Abstract
OBJECTIVE
The purpose of this study was to evaluate the predictive power of the screening questionnaires including Epworth Sleepiness Scale (ESS), Berlin questionnaire (BQ) and STOP-Bang questionnaire (SBQ) to identify the high-risk patients for OSA in a sleep clinic setting considering age, gender and comorbidities.
MATERIAL AND METHODS
1003 patients who admitted to our sleep center with the preliminary diagnosis of OSA between June 2016–May 2018 were included in the study. All patients underwent in-lab polysomnographic examination and filled out ESS, Berlin and STOP-Bang questionnaires. Predictive parameters for each screening questionnaires were calculated.
RESULTS
For apnea-hypopnea index (AHI) ≥5/h, the sensitivity and the specificity of the EES, BQ and SBQ were 50.6%, 89.8%, 97.9% and 56.6%, 27.3%, 16.2% respectively. The STOP-Bang questionnaire had the highest sensitivity in both males and females (99.1%, 94.8% respectively) and in the different age groups (97.3% for ≥45 age-group, 99.2% for ≥65 age-group). In the groups of patients with hypertension, diabetes mellitus, coronary artery disease, chronic obstructive pulmonary disease and asthma, the sensitivity of the STOP-Bang questionnaire was 99.5%, 100%, 99.5%, 100%, 97.4%, respectively.
CONCLUSION
The STOP-Bang questionnaire had the highest sensitivity for detecting high-risk patients for OSA in a sleep clinic setting. STOP-Bang questionnaire was superior to the Berlin questionnaire and ESS in the different groups of age, gender, and comorbidities. Considering the close relationship between OSA and comorbidities, it is critical to screen patients in terms of OSA in outpatient clinics of internal medicine, cardiology, and chest disease departments. The STOP-Bang questionnaire, with its high sensitivity, may be useful for screening OSA. However, the low specificity should be improved in the questionnaire.
Keywords: OSA, STOP-Bang, Berlin Questionnaire, ESS, Screening questionnaires
INTRODUCTION
Obstructive sleep apnea (OSA) is the most common sleep-disordered breathing, characterized by recurrent obstruction of the upper airway during sleep. It has been estimated that 1 in 5 adults has at least mild OSA and 1 in 15 has at least moderate [1]. Previous studies demonstrated that 80% of individuals with moderate-to-severe OSA may remain undiagnosed and furthermore untreated [2]. Untreated OSA is associated with serious adverse health consequences, including hypertension (HT), coronary artery disease (CAD), stroke, neurocognitive dysfunction, and metabolic syndrome [3]. OSA is also a preventable risk factor for motor vehicle accidents [4]. Considering the growing prevalence rates and adverse health consequences of OSA, it is critical to identify high-risk patients for OSA [5]. The gold standard diagnostic method for OSA is overnight polysomnography (PSG). However, PSG is an expensive and time-consuming process. Long waiting periods for polysomnographic studies are still an important problem for the diagnosis of OSA [2]. To assist in managing long waiting lists by identifying patients at high risk for OSA, several screening questionnaires have been developed. Screening questionnaires can be used to prioritize patients for PSG [6]. Depending on the population to which the questionnaire is applied, characteristics of the ideal screening tool can differ. What is expected from an ideal screening questionnaire in a sleep clinic population is high sensitivity with an acceptable specificity. It is important to avoid missing cases in sleep clinic population [7]. The Epworth Sleepiness Scale (ESS), Berlin Questionnaire (BQ), and STOP-Bang questionnaire are the most popular screening questionnaires used for the detection of patients at high risk for OSA [8].
The purpose of this study was to evaluate the predictive power of the screening questionnaires including the ESS, Berlin, and STOP-Bang to identify high-risk patients for OSA in a sleep clinic setting with consideration to age, gender differences, and comorbidities.
MATERIAL AND METHODS
Subjects
This was a cross-sectional study. Adult patients who applied to the sleep center of Dr. Suat Seren Training and Research Hospital with the presumptive diagnosis of OSA between June 2016 and May 2018 were enrolled in the study. Inclusion criteria were as follows: age over 18 years, OSA symptoms (snoring and/or witnessed apnea and/or excessive daytime sleepiness), no previous diagnosis and treatment of OSA, no previous diagnosis of other sleep disorders, completion of questionnaires, personal constant for PSG examination, and participate in study. Patients who did not meet these criteria were excluded from the study. Additionally, patients with the sleep efficacy less than 60% at the PSG examination, who has other sleep disorders (central sleep apnea syndrome, sleep-related movement disorders, insomnia, parasomnias) active psychiatric disorder, and inability to complete questionnaires were also excluded from the study. History of chronic illness of all patients was recorded.
