) Close up of the cotyledon in (E) showing asymmetric divisions in the stomatal lineage (highlighted in red). (G) BASL polar distribution (in green) is required to make the divisions asymmetric. In a basl mutant divisions of the stomatal lineage are symmetric and lead to the formation of stomatal clusters (Based on results from [68,71,87]). (H) Arabidopsis thaliana seed. (I) In wild type embryos the presence of an auxin response is associated with asymmetric divisions. In transgenic lines expressing a negative regulator of auxin signaling (pRPS5A::bdl), these embryonic divisions become symmetric and are reminiscent of the conformation adopted by soap film in quadrant (based on result from [5,30]). (J) Schematic representation of the spontaneous formation of soap film in a quadrant (based on results from [5]). (K) Glandular trichome of Pilea glaucophylla exhibiting a division pattern similar to (J) and pRPS5A::bdl.
Beside longitudinal divisions, asymmetric divisions represent another type of deviation from the shortest symmetric path. Asymmetric cell divisions are found in distinct contexts during plant development, for instance within the early steps of the stomatal lineage in Arabidopsis [57–59], during the formation of subsidiary cells in monocotyledons [60] or the first steps of lateral roots development [61–63]. In Arabidopsis, the very first division giving rise to the suspensor and embryo lineages or divisions leading to the formation of protodermal layers between the 8 and 16 cells stage of the embryo are asymmetric [64,65]. In the case of the division to form the protoderm the division plane still passes through the centroid of the cell [66] but does not produce daughter cells of equal volume. The division plane is a possible surface area minimisation [66], however, there is evidence that other factors influence its placement, perhaps to ensure its selection over other minimal surfaces [30,65,67]. Polarly distributed proteins and auxin signalling are the two main factors commonly associated with asymmetric divisions in various developmental contexts.
In the amplifying divisions of the stomata lineage, the nucleus and division plane are displaced from the centroid of the cell. The polar distribution of BREAKING OF ASYMMETRY IN THE STOMATAL LINEAGE (BASL) and its interacting members BREVIX RADIX (BRX) or BRX-LIKE (BRXL) is essential to ensure the asymmetry of divisions in the stomatal lineage. BASL is present in a crescent in meristemoids, and its polarity switches following division to generate the characteristic spiral pattern seen in stomatal lineages. BASL triggers nucleus migration away from its location prior to cell division and then towards its location following division with the new cell wall forming away from the cell edge where BASL is enriched [68,69] (Figure 2E–G). Defective expression of BASL or BRX and BRXL family members leads to symmetric divisions in the stomatal lineage [70,71]. In Maize, the polar distribution of PANGLOSS receptors like kinase (PAN1 and PAN2) in the subsidiary mother cell is essential for setting the asymmetry of the division which produces the subsidiary cells [72,73]. The importance of protein polarity for asymmetric cell division could be wider than these specific contexts, indeed recently identified polar proteins of the SOSEKI family are widely expressed in plants and ectopic expression of their members leads to the formation of asymmetric divisions in various organs [74,75]. In addition to polar proteins, auxin signalling has been shown to be associated with the formation of asymmetric divisions.
