Abstract
Ion Mobility (IM) coupled to mass spectrometry (MS) is a useful tool for separating species of interest out of small quantities of heterogenous mixtures via a combination of m/z and molecular shape. While tandem MS instruments are common, instruments which employ tandem IM are less so with the first commercial IM–MS instrument capable of multiple IM selection rounds being released in 2019. Here we explore the history of tandem IM instruments, recent developments, the applications to biological systems and expected future directions.
Keywords: ion mobility, mass spectrometry, protein structure, tandem ion mobility
Introduction
Mass spectrometry (MS) was once the purview of select chemists and physicists, but is now one of the most widely used analytical methods used by researchers in many other scientific disciplines. It is used to analyse a range of analytes from biopharmaceuticals [1, 2] to explosives [3], and used in fields ranging from life-sciences to ecology [4] and petroleomics [5]. MS has also been an integral part of many space missions [6].
A major key to the success of MS has been the ability to perform tandem MS experiments whereby ions of interest are selected during the first MS analysis stage, dissociated (using a number of different approaches such as collision-induced dissociation [7, 8], electron capture dissociation [9] and infrared photodissociation [10, 11]), with the fragments analysed during the second stage of MS. Tandem MS therefore allows the detailed structural characterisation of ions and has been an integral part of proteomics and other omics fields.
Ion mobility (IM) and, in particular, ion mobility coupled to mass spectrometry (IM–MS), appears to be heading in the same direction. An increasing number of practitioners are using it as part of their analytical toolbox, especially since the development of commercial instrumentation in 2006 [12]. IM functions by separating molecules according to their m/z and reduced mobility K in a neutral buffer gas. IM–MS can be used to calculate the momentum transfer collision cross section (CCS) [13] of an analyte, a descriptor closely related to its three-dimensional structure. Used in conjunction with theoretically calculated CCS values, structures for species of interest can be assigned to the experimental data. IM–MS also provides an extra level of analytical separation which can be used to distinguish isobaric species, something not possible using mass spectrometry alone.
Analysis of biological molecules using IM–MS has provided new insights into systems that are not easily studied by other biophysical and structural approaches. The resulting mobility spectra however can be very complex, especially for proteins, and increases in the IM resolving power alone cannot resolve the multiple overlapping conformational families present in the spectra [14, 15]. In an analogous approach to tandem MS, tandem IM coupled to MS can overcome the limitations of single IM analyses, especially when ions are activated between the two IM rounds.
In this review, we introduce the fundamentals of ion mobility and discuss different ion mobility technologies. We then outline the history of the development of tandem ion mobility–mass spectrometry and discuss expected future advances especially with regards to the analysis of biological molecules.
Ion mobility
Ion mobility (IM), or ion mobility spectrometry (IMS), is rooted in the same physics which birthed mass-spectrometry over 100 hundred years ago and predates MS [16]. In the archetypal drift-tube ion mobility spectrometry (DTIMS) setup, ions moving through a neutral gas under a constant electric field experience a reduced mobility, as collisions occur between the ion and the buffer gas. Ions of the same mass and charge, but different three-dimensional structures, will have different reduced mobilities through the buffer gas: more extended structures will undergo a greater number of collisions and so will take longer to traverse the drift-region than a compact structure. This reduced mobility K can be used to calculate a collision cross-section (CCS) according to the Mason–Schamp equation [17]:
where Ω is CCS, e is the elementary charge, z is the charge state of the analyte ion, N is the density of the drift-gas, µ the reduced mass of the collision ion, kB the Boltzmann constant at temperature T.
The CCS can be used as an effective empirical factor for a variety of biological systems. For instance, in the field of glycomics, it is inherently difficult to assign structures due to the wide variety of isomerism which occurs, however CCS has been used to differentiate between different glycan structures with differential branching [18]. In small molecule analysis CCS can be used as an additional identification factor [19]. IM has also been used in conjunction with native MS to study the dynamics of protein structures [20], fibril formation in amyloid-β [21], and investigate the structural dynamics of disordered proteins — a notoriously difficult system to work with [22–24].
Since the development of DTIMS, there have been numerous other IM methods developed, such as ones where the CCS can be measured directly from reduced mobility: differential mobility analysis (DMA) [25], overtone mobility spectrometry (OMS) [26] and transversal modulation IMS (TMIMS) [27], or where it can be obtained through the use of calibrants [28]: travelling wave IMS (TWIMS) [29] and trapped IMS (TIMS) [30]. IM methods have been discussed in detail elsewhere [13], however we will briefly revise here how different IM methods function.
As previously described, DTIMS functions by applying a constant electric field across the drift-region and therefore the velocity of analyte ions can be described by the Mason–Schamp equation. TWIMS functions by inducing electric fields in consecutive plates within stacked ring ion guides (SRIGs) to create an electrical wave upon which ions ‘surf’ [12, 29, 31]. Both DTIMS and TWIMS are described as time-dispersive as their mobility is defined as the time it takes for each ion to traverse the drift-region with a static gas. Both DMA [25] and field asymmetric waveform IMS (FAIMS) [32, 33] utilise gas which flows perpendicular to a modulated electric field, ions which have a certain mobility are able to traverse the drift-region and pass through an aperture. The difference between DMA and FAIMS is that DMA applies a constant electric field, which is increased or decreased incrementally to obtain an IM spectrum. In comparison FAIMS applies pulses of opposing electrical fields, and so CCS cannot be calculated, unlike with DMA. These are described as spatially dispersive. TIMS utilises an electric field which opposes a gas flow to trap analyte ions. The gating field can then be dropped incrementally and the ions traverse the drift-region. The ions with the largest CCS experience the greatest force through the drift-region [30, 34]. Due to the elution of the ions from the trap being controlled by their movement against the electric field, TIMS can be described as field-dispersive. Recently a new U-shaped mobility analyser (UMA) was developed which features flow and counter-flow separation, comparable to a mixture of DMA and TIMS separation [35].