All patients underwent in-lab PSG examinations and completed the ESS, Berlin, and STOP-Bang questionnaires.
Screening Questionnaires
The ESS was originally designed to evaluate daytime sleepiness. However, it has been recommended as a tool for screening OSA. The ESS is a questionnaire with eight questions and responses on a 4-point Likert format (0–3). The score ranges from 0 to 24 and ESS scores ≥10 indicate excessive daytime sleepiness and high risk for OSA [9].
The BQ consists of 10 questions arranged in three categories. The first category comprises five questions about snoring and cessation of breathing, the second category comprises four questions about daytime sleepiness and fatigue/tiredness, and the last category comprises information about the presence of systemic arterial HT and obesity. Persistent symptoms (>3–4 times/week) in at least two or more questions are considered as positive for categories 1 and 2. The third category is considered positive if there is a history of high blood pressure or a body mass index (BMI) >30 kg/m2. Patients with a positive score in two or more categories were defined as having high risk for OSA [10].
The STOP-Bang questionnaire was first developed to screen surgical patients for OSA [11]. It is now widely used in the general population, the sleep clinic population, and other different populations to identify high-risk patients for OSA. The STOP-Bang questionnaire comprises eight questions, four of which are subjective (snoring, tiredness, observed apnea, and high blood pressure), and four pertain to demographics (BMI >35 kg/m2, age >50 years, neck circumference >40 cm, and male gender). The total score ranges from 0 to 8. Answering yes to three or more questions is considered as high risk for OSA [12]. We used valid Turkish versions of the three questionnaires [13–15].
In-Laboratory PSG
The diagnosis of OSA was made using in-lab polysomnographic examinations (Comet Grass Telefactor, version 4.5.3). PSG included electroencephalography, electrooculography, submental electromyography, anterior tibialis electromyography, electrocardiography, finger pulse oximeter, thoraco-abdominal movements, airflow (nasal pressure cannula), oronasal thermistor, and digital microphone for snoring detection. PSG recordings were analyzed by a physician experienced in sleep disorders using TWin EEG/PSG Software. Scoring of the sleep and respiratory events was performed according to the standard criteria of the American Academy of Sleep Medicine [16]. The apnea-hypopnea index (AHI) was defined as the total number of apnea and hypopnea events per hour. The diagnosis of OSA was defined by AHI.
Statistical Analysis
The Statistical Package for Social Sciences version 25.0 (IBM SPSS Corp.; Armonk, NY, USA) software package was used to analyze the data. The demographic data are presented with descriptive statistics. Numeric data are given as mean±standard deviation (SD), and frequency data are given as number and percentage (%). The concordance of numeric variables with normal distribution was evaluated using the Shapiro-Wilk test. For data with normal distribution, Student’s t test was used to compare two groups. Cross tabulation was used for categorical data and Chi-square analysis was performed. PSG was considered as the gold standard and the sensitivity, specificity, positive predictive values (PPVs), negative predictive values (NPVs), likelihood ratio positive (LR+), and likelihood ratio negative (LR−) values of the ESS, BQ, and STOP-Bang questionnaires according to specific cut-off values were calculated. Receiver-operating characteristic (ROC) curves were constructed to assess the ESS, BQ, and STOP-Bang questionnaires regarding their likelihood to predict high risk for OSA. Correlation between the questionnaires was analyzed using Spearman’s correlation coefficient. Multiple logistic regression analysis was performed to identify risk factors that affected OSA. All tests were two-sided and statistical significance was assumed when p<0.05.
The study was performed in accordance with the criteria of the Declaration of Helsinki. The study was approved by the local research ethics committee (Date: 12.05.2016, Number: 5300). All participants included in the signed a written informed consent form.
RESULTS
A total of 1,003 patients were included in the study, comprising 698 (69.6%) males and 305 (30.4%) females. The mean age of the study population was 50.65±11.38 years. Table 1 demonstrates the demographic and anthropometric characteristics of the study population. According to the PSG results, 229 patients (22.8%) had mild OSA (AHI=5–14.9), 256 (25.52%) had moderate OSA (AHI=15–29.9), and 419 (41.74%) patients had severe OSA (AHI≥30). In total, 90.12% of the study population had an AHI≥5 events/h.
Table 1.