Auxin is involved in many aspects of plant development from early embryogenesis to the late developmental stages of lateral organs. In many of these developmental processes, divisions associated with auxin signalling are asymmetric. For instance, while high auxin signalling is associated with the asymmetric divisions of the stomatal lineage leading to the guard mother cell formation, the last symmetric division giving rise to the guard cells is preceded by a decrease in auxin responses [76]. Another developmental process where auxin is associated with asymmetric division is the formation of lateral roots (LR). LR are initiated from the pericycle by a pair of anticlinal asymmetric divisions taking place after a local auxin response maxima [77–79]. Pericycle divisions can be triggered by laser ablation of endodermal cells, but only concomitant auxin application can recapitulate the anticlinal asymmetric division [80]. This relationship between auxin and asymmetric divisions is further illustrated by genetic approaches in the embryo. Defective alleles of AUXIN RESPONSE FACTOR 5 are widely affected in auxin responses and presents a shift from asymmetric to symmetric division during embryonic ground tissue initiation [81]. Consistently, ectopic expression of the stabilized negative regulator of auxin responses bodenlos/iaa12 directs symmetric divisions instead of asymmetric at the 8 to 16 cell transition [30] (Figure 2H–I). These symmetric divisions are reminiscent of the conformation adopted by soap films in quadrant (Figure 2J) and the resulting pattern is frequently observed in other globular structures such as glandular trichomes (Figure 2K) [5]. This similar pattern between constrained soap film, glandular trichomes, and auxin response deficient embryos suggests that auxin is able to shift divisions from symmetric to asymmetric. Interestingly auxin triggered asymmetric divisions seem to require local tuning of microtubule stability both in embryonic and lateral root development. Indeed the auxin triggered asymmetric division of pericycle cells requires functional microtubule severing protein KATANIN [80] and pericycle divisions are misplaced in katanin mutants [82]. In addition, a study combining modelling and imaging demonstrated that cell divisions in the embryo could be predicted from the observed CMT orientation. In turn, the CMT orientation prior to division could be predicted by cell geometry with the addition of a cell-edge triggered catastrophe and microtubule stabilization at a selected cell-face. The authors proposed that auxin is the factor responsible for tuning the stability of microtubules at the faces of the cells [67]. Auxin, like mechanical stress and polarity, is a factor that can impact the division plane orientation, but the processes involving these factors are highly intertwined as illustrated by the influence of mechanical stress on both polarly distributed proteins BRXL [83] or auxin transporter PIN FORMED 1 (PIN1) [22,84,85] and microtubules dynamics [85]. While microtubule dynamics are not required for mediating mechanical-stress triggered changes in BRXL or PIN1 polarity [83,85], BASL and BRX effects on pre-division nuclear migration and division asymmetry do require functional microtubule dynamics [69]. Microtubules appear to be central in the integration of multiple signals in the process of division path selection.
Conclusion
Studies spanning several decades have highlighted the fundamental role of microtubules and associated structures in defining cell division orientation. Distinct factors like mechanical stress, polar proteins, and auxin signalling have been described in deviating the division path from the shortest symmetric one. One challenge for the future will be to elucidate the mechanism by with these factor acts on the main structures involved in cell division orientation. Another challenge for the future is to unify the proposed rules for cell division into a common conceptual framework and to understand their biological significance.
Perspectives
Importance to the field: Ultimately all cells result from cell division. As plant cell movements are restricted by cell walls, the orientation of cell divisions determines the organisation of tissues, however, after more than a century of research, the precise mechanism of cell division orientation and the factors acting on it remains an open question.
Current thinking: Improvements in live-imaging, image analyses, computational modelling as well as the establishment of new model species continuously increase our understanding of cell division orientation, however, none of the existing rules can explain all of the observed divisions. Auxin signalling, mechanical stress, and polarly distributed proteins are commonly associated with divisions not following the shortest path.
Future directions: An important challenge for the future would be to determine if there is a universal rule for determining cell division orientation and if not, to identify the mechanism by which cell division orientation is changed. Another important step would be to clarify the function of the PPB.
Acknowledgements
We thank Magalie Uyttewaal (IJPB, INRAE, Versailles France) for kindly provided the meristem image in Figure 2. L.S and S.R are funded by the Gatsby Charitable Foundation (GAT3395/CDE)., S.R is funded by the Royal Society URF (URF\R1\180196).