An important consideration of IM–MS instruments is the resolving power: the ability for an instrument to separate two closely eluting species. Simplistically, resolving power R can be described as:
Where td is drift time at the peak top and w is the full-width half maximum of the peak [36]. It has also been described in terms of td/Δtd or CCS/ΔCCS. The definitions where R is based on td are applicable to IM techniques where td scales proportionally with CCS, which is not the case for techniques such as TWIMS and TIMS. Therefore, the definition CCS/ΔCCS should be considered the most accurate definition of R. Resolving power calculations can be optimised by using more experimental factors, however they are IM method dependent. For instance in DTIMS the R can be increased by increasing the electric field strength, or in TWIMS by altering the velocity of the wave [37].
Ion mobility–mass spectrometry (IM–MS) has been a rapidly expanding field since the 1990s, when publications became more numerous, and has been increasing at an exponential rate (Figure 1A). Multiple mass spectrometry companies have released their own IM–MS instruments, such as the first commercial IM–MS instrument the Synapt (Waters Corp, UK), the FAIMS Quantum (Thermo), 6560 IM QToF (Agilent), ABSciex Selexion (Sciex), IMS-TOF (Tofwerk), and the TIMS-ToF (Bruker).
Figure 1. History and Development of IM-MS and Tandem IM.
(A) History of IM–MS and Tandem-IM publications, from searches on Web of Science for topics: IM–MS; Tandem-IM. Astrices represent where there were fewer than 10 publications per year (B) Timeline of advances of IM, IM–MS and Tandem-IM, with the dating from 1960–2020. References, in chronological order: [16, 25, 27, 29, 30, 32, 33, 35, 38–49].
Development of tandem IM
Another field which has been steadily growing is tandem IM (Figure 1A), whereby multiple rounds of mobility selection can take place, analogous to tandem MS where successive rounds of mass analysis can occur. Tandem MS instruments can provide useful parallels which can be applied to the description of tandem IM instruments, highlighted in the comparison between triple quadrupole and quadrupole ion trap MS instruments [50]. Triple quadrupoles are tandem-in-space where ion selection and activation occur in separate regions of the instrument. Quadrupole ion traps, where ions are accumulated in a trap and then analysis and fragmentation occur in the same space, are tandem-in-time. Both tandem-in-space and tandem-in-time IM–IM instruments have been developed. It should be noted that in this review we will only be discussing tandem IM coupled to mass-spectrometry; other types of tandem IM, such as the stand-alone DMA–DMA [51], FAIMS–DMA [52] and the DMA–DTIMS [38] used for aerosol analysis, will not be discussed.
The first tandem IM instrument was developed in 2001 by the National Measurements Standards of Canda and was a multi-FAIMS separation system, where three FAIMS cells were arranged perpendicularly. The first and the third FAIMS cells acted as separation cells, while the second acted as a trapping cell [53]. The instrument was developed in order to increase the limits of detection of small analyte ions through selection and accumulation of an ion species, which could then be introduced as a single ion cloud.
The applications of FAIMS in tandem IM was expanded to be coupled to a DTIMS cell in 2005 [39]. The FAIMS cell was used to scan through different mobilities using a shifting compensation voltage, which allowed greater sensitivity in the DTIMS cell. This was used initially for increasing the limits of detection for complex peptide mixtures, and was used in 2006 to study the protein conformations of ubiquitin, making it the first use of tandem IM for native proteins [39, 54]. The DTIMS separation post-FAIMS was able to show a greater number of measurable conformers than had been seen previously [54–58].
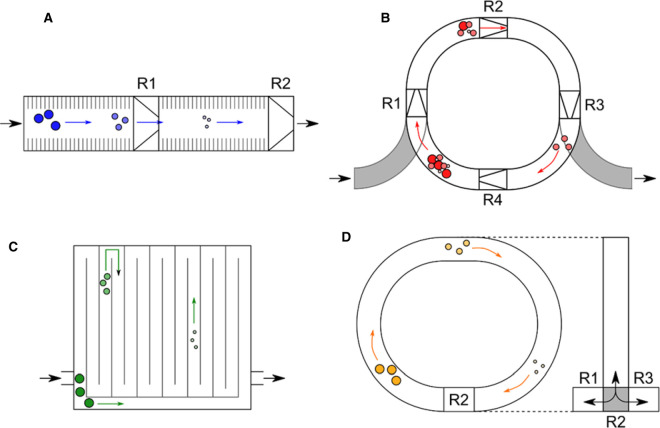
At a similar time in 2006, the Clemmer group developed a tandem DTIMS instrument, comprising separate drift-tubes flanked by funnel gating systems with a total R ∼ 500 (∼50 for the first DTIMS section and ∼10 for the second) (Figure 2A) [40, 59]. This geometry allowed a variety of experiments to be performed, depending on how the gating systems (Figure 2A, R1 and R2) were used. R1 or R2 could allow full ion transmission, which resulted in greater IM resolution for all species; as gating devices allowing only a single species to be transmitted for mobility separation; or as an ion activation cell, allowing fragmentation pre- or post-mobility. The same year a three drift-cell IM–MS instrument, using the same geometry of drift-cell flanked by ion funnels, was developed by the same group [41]. These geometries could be used flexibly and the first experiments were initially used to increase peptide fragmentation coverage by using the mobility selection and activation [40, 41, 60].
Figure 2. Example geometries of select tandem-IM instruments.
(A) tandem DTIMS presented by Koeniger et al. [40] where ion packets can be transmitted, selected or activated in regions R1 and R2 (B) ion cyclotron presented by Merenbloom et al. [61] where ion packets of particular mobilities are selected for in regions R1–R4 (C) SUPER-SLIM presented by Deng et al. [43] where ion packets can be pushed to either travel through or bypass a serpentine IM track D) cyclic IM-QToF presented by Giles et al. [47] where ion packets can be stored and activated in regions R1 and R3, and introduced to the cyclic IM guide and activated in region R2.
These linear IM–IM instruments were also used for native MS analysis of well-characterised proteins such as ubiquitin [14]. By taking variable mobility selections and activating them it was possible to directly track stable and unstable precursor conformations and their transitional and final states. This sort of methodology is of particular interest to those who study protein unfolding or misfolding, as the majority of other structural biology techniques are only able to measure ensemble states. Indeed these systems were used to develop a new method of ion mobility separation: overtone mobility spectrometry (OMS) [62]. One observation of utilising the funnel regions present in the linear tandem DTIMS instrument for mobility selection was an increase in resolution. Ions which have the same resonance as the frequency of the gating funnel have a mobility resolution which is higher than that of the resolving power given by the frequency, hence the designation overtone [26, 62–64].