Demographic and anthropometric characteristics of the study population (n=1,003; data are depicted as mean [SD] or number [%])
| Variables | AHI<5 | AHI≥5 | p |
|---|---|---|---|
| Number (%) | 99 (9.88) | 904 (90.12) | |
| Male gender, N (%) | 46 (46.5) | 652 (72.1) | <0.001 |
| Age, year, mean (SD) | 44.2 (11.1) | 51.3 (11.1) | <0.001 |
| BMI, kg/m2, mean (SD) | 29 (5.6) | 32.5 (5.9) | <0.001 |
| Neck circumference, cm, mean (SD) | 39.1 (3.03) | 41.6 (3.3) | <0.001 |
| Smoker, N (%) | 45 (45.5) | 381 (42.1) | 0.527 |
| Comorbidities, N (%) | |||
| Hypertension | 24 (24.2) | 365 (40.4) | 0.002 |
| Diabetes mellitus | 14 (14.1) | 223 (24.7) | 0.010 |
| CAD | 7 (7.1) | 117 (12.9) | 0.092 |
| CHF | 0 (0) | 24 (2.7) | 0.101 |
| Valvular heart disease | 3 (3) | 23 (2.5) | 0.773 |
| Arrhythmia | 8 (8.1) | 69 (7.6) | 0.874 |
| Asthma | 6 (6.1) | 78 (8.6) | 0.381 |
| COPD | 6 (6.1) | 84 (9.3) | 0.285 |
| CRF | 0 (0) | 10 (1.1) | 0.293 |
| SVD | 1 (1) | 10 (1.1) | 0.931 |
| Hypothyroidism | 6 (6.1) | 84 (9.3) | 0.764 |
| Depression | 16 (16.2) | 60(6.6) | 0.001 |
| Cancer | 3 (3) | 11 (1.2) | 0.144 |
BMI: body mass index; CHF: congestive heart failure; COPD: chronic obstructive pulmonary disease; CRF: chronic renal failure; CAD: coronary arterial disease; SVD: cerebrovascular disease
Using a cut-off of AHI≥5 events/h, the sensitivity of the ESS, Berlin, and STOP-Bang questionnaires was 50.6%, 89.8%, and 97.9%, respectively. The STOP-Bang questionnaire had the highest sensitivity, but also the lowest specificity. The sensitivity and specificity were calculated for AHI≥15 events/h but yielded similar results to AHI≥5 events/h (Table 2).
Table 2.
Predictive parameters for the ESS, Berlin, and STOP-Bang questionnaires for OSA
| Variables | ESS (%) | Berlin (%) | STOP-Bang (%) |
|---|---|---|---|
| AHI≥5 events/h | |||
| Sensitivity | 50.6 | 89.8 | 97.9 |
| Specificity | 56.6 | 27.3 | 16.2 |
| PPV | 91.4 | 91.9 | 91.4 |
| NPV | 11.1 | 22.7 | 45.7 |
| LR positive | 1.165 | 1.235 | 1.168 |
| LR negative | 0.872 | 0.373 | 0.129 |
| AHI≥15 events/h | |||
| Sensitivity | 53.9 | 89.3 | 98.5 |
| Specificity | 58.5 | 14.3 | 7.6 |
| PPV | 72.8 | 68.2 | 68.7 |
| NPV | 38.2 | 39.5 | 71.4 |
| LR positive | 1.301 | 1.042 | 1.066 |
| LR negative | 0.788 | 0.748 | 0.197 |
ESS: Epworth Sleepiness Scale; PPV: positive predictive value; NPV: negative predictive value; LR: Likelihood ratio
When the discriminative power of the questionnaires was evaluated considering the gender differences, the STOP-Bang questionnaire had the highest sensitivity in both males and females (99.1% and 94.8, respectively; Table 3). The ESS had the highest specificity in both genders (60.9% males and 52.6% females).
Table 3.
Predictive parameters for the ESS, Berlin, STOP-Bang Questionnaires for OSA (AHI≥5 events/h), considering gender differences
| Variables | ESS (%) | Berlin (%) | STOP-Bang (%) |
|---|---|---|---|
| Male gender | |||
| Sensitivity | 53.7 | 89.9 | 99.1 |
| Specificity | 60.9 | 30.4 | 8.7 |
| PPV | 95.1 | 94.8 | 93.9 |
| NPV | 8.5 | 17.5 | 40 |
| LR positive | 1.373 | 1.291 | 1.085 |
| LR negative | 0.760 | 0.332 | 0.103 |
| Female gender | |||
| Sensitivity | 42.5 | 89.7 | 94.8 |
| Specificity | 52.8 | 24.5 | 22.6 |
| PPV | 81.1 | 85 | 85.4 |
| NPV | 16.2 | 33.3 | 48 |
| LR positive | 0.900 | 1.190 | 1.224 |
| LR negative | 1.089 | 0.420 | 0.230 |
ESS: Epworth Sleepiness Scale; PPV: positive predictive value; NPV: negative predictive value; LR: Likelihood ratio
Table 4 shows the predictive parameters for the ESS, Berlin, and STOP-Bang questionnaires in the different age groups. The questionnaire with the highest sensitivity in the patient group aged 45 years and older was the STOP-Bang (97.3%). This was followed by the BQ and ESS. In the group of patients aged 65 years and older, it was observed that there was a decrease in the sensitivity of the BQ and ESS, whereas the sensitivity of the STOP-Bang increased to 99.2%.