Abbreviations
- BASL
BREAKING OF ASYMMETRY IN THE STOMATAL LINEAGE
- BRX
BREVIX RADIX
- BRXL
BRX-LIKE
- CDZ
Cortical Division Zone
- LR
Lateral Roots
- MTs
Microtubules
- PAN1
PANGLOSS1
- PAN2
PANGLOSS2
- PHGAPS
PLECKSTRIN HOMOLOGY GAPs
- PIN1
PIN FORMED 1
- POK1/2
PHRAGMOPLAST ORIENTING KINESIN1/2
- PP2A
PROTEIN PHOSPHATASE 2A
- PPB
Pre-Prophase Band of microtubules
- SAM
Shoot Apical Meristem
- TAN1
TANGLED1
- TON1
TONNEAU1
- TRM
TON1 RECRUITING MOTIF
- TTP
TON1-TRM-PP2A
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Author Contribution
L.S. and S.R. conceived the review, L.S. wrote the manuscript and made the figure with input and editing from S.R.
Open Access
Open access for this article was enabled by the participation of University of Cambridge in an all-inclusive Read & Publish pilot with Portland Press and the Biochemical Society under a transformative agreement with JISC.
References
- 1.Hofmeister W. (1863) Zusatze und Berichtigungen zu den 1851 veroffentlichen Untersuchungen der Entwicklung hoherer Kryptogamen. Jahrb Wiss Bot 3, 259–293 [Google Scholar]
- 2.Sachs J. (1878) Ueber die anordnung der zellen in jüngsten pflanzentheilen. Arbeiten d. Bot. Inst 2, 46–104 [Google Scholar]
- 3.Errera L. (1886) Sur une condition fondamentale d’équilibre des cellules vivantes. Annales de la Société belge de microscopie 103, 822–824 [Google Scholar]
- 4.Flanders D.J., Rawlins D.J., Shaw P.J. and Lloyd C.W. (1990) Nucleus-associated microtubules help determine the division plane of plant epidermal cells: avoidance of four-way junctions and the role of cell geometry. J. Cell Biol. 110, 1111–1122 10.1083/jcb.110.4.1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besson S. and Dumais J. (2011) Universal rule for the symmetric division of plant cells. Proc. Natl. Acad. Sci. U.S.A. 108, 6294–6299 10.1073/pnas.1011866108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter R., Sánchez-Corrales Y.E., Hartley M., Grieneisen V.A. and Marée A.F.M. (2017) Pavement cells and the topology puzzle. Development 144, 4386–4397 10.1242/dev.157073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibson W.T., Veldhuis J.H., Rubinstein B., Cartwright H.N., Perrimon N., Brodland G.W. et al. (2011) Control of the mitotic cleavage plane by local epithelial topology. Cell 144, 427–438 10.1016/j.cell.2010.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson M.D.B., Duran-Nebreda S., Kierzkowski D., Strauss S., Xu H., Landrein B. et al. (2019) Global topological order emerges through local mechanical control of cell divisions in the Arabidopsis shoot apical meristem. Cell Syst. 8, 53–65.e3 10.1016/j.cels.2018.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinnott E.W. and Bloch R. (1940) Cytoplasmic behavior during division of vacuolate plant cells. Proc. Natl. Acad. Sci. U.S.A. 26, 223–227 10.1073/pnas.26.4.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venverloo C.J., Hovenkamp P.H., Weeda A.J. and Libbenga K.R. (1980) Cell division in nautilocalyx explants I. Phragmosome, preprophase band and plane of division. Z. Pflanzenphysiol. 100, 161–174 10.1016/S0044-328X(80)80209-1 [DOI] [Google Scholar]
- 11.Traas J.A., Doonan J.H., Rawlins D.J., Shaw P.J., Watts J. and Lloyd C.W. (1987) An actin network is present in the cytoplasm throughout the cell cycle of carrot cells and associates with the dividing nucleus. J. Cell Biol. 105, 387–395 10.1083/jcb.105.1.387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoppin V., Vantard M., Schmit A.C. and Lambert A.M. (1994) Isolated plant nuclei nucleate microtubule assembly: the nuclear surface in higher plants has centrosome-like activity. Plant Cell 6, 1099–1106 10.2307/3869888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pickett-Heaps J.D. and Northcote D.H. (1966) Organization of microtubules and endoplasmic reticulum during mitosis and cytokinesis in wheat meristems. J. Cell Sci. 1, 109–120 PMID: [DOI] [PubMed] [Google Scholar]