OMS was limited by the number of funnels which an ion packet could pass through, so efforts were made to create an IM–MS instrument with a circular drift-region [61]. This has clear advantages over linear instruments as the total drift distance can be incrementally increased. The ion cyclotron described in 2009 had funnels connecting curved DTIMS regions and allowed refocusing of ions to prevent loss of signal (Figure 2B) [61]. The electric fields across these regions is applied at a selected frequency, and the field is switched between each segment and funnel. The function of this is that only ions with mobilities resonant to the frequency of field application are able to proceed to the following segment, whilst others are removed. Simplistically, ‘trimming’ of the ion packet occurs by the gating funnels to allow selection of a single mobility species and increasing the mobility resolution of that species [61]. While the trimming of the ion packets increased the mobility resolution up to R > 1000, possibly artificially, the sensitivity of the system is reduced due to the continual loss of ions due to non-resonance with the drift-field frequency. The sensitivity of the ion cyclotron can be increased by using a different trapping mechanism allowing multiple ion packets to be introduced prior to mobility separation [65]. Another limitation of OMS and the cyclotron in particular is that the only ions which have a stable conformation can be transmitted [66] and early work by the Clemmer group specifically showed that the conformations of proteins in the gas phase can decay over time-scale of milliseconds under certain conditions [58, 67, 68].
A decade of expansion
In the past decade many advances have been made in tandem IM instrumentation. Transversal modulation IMS (TMIMS) [27] for example operates at atmospheric pressures, and similarly to DMA analysers, utilises electric fields. Perpendicular and oscillating fields create a stable trajectory for ions of a particular mobility. During the first publication a tandem TMIMS configuration cell was tested, which was then coupled at the front end of a mass-spectrometer, showing a resolving power of R > 80 [27, 49].
Developed later were structures for lossless ion manipulation (SLIM) [42], which formed the basis of future tandem IM devices such as the SUPER [43] and M-SLIM [44] instruments discussed later. These structures were based on TWIMS, and utilised modified RF amplitudes and DC guarding voltages in order to allow manipulation of the ion packet with almost completely negligible transmission losses. This technology could move ion packets orthogonally into ion traps before selecting species for further mobility separation. This was then explored further to create a serpentine ultralong path with extended routing (SUPER) [43]. This involved a S-shape path of SLIM transmission which could allow full or partial routing of an ion packet through a region of ∼18 m in length (Figure 2C). This could be performed multiple times allowing ion separation lengths of a 1 km, with a resolving power of R ∼ 1860 being reported. [43] In this instrument, protein conformations were found to be stable in the gas-phase for much longer periods than previously reported. This is most likely due to the specific instrument used for IM analysis, as sections of it operated at a lower pressure [69]. This was used in order to measure the mobility differences present in molecules with differential isotopic abundances.
Not discussed in this review at length, has been the application of tandem IM to aerosol analysis. Tandem DMA [51] and FAIMS instruments can often be used stand-alone coupled to detectors, such as a condensation particle counter [70] (CPC) or a Raman spectroscopy [71]. In 2018 a tandem DMA cell, modified for fragmentation of highly thermally stable volatile compounds, was coupled to a triple-quadrupole, and then faraday cup detector system [45]. These detectors allowed proof-of-concept for the fragmentation and identification of a variety of explosive compounds through the use of IM alone.
A tandem trapped ion mobility spectrometer (TIMS) has also been developed [46], which is comparable in geometry to the tandem DTIMS instruments [40, 41] discussed previously (Figure 2A). Two TIMS cells are aligned with an interface capable of gating and activation, allowing mobility selection of sub-species and ion activation. A novel aspect of TIMS is that the geometry allows an increase in the duty-cycle of fragmentation. This is through the accumulation of ions in the entrance funnel prior to single stage mobility analysis and subsequent fragmentation [72], meaning that in a tandem TIMS instrument the serial fragmentation capabilities could be greatly increased. These sorts of applications, along with the initial tandem DTIMS experiments [40, 41, 60], allow an increase in the information which can be gained from each injection, as there can be multiple rounds of separation and fragmentation at a peptide level. The implications for ‘bottom-up’ proteomics is that there will be increased sensitivity and resolution for samples due to the multiple rounds of separation in the instrument.
A new geometry capable of flexible tandem IM experiments was announced in 2018, and then released as part of a commercial IM–MS system: the cyclic IM QToF system from Waters Corp [47, 73–75]. The geometry presented in the cyclic instrument is unlike the linear tandem DTIMS or ion cyclotron presented previously [40, 41, 61]. The ion mobility cell is made of curved TWIMS sections, which sits orthogonally to the direction of the ion path. Ion packets can be introduced via a multi-directional array, which allows multiple modes of operation. While it may appear superficially similar to the ion cyclotron, it operates in a significantly different manner, allowing ion storage and tandem-in-time IM analysis.
Initially, the IM resolution can be scaled incrementally by increasing the number of passes an ion packet experiences in the drift-region, which was used to successfully differentiate between inverse peptides SDGRG and GRGDS after 100 passes with an R ∼ 750 [47]. However more complex analyses can be performed. The section which sits in-line with the cyclic IM cell is called the array (Figure 2D, R3), and it is flanked by both ‘pre-array’ and ‘post-array’ storage regions. While ion packets are traversing the drift-region, subsections of the ion packet, upon crossing the array, can be ‘sliced’ out and stored for further analysis in the pre-array region, allowing expulsion of the remaining ions from the drift-region for mass analysis, whereby the retained ions can be analysed for IM. These slices can be analysed by more multi-pass or by collisional activation (CA). While multi-pass analysis has been shown to be very effective for increasing the resolution of small molecules [75], it is not as effective for larger systems such as proteins [15]. With larger systems, performing slice-CA has been shown to be far more effective for distinguishing between overlapping mobility species, as arrival time distributions for proteins appear to be more diffuse due to the inherent flexibility within these systems (Eldrid et al. [76] under review).
