Abstract
Objectives
The aim of this study is to investigate the hearing outcomes of cochlear implantation (CI) in patients with hearing loss who had received radiotherapy for nasopharyngeal cancer (NPC). The study compared speech perception in patients who had prior radiotherapy with those who did not receive radiotherapy.
Methods
Eighty‐eight Cantonese speaking adult patients who had profound sensorineural hearing loss and received CI from 1995 to 2015 at the Chinese University of Hong Kong CI‐center were studied. Twenty‐five patients had history of NPC and radiotherapy were determined as the exposed group, while 63 patients of mixed etiologies but with no history of radiotherapy were included in the control group. The Hong Kong Speech Perception Test Manual (HKSPTM) scores preoperatively, at 6, 12, and 24 months postoperatively were used to assess hearing performance. The HKSPTM consisted categories of speech recognition, word recognition, and tone perception.
Results
No statistical significance differences were found at the four time‐points in the three categories of HKSPTM between the two groups.
Conclusion
CI is a clinically effective intervention and good rehabilitative option for hearing restoration in NPC patients with hearing impairment. Further studies with greater sample size and additional pathological studies on the pathophysiology of hearing loss in this subgroup of patients may provide supplementary information for clinicians when counseling for CI.
Level of evidence
4.
Keywords: cochlear implants, nasopharyngeal cancer, postirradiation deafness, sensorineural hearing loss
This article describes the clinical outcomes of post‐radiated deafened adults receiving cochlear implant in comparison to non‐radiated patients. Clinical outcomes of the study group is similar or superior to the control group in speech perception.
1. INTRODUCTION
Nasopharyngeal carcinoma (NPC) mainly affects East Asians, especially southern Chinese, 1 and is highly prevalent in Hong Kong. Radiotherapy is the mainstay therapeutic modality. It results in a high long‐term survival rate but has short‐term complications, which are generally acceptable. 2 Otological complications, especially hearing impairment, remain the most common complication in long‐term survivors. Conductive hearing loss is generally related to middle ear effusion or chronic suppurative otitis media. Sensorineural (cochlear) hearing loss often occurs progressively or suddenly late after irradiation, even though the retrocochlear auditory pathways remain functionally intact. 1 Previous studies in our center and by others showed that cochlear implantation (CI) was a safe and effective option for hearing rehabilitation for patients with postirradiation profound sensorineural hearing loss. 1 , 2 , 3 , 4 Yue et al 3 summarized the outcomes of fitting cochlear implants in four NPC patients and 32 non‐NPC patients. They reported that encouraged overall postoperative outcomes in speech‐perception tests, 81.4% (±13.7) of sentence recognition in NPC group and 42.3% (±37.8) in non‐NPC group were observed. Yue et al suggested that the radiation damage may be mostly limited to the organ of Corti. However, there was still a concern that damage to the cochlear nerves and central pathway was an important factor in radiation‐induced deafness, explaining the mixed results seen in implant recipients. This case‐control study was designed to identify the efficacy of CI in a consecutive series of patients in a single setting. The aim of this study was to investigate the hearing outcomes of CI in patients with hearing loss who had received radiotherapy for NPC. The main objective was to compare speech perception in patients who had prior radiotherapy with those who had not had received radiotherapy.
2. MATERIALS AND METHOD
This was a retrospective case‐control study. All adult patients who had received cochlear implants at the Chinese University of Hong Kong cochlear implant center were reviewed. From 1995 to 2015, a total of 88 adults with bilateral profound sensorineural hearing loss of mixed etiologies had undergone CI. Among 88 adults, 25 patients were diagnosed with NPC with profound sensorineural hearing loss following radiotherapy underwent CI, and 63 patients with profound sensorineural with no history of radiation therapy who had undergone CI were included as the control group. The background information of subjects is summarized in Table 1.
TABLE 1.
Background information of subjects
| NPC group | Non‐NPC group | |
|---|---|---|
| Number of subjects | 25 | 63 |
| Sex | males: 14; females: 11 | males:37; females: 26 |
| Age at implantation (years) | 57.76 (±7.95) | 53.68 (±10.23) |
| Duration of profound hearing loss (years) | 7.93 (±6.36) | 7.95 (±8.36) |
| Pure‐tone average (0.5, 1, 2 kHz) | 115 dBHL | 113 dBHL |
Note: Results are presented as mean ± (SD).
Abbreviations: dBHL, decibels hearing level; kHz, kilohertz; NPC, nasopharyngeal cancer.