- 14.Caillaud M.-C. (2019) Anionic lipids: A pipeline connecting key players of plant cell division. Front. Plant. Sci. 10, 419 10.3389/fpls.2019.00419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunning B.E.S. and Sammut M. (1990) Rearrangements of microtubules involved in establishing cell division planes start immediately after DNA synthesis and are completed just before mitosis. Plant Cell 2, 1273–1282 10.2307/3869345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miart F., Desprez T., Biot E., Morin H., Belcram K., Höfte H. et al. (2014) Spatio-temporal analysis of cellulose synthesis during cell plate formation in Arabidopsis. Plant J. 77, 71–84 10.1111/tpj.12362 [DOI] [PubMed] [Google Scholar]
- 17.Strasburger E. (1882) Ueber den theilungsvorgang der zellkerne und das verhältniss der kerntheilung zur zelltheilung. Archiv für Mikroskopische Anatomie 21, 476–590 10.1007/BF02952628 [DOI] [Google Scholar]
- 18.Bajer A. (1965) Cine micrographic analysis of cell plate formation in endosperm. Exp. Cell Res. 37, 376–398 10.1016/0014-4827(65)90186-2 [DOI] [PubMed] [Google Scholar]
- 19.Smertenko A., Hewitt S.L., Jacques C.N., Kacprzyk R., Liu Y., Marcec M.J. et al. (2018) Phragmoplast microtubule dynamics - a game of zones. J. Cell Sci. 131, jcs203331 10.1242/jcs.203331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucas J.R. and Sack F.D. (2012) Polar development of preprophase bands and cell plates in the Arabidopsis leaf epidermis. Plant J. 69, 501–509 10.1111/j.1365-313X.2011.04809.x [DOI] [PubMed] [Google Scholar]
- 21.Guérin A., Gravelle S. and Dumais J. (2016) Forces behind plant cell division. Proc. Natl. Acad. Sci. U.S.A. 113, 8891–8893 10.1073/pnas.1609309113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamant O., Heisler M.G., Jönsson H., Krupinski P., Uyttewaal M., Bokov P. et al. (2008) Developmental patterning by mechanical signals in Arabidopsis. Science 322, 1650–1655 10.1126/science.1165594 [DOI] [PubMed] [Google Scholar]
- 23.Hamant O., Inoue D., Bouchez D., Dumais J. and Mjolsness E. (2019) Are microtubules tension sensors? Nat. Commun. 10, 2360 10.1038/s41467-019-10207-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson S. and Kuhlemeier C. (2018) Global compression reorients cortical microtubules in arabidopsis hypocotyl epidermis and promotes growth. Curr. Biol. 28, 1794–1802.e2 10.1016/j.cub.2018.04.028 [DOI] [PubMed] [Google Scholar]
- 25.Verger S., Long Y., Boudaoud A. and Hamant O. (2018) A tension-adhesion feedback loop in plant epidermis. eLife 7, e34460 10.7554/eLife.34460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lloyd C.W. (1991) Laser microsurgery demonstrates that cytoplasmic strands anchoring the nucleus across the vacuole of premitotic plant cells are under tension. Implications for division plane alignment. Development 113, 931–939 [Google Scholar]
- 27.Ashkin A. and Dziedzic J.M. (1989) Internal cell manipulation using infrared laser traps. Proc. Natl. Acad. Sci. U.S.A. 86, 7914–7918 10.1073/pnas.86.20.7914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez P., Allsman L.A., Brakke K.A., Hoyt C., Hayes J., Liang H. et al. (2018) Predicting division planes of three-dimensional cells by soap-film minimization. Plant Cell 30, 2255–2266 10.1105/tpc.18.00401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin Q. and Hasenstein K.H (2009) Cytoskeletal changes during spermatogenesis in chara antheridia In The Plant Cytoskeleton: A Key Tool for Agro-Biotechnology (Blume Y.B., Baird W.V., Yemets A.I., Breviario D., eds), pp. 129–142, Springer, Dordrecht, the Netherlands [Google Scholar]