Table 4.
Predictive Parameters for the ESS, Berlin, and STOP-Bang Questionnaires for OSA (AHI≥5 events/h), considering age (≥45 y and ≥65 y)
| Variables | ESS (%) | Berlin (%) | STOP-Bang (%) |
|---|---|---|---|
| Age≥45 y | |||
| Sensitivity | 45.8 | 88.3 | 97.3 |
| Specificity | 40.9 | 12.3 | 5.2 |
| PPV | 63.6 | 69.5 | 69.8 |
| NPV | 25 | 31.9 | 45.7 |
| LR positive | 0.774 | 1.006 | 1.026 |
| LR negative | 1.325 | 0.951 | 0.519 |
| Age≥65 y | |||
| Sensitivity | 42.5 | 82.5 | 99.2 |
| Specificity | 49.2 | 11.1 | 3.9 |
| PPV | 10.2 | 11.2 | 12.3 |
| NPV | 86.3 | 82.4 | 97.1 |
| LR positive | 0.836 | 0.928 | 1.032 |
| LR negative | 1.195 | 1.576 | 0.205 |
y: years.
ESS: Epworth Sleepiness Scale; PPV: positive predictive value; NPV: negative predictive value; LR: Likelihood ratio
Table 5 shows the sensitivity and specificity values of the ESS, Berlin, and STOP-Bang questionnaires in the groups of patients with and without comorbidities. Of the study population, 389 patients had HT, 237 patients had diabetes mellitus (DM), 124 patients had CAD, 90 patients had chronic obstructive pulmonary disease (COPD), and 84 patients had asthma. In total, 362 (36%) patients had no additional disease. Questionnaires were individually evaluated in the groups with HT, DM, CAD, COPD, and asthma. The STOP-Bang questionnaire had the highest sensitivity in all groups, regardless of the presence of comorbidities. This was followed by the BQ. When multiple logistic regression analysis was performed by adding all comorbidities, HT was found to increase the risk for OSA 2.03 times (95% confidence interval [CI]: 1.20–3.43, P=0.008) independent of additional diseases.
Table 5.
Predictive parameters for the ESS, Berlin, STOP-Bang questionnaires for OSA in patients with and without comorbidities
| Variables | ESS (%) | Berlin (%) | STOP-Bang (%) |
|---|---|---|---|
| No comorbidity (n=362) | |||
| Sensitivity | 47.9 | 84.6 | 96.6 |
| Specificity | 63.2 | 42.1 | 21.1 |
| PPV | 91.7 | 92.6 | 91.3 |
| NPV | 12.4 | 24.2 | 42.1 |
| Hypertension (n=389) | |||
| Sensitivity | 52.1 | 94.5 | 99.5 |
| Specificity | 45.8 | 8.3 | 4.2 |
| PPV | 93.6 | 94 | 94 |
| NPV | 5.9 | 9.1 | 33.3 |
| DM (n=237) | |||
| Sensitivity | 56.5 | 91.9 | 100 |
| Specificity | 42.9 | 21.4 | 0 |
| PPV | 94 | 94.9 | 94.1 |
| NPV | 5.8 | 14.3 | 0 |
| CAD (n=124) | |||
| Sensitivity | 55.6 | 89.7 | 99.5 |
| Specificity | 57.1 | 0 | 4.2 |
| PPV | 95.6 | 93.8 | 94 |
| NPV | 7.1 | 0 | 33.3 |
| COPD (n=90) | |||
| Sensitivity | 52.4 | 92.9 | 100 |
| Specificity | 83.3 | 33.3 | 16.7 |
| PPV | 97.8 | 95.1 | 94.4 |
| NPV | 11.1 | 25 | 100 |
| Asthma (n=84) | |||
| Sensitivity | 55.1 | 92.3 | 97.4 |
| Specificity | 33.3 | 16.7 | 0 |
| PPV | 91.5 | 93.5 | 92.7 |
| NPV | 5.4 | 14.3 | 0 |
ESS: Epworth Sleepiness Scale; PPV: positive predictive value; NPV: negative predictive value; DM: Diabetes mellitus; CAD: Coronary artery disease; COPD: Chronic obstructive pulmonary disease
In all analyses, STOP-Bang questionnaire had the highest sensitivity, whereas its specificity was generally low. Therefore, some analyses were performed using different thresholds for STOP-Bang (see Table 6). When the STOP-Bang score threshold was 4 and above, the sensitivity was found as 74.6%, specificity 63.6%, PPV 94.9, and LR+ 2.049 for AHI≥5 events/h. When the STOP-Bang score threshold was raised to 5 and above, specificity (87.9%) showed an increase, but there was a significant decrease in sensitivity (45%).