One of the limitations of the systems which are able to perform multi-pass analysis (cIM and SUPER-SLIM) is from ‘roll-over’, whereby during the multiple passes of the mobility separations, high-mobility ions overtake low-mobility ions, leading to an overlap in data and artefacts. To overcome this, multi-level SLIM [42, 43] (M-SLIM) was developed in 2020 where ions can be pushed vertically to different separation levels [44]. This cleverly circumvents collecting data over a wide mobility range without performing mobility selection (i.e. slicing in the cIMs) as higher mobility ions are elevated to undergo multiple passes separately from lower mobility species. Each of these SUPER-SLIM levels can elute ions towards mass-detection separately, preventing ion packets becoming mixed post-separation.
What's on the horizon?
Currently there is a single commercially available tandem IM–MS instrument available, the cyclic IMS QToF from Waters [47] however Bruker is developing a tandem TIMS ToF, and the SLIMS technology is being commercialised by MOBILion [46]. The release of the cyclic IM QToF is of considerable interest to the IM–MS community, as in a single year, it has lead to an almost global doubling of the number of MS instruments capable of tandem IM, which were previously built in-house by highly specialised groups. We expect the field of tandem IM to increase in popularity and research output due to the expansion of the number of instruments present.
This greater accessibility of tandem IM will likely aid research in diverse fields. In particular fields which deal with highly heterogenous mixtures such as glycomics, lipidomics or petroleomics seem to be ideal targets. A recent tandem IM study on glycans showed that specific mobility species produced fragments with specific isotopic shifts, allowing identification of anomeric forms of saccharides without derivatisation [75].
DTIMS remained a standalone technique for almost 70 years before coupling with MS, and since then the field has developed to include a diverse set of separation techniques, and is commonly coupled to analytical instrumentation. Tandem IM instruments were developed and a flurry of research in the following decade has lead to significant technical advances, through creation of instruments of increased resolving power capable of measuring systems on the scale of 100s of milliseconds to seconds [15, 77]. This now brings ion mobility into the time-scale of protein folding events, something which was not available two decades ago during the nascent combination of IM and MS [58, 67, 68].
We expect the latest innovations will be incorporated into a variety of systems, allowing a variety of data collection methods to either increase the resolution of the data gathered by IM and IM–IM methods, or allow deep probing of particular subsets such as with tandem ion mobility coupled to activation of ions between successive IM rounds. It is also to be expected that these advances will be coupled with a variety of spectroscopic or fragmentation methods which will be applicable to multiple systems. There are already publications showing that a combination of IM and top-down fragmentation methods can give detailed structural information [78]. These dissociation techniques, such as infrared photodissociation (IR-PD), ultraviolet photodissociation (UV-PD) and electron capture dissociation (ECD), are proven to allow characterisation of a variety of systems to a greater degree than before [79]. Similarly the use of infrared spectroscopy has been combined with IM to give detailed conformational information on analytes [80, 81]. We can only expect the scientific output using these instruments to increase and diversify.
While the instruments developed so far allow for tandem IM experiments it is worth noting that IM can be performed to the nth level, IMN, experiments are also possible with some of the instruments described [15, 43, 44, 47]. Again in analogy to MSN we expect that such IMN experiments will also allow even further probing of structural ensembles.
As with the development of any new technology we also anticipate the development of new software. Processing and visualisation of the multi-dimensional data obtained from these experiments is complex using existing software [82].
In this review we have discussed the development and expansion of tandem IM and its' applications. The field has increased in areas of research output, applications and instrumentation developed, and very encouraging results show that it has become a highly useful analytical tool. We expect tandem ion mobility to experience the same uptake that occurred with the commercialisation of IM, which is now becoming standard addition to MS instrumentation. As with MS we expect IM to become ubiquitous in laboratories around the world.
Perspectives
Tandem IM coupled to mass spectrometry enables a variety of experiments to be carried out for probing in detail the conformation of ions. We have seen the most application of this technology in the biochemistry and structural biology fields. Tandem IM coupled with ion activation processes is able to give new insights on systems such as glycan structure [75] or protein conformation and unfolding [14, 76].
Tandem IM is currently experiencing a growth of interest (Figure 1A), possibly due to the availability of instrumentation, with analytical instrumentation companies having released or are preparing to release tandem IM–MS products [46, 47].
As the instrumentation becomes more readily available we expect to see new fragmentation techniques coupled to tandem IM. New software to analyse the multi-dimensional data will also need to be developed, as multi-dimensional data-sets are produced. Further work should ensure tandem IM–MS is made accessible to as many researchers as possible, as its potential advantages for many fields of research has not been fully realised yet.
Abbreviations
- CA
collisional activation
- CCS
collision cross section
- DMA
differential mobility analysis
- DTIMS
drift-tube ion mobility spectrometry
- FAIMS
field asymmetric waveform IMS
- IM
ion mobility
- IMS
ion mobility spectrometry
- MS
mass spectrometry
- OMS
overtone mobility spectrometry
- SLIM
structures for lossless ion manipulation
- TIMS
trapped IMS
- TMIMS
transversal modulation IMS
- TWIMS
travelling wave IMS
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
C.E. is funded by a BBSRC iCASE Award with Waters BB/L015382/1.
Author Contribution
C.E. and K.T. wrote the manuscript.
Open Access
Open access for this article was enabled by the participation of University College London in an all-inclusive Read & Publish pilot with Portland Press and the Biochemical Society under a transformative agreement with JISC.