- 30.Yoshida S., de Reuille P B., Lane B., Bassel G.W., Prusinkiewicz P., Smith R.S. et al. (2014) Genetic control of plant development by overriding a geometric division rule. Dev. Cell 29, 75–87 10.1016/j.devcel.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 31.Azimzadeh J., Nacry P., Christodoulidou A., Drevensek S., Camilleri C., Amiour N. et al. (2008) Arabidopsis TONNEAU1 proteins are essential for preprophase band formation and interact with centrin. Plant Cell 20, 2146–2159 10.1105/tpc.107.056812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaefer E., Belcram K., Uyttewaal M., Duroc Y., Goussot M., Legland D. et al. (2017) The preprophase band of microtubules controls the robustness of division orientation in plants. Science 356, 186–189 10.1126/science.aal3016 [DOI] [PubMed] [Google Scholar]
- 33.Chan J., Calder G., Fox S. and Lloyd C. (2005) Localization of the microtubule end binding protein EB1 reveals alternative pathways of spindle development in Arabidopsis suspension cells. Plant Cell 17, 1737–1748 10.1105/tpc.105.032615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lloyd C. and Chan J. (2006) Not so divided: the common basis of plant and animal cell division. Nat. Rev. Mol. Cell Biol. 7, 147–152 10.1038/nrm1831 [DOI] [PubMed] [Google Scholar]
- 35.Ôta T. (1961) The role of cytoplasm in cytokinesis of plant cells. Cytologia (Tokyo) 26, 428–447 10.1508/cytologia.26.428 [DOI] [Google Scholar]
- 36.Gunning B.E. and Wick S.M. (1985) Preprophase bands, phragmoplasts, and spatial control of cytokinesis. J. Cell Sci. Suppl. 2, 157–179 10.1242/jcs.1985.Supplement_2.9 [DOI] [PubMed] [Google Scholar]
- 37.Arima K., Tamaoki D., Mineyuki Y., Yasuhara H., Nakai T., Shimmen T. et al. (2018) Displacement of the mitotic apparatuses by centrifugation reveals cortical actin organization during cytokinesis in cultured tobacco BY-2 cells. J. Plant Res. 131, 803–815 10.1007/s10265-018-1047-4 [DOI] [PubMed] [Google Scholar]
- 38.Palevitz B.A. and Hepler P.K. (1974) The control of the plane of division during stomatal differentiation in Allium. Chromosoma 46, 297–326 10.1007/BF00284884 [DOI] [Google Scholar]
- 39.Livanos P. and Müller S. (2019) Division plane establishment and cytokinesis. Annu. Rev. Plant Biol. 70, 239–267 10.1146/annurev-arplant-050718-100444 [DOI] [PubMed] [Google Scholar]
- 40.Müller S., Han S. and Smith L.G. (2006) Two kinesins are involved in the spatial control of cytokinesis in Arabidopsis thaliana. Curr. Biol. 16, 888–894 10.1016/j.cub.2006.03.034 [DOI] [PubMed] [Google Scholar]
- 41.Stöckle D., Herrmann A., Lipka E., Lauster T., Gavidia R., Zimmermann S. et al. (2016) Putative RopGAPs impact division plane selection and interact with kinesin-12 POK1. Nat. Plants 2, 16120 10.1038/nplants.2016.120 [DOI] [PubMed] [Google Scholar]