Table 6.
Predictive parameters for various STOP-Bang thresholds to predict OSA
| Variables | STOP-Bang≥3 | STOP-Bang≥4 | STOP-Bang≥5 | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| AHI ≥5 | AHI ≥15 | AHI ≥5 | AHI ≥15 | AHI ≥5 | AHI ≥15 | |
| Sensitivity (%) | 97.5 | 98.5 | 74.6 | 77.3 | 45 | 49.8 |
| Specificity (%) | 16.2 | 7.6 | 63.6 | 42.7 | 87.9 | 74.7 |
| PPV (%) | 91.4 | 68.7 | 94.9 | 73.5 | 97.1 | 80.2 |
| NPV (%) | 45.7 | 71.4 | 21.5 | 47.8 | 14.9 | 42 |
| LR positive | 1.163 | 1.066 | 2.049 | 1.349 | 3.719 | 1.968 |
| LR negative | 0.154 | 0.197 | 0.399 | 0.531 | 0.625 | 0.672 |
PPV: positive predictive value; NPV: negative predictive value; LR: Likelihood ratio
As an alternative to the questionnaires, we evaluated the predictive values for high-risk OSA when the three basic OSA symptoms (snoring, witnessed apnea, and daytime sleepiness) were all positive. When the three symptoms were positive, sensitivity was 74.8%, specificity 47.5%, PPV 92.9%, NPV 17.1%, LR+ 1.424, and LR− 0.530, for AHI≥5 events/h. The sensitivity and specificity were 76.7% and 36% for AHI≥15 events/h, respectively.
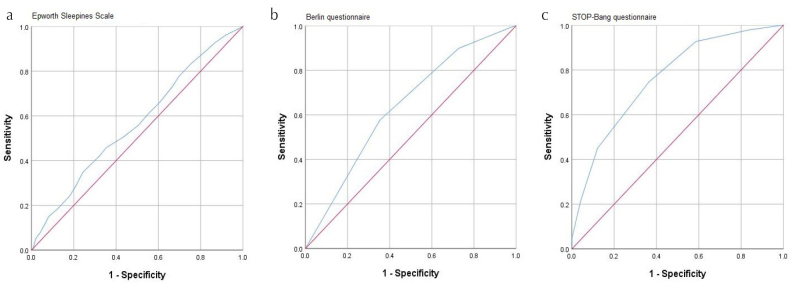
ROC curves for the ESS, Berlin, and STOP-Bang questionnaires were constructed (see Figure 1). The area under the ROC curve (AUC) (95% CI) for the ESS, Berlin, and STOP-Bang was 0.565 (95% CI 0.506–0.624), 0.637 (95% CI 0.576–0.697), 0.763 (95% CI 0.713–0.814), respectively (for AHI≥5 events/h). The Spearman’s correlation coefficient between the ESS and BQ, the ESS and STOP-Bang, and BQ and STOP-Bang were 0.20, 0.21, and 0.46, respectively (p<0.001).
Figure 1. a–c.
Receiver operator characteristic (ROC) curves for Epworth Sleepiness Scale (a), Berlin (b), and STOP-Bang questionnaires for AHI≥5/h (c)
DISCUSSION
In this study, we evaluated the diagnostic power of screening questionnaires including the ESS, Berlin, and STOP-Bang to identify high-risk patients for OSA in a sleep clinic setting. In addition, we also assessed the predictive value of the questionnaires considering age, gender differences, and comorbidities. It was clearly shown that the STOP-Bang questionnaire had excellent sensitivity for detecting high-risk patients for OSA, even in the different groups of age, gender, and comorbidities. However, the low specificity should be improved in the questionnaire.