References
- 1.Rosati S., Yang Y., Barendregt A. and Heck A.J.R. (2014) Detailed mass analysis of structural heterogeneity in monoclonal antibodies using native mass spectrometry. Nat. Protoc. 9, 967–976 10.1038/nprot.2014.057 [DOI] [PubMed] [Google Scholar]
- 2.Deng B., Lento C. and Wilson D.J. (2016) Hydrogen deuterium exchange mass spectrometry in biopharmaceutical discovery and development: a review. Anal. Chim. Acta 940, 8–20 10.1016/j.aca.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 3.Evans C.S., Sleeman R., Luke J. and Keely B.J. (2002) A rapid and efficient mass spectrometric method for the analysis of explosives. Rapid Commun. Mass Spectrom. 16, 1883–1891 10.1002/rcm.799 [DOI] [PubMed] [Google Scholar]
- 4.Matta M.E., Orland I.J., Ushikubo T., Helser T.E., Black B.A. and Valley J.W. (2013) Otolith oxygen isotopes measured by high-precision secondary ion mass spectrometry reflect life history of a yellowfin sole (Limanda aspera). Rapid Commun. Mass Spectrom. 27, 691–699 10.1002/rcm.6502 [DOI] [PubMed] [Google Scholar]
- 5.Cho Y., Ahmed A., Islam A. and Kim S. (2015) Developments in FT-ICR ms instrumentation, ionization techniques, and data interpretation methods for petroleomics. Mass Spectrom. Rev. 34, 248–263 10.1002/mas.21438 [DOI] [PubMed] [Google Scholar]
- 6.Mahaffy P.R., Webster C.R., Atreya S.K., Franz H., Wong M., Conrad P.G. et al. (2013) Abundance and isotopic composition of gases in the martian atmosphere from the curiosity rover. Science 341, 263–266 10.1126/science.1237966 [DOI] [PubMed] [Google Scholar]
- 7.Haddon W.F. and McLafferty F.W. (1968) Metastable Ion characteristics. VII. collision-Induced metastables. J. Am. Chem. Soc. 90, 4745–4746 10.1021/ja01019a053 [DOI] [Google Scholar]
- 8.Jennings K.R. (1968) Colllision-induced decompositions of aromatic ions. Int. J. Mass Spectrom. 1, 227–235 10.1016/0020-7381(68)85002-8 [DOI] [Google Scholar]
- 9.Zubarev R.A., Kelleher N.L. and McLafferty F.W. (1998) Electron capture dissociation of multiply charged protein cations. A nonergodic process. J. Am. Chem. Soc. 120, 3265–3266 10.1021/ja973478k [DOI] [PubMed] [Google Scholar]
- 10.Baykut G., Watson C.H., Weller R.R. and Eyler J.R. (1985) Infrared multiphoton dissociation of some oxygen-containing hydrocarbon ions. differentiation of isomeric ion structures in the gas phase. J. Am. Chem. Soc. 107, 8036–8042 10.1021/ja00312a040 [DOI] [Google Scholar]
- 11.Little D.P., Speir J.P., Senko M.W., O'Connor P.B. and McLafferty F.W. (1994) Infrared multiphoton dissociation of large multiply charged ions for biomolecule sequencing. Anal. Chem. 66, 2809–2815 10.1021/ac00090a004 [DOI] [PubMed] [Google Scholar]
- 12.Pringle S.D., Giles K., Wildgoose J.L., Williams J.P., Slade S.E., Thalassinos K. et al. (2007) An investigation of the mobility separation of some peptide and protein ions using a new hybrid quadrupole/travelling wave IMS/oa-ToF instrument. Int. J. Mass Spectrom. 261, 1–12 10.1016/j.ijms.2006.07.021 [DOI] [Google Scholar]
- 13.Gabelica V., Shvartsburg A.A., Afonso C., Barran P., Benesch J.L.P., Bleiholder C. et al. (2019) Recommendations for reporting ion mobility mass spectrometry measurements. Mass Spectrom. Rev. 38, 291–320 10.1002/mas.21585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koeniger S.L. and Clemmer D.E. (2007) Resolution and structural transitions of elongated states of ubiquitin. J. Am. Soc. Mass Spectrom. 18, 322–331 10.1016/j.jasms.2006.09.025 [DOI] [PubMed] [Google Scholar]
- 15.Eldrid C., Ujma J., Kalfas S., Tomczyk N., Giles K., Morris M. et al. (2019) Gas phase stability of protein ions in a cyclic ion mobility spectrometry traveling wave device. Anal. Chem. 91, 7554–7561 10.1021/acs.analchem.8b05641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeleny J. (1898) VI. On the ratio of the velocities of the two ions produced in gases by Röntgen radiation; and on some related phenomena. London, Edinburgh. Dublin Philos. Mag. J. Sci. 46, 120–154 10.1080/14786449808621173 [DOI] [Google Scholar]
- 17.Revercomb H.E. and Mason E.A. (1975) Theory of plasma chromatography/gaseous electrophoresis. A review. Anal. Chem. 47, 970–983 10.1021/ac60357a043 [DOI] [Google Scholar]
- 18.Barroso A., Giménez E., Konijnenberg A., Sancho J., Sanz-Nebot V. and Sobott F. (2018) Evaluation of ion mobility for the separation of glycoconjugate isomers due to different types of sialic acid linkage, at the intact glycoprotein, glycopeptide and glycan level. J. Proteomics 173, 22–31 10.1016/j.jprot.2017.11.020 [DOI] [PubMed] [Google Scholar]
- 19.Mairinger T., Causon T.J. and Hann S. (2018) The potential of ion mobility–mass spectrometry for non-targeted metabolomics. Curr. Opin. Chem. Biol. 42, 9–15 10.1016/j.cbpa.2017.10.015 [DOI] [PubMed] [Google Scholar]
- 20.Shelimov K.B., Clemmer D.E., Hudgins R.R. and Jarrold M.F. (1997) Protein structure in vacuo: gas-phase conformations of BPTI and cytochrome c. J. Am. Chem. Soc. 119, 2240–2248 10.1021/ja9619059 [DOI] [Google Scholar]
- 21.Bernstein S.L., Dupuis N.F., Lazo N.D., Wyttenbach T., Condron M.M., Bitan G. et al. (2009) Amyloid-β 2 protein oligomerization and the importance of tetramers and dodecamers in the aetiology of Alzheimer's disease. Nat. Chem. 1, 326–331 10.1038/nchem.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beveridge R., Covill S., Pacholarz K.J., Kalapothakis J.M.D., Macphee C.E. and Barran P.E. (2014) A mass-spectrometry-based framework to define the extent of disorder in proteins. Anal. Chem. 86, 10979–10991 10.1021/ac5027435 [DOI] [PubMed] [Google Scholar]