- 42.Lipka E., Gadeyne A., Stöckle D., Zimmermann S., De Jaeger G., Ehrhardt D.W. et al. (2014) The phragmoplast-orienting kinesin-12 class proteins translate the positional information of the preprophase band to establish the cortical division zone in Arabidopsis thaliana. Plant Cell 26, 2617–2632 10.1105/tpc.114.124933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez P., Dixit R., Balkunde R.S., Zhang A., O'Leary S.E., Brakke K.A. et al. (2020) TANGLED1 mediates microtubule interactions that may promote division plane positioning in maize. J. Cell Biol. 219, e201907184 10.1083/jcb.201907184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herrmann A., Livanos P., Lipka E., Gadeyne A., Hauser M.-T., Van Damme D. et al. (2018) Dual localized kinesin-12 POK2 plays multiple roles during cell division and interacts with MAP65-3. EMBO Rep. 19, e46085 10.15252/embr.201846085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Panteris E., Adamakis I.-D.S., Voulgari G. and Papadopoulou G. (2011) A role for katanin in plant cell division: microtubule organization in dividing root cells of fra2 and lue1 Arabidopsis thaliana mutants. Cytoskeleton (Hoboken) 68, 401–413 10.1002/cm.20522 [DOI] [PubMed] [Google Scholar]
- 46.Komis G., Luptovčiak I., Ovečka M., Samakovli D., Šamajová O. and Šamaj J. (2017) Katanin effects on dynamics of cortical microtubules and mitotic arrays in Arabidopsis thaliana revealed by advanced live-cell imaging. Front. Plant Sci. 8, 866 10.3389/fpls.2017.00866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sasaki T., Tsutsumi M., Otomo K., Murata T., Yagi N., Nakamura M. et al. (2019) A novel katanin-tethering machinery accelerates cytokinesis. Curr. Biol. 29, 4060–4070.e3 10.1016/j.cub.2019.09.049 [DOI] [PubMed] [Google Scholar]
- 48.Smith L.G., Hake S. and Sylvester A.W. (1996) The tangled-1 mutation alters cell division orientations throughout maize leaf development without altering leaf shape. Development 122, 481–489 PMID: [DOI] [PubMed] [Google Scholar]
- 49.Louveaux M., Julien J.-D., Mirabet V., Boudaoud A. and Hamant O. (2016) Cell division plane orientation based on tensile stress in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 113, E4294–E4303 10.1073/pnas.1600677113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwiatkowska D. (2006) Flower primordium formation at the Arabidopsis shoot apex: quantitative analysis of surface geometry and growth. J. Exp. Bot. 57, 571–580 10.1093/jxb/erj042 [DOI] [PubMed] [Google Scholar]
- 51.Kwiatkowska D. (2008) Flowering and apical meristem growth dynamics. J. Exp. Bot. 59, 187–201 10.1093/jxb/erm290 [DOI] [PubMed] [Google Scholar]
- 52.Kwiatkowska D. and Routier-Kierzkowska A.-L. (2009) Morphogenesis at the inflorescence shoot apex of Anagallis arvensis: surface geometry and growth in comparison with the vegetative shoot. J. Exp. Bot. 60, 3407–3418 10.1093/jxb/erp176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwiatkowska D. and Dumais J. (2003) Growth and morphogenesis at the vegetative shoot apex of Anagallis arvensis L. J. Exp. Bot. 54, 1585–1595 10.1093/jxb/erg166 [DOI] [PubMed] [Google Scholar]
- 54.Lintilhac P.M. and Vesecky T.B. (1984) Stress-induced alignment of division plane in plant tissues grown in vitro. Nature 307, 363–364 10.1038/307363a0 [DOI] [Google Scholar]
- 55.Goodbody K.C. and Lloyd C.W. (1990) Actin filaments line up across Tradescantia epidermal cells, anticipating wound-induced divison planes. Protoplasma 157, 92–101 10.1007/BF01322641 [DOI] [Google Scholar]
- 56.Venverloo C.J. (1990) Regulation of the plane of cell division in vacuolated cells. Protoplasma 155, 85–94 10.1007/BF01322618 [DOI] [Google Scholar]
- 57.Landré P. (1972) Origine et développement des épidermes cotylédonaires et foliaires de la Moutarde (Sinapis alba L.). Différenciation ultrastructurale des stomates. Ann. Sci. Nat. Bot. Biol. Veg. 12, 247–322 [Google Scholar]