In 25 cochlear implantees with history of NPC, 22 received 2D RT and 3 received IMRT. The dose to NP was similar to cochlear in the era of 2D RT with 30 to 33 fractions between 60 and 66 Gy. For patients receiving IMRT, the cochlear dose were constrained to 50 to 55 Gy.
Yue et al 3 found that patients with congenital profound deafness in the non‐NPC control group had a higher individual differences due to the heterogeneity of etiologies. As a result, patients with congenital profound deafnessin our study were excluded from the control group.
The Hong Kong Speech Perception Test Manual (HKSPTM) 5 was administered to all subjects preoperatively, and then at 6, 12, and 24 months postoperatively. However, some of the subjects did not attend at all postoperatively, or some of them were absent in one or two sessions. Thus, the number of subjects in different tests were not the same and listed in Tables 2 and Table 3. The follow‐up period varied from 3 to 52 months. All subjects in this study speak Cantonese as their mother tongue. Speech perception tests were administered by a certified team of audiologists and conducted in an audiometric booth. This study has obtained approval by the Ethical Committee of the Chinese University of Hong Kong. All subjects were informed and consented for the collection of data for outcome review before surgery.
TABLE 2.
Number of subjects completed each speech perception test
| NPC group | Non‐NPC group | |
|---|---|---|
| Speech recognition test | n = 19 | n = 62 |
| Word recognition test | n = 17 | n = 52 |
| Tone perception test | n = 15 | n = 59 |
Abbreviation: NPC, nasopharyngeal cancer.
TABLE 3.
Number of subjects completed each speech perception test at 4 time‐points
| NPC group | Non‐NPC group | |||||||
|---|---|---|---|---|---|---|---|---|
| Preoperatively | 6 months | 12 months | 24 months | Preoperatively | 6 months | 12 months | 24 months | |
| Speech recognition test | n = 25 | n = 23 | n = 19 | n = 20 | n = 63 | n = 63 | n = 63 | n = 62 |
| Word recognition test | n = 25 | n = 22 | n = 19 | n = 18 | n = 63 | n = 55 | n = 55 | n = 56 |
| Tone perception test | n = 25 | n = 20 | n = 15 | n = 16 | n = 63 | n = 61 | n = 60 | n = 61 |
Abbreviation: NPC, nasopharyngeal cancer.
3. HKSPTM
The Hong Kong Speech Perception Test Manual (HKSPTM) is developed by the audiologists and speech therapists from the three main cochlear implant centers in Hong Kong. The aims at developing a unified protocol for assessing speech perception ability of Cantonese speaking cochlear implant candidates.
Except the environmental sound identification, these parameters for measurement was based on the segmental and suprasegmental features of Cantonese. Speech perception ability in various linguistic levels, including predictable and unpredictable words and sentences were also assessed, so as to give a full spectrum of implantee's speech perception performance before and after implantation.
The HKSPTM includes a speech recognition test, a word recognition test and a tone perception test. For speech recognition test and word recognition test, subjects were required to repeat the sentence/word in a forced choice manner. The tests were conducted in a soundproof room without noise competition. The orientation of the subject was at zero‐degrees azimuth and 1 m from the speaker. The stimulus was presented at 65 dB(A). Three practice items were listed at the beginning of each test and were completed by the subject before the test was administered. One repetition of the stimulus was allowed upon the subject's request. For the tone perception test, the subjects were required to read aloud the four response choices for each item.
Statistical tests were performed using SPSS 22.0. The differences in age, gender, implant type, and implant side among the subjects, were tested using independent t‐test and Chi‐square χ2 test. The mean values and SDs for both NPC and control groups were compared using an independent t‐test to ascertain if there was a significant difference in implant age and duration of profound hearing loss. Mixed ANOVA with between‐ and within‐subject factor were used to determine the significance of differences in NPC group and control group. Statistical significance was considered to be a p value of <.05.
4. RESULTS
A total of 88 patients (25 NPC patients and 63 control patients) were included in the study. All patients had undergone successful implantation with complete insertion of multichannel electrodes. No major surgical complications were reported. The follow‐up period was 24 months postoperatively. Table 4 depict the comparison of age, implant age and duration of profound hearing loss for the NPC and control groups. Independent t‐test revealed the NPC group was implanted at an older age, t(56.48) = 1.99, P = .051. No significant difference with age and duration of profound hearing loss was found in both groups, t(45.32) = 0.791, P = .433 and t(47.64) = −0.085, P = .933, respectively. Table 5 present the comparison of gender, implant side and CI model for the NPC and control groups. Chi‐square test revealed no statistical significance with gender, implant side and implant model, Pearson χ2 (1) = 0.055, P = .815; Pearson χ2 (1) = 0.987, P = .321, and Pearson χ2 (2) = 0.958, P = .620, respectively.