When the cut-off for the diagnosis of OSA was regarded as AHI≥5, STOP-Bang had the highest sensitivity, AUC, and PPV (97.9%, 0.763 (95% CI 0.713–0.814), 91.4%, respectively). In the study by Pataka et al. [17], which examines patients admitted to a sleep clinic, the STOP-Bang had the highest sensitivity (97.6%), the largest AUC (0.73; 95% CI 0.7–0.76), and best OR (5.9; 95% CI 3.6–9.5), but the lowest specificity (12.7%) for AHI≥15. Similarly, in the study conducted by Kim et al. [7], the STOP-Bang questionnaire had the highest sensitivity, but its specificity was very low. In another study, the sensitivity and specificity for the screening tools were as follows: Berlin 88% and 25%, STOP-Bang 90%, and 25%, respectively [18]. The results in the literature are consistent with the results of our study.
Considering age and gender, our results demonstrated that STOP-Bang had the highest sensitivity for predicting OSA. STOP-Bang questionnaire was followed by the BQ in terms of sensitivity in both gender and age groups. The sensitivity of STOP-Bang was higher in the male gender than in the female gender. The fact that male gender is a variable of the STOP-Bang questionnaire may have affected this situation. In the study conducted by Mou et al. [19], the efficacy of the STOP-Bang questionnaire was evaluated in identifying high-risk OSA patients with consideration to gender differences. As a result, the specificity was found to be low, especially in men, and alternative models were recommended [19]. The specificity of STOP-Bang was also lower in male gender than in female gender according to our data. When the age variable was evaluated, STOP-Bang had the highest sensitivity in patients older than 65 years.
The presence of comorbidities can affect the performance of diagnostic tests [20]. In our study, the efficacy of the ESS, Berlin, and STOP-Bang questionnaires in the patient groups with and without comorbidities was examined. STOP-Bang was found to have the highest sensitivity in the analyses based on comorbidities. STOP-Bang is highly successful in identifying high-risk patients in terms of OSA in HT, DM, CAD, asthma, and COPD groups, as well as in the non-comorbidity group. The results were excellent in terms of sensitivity, but the specificity rates were not at the acceptable levels. To our knowledge, this study is the first to assess different comorbidities, screening questionnaires, and OSA altogether. In a study conducted to determine the best screening questionnaire for OSA in patients with COPD, the sleep apnea clinical score, ESS, and BQ were performed, and the sensitivity rates were reported as 60%, 40%, and 60%, respectively [21]. Lu et al. [22] assessed the predictive performance of the Berlin and STOP-Bang questionnaires for OSA in patients with asthma. It was demonstrated that STOP-Bang questionnaire had better predictive performance than the BQ for identifying moderate and severe OSA in patients with asthma. Margallo et al. [23] evaluated the performance of the BQ in patients with resistant HT in detecting OSA and revealed that the BQ had low accuracy in detecting patients with OSA. Contrary to these data, in this study, the BQ had high sensitivity for predicting OSA in patients with HT. In another study, the STOP-Bang questionnaire was performed for screening OSA in patients with metabolic syndrome and the sensitivity and specificity were found as 86.36% and 50.94%, respectively [24]. In our study, HT was found to double the risk for OSA in multiple logistic regression analysis including additional diseases. Today, the association between OSA and HT is clearly presented. The Wisconsin Sleep Cohort Study showed that moderate or severe OSA had a 3-fold increased risk for the presence of HT [25].
For most diagnostic tools such as STOP-Bang, there is a tradeoff between sensitivity and specificity. As the cut-off value shifts to increase specificity, sensitivity decreases. In this study, when the STOP-Bang score threshold was raised from ≥3 to ≥4, the sensitivity decreased to 74.6% and the specificity increased to 63.6% (AHI≥5 events/h). Although the sensitivity was in an acceptable range despite the decrease, there was a marked increase in the specificity. When the STOP-Bang score threshold of ≥3 and ≥4 were compared in terms of PPV and LR+, PPV increased from 91.4% to 94.9% and LR+ from 1.163 to 2.049. Sensitivity sharply decreased for thresholds of 5 and above.
What happens when we question only three basic symptoms (snoring, witnessed apnea, and excessive daytime sleepiness) of OSA instead of surveys? As it was demonstrated, even the presence of three basic OSA symptoms was more predictive than the ESS in predicting OSA. In a study conducted by Ulasli et al., ESS was found to be a weak predictor for OSA [26]. In studies involving patient populations admitted to sleep clinics, sensitivity and specificity values for the ESS ranged from 31.3% to 72.55%, and from 53.3% to 75% for AHI≥5 events/h, respectively [17, 27–29]. Except for the study conducted by El-Sayet et al. [27], the ESS seems to be a poor questionnaire for predicting OSA, as it was in our study. The ESS was originally designed to assess the risk of daytime sleepiness, and maybe it should remain as such.