- 58.Yang M. and Sack F.D. (1995) The too many mouths and four lips mutations affect stomatal production in Arabidopsis. Plant Cell 7, 2227–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nadeau J.A. and Sack F.D. (2002) Stomatal development in Arabidopsis. Arabidopsis Book 1, e0066 10.1199/tab.0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stebbins G.L. and Shah S.S. (1960) Developmental studies of cell differentiation in the epidermis of monocotyledons. Dev. Biol. 2, 477–500 10.1016/0012-1606(60)90050-6 [DOI] [PubMed] [Google Scholar]
- 61.Casimiro I., Marchant A., Bhalerao R.P., Beeckman T., Dhooge S., Swarup R. et al. (2001) Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13, 843–852 10.1105/tpc.13.4.843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lucas M., Kenobi K., von Wangenheim D., Voβ U., Swarup K., De Smet I. et al. (2013) Lateral root morphogenesis is dependent on the mechanical properties of the overlaying tissues. Proc. Natl. Acad. Sci. U.S.A. 110, 5229–5234 10.1073/pnas.1210807110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.von Wangenheim D., Fangerau J., Schmitz A., Smith R.S., Leitte H., Stelzer E.H.K. et al. (2016) Rules and self-organizing properties of post-embryonic plant organ cell division patterns. Curr. Biol. 26, 439–449 10.1016/j.cub.2015.12.047 [DOI] [PubMed] [Google Scholar]
- 64.Mansfield S.G. and Briarty L.G. (1991) Early embryogenesis in Arabidopsis thaliana. II. The developing embryo. Can. J. Bot. 69, 461–476 10.1139/b91-063 [DOI] [Google Scholar]
- 65.Capron A., Chatfield S., Provart N. and Berleth T. (2009) Embryogenesis: pattern formation from a single cell. Arabidopsis Book 7, e0126 10.1199/tab.0126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moukhtar J., Trubuil A., Belcram K., Legland D., Khadir Z., Urbain A. et al. (2019) Cell geometry determines symmetric and asymmetric division plane selection in Arabidopsis early embryos. PLoS Comput. Biol. 15, e1006771 10.1371/journal.pcbi.1006771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chakrabortty B., Willemsen V., de Zeeuw T., Liao C.-Y., Weijers D., Mulder B. et al. (2018) A plausible microtubule-based mechanism for cell division orientation in plant embryogenesis. Curr. Biol. 28, 3031–3043.e2 10.1016/j.cub.2018.07.025 [DOI] [PubMed] [Google Scholar]
- 68.Robinson S., de Reuille P B., Chan J., Bergmann D., Prusinkiewicz P. and Coen E. (2011) Generation of spatial patterns through cell polarity switching. Science 333, 1436–1440 10.1126/science.1202185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muroyama A., Gong Y. and Bergmann D.C. (2020) Opposing, polarity-driven nuclear migrations underpin asymmetric divisions to pattern Arabidopsis stomata. Curr. Biol. 30, 4467–4475.e4 10.1016/j.cub.2020.08.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rowe M.H., Dong J., Weimer A.K. and Bergmann D.C. (2019) A plant-specific polarity module establishes cell fate asymmetry in the Arabidopsis stomatal lineage. BioRxiv 10.1101/614636 [DOI] [Google Scholar]
- 71.Dong J., MacAlister C.A. and Bergmann D.C. (2009) BASL controls asymmetric cell division in Arabidopsis. Cell 137, 1320–1330 10.1016/j.cell.2009.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cartwright H.N., Humphries J.A. and Smith L.G. (2009) PAN1: a receptor-like protein that promotes polarization of an asymmetric cell division in maize. Science 323, 649–651 10.1126/science.1161686 [DOI] [PubMed] [Google Scholar]
- 73.Facette M.R., Park Y., Sutimantanapi D., Luo A., Cartwright H.N., Yang B. et al. (2015) The SCAR/WAVE complex polarizes PAN receptors and promotes division asymmetry in maize. Nat. Plants 1, 14024 10.1038/nplants.2014.24 [DOI] [PubMed] [Google Scholar]