- 23.Dickinson E.R., Jurneczko E., Pacholarz K.J., Clarke D.J., Reeves M., Ball K.L. et al. (2015) Insights into the conformations of three structurally diverse proteins: cytochrome c, p53, and MDM2, provided by variable-temperature Ion mobility mass spectrometry. Anal. Chem. 87, 3231–3238 10.1021/ac503720v [DOI] [PubMed] [Google Scholar]
- 24.Ridgway Z., Lee K.-H., Zhyvoloup A., Wong A., Eldrid C., Hannaberry E. et al. (2020) Analysis of baboon IAPP provides insight into amyloidogenicity and cytotoxicity of human IAPP. Biophys. J. 118, 1142–1151 10.1016/j.bpj.2019.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reischl G.P. (1991) Measurement of ambient aerosols by the differential mobility analyzer method: concepts and realization criteria for the size range between 2 and 500 nm. Aerosol Sci. Technol. 14, 5–24 10.1080/02786829108959467 [DOI] [Google Scholar]
- 26.Valentine S.J., Stokes S.T., Kurulugama R.T., Nachtigall F.M. and Clemmer D.E. (2009) Overtone mobility spectrometry: Part 2. theoretical considerations of resolving power. J. Am. Soc. Mass Spectrom. 20, 738–750 10.1016/j.jasms.2009.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vidal-De-Miguel G., MacÍa M. and Cuevas J. (2012) Transversal modulation ion mobility spectrometry (TM-IMS), a new mobility filter overcoming turbulence related limitations. Anal. Chem. 84, 7831–7837 10.1021/ac301127u [DOI] [PubMed] [Google Scholar]
- 28.May J.C., Morris C.B. and McLean J.A. (2017) Ion mobility collision cross section compendium. Anal. Chem. 89, 1032–1044 10.1021/acs.analchem.6b04905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giles K., Pringle S.D., Worthington K.R., Little D., Wildgoose J.L. and Bateman R.H. (2004) Applications of a travelling wave-based radio-frequency-only stacked ring ion guide. Rapid Commun. Mass Spectrom. 18, 2401–2414 10.1002/rcm.1641 [DOI] [PubMed] [Google Scholar]
- 30.Fernandez-Lima F.A., Kaplan D.A. and Park M.A. (2011) Note: integration of trapped ion mobility spectrometry with mass spectrometry. Rev. Sci. Instrum. 82, 126106 10.1063/1.3665933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giles K., Williams J.P. and Campuzano I. (2011) Enhancements in travelling wave ion mobility resolution. Rapid Commun. Mass Spectrom. 25, 1559–1566 10.1002/rcm.5013 [DOI] [PubMed] [Google Scholar]
- 32.Gorshkov M.P. (1982) USSR Inventors Certificate no. 966583. Byull. Izobr
- 33.Buryakov I.A., Krylov E V., Nazarov E.G. and Rasulev U.K. (1993) A new method of separation of multi-atomic ions by mobility at atmospheric pressure using a high-frequency amplitude-asymmetric strong electric field. Int. J. Mass Spectrom. Ion Process. 128, 143–148 10.1016/0168-1176(93)87062-W [DOI] [Google Scholar]
- 34.Fernandez-Lima F., Kaplan D.A., Suetering J. and Park M.A. (2011) Gas-phase separation using a trapped ion mobility spectrometer. Int. J. Ion Mobil. Spectrom. 14, 93–98 10.1007/s12127-011-0067-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang K., Qiu R., Zhang X., Gillig K.J. and Sun W. (2020) U-Shaped mobility analyzer: a compact and high-Resolution counter-Flow Ion mobility spectrometer. Anal. Chem. 92, 8356–8363 10.1021/acs.analchem.0c00868 [DOI] [PubMed] [Google Scholar]
- 36.Siems W.F., Wu C., Tarver E.E., Hill H.H., Larsen P.R. and McMinn D.G. (1994) Measuring the resolving power of Ion mobility spectrometers. Anal. Chem. 66, 4195–4201 10.1021/ac00095a014 [DOI] [Google Scholar]
- 37.Dodds J.N., May J.C. and McLean J.A. (2017) Correlating resolving power, resolution, and collision cross section: unifying cross-Platform assessment of separation efficiency in ion mobility spectrometry. Anal. Chem. 89, 12176–12184 10.1021/acs.analchem.7b02827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oberreit D.R., McMurry P.H. and Hogan C.J. (2014) Analysis of heterogeneous uptake by nanoparticles via differential mobility analysis-drift tube ion mobility spectrometry. Phys. Chem. Chem. Phys. 16, 6968–6979 10.1039/C3CP54842B [DOI] [PubMed] [Google Scholar]
- 39.Tang K., Li F., Shvartsburg A.A., Strittmatter E.F. and Smith R.D. (2005) Two-dimensional gas-phase separations coupled to mass spectrometry for analysis of complex mixtures. Anal. Chem. 77, 6381–6388 10.1021/ac050871x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koeniger S.L., Merenbloom S.I., Valentine S.J., Jarrold M.F., Udseth H.R., Smith R.D. et al. (2006) An IMS-IMS analogue of MS-MS. Anal. Chem. 78, 4161–4174 10.1021/ac051060w [DOI] [PubMed] [Google Scholar]
- 41.Merenbloom S.I., Koeniger S.L., Valentine S.J., Plasencia M.D. and Clemmer D.E. (2006) IMS-IMS and IMS-IMS-IMS/MS for separating peptide and protein fragment ions. Anal. Chem. 78, 2802–2809 10.1021/ac052208e [DOI] [PubMed] [Google Scholar]