There are notable limitations to our study. Our study population consists of patients who were admitted to a sleep clinic. It will not be appropriate to reflect our results to the general population. Although the prevalence of OSA was high in the sleep clinic population, the number of patients with AHI≤5 events/h remained relatively low. Therefore, the characteristics of the false-negative patient group were not compared with other groups. Additionally, it should be addressed that there are also some limitations regarding screening tools design and cultural adaptation to Turkish people, such as the question regarding falling asleep while waiting for the traffic light while driving in the Epworth scale and BQ is not entirely consistent with the current situation in Turkey. Patients who do not drive cannot answer these questions.
In conclusions, the STOP-Bang questionnaire had the highest sensitivity for detecting high-risk patients for OSA in a sleep clinic setting. Furthermore, the STOP-Bang questionnaire was superior to the BQ and ESS in the different groups of age, gender, and comorbidities including HT, DM, CAD, COPD, and asthma. Considering the close relationship between OSA and comorbidities, it is critical to screen patients in terms of OSA in outpatient clinics of internal medicine, cardiology, and chest disease departments. The STOP-Bang questionnaire, with its high sensitivity, may be useful for screening OSA. However, modifications must be made to increase its specificity. Increasing the STOP-Bang score threshold from 3 to 4 for high-risk OSA might be an alternative.
MAIN POINTS.
Compare to Epworth Sleepiness Scale (ESS) and Berlin Questionnaire (BQ), STOP-Bang questionnaire had the highest sensitivity for detecting high-risk patients for obstructive sleep apnea (OSA) in a sleep clinic setting.
STOP-Bang questionnaire was also superior to the BQ and ESS in the different groups of age, gender, and comorbidities including hypertension, diabetes mellitus, coronary artery disease, chronic obstructive pulmonary disease, and asthma.
The STOP-Bang questionnaire, with its high sensitivity, may be useful for screening OSA. However, modifications must be made to increase its specificity.
Footnotes
Ethics Committee Approval: Ethics Committee approval for the study was obtained from the Ethics Committee of Dr. Suat Seren Training and Research Hospital (Date: 12.05.2016, Number: 5300).
Informed Consent: Written informed consent was obtained from the all participants included in the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - B.O.A., Z.Z.U.H.; Design - B.O.A., Z.Z.U.H.; Supervision - Z.Z.U.H., M.N.O.; Resources - B.O.A.; Materials - B.O.A.; Data Collection and/or Processing - B.O.A; Analysis and/or Interpretation - B.O.A., M.N.O.; Literature Search - B.O.A.; Writing Manuscript - B.O.A., Z.Z.U.H.; Critical Review - B.O.A., Z.Z.U.H., M.N.O.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Flemons WW, Douglas NJ, Kuna ST, et al. Access to Diagnosis and Treatment of Patients with Suspected Sleep Apnea. Am J Respir Crit Care Med. 2004;169:668–72. doi: 10.1164/rccm.200308-1124PP. [DOI] [PubMed] [Google Scholar]
- 3.Stansbury RC, Strollo P. Clinical manifestations of sleep apnea. J Thorac Dis. 2015;7:E298–310. doi: 10.3978/j.issn.2072-1439.2015.09.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tregear S, Reston J, Schoelles K, et al. Obstructive sleep apnea and risk of motor vehicle crash: Systematic review and meta-analysis. J Clin Sleep Med. 2009;5:573–81. doi: 10.5664/jcsm.27662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prasad KT, Sehgal IS, Agarwal R, et al. Assessing the likelihood of obstructive sleep apnea: a comparison of nine screening questionnaires. Sleep Breath. 2017;21:909–17. doi: 10.1007/s11325-017-1495-4. [DOI] [PubMed] [Google Scholar]
- 6.Amra B, Rahmati B, Soltaninejad F, Feizi A. Screening Questionnaires for Obstructive Sleep Apnea: An Updated Systematic Review. Oman Med J. 2018;33:184–92. doi: 10.5001/omj.2018.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim B, Lee EM, Chung YS, et al. The utility of three screening questionnaires for obstructive sleep apnea in a sleep clinic setting. Yonsei Med J. 2015;56:684–90. doi: 10.3349/ymj.2015.56.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiu HY, Chen PY, Chuang LP, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: A bivariate meta-analysis. Sleep Med Rev. 2017;36:57–70. doi: 10.1016/j.smrv.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Johns MW. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 10.Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 11.Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: A tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812–21. doi: 10.1097/ALN.0b013e31816d91b5. [DOI] [PubMed] [Google Scholar]