- 74.Yoshida S., van der Schuren A., van Dop M., van Galen L., Saiga S., Adibi M. et al. (2019) A SOSEKI-based coordinate system interprets global polarity cues in Arabidopsis. Nat. Plants 5, 160–166 10.1038/s41477-019-0363-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Dop M., Fiedler M., Mutte S., de Keijzer J., Olijslager L., Albrecht C. et al. (2020) DIX domain polymerization drives assembly of plant cell polarity complexes. Cell 180, 427–439.e12 10.1016/j.cell.2020.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Le J., Liu X.-G., Yang K.-Z., Chen X.-L., Zou J.-J., Wang H.-Z. et al. (2014) Auxin transport and activity regulate stomatal patterning and development. Nat. Commun. 5, 3090 10.1038/ncomms4090 [DOI] [PubMed] [Google Scholar]
- 77.Malamy J.E. and Benfey P.N. (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124, 33–44 PMID: [DOI] [PubMed] [Google Scholar]
- 78.Dubrovsky J.G., Doerner P.W., Colón-Carmona A. and Rost T.L. (2000) Pericycle cell proliferation and lateral root initiation in Arabidopsis. Plant Physiol. 124, 1648–1657 10.1104/pp.124.4.1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dubrovsky J.G., Sauer M., Napsucialy-Mendivil S., Ivanchenko M.G., Friml J., Shishkova S. et al. (2008) Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc. Natl. Acad .Sci. U.S.A. 105, 8790–8794 10.1073/pnas.0712307105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marhavý P., Montesinos J.C., Abuzeineh A., Van Damme D., Vermeer J.E.M., Duclercq J. et al. (2016) Targeted cell elimination reveals an auxin-guided biphasic mode of lateral root initiation. Genes Dev. 30, 471–483 10.1101/gad.276964.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Möller B.K., Ten Hove C.A., Xiang D., Williams N., López L.G., Yoshida S. et al. (2017) Auxin response cell-autonomously controls ground tissue initiation in the early Arabidopsis embryo. Proc. Natl. Acad. Sci. U.S.A. 114, E2533–E2539 10.1073/pnas.1616493114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ovečka M., Luptovčiak I., Komis G., Šamajová O., Samakovli D. and Šamaj J. (2020) Spatiotemporal pattern of ectopic cell divisions contribute to mis-shaped phenotype of primary and lateral roots of katanin1 mutant. Front. Plant Sci. 11, 734 10.3389/fpls.2020.00734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bringmann M. and Bergmann D.C. (2017) Tissue-wide mechanical forces influence the polarity of stomatal stem cells in Arabidopsis. Curr. Biol. 27, 877–883 10.1016/j.cub.2017.01.059 [DOI] [PubMed] [Google Scholar]
- 84.Nakayama N., Smith R.S., Mandel T., Robinson S., Kimura S., Boudaoud A. et al. (2012) Mechanical regulation of auxin-mediated growth. Curr. Biol. 22, 1468–1476 10.1016/j.cub.2012.06.050 [DOI] [PubMed] [Google Scholar]
- 85.Heisler M.G., Hamant O., Krupinski P., Uyttewaal M., Ohno C., Jönsson H. et al. (2010) Alignment between PIN1 polarity and microtubule orientation in the shoot apical meristem reveals a tight coupling between morphogenesis and auxin transport. PLoS Biol. 8, e1000516 10.1371/journal.pbio.1000516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Louveaux M., Rochette S., Beauzamy L., Boudaoud A. and Hamant O. (2016) The impact of mechanical compression on cortical microtubules in Arabidopsis: a quantitative pipeline. Plant J. 88, 328–342 10.1111/tpj.13290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Y., Wang P., Shao W., Zhu J.-K. and Dong J. (2015) The BASL polarity protein controls a MAPK signaling feedback loop in asymmetric cell division. Dev. Cell 33, 136–149 10.1016/j.devcel.2015.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]