- 42.Webb I.K., Garimella S.V.B., Tolmachev A V., Chen T.C., Zhang X., Norheim R V. et al. (2014) Experimental evaluation and optimization of structures for lossless ion manipulations for ion mobility spectrometry with time-of-flight mass spectrometry. Anal. Chem. 86, 9169–9176 10.1021/ac502055e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deng L., Webb I.K., Garimella S.V.B., Hamid A.M., Zheng X., Norheim R V. et al. (2017) Serpentine ultralong path with extended routing (SUPER) high resolution traveling wave ion mobility-MS using structures for lossless Ion manipulations. Anal. Chem. 89, 4628–4634 10.1021/acs.analchem.7b00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hollerbach A.L., Li A., Prabhakaran A., Nagy G., Harrilal C.P., Conant C.R. et al. (2020) Ultra-high-resolution ion mobility separations over extended path lengths and mobility ranges achieved using a multilevel structures for lossless ion manipulations module. Anal. Chem. 92, 7972–7979 10.1021/acs.analchem.0c01397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amo-González M., Carnicero I., Pérez S., Delgado R., Eiceman G.A., Fernández De La Mora G. et al. (2018) Ion mobility spectrometer-fragmenter-ion mobility spectrometer analogue of a triple quadrupole for high-resolution Ion analysis at atmospheric pressure. Anal. Chem. 90, 6885–6892 10.1021/acs.analchem.8b01086 [DOI] [PubMed] [Google Scholar]
- 46.Liu F.C., Ridgeway M.E., Park M.A. and Bleiholder C. (2018) Tandem trapped ion mobility spectrometry. Analyst 143, 2249–2258 10.1039/C7AN02054F [DOI] [PubMed] [Google Scholar]
- 47.Giles K., Ujma J., Wildgoose J., Pringle S., Richardson K., Langridge D. et al. (2019) A cyclic ion mobility-Mass spectrometry system. Anal. Chem. 91, 8564–8573 10.1021/acs.analchem.9b01838 [DOI] [PubMed] [Google Scholar]
- 48.McAfee K.B. and Edelson D. (1963) Identification and mobility of ions in a townsend discharge by time-resolved mass spectrometry [8]. Proc. Phys. Soc. 81, 382–384 10.1088/0370-1328/81/2/125 [DOI] [Google Scholar]
- 49.Vidal-de-Miguel G., Macía M., Barrios C. and Cuevas J. (2015) Transversal modulation Ion mobility spectrometry (IMS) coupled with mass spectrometry (MS): exploring the IMS-IMS-MS possibilities of the instrument. Anal. Chem. 87, 1925–1932 10.1021/ac504178n [DOI] [PubMed] [Google Scholar]
- 50.Johnson J V., Yost R.A., Kelley P.E. and Bradford D.C. (1990) Tandem-in-space and tandem-in-time mass spectrometry: triple quadrupoles and quadrupole ion traps. Anal. Chem. 62, 2162–2172 10.1021/ac00219a003 [DOI] [Google Scholar]
- 51.Rader D.J. and McMurry P.H. (1986) Application of the tandem differential mobility analyzer to studies of droplet growth or evaporation. J. Aerosol Sci. 17, 771–787 10.1016/0021-8502(86)90031-5 [DOI] [Google Scholar]
- 52.Menlyadiev M.R., Tarassov A., Kielnecker A.M. and Eiceman G.A. (2015) Tandem differential mobility spectrometry with ion dissociation in air at ambient pressure and temperature. Analyst 140, 2995–3002 10.1039/C4AN02159B [DOI] [PubMed] [Google Scholar]
- 53.Guevremont R., Ding L., Ells B., Barnett D.A. and Purves R.W. (2001) Atmospheric pressure ion trapping in a tandem FAIMS-FAIMS coupled to a TOFMS: studies with electrospray generated gramicidin S ions. J. Am. Soc. Mass Spectrom. 12, 1320–1330 10.1016/S1044-0305(01)00321-X [DOI] [PubMed] [Google Scholar]
- 54.Shvartsburg A.A., Li F., Tang K. and Smith R.D. (2006) Characterizing the structures and folding of free proteins using 2-D gas-phase separations: observation of multiple unfolded conformers. Anal. Chem. 78, 3304–3315 10.1021/ac060283z [DOI] [PubMed] [Google Scholar]
- 55.Valentine S.J., Counterman A.E. and Clemmer D.E. (1997) Conformer-dependent proton-transfer reactions of ubiquitin ions. J. Am. Soc. Mass Spectrom. 8, 954–961 10.1016/S1044-0305(97)00085-8 [DOI] [Google Scholar]
- 56.Purves R.W., Barnett D.A., Ells B. and Guevremont R. (2000) Investigation of bovine ubiquitin conformers separated by high-field asymmetric waveform ion mobility spectrometry: cross section measurements using energy-loss experiments with a triple quadrupole mass spectrometer. J. Am. Soc. Mass Spectrom. 11, 738–745 10.1016/S1044-0305(00)00136-7 [DOI] [PubMed] [Google Scholar]
- 57.Purves R.W., Barnett D.A., Ells B. and Guevremont R. (2001) Elongated conformers of charge states +11 to +15 of bovine ubiquitin studied using ESI-FAIMS-MS. J. Am. Soc. Mass Spectrom. 12, 894–901 10.1016/S1044-0305(01)00272-0 [DOI] [PubMed] [Google Scholar]
- 58.Myung S., Badman E.R., Lee Y.J. and Clemmer D.E. (2002) Structural transitions of electrosprayed ubiquitin ions stored in an ion trap over ∼10 ms to 30 s. J. Phys. Chem. A 106, 9976–9982 10.1021/jp0206368 [DOI] [Google Scholar]
- 59.Shaffer S.A., Prior D.C., Anderson G.A., Udseth H.R. and Smith R.D. (1998) An Ion funnel interface for improved Ion focusing and sensitivity using electrospray ionization mass spectrometry. Anal. Chem. 70, 4111–4119 10.1021/ac9802170 [DOI] [PubMed] [Google Scholar]
- 60.Merenbloom S.I., Bohrer B.C., Koeniger S.L. and Clemmer D.E. (2007) Assessing the peak capacity of IMS-IMS separations of tryptic peptide ions in He at 300 K. Anal. Chem. 79, 515–522 10.1021/ac061567m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Merenbloom S.I., Glaskin R.S., Henson Z.B. and Clemmer D.E. (2009) High-resolution ion cyclotron mobility spectrometry. Anal. Chem. 81, 1482–1487 10.1021/ac801880a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kurulugama R.T., Nachtigall F.M., Lee S., Valentine S.J. and Clemmer D.E. (2009) Overtone mobility spectrometry: Part 1. experimental observations. J. Am. Soc. Mass Spectrom. 20, 729–737 10.1016/j.jasms.2008.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valentine S.J., Kurulugama R.T. and Clemmer D.E. (2011) Overtone mobility spectrometry: Part 3. On the origin of peaks. J. Am. Soc. Mass Spectrom. 22, 804–816 10.1007/s13361-011-0087-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kurulugama R.T., Nachtigall F.M., Valentine S.J. and Clemmer D.E. (2011) Overtone mobility spectrometry: Part 4. OMS-OMS analyses of complex mixtures. J. Am. Soc. Mass Spectrom. 22, 2049–2060 10.1007/s13361-011-0217-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glaskin R.S., Ewing M.A. and Clemmer D.E. (2013) Ion trapping for ion mobility spectrometry measurements in a cyclical drift tube. Anal. Chem. 85, 7003–7008 10.1021/ac4015066 [DOI] [PubMed] [Google Scholar]