- 12.Nagappa M, Liao P, Wong J, et al. Validation of the stop-bang questionnaire as a screening tool for obstructive sleep apnea among different populations: A systematic review and meta-Analysis. PLoS One. 2015;10:e0143697. doi: 10.1371/journal.pone.0143697. doi: 10.1371/journal.pone.0143697. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izci B, Ardic S, Firat H, et al. Reliability and validity studies of the Turkish version of the Epworth Sleepiness Scale. Sleep Breath. 2008;12:161–8. doi: 10.1007/s11325-007-0145-7. [DOI] [PubMed] [Google Scholar]
- 14.Yüceege M, Fırat H, Sever Ö, et al. The effect of adding gender item to Berlin Questionnaire in determining obstructive sleep apnea in sleep clinics. Ann Thorac Med. 2015;10:25–8. doi: 10.4103/1817-1737.146856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.car HV, Kaya A, Yucel F, et al. Validation of the STOP-Bang Questionnaire: An Obstructive Sleep Apnoea Screening Tool in Turkish Population. Turkish J Anesth Reanim. 2013;41:115–12. doi: 10.5152/TJAR.2013.46. [DOI] [Google Scholar]
- 16.Berry RB, Brooks R, Gamaldo CE, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Darien, IL: American Academy of Sleep Medicine; 2016. Version 2.3 www.aasmnet.org. [Google Scholar]
- 17.Pataka A, Daskaloqoulou E, Kalamaras G, et al. Evaluation of five different questionnaires for assessing sleep apnea syndrome in a sleep clinic. Sleep Med. 2014;15:776–81. doi: 10.1016/j.sleep.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Pereira EJ, Driver HS, Stewart SC, et al. Comparing a combination of validated questionnaires and level III portable monitor with polysomnography to diagnose and exclude sleep apnea. J Clin Sleep Med. 2013;9:1259–66. doi: 10.5664/jcsm.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mou J, Pflugeisen BM, Crick BA, et al. The discriminative power of STOP-Bang as a screening tool for suspected obstructive sleep apnea in clinically referred patients: considering gender differences. Sleep Breath. 2019;23:65–75. doi: 10.1007/s11325-018-1658-y. [DOI] [PubMed] [Google Scholar]
- 20.utjes AWS, Reitsma JB, Vandenbroucke JP, et al. Case-control and two-gate designs in diagnostic accuracy studies. Clin Chem. 2005;51:1335–41. doi: 10.1373/clinchem.2005.048595. [DOI] [PubMed] [Google Scholar]
- 21.Faria AC, da Costa CH, Rufino R. Sleep apnea clinical score, Berlin questionnaire, or Epworth sleepiness scale: Which is the best obstructive sleep apnea predictor in patients with COPD? Int J Gen Med. 2015;8:275–81. doi: 10.2147/IJGM.S86479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu H, Fu C, Li W, et al. Screening for obstructive sleep apnea syndrome in asthma patients: A prospective study based on Berlin and STOP-Bang questionnaires. J Thorac Dis. 2017;9:1945–58. doi: 10.21037/jtd.2017.06.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Margallo VS, Muxfeldt ES, Guimarães GM, et al. Diagnostic accuracy of the Berlin questionnaire in detecting obstructive sleep apnea in patients with resistant hypertension. J Hypertens. 2014;32:2030–7. doi: 10.1097/HJH.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 24.Teng Y, Xiong Y, Wang N. The applications of the STOP-Bang questionnaire in screening obstructive sleep apnea in patients with metabolic syndrome. Chin J Tuberc Respir Dis. 2015;38:461–6. [PubMed] [Google Scholar]
- 25.Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 26.Ulasli SS, Gunay E, Koyuncu T, et al. Predictive value of Berlin Questionnaire and Epworth Sleepiness Scale for obstructive sleep apnea in a sleep clinic population. Clin Respir J. 2014;8:292–6. doi: 10.1111/crj.12070. [DOI] [PubMed] [Google Scholar]
- 27.El-Sayed IH. Comparison of four sleep questionnaires for screening obstructive sleep apnea. Egypt J Chest Dis Tuberc. 2012;61:433–44. doi: 10.1016/j.ejcdt.2012.07.003. [DOI] [Google Scholar]
- 28.Vana K, Silva G, Goldberg R. Predictive abilities of the STOP-Bang and Epworth Sleepiness Scale in identifying sleep clinic patients at high risk for obstructive sleep apnea. Res Nurs Health. 2013;36:84–94. doi: 10.1002/nur.21512. [DOI] [PubMed] [Google Scholar]
- 29.Prasad KT, Sehgal IS, Agarwal R, et al. Assessing the likelihood of obstructive sleep apnea: a comparison of nine screening questionnaires. Sleep Breath. 2017;21:909–17. doi: 10.1007/s11325-017-1495-4. [DOI] [PubMed] [Google Scholar]