Abstract
Multiple micronutrient deficiencies (MNDs) co‐exist, often because of poor intakes and adversely impact health. Habitual diets were assessed in 300 school children (6–17 years old) recruited from two government schools by simple random sampling. Probability of adequacy (PA) for 11 micronutrients and mean probability of adequacy (MPA) was calculated. Haemoglobin, plasma ferritin, folic acid, vitamin B12 and C‐reactive protein were estimated. Descriptive statistics and regression analysis were used to estimate magnitude and factors associated with MNDs. The contribution of fortified foods and/or supplements in addressing inadequacies and excessive intakes was modelled. The PA ranged from 0.04 for folate to 0.70 for zinc, and the MPA was 0.27. Prevalence of anaemia (53%), iron deficiency (57%; ID), iron deficiency anaemia (38%; IDA), folate deficiency (24%) and B12 deficiency (43%) was high. Dietary inadequacy of iron, zinc and a low MPA was associated with anaemia and IDA. Inclusion of double fortified salt (DFS), fortified rice (FR) or iron folic acid (IFA) supplements individually in habitual diet reduced probability of iron inadequacy significantly from 82% to ≤13%. Inclusion of DFS and FR simultaneously led to disappearance of iron inadequacy, but risk of excessive intake increased to 16%. Inclusion of DFS, FR and IFA together increased risk of excess iron intake to 40%. Nevertheless, intakes of folate and B12 remained inadequate even with FR and/or IFA. These results indicate a high risk of dietary MNDs in children and suggest need for more systematic intake measurements in representative sample and adjustment of iron dosages to avoid excessive intakes.
Keywords: adolescents, anaemia, children, food and nutrient intake, food fortification, India iron deficiency, iron deficiency anaemia, iron folic acid supplementation, mean probability of adequacy, micronutrient deficiencies, probability of adequacy
Key messages.
The risk of dietary inadequacies and biochemical deficiencies of haemoglobin, plasma ferritin, folic acid and vitamin B12 is high in school‐age (6–17 years) children in a peri‐urban district of South India.
Individual dietary inadequacies of iron and zinc and overall inadequacy of multiple micronutrients are associated with anaemia and IDA.
Food fortification or IFA supplementation fills the gaps in dietary inadequacies of iron.
Consumption of multiple fortified foods with or without IFA supplements simultaneously along with habitual diets may lead to excessive intakes of iron.
Assessment of micronutrient inadequacies at population level and adjustment of nutrient levels in fortified foods and/or supplements needs to be considered.
1. INTRODUCTION
Micronutrients are required for optimal cellular and metabolic functions in the body (Pfeiffer et al., 2013; Shergill‐Bonner, 2017). Multiple micronutrient deficiencies (MNDs) are reported more frequently among pregnant women and children below 5 years of age and adversely impact growth, development, cognition, general health and productivity (Bailey, West, & Black, 2015; Muthayya et al., 2013; Walker et al., 2007). The recent Comprehensive National Nutrition Survey conducted in India indicated a high prevalence of anaemia (24–41%), iron deficiency (17–32%), vitamin B12 deficiency (14–31%) and folate deficiency (23–37%) among children and adolescents (CNNS, 2019). Low food diversity, poor diet intakes and low bioavailability combined with high physiological demands and frequent infections contribute to the aetiology of MNDs (Burchi, Fanzo, & Frison, 2011; Gibson, Bailey, Gibbs, & Ferguson, 2010; Pasricha, Armitage, Prentice, & Drakesmith, 2018). Alleviation of MNDs is therefore critical and is globally addressed through intervention strategies that include supplementation, fortification and food diversification (Gibson, 2011; Girard, Self, McAuliffe, & Olude, 2012; Radhika et al., 2011; Sivakumar et al., 2006). However, the burden of MNDs, particularly the dietary inadequacies among school‐age children, has been less studied in India, using the more robust probability approach (Ahluwalia, 2002; Kehoe et al., 2014; Radhika, Swetha, Kumar, Krishna, & Laxmaiah, 2018).