- 66.Lee S., Ewing M.A., Nachtigall F.M., Kurulugama R.T., Valentine S.J. and Clemmer D.E. (2010) Determination of cross sections by overtone mobility spectrometry: evidence for loss of unstable structures at higher overtones. J. Phys. Chem. B. 114, 12406–12415 10.1021/jp1060123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Badman E.R., Hoaglund-Hyzer C.S. and Clemmer D.E. (2001) Monitoring structural changes of proteins in an ion trap over ∼10–200 ms: unfolding transitions in cytochrome c ions. Anal. Chem. 73, 6000–6007 10.1021/ac010744a [DOI] [PubMed] [Google Scholar]
- 68.Badman E.R., Myung S. and Clemmer D.E. (2005) Evidence for unfolding and refolding of gas-phase cytochrome c ions in a paul trap. J. Am. Soc. Mass Spectrom. 16, 1493–1497 10.1016/j.jasms.2005.04.013 [DOI] [PubMed] [Google Scholar]
- 69.Allen S.J., Giles K., Gilbert T. and Bush M.F. (2016) Ion mobility mass spectrometry of peptide, protein, and protein complex ions using a radio-frequency confining drift cell. Analyst 141, 884–891 10.1039/C5AN02107C [DOI] [PubMed] [Google Scholar]
- 70.Russell L.M., Flagan R.C. and Seinfeld J.H. (1995) Asymmetric instrument response resulting from mixing effects in accelerated DMA-CPC measurements. Aerosol Sci. Technol. 23, 491–509 10.1080/02786829508965332 [DOI] [Google Scholar]
- 71.Moisala A., Nasibulin A.G., Shandakov S.D., Jiang H. and Kauppinen E.I. (2005) On-line detection of single-walled carbon nanotube formation during aerosol synthesis methods. Carbon N. Y. 43, 2066–2074 10.1016/j.carbon.2005.03.012 [DOI] [Google Scholar]
- 72.Meier F., Beck S., Grassl N., Lubeck M., Park M.A., Raether O. et al. (2015) Parallel accumulation-serial fragmentation (PASEF): multiplying sequencing speed and sensitivity by synchronized scans in a trapped ion mobility device. J. Proteome Res. 14, 5378–5387 10.1021/acs.jproteome.5b00932 [DOI] [PubMed] [Google Scholar]
- 73.McCullagh M., Giles K., Richardson K., Stead S. and Palmer M. (2019) Investigations into the performance of travelling wave enabled conventional and cyclic ion mobility systems to characterise protomers of fluoroquinolone antibiotic residues. Rapid Commun. Mass Spectrom. 33, 11–21 10.1002/rcm.8371 [DOI] [PubMed] [Google Scholar]
- 74.Giles K., Ujma J., Wildgoose J., Green M.R., Richardson K., Langridge D. et al. (2017) Design and Performance of a Second-Generation Cyclic Ion Mobility Enabled Q-ToF 65th ASMS Conf. Mass Spectrom. Allied Top [Google Scholar]
- 75.Ujma J., Ropartz D., Giles K., Richardson K., Langridge D., Wildgoose J. et al. (2019) Cyclic ion mobility mass spectrometry distinguishes anomers and open-Ring forms of pentasaccharides. J. Am. Soc. Mass Spectrom. 30, 1028–1037 10.1007/s13361-019-02168-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eldrid C., Ujma J., Britt H., Cragnolini T., Kalfas S., Cooper-Shepherd D. et al. (2020) Cyclic ion mobility: collision activation experiments elucidate protein behaviour in the Gas-Phase. ChemRxiv 10.26434/chemrxiv.11687100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Allen S.J., Eaton R.M. and Bush M.F. (2017) Structural dynamics of native-Like ions in the Gas phase: results from tandem Ion mobility of cytochrome c. Anal. Chem. 89, 7527–7534 10.1021/acs.analchem.7b01234 [DOI] [PubMed] [Google Scholar]
- 78.Williams J.P., Morrison L., Hughes C.J., Beckman J., Voinov V.G., Lermyte F. et al. (2019) Structural Characterisation of Intact Proteins using Electron Capture Dissociation within an Ion Mobility enabled TOF. 10000
- 79.Williams J.P., Morrison L.J., Brown J.M., Beckman J.S., Voinov V.G. and Lermyte F. (2020) Top-down characterization of denatured proteins and native protein complexes using electron capture dissociation implemented within a modified ion mobility-Mass spectrometer. Anal. Chem. 92, 3674–3681 10.1021/acs.analchem.9b04763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Masson A., Kamrath M.Z., Perez M.A.S., Glover M.S., Rothlisberger U., Clemmer D.E. et al. (2015) Infrared spectroscopy of mobility-selected H+-Gly-Pro-Gly-Gly (GPGG). J. Am. Soc. Mass Spectrom. 26, 1444–1454 10.1007/s13361-015-1172-4 [DOI] [PubMed] [Google Scholar]
- 81.Voronina L., Masson A., Kamrath M., Schubert F., Clemmer D., Baldauf C. et al. (2016) Conformations of prolyl-peptide bonds in the bradykinin 1–5 fragment in solution and in the gas phase. J. Am. Chem. Soc. 138, 9224–9233 10.1021/jacs.6b04550 [DOI] [PubMed] [Google Scholar]
- 82.Allison T.M., Barran P., Benesch J.L.P., Cianferani S., Degiacomi M.T., Gabelica V. et al. (2020) Software requirements for the analysis and interpretation of native Ion mobility mass spectrometry data. Anal. Chem. 92, 10881–10890 10.1021/acs.analchem.9b05792 [DOI] [PubMed] [Google Scholar]