TABLE 4.
Depict the comparison of age characteristics for NPC and Control groups
| t‐test for equality of means | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Levene's test for equality of variances | t | df | Sig. (2‐tailed) | Mean difference | Std. error difference | 95% Confidence interval of the difference | |||
| F | Sig. | Lower | Upper | ||||||
| Age | 0.164 | 0.686 | 0.791 | 86 | 0.433 | 1.541 | 1.947 | −2.380 | 5.462 |
| Implant age | 2.062 | 0.155 | 1.992 | 86 | 0.051 | 4.077 | 2.047 | −0.22 | 8.177 |
| Duration of profound loss | 1.743 | 0.190 | −0.085 | 83 | 0.933 | −1.447 | 1.710 | −3.584 | 3.295 |
Abbreviations: df, degree of freedom; Sig, significance; Std. error difference = Standard error difference; t, t value in t‐test.
TABLE 5.
The comparison of gender, implant side, and CI model for NPC and control groups
| Gender | CI_side | CI_model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | P‐value | Left | Right | P‐value* | Cochlear | AB | Med‐El | P‐value* | |
| NPC | 14 | 11 | P = .815 | 12 | 13 | p = .321 | 13 | 12 | 0 | P = .0.620 |
| Non‐NPC | 37 | 26 | 23 | 40 | 37 | 24 | 1 | |||
| Total | 51 | 37 | 35 | 53 | 50 | 36 | 1 | |||
P values are based on Chi‐square test, with P <.05 indicating statistical significance.
Abbreviations: AB, Advance bionics (Calnifornia, USA); CI, cochlear implantation; Cochlear, cochlear limited (Sydney, Australia); Med‐El, Med‐El (Innsbruck, Austria); NPC, nasopharyngeal cancer.
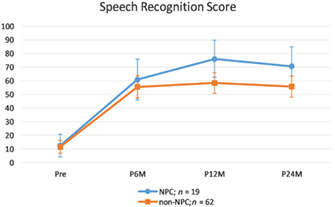
4.1. Comparison of speech recognition between NPC and control group (NPC (n = 19) vs n‐NPC (n = 62))
Multivariate results suggested a significant main effect of time, Wilks' Lambda = 0.34, F (3,77) = 49.20, P <.001, and insignificant interaction effect between time and group, Wilks' Lambda = 0.91, F (3,77) = 2.64, P = .056. Main effect of group was insignificant, F (1,79) = 2.81, P = .097. For the main effect of time, speech recognition scores increased from preoperatively to 6 months postoperatively (mean difference = 46.24, P <.001), and from 6 to 12 months postoperatively (mean difference = 9.00, P <.007), but leveled off from 12 months to 24 months postoperatively (mean difference = −3.99, P = .526), as revealed by Bonferroni‐adjusted paired t‐tests.
Simple effect of group (ie, NPC vs Control) was explored at each point of time using independent t‐tests with adjusted alpha level of 0.0125 (0.05/4). No statistical differences were found at preoperatively and 6 months postoperatively., At 12 and 24 months postoperatively although the same trend existed the difference did not approach statistical significance, t(39.99) = 2.71, P = .046; and t(40.16) = 2.18, P = .036, respectively.(Figure 1).
FIGURE 1.
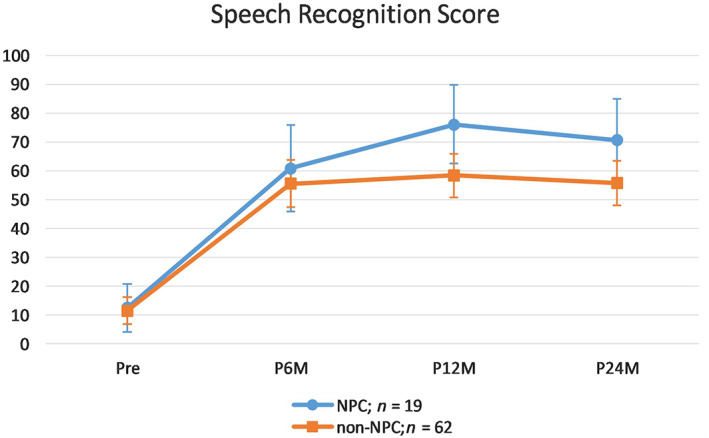
Comparison of speech recognition score between NPC and non‐NPC patients. NPC, nasopharyngeal cancer
4.2. Comparison of word recognition between NPC and Control Group (NPC (n = 17) vs n‐NPC (n = 52))
Multivariate results suggested a significant main effect of time, Wilks' Lambda = 0.27, F (3,65) = 57.45, P <.001, and insignificant interaction effect between time and group, Wilks' Lambda = 0.96, F (3,65) = 0.87, P = .462. Main effect of group was insignificant, F (1,67) = 0.093, P = .762, For the main effect of time, word recognition scores increased from preoperatively to 6 months postoperatively (mean difference = 36.08, P <.001), and from 6 to 12 months postoperatively, mean difference = 15.31, P <.001), but leveled off from 12 to 24 months postoperatively (mean difference = −0.57, P = 1.00), as revealed by Bonferroni‐adjusted paired t‐tests.