The current impetus of the government of India as a policy is on the provision of prophylactic iron folic acid (IFA) supplements, which is one of the six interventions as part of Anaemia Mukth Bharat (ABM) Programme (Bhatia, Sahoo, & Parida, 2018; MoHFW‐GoI, 2018), that envisages to reach out to six types of beneficiaries (children 6–59 months: 1‐ml IFA syrup providing 20‐mg elemental iron and 100‐μg folic acid, biweekly; children 5–9 years: one IFA tablet providing 45‐mg iron and 400‐μg folic acid, weekly; adolescent girls and boys 10–19 years and women of reproductive age 20–49 years: one tablet providing 60‐mg iron and 500‐μg folic acid, weekly; pregnant women: one tablet providing 60‐mg iron and 500‐μg folic acid, daily for minimum 180 days from the second trimester; and lactating mothers of 0‐ to 6‐month child: one tablet providing 60‐mg iron and 500‐μg folic acid, daily for 180 days postpartum).
Further, the Food Safety and Standards Authority of India (FSSAI) has established guidelines to fortify five staple foods with essential micronutrients, namely, salt with iodine alone (15–30 ppm) named iodized salt or salt with iodine (15–30 ppm) and iron (850–1000 ppm) named iron fortified iodized salt or double fortified salt (DFS); whole wheat flour (atta), refined wheat flour (maida) and raw rice with iron (14–21.25 mg kg−1 as Na Fe EDTA or 28–42.5 mg kg−1 as other prescribed iron fortificants), folic acid (75–125 μg kg−1) and vitamin B12 (0.75–1.25 μg kg−1) named fortified atta, fortified maida and fortified raw rice respectively; vegetable oil with vitamin A/retinol equivalents (6–9.9 μg g−1) and vitamin D (0.11–0.16 μg g−1), named fortified oil; and milk (toned milk, double toned milk, skimmed milk and standardized milk) with vitamin A/retinol equivalents (270–450 μg L−1) and vitamin D (5–7.5 μg L−1), named fortified milk. Additionally, wheat flour and raw rice may also be fortified with six other micronutrients (zinc, vitamins A, B1, B2, B3 and B6) either singly or in combination at the prescribed limits (FSSAI, 2018).
The Indian government has more recently (‘FFRC‐FSSAI,’ 2019) intensified policy changes to promote fortified staples in the safety net food security programs such as Public Distribution System (PDS), Mid‐day Meal (MDM) Programme and Integrated Child Development Scheme (ICDS). In this context, there is a need to quantify the usual intakes from habitual diets and contribution of fortified foods and/or supplements towards inadequate (below estimated average requirements, EAR) or excessive (above tolerable upper limits, TULs) micronutrient intakes (Guamuch et al., 2014) in the target beneficiaries. In the current study, we assessed the magnitude and factors associated with dietary and subclinical MNDs among MDM beneficiaries, that is, school‐age children (6–17 years). In addition, we also modelled the contribution of fortified rice, DFS and IFA supplements, either singly or in combination, towards dietary adequacies or excessive intakes of iron, folic acid and vitamin B12.
2. METHODS
2.1. Source of data and study population
For the current analysis, the baseline data collected during the screening and enrolment phase (between June and July 2017) of the study that assessed the efficacy of multiple micronutrient fortified rice in reducing anaemia and improving the micronutrient status among school children (6–17 years) were used (Radhika et al., Unpublished data). Briefly, the study was carried out in two randomly selected government‐aided schools, which serve MDM to children, in Rangareddy district, Telangana, South India. The schools are located within 5‐km distance in the same area and cater to the children of similar socio‐economic status. Each school had independent primary school and high school units within the same premises. A total of 2,024 school children were screened, and the eligible children satisfying the inclusion criteria were enrolled and randomized into two groups, the control group and the experimental group. The desired sample size was 1,260 children (630 children in each group; 6–17 years). Dietary data were collected in a subsample of 300 children (150 children from each of the two schools) selected using simple random sampling. Care was taken to ensure comparable distribution of gender (boys and girls; χ 2 = 1.091, P = .296), age groups (6–9, 10–13 and 14–17 years; χ 2 = 2.444, P = .295), proportion of stunting (χ 2 = 0.760, P = .383), thinness (χ 2 = 0.160, P = .689) and anaemia (χ 2 = 0.210, P = .649) among children between the two schools. The written informed assent or consent from children and consent from their parents/guardians were obtained before screening and enrolling the children.
2.2. Socio‐demographic and anthropometric data
Prior to initiation of the study, all the research investigators were trained for 3 weeks by subject experts from Indian Council of Medical Research‐National Institute of Nutrition (ICMR‐NIN) in all the study procedures that included both classroom and practical sessions in another school not involved in the study. The trained field investigators collected the data on social demographics, household income and selected household and WASH facilities (chosen based on literature and logistic feasibility), by conducting household interviews and recording on paper‐based pretested questionnaires. The questionnaires were translated to local languages (telugu, hindi and urdu) as suggested by the World Health Organization (WHO) (available from: https://www.who.int/substance_abuse/research_tools/translation/en/), to obtain conceptual and cultural equivalence and thereafter used in the study.
The field investigators were trained by the in‐house expert anthropometrist on measuring height and weight in school children following standard techniques (WHO, 1995; WHO, 2005b) to the nearest 0.1 cm and 0.1 kg using seca 213 stadiometer and seca 813 weighing scale, respectively. Standardization was conducted in a convenience sample of 24 school children (12 of 6–9 years and 12 of 10–15 years) using repeat measures protocol (Stomfai et al., 2011; WHO MGRS Group, 2006) until acceptable intra‐ and inter‐individual reliability within and between field investigators and relative to the expert anthropometrist was obtained, that is, intra‐ and inter‐individual technical error of measurement (TEM) of investigators within 95% precision margin of the expert (within ±2 times the expert's TEM), relative TEM (%TEM) of <1.5% for intra‐individual reliability and <2% for inter‐individual reliability, a coefficient of variation (CV) of < 5% and the reliability coefficient (R) of >95%. The maximum allowable difference between a given pair of measurements was 100 g for weight and 7 mm for height. In the actual study, heights and weights were measured in duplicates in all children. Resampling was done in about 10% of children, and measurements by another trained investigator and expert anthropometrist were done using the same equipment and standard techniques. The equipment were calibrated with certified weights and measures, before use on each day, during training and data collection period in the study. The height‐for‐age z score (HAZ) and body mass index (BMI)‐for‐age z score (BAZ) were computed using WHO growth references and Anthroplus software (WHO, 2009). Mild to moderate thinness and stunting were defined as BAZ and HAZ between 2 and 3 z scores below the median and overweight/obesity defined as BAZ greater than 1 z score above the median of the WHO reference population.
2.3. Dietary intake data
Trained nutritionists assessed food intake in all the children using multiple‐pass 24‐h diet recall method (24HR). In approximately 35% children, the 24HR was repeated on three non‐consecutive days (two week days and one weekend day) within a reference week to calculate usual intakes by adjusting for intra‐individual variations. The women involved in preparation and serving food in the household were asked to describe all foods and beverages consumed by the target child during the preceding 24‐h, including time of consumption, sources where the food was obtained, quantities of ingredients used, total cooked content and portion size consumed. As the target children will also consume MDM in the school as well as foods at other away‐from‐home outlets, all diet assessments were planned and scheduled when the child was also present in the household. The adolescent child (10–17 years) responded to questions on type and amount of food consumed at school or outside. However, for younger children (6–9 years), the caregiver was the primary respondent. Further, the type and amount of MDM consumed by children in the school was measured by field investigators and data included in the analysis. The recipe information of composite dishes was enquired, and the amounts of each ingredient used and available in the household were measured and recorded using the seca culina 852 electronic kitchen scale with a precision of 1‐g. A set of 14 visual aids, 12 standard cups and two spoons that were similar to common household utensils were used to help the respondent estimate the cooked volume and portion sizes consumed by the child. If the ingredients were not available at home, the nutritionist enquired and recorded the reported size, number and quantity of ingredients used in terms of standard cups and spoons, the weights of which were estimated from an existing standard list that was developed in‐house. These weights are imputed, whenever the nutritionist is unable to estimate the actual weight of the ingredients onsite.
2.4. Usual intake estimation
The usual intake distributions for food intakes were estimated in a measurement error model using simple linear regression adjusting for the intra‐person, day‐to‐day variance of the observed food intakes obtained from the subsample of children from the same study (Joseph & Carriquiry, 2010). As the intake distributions were skewed, the data were transformed using Box Cox transformation to obtain symmetrical distributions. The best unbiased linear estimates of the intake for each child for each food (kth) consumed was estimated using the three repeated 24HRs. The usual intake of each food for each child was estimated as a function of the average intake of the same food in the three 24HRs of the subsample of children and the intake of the same food from one 24HR in rest of the children using the equation, UI k = I k + e k , where UI k is the estimate of the usual intake of the kth food, e k is the error term and I k is the average intake estimated from the three 24HRs, as a function of the dietary intake from 1‐day recall, that is, I k = (a k + b k intake of one 24HR), where a k and b k were estimated using the principle of the least squares method. The macronutrients and micronutrients in the kth food were computed using the Indian Food Composition Tables (IFCT) Database as the primary source for nutrient values (Longvah, Anantan, Bhaskarachary, & Venkaiah, 2017). The database was expanded to include recipe information on most commonly consumed foods, MDMs offered at school, away‐from‐home foods, ready‐to‐eat snacks and processed foods. For vitamin B12 values and foods not reported in IFCT, the nutrient values from the previous edition of the Nutrient Values of Indian Foods (NVIF) were used (Gopalan et al., 1989). The food and nutrient database of the United States Department of Agriculture (USDA, 2015) was used for those foods that did not have a nutrient value in the IFCT and NVIF. To ensure minimum variation between the databases, the nutrient values of some common foods were compared after correction for moisture values, and the variations were found to be in the range of 10–20%.
2.5. Determination of probability of adequacy and mean probability of adequacy
The probability of adequacy (PA) was calculated for eleven essential micronutrients (vitamin A, vitamin C, thiamin, riboflavin, niacin, dietary folate, B12, B6, calcium, iron and zinc). Age and gender specific, EARs proposed by the Institute of Medicine (IOM), USA (IOM, 1998; IOM, 2000a; IOM, 2001 and IOM, 2011) were used to calculate the probability of adequacy (PA) of micronutrients other than zinc. The EAR of iron was adjusted for 10% bioavailability, close to the reported bioavailability of iron from rice‐based meals in adolescent girls (Nair et al., 2013). To calculate the EAR of zinc, the recommendation proposed for refined/mixed diet by the International Zinc Consultative Group (IZiNCG, 2004) was used, because a refined cereal‐based diet is typically consumed in South India (Table S1). The coefficient of variation (CV) of requirements proposed by WHO (WHO, 2005a) and IZiNCG (IZiNCG, 2004) was used to calculate the standard deviation (SD) of requirements (SD = CV × EAR). The PA was calculated as the probability that a child's usual intake was above EAR, using the ‘CDFNORM’ function in SPSS software (IOM, 2000b). The mean probability of micronutrient adequacy (MPA) for each child was calculated as a mean of probability of adequacies of all 11 micronutrients. The prevalence of micronutrient inadequacy was defined as MPA < 0.5 (Becquey & Martin‐Prevel, 2010). The macronutrient distribution range was calculated as the percentage of energy obtained from the amount of carbohydrates, protein and fats consumed and compared with the Acceptable Macronutrient Distribution Range (AMDR) proposed by WHO (WHO, 2003). Estimated Energy Requirements (EERs) were calculated for each child using equations for basal metabolic rate, estimated from the individual's age, gender and weight and considering the additional demads for growth and habitual moderate physical activity (FAO/WHO/UNU, 2004).
2.6. Sample collection and biochemical estimation methods
Venous blood samples (4 ml) were collected in heparin tubes from the children in the morning, and plasma was separated and stored at −80°C. Haemoglobin in the whole blood was estimated by direct cyanmethaemoglobin kit (Hemocor‐D, Coral Clinical Systems, India). Plasma ferritin (Calbiotech, El Cajon, CA, USA) and C‐reactive protein (CRP; [R&D System, Minneapolis, MN, USA]) were estimated by the Enzyme Linked Immuno Sorbent Assay (ELISA) method. Plasma concentrations of B12 and folate were determined by dual count no‐boil Radio Immuno Assay (RIA) kit (Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA). Anaemia was defined in terms of haemoglobin values, 8–11.5 g dL−1 in children 6–11 years and 8–12 g dL−1 in children aged ≥12 years. Ferritin concentrations <15 μg L−1 were considered as iron deficient (ID), and ferritin concentrations <15 μg L−1 along with anaemia were considered to have iron deficiency anaemia (IDA) (WHO, 2017). Ferritin values were excluded if CRP concentrations were >5 mg L−1. The concentrations of B12 < 150 pmol L−1 and folate < 10 nmol L−1 were considered as deficient (WHO & FAO, 2006; Shalini et al., 2019). A set of three in‐house quality control samples were analysed along with each set of samples for all the analytes, and the inter/intra‐assay variation was always <5%.
2.7. Data analysis
Descriptive statistics, mean and SD, were used for normally distributed variables. The differences in mean values were tested using one‐way analysis of variance (ANOVA) with post‐hoc least significant difference (LSD) test for multiple comparisons. For skewed distributions, median values with inter quartile range (IQR) are reported particularly for food groups, nutrient intakes and biomarkers. The differences were tested using the non‐parametric Kruskal–Wallis test for comparison between more than two groups. The Chi square test was used to investigate the association of categorical outcome variables (dietary micronutrient inadequacy, anaemia, ID, IDA, folic acid and vitamin B12 deficiency) and the independent dietary and non‐dietary factors. Logistic regression analysis with crude and adjusted odds ratios was used to test the strength and direction of the association (Hosmer, Lemeshow, & Sturdivant, 2013). Multivariate logistic regression, with models adjusted for age, gender and dietary energy, was used to examine the association of various factors with dietary micronutrient inadequacy and also to examine the association of overall as well as individual dietary micronutrient adequacies with anaemia, ID, IDA, folate deficiency and vitamin B12 deficiency. Statistical significance was considered at P < .05, and all tests done were two sided. The data were analysed using SPSS software package (version 19.0, SPSS Inc, Chicago, IL, USA).
2.8. Ethical considerations
The study is approved by the Institutional Ethical Committee of the ICMR‐NIN, Hyderabad, India (IEC Protocol Number: 10/I/2017) and is registered with the Clinical Trials Registry, India (CTRI Trial Registration Number: CTRI/2017/11/010655).
3. RESULTS
3.1. Study population characteristics
The mean age (SD) of the children was 11.7 (2.45) years and was similar between boys and girls. A significantly higher proportion (27%) of younger children (6–9 years) were thin compared with older children (10–13 years: 18% and 14–17 years: 10%), while there were no gender differences. Prevalence of stunting was similar in all age groups and was independent of gender. The prevalence of overweight/obesity was 5%. Ninety percent of the households were nuclear families, 49% belonged to either scheduled caste or scheduled tribes, 56% had an own house, 51% had a separate kitchen and 85% of the study population were Hindus. Majority of the parents were uneducated, whereas 21% of the mothers were homemakers. About 66% of mothers and 80% of fathers were daily‐wage labourers. Majority of households (≥93%) had access to improved household facilities such as electricity, non‐solid cooking fuel (Liquid Petroleum Gas), piped water and improved toilet (Table 1). The mean family income was INR 15,054 per month (approximately USD 198.08). About 91% of the households habitually consumed a mixed diet.
TABLE 1.
Characteristics of the study population
| Characteristic | Study children (N = 300) |
|---|---|
| Age and gender distribution in the study population | |
| Age, years, mean ± SD | 11.74 ± 2.45 |
| 6–9 years, n (%) | 54 (18.00) |
| 10–13 years, n (%) | 174 (58.00) |
| 14–17 years, n (%) | 72 (24.00) |
| Girls, n (%) | 165 (55.00) |
| Boys, n (%) | 135 (45.00) |
| Anthropometry of study children | |
| Weight, kg, mean ± SD | 33.39 ± 10.78 |
| Height, cm, mean ± SD | 141.51 ± 14.72 |
| Stunted (HAZ <−2 to <−3), n (%) | 43 (14.33) |
| Thin (BAZ <−2 to −3), n (%) | 53 (17.67) |
| Overweight/obese (BAZ >+1), n (%) | 15 (5.00) |
| Religion and community, n (%) | |
| Hindus | 251 (84.51) |
| Muslims and others | 46 (15.49) |
| Scheduled caste and scheduled tribes | 146 (49.16) |
| Backward caste and others communities | 151 (50.84) |
| Family income per month, n (%) | |
| High income (≥ INR 16,722) or (≥ USD 220.03) | 101 (34.01) |
| Middle income (INR 10,840–INR 16,722) or (USD 142.63 ‐ USD 220.02) | 98 (33.00) |
| Low income (< INR 10,840) or (< USD 142.63) | 98 (33.00) |
| Level of education and occupation of parents, n (%) | |
| Mother—uneducated | 197 (65.70) |
| Mother—primary education (1st to 5th standard) | 40 (13.30) |
| Mother—upper primary education & above (6th standard and above) | 63 (21.00) |
| Father—uneducated | 142 (47.30) |
| Father—primary education (1st to 5th standard) | 56 (18.70) |
| Father—upper primary education & above (6th standard and above) | 102 (34.00) |
| Mother—homemaker | 60 (20.55) |
| Mother—housemaid or daily wage labour | 194 (66.44) |
| Mother—service, business or other occupation | 38 (13.01) |
| Father—daily wage labour | 212 (80.30) |
| Father—service, business or other occupation | 52 (19.70) |
| Household facilities, n (%) | |
| Ownership of house | 167 (56.23) |
| Presence of separate kitchen | 151 (50.84) |
| Access to improved non‐solid cooking fuel | 279 (93.94) |
| Presence of electricity | 296 (99.66) |
| Access to improved source of drinking water | 281 (94.61) |
| Access to improved toilet facility | 277 (93.27) |
Note: Values are n (%) or mean ± SD.