Simple effect of group (ie, NPC vs Control) was explored at each point of time using independent t‐tests with adjusted alpha level of .0125 (.05/4). No statistical differences were found between groups at each of the four time points (Figure 2).
FIGURE 2.
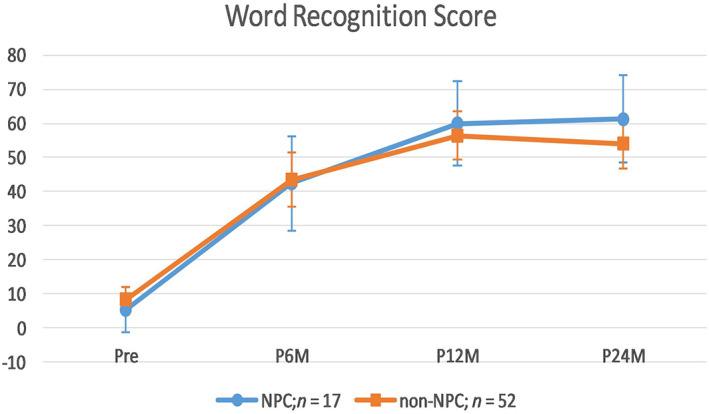
Comparison of word recognition score between NPC and non‐NPC patients. NPC, nasopharyngeal cancer
4.3. Comparison of tone perception between NPC and control group (NPC (n = 15) vs n‐NPC (n = 59))
Multivariate results suggested a significant main effect of time, Wilks' Lambda = 0.47, F (3,70) = 26.82, P <.001, and insignificant interaction effect between time and group, Wilks' Lambda = 0.96, F (3,70) = 0.86, P = .464. Main effect of group was insignificant, F (1,72) = 0.79, P = .376. For the main effect of time, tone perception scores increased from preoperatively to 6 months postoperatively (mean difference = 29.47, P <.001), leveled off from 6 to 12 months postoperatively (mean difference = 0.37, P = 1.00), but increased again from 12 to 24 months postoperatively (mean difference = 5.14, P = .189), as revealed by Bonferroni‐adjusted paired t‐tests.
Simple effect of group (ie, NPC vs Control) was explored at each point of time using independent t‐tests with adjusted alpha level of .0125 (.05/4). No statistical differences were found at all time‐points; while at 24 months postoperatively a nonsignificant trend for better performance with the NPC group existed, t(38.18) = 1.92, P = .062 (Figure 3).
FIGURE 3.
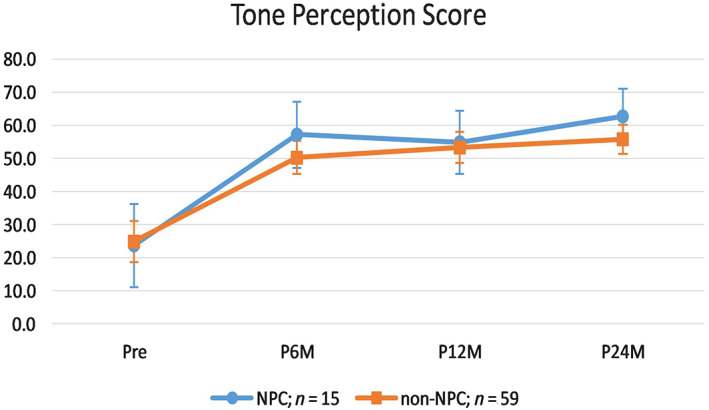
Comparison of tone perception score between NPC and non‐NPC scores. NPC, nasopharyngeal cancer
5. DISCUSSION
Agreement on the mechanisms of radiotherapy‐induced sensorineural hearing loss has not yet been reached. It was believed that radiation led to inner‐ear vascular insufficiency, damage to the organ of Corti and inflammation‐induced damage to the cochlear nerve. 6 Profound deafness has been observed in some postirradiated patients years after their initial radiotherapy. 7 If multiple etiologies are involved in patients' profound hearing loss, the outcomes of CI will not be comparable to that of other etiologies.
This retrospective study confirmed that CI can be an option for restoring hearing in patients who have received radiotherapy for NPC. The CI patients' open‐set speech recognition steadily improved throughout the 24 months of follow‐up and remained good and stable 2 years postoperatively in both the case and control groups. The NPC implantees experienced improvement in speech perception tests, which contributed to an improvement in quality of life for these cancer survivors.
Contrary to the common belief that radiation may damage neurological pathways and thus impede electrical stimulation (ie, retrocochlear hearing loss). In the present study, there was no statistical differences in speech perception tests between NPC and control groups at each of the four time points. The two groups performed similarly in speech perception tests over the 2‐year period.
The limited number of studies to support CI in irradiated ears 1 , 3 and a lack of histopathological studies of these ears has resulted in confusing messages regarding advising irradiated patients to undergo CI. Several factors could have contributed to the consistently good performance in this group of patients. Hearing loss in postirradiated NPC patients is mostly from hair cell damage due to ischemia rather than cochlear nerve degeneration. The irradiation compromises the vascular supply resulting in damage to the stria vascularis. The neurological pathway from hair cells to the central auditory system must remain largely intact. As the conducting function of the cochlear nerve is a crucial factor that determines the outcomes of CI, neural integrity predicts good performance after CI.
Limitation of this study included relatively small number on the NPC group when compare to the non NPC (control group). The default to follow up rate is also high since we only included those patients who have completed all the follow ups in the final calculations.
6. CONCLUSION
This retrospective study investigated the effectiveness of CI in postirradiated NPC patients with hearing impairment in comparison to non‐NPC patients with profound sensorineural hearing loss. Our results show that CI is a clinically effective intervention and good rehabilitative option for hearing restoration in NPC and non‐NPC patients with hearing impairment. We propose that radiotherapy induces structural damage in the cochlea, mainly to hair cells, and the function of the cochlear nerve is mostly preserved, thus to similar performance in both groups. We can now confidently recommend CI for the restoration of hearing in postirradiated patients with significant hearing impairment. There should be future studies with greater sample size and preferably some pathological studies on the exact pathophysiology of hearing loss in NPC post irradiated patients.
CONFLICT OF INTEREST
There are no known conflicts of interest associated with this publication.
Chang WW, Yeung KN, Luk BP, Leung KK, Sung JK, Tong MC. Cochlear implantation in postirradiated ears: A case‐control comparative study. Laryngoscope Investigative Otolaryngology. 2020;5:1163–1167. 10.1002/lio2.486
BIBLIOGRAPHY
- 1. Soh JM, D'Souza VD, Sarepaka GK, et al. Cochlear implant outcomes: a comparison between irradiated and non‐irradiated ears. Clin Exp Otorhinolaryngol. 2012;5:93‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chua YK, Tan KK. Successful rehabilitation with cochlear implant in post‐irradiation induced hearing loss in nasopharyngeal carcinoma patient. Annals Acad Med. 2007;36:74‐77. [PubMed] [Google Scholar]
- 3. Yue V, Leung EKS, Wong TKC, Tong MCF, van Hasselt CA. Cochlear implantation for post‐irradiation deafness. Cochlear Implants Int. 2004;5:165‐168. [DOI] [PubMed] [Google Scholar]
- 4. Low WK, Gopal K, Goh LK, Fong KW. Cochlear implantation in post‐irradiated ears: outcomes and challenges. The Laryngoscope. 2006;116:1258‐1262. [DOI] [PubMed] [Google Scholar]
- 5. Cochlear Implant Working Group . Hong Kong Speech Perception Test Manual (HKSPTM). Hospital Authority, Hong Kong; 2000.
- 6. Young YH, Lou PJ. Post‐irradiation sudden deafness. J Laryngol Otol. 1999;113:815‐817. [DOI] [PubMed] [Google Scholar]
- 7. Gibb A, Loh K. The role of radiation in delayed hearing loss in nasopharyngeal carcinoma. J Laryngol Otol. 2000;114(2):139‐144. [DOI] [PubMed] [Google Scholar]