Abbreviations: BAZ, body mass index for age z score; HAZ, height for age z score; INR, Indian Rupee; SD, standard deviation; USD, United States Dollar.
3.2. Food group consumption and dietary micronutrient adequacies
The children consumed a diverse diet. The median (IQR) number of food groups consumed by study children was 13 (11, 13) and was similar in all age groups, with a minimum of eight food groups consumed by all children and ≥11 food groups consumed by 87% of the children. However, inequalities are seen in the median consumption of different food groups (Table S2) and nutrient adequacies (Table 2).
TABLE 2.
Probability of adequacy of micronutrient intakes among different age groups
| Micronutrient | 6–9 years (n = 54) | 10–13 years (n = 174) | 14–17 years (n = 72) | Pooled (N = 300) | P value |
|---|---|---|---|---|---|
| PA‐vitamin A | 0.10 ± 0.26a | 0.10 ± 0.26a | 0.03 ± 0.10a | 0.08 ± 0.23 | 0.059 |
| PA‐vitamin C | 0.43 ± 0.46a | 0.35 ± 0.45a | 0.17 ± 0.32b | 0.32 ± 0.43 | 0.001 ** |
| PA‐thiamine | 0.50 ± 0.46a | 0.47 ± 0.43a | 0.21 ± 0.37b | 0.41 ± 0.44 | <0.001 *** |
| PA‐riboflavin | 0.31 ± 0.40a | 0.13 ± 0.28 b | 0.05 ± 0.18 c | 0.15 ± 0.30 | <0.001 *** |
| PA‐niacin | 0.59 ± 0.40a | 0.52 ± 0.41a | 0.33 ± 0.39b | 0.48 ± 0.41 | 0.001 ** |
| PA‐dietary folate | 0.09 ± 0.27a | 0.04 ± 0.15b | 0.01 ± 0.07b | 0.04 ± 0.17 | 0.030 * |
| PA‐vitamin B12 | 0.10 ± 0.30a | 0.07 ± 0.25a | 0.06 ± 0.24a | 0.07 ± 0.26 | 0.640 |
| PA‐vitamin B6 | 0.70 ± 0.41a | 0.56 ± 0.43 b | 0.28 ± 0.38c | 0.52 ± 0.44 | <0.001 *** |
| PA‐calcium | ND | ND | ND | ND | ND |
| PA‐iron | 0.24 ± 0.38a | 0.20 ± 0.35a | 0.09 ± 0.24b | 0.18 ± 0.34 | 0.017 * |
| PA‐zinc | 0.85 ± 0.30a | 0.77 ± 0.35a | 0.41 ± 0.41b | 0.70 ± 0.39 | <0.001 *** |
| MPA | 0.36 ± 0.22a | 0.29 ± 0.20 b | 0.15 ± 0.17 c | 0.27 ± 0.21 | <0.001 *** |
Note: Values are mean ± standard deviation. Mean values between age groups were compared by one‐way ANOVA with post‐hoc LSD test. Significant differences in mean values between age groups are indicated by different superscript letters a, b and c.
Abbreviations: ANOVA, analysis of variance; LSD, least significant disfference; MPA, mean probability of adequacy; ND, not determinable because PA was less than 0.001; PA, probability of adequacy.
Significantly different at P ≤ .05.
Significantly different at P ≤ .01.
Significantly different at P ≤ .001.
Overall, 96–98% of children consumed foods from the vegetable group; however, the median daily intakes were very low (5‐g leafy vegetables, 25‐g roots and tubers and 60‐g other vegetables). The pulse and legume group was consumed by 81% of the children, but the consumption level was only 12 g day−1. About 8%, 17% and 26% of children consumed dairy, fruits and flesh foods, respectively, whereas 69% consumed eggs. The primary source of egg was the MDM provided in schools. In contrast, the median cereal consumption (predominantly rice) was 328 g day−1. Almost all children (96%) consumed ready‐to‐eat away‐from‐home foods on the day prior to the survey (Table S2).
The younger children (6–9 years) consumed a significantly lower amount of foods from all the food groups, dietary energy and micronutrients compared with older children (10–13 years and 14–17 years) (Tables S2 and S3). Consequently, the macronutrient and micronutrient intakes differed significantly between the age groups. Though the dietary energy intake was higher (1,950 kcal vs. 1,603 kcal day−1) in older children (10–17 years), almost 26% of them consumed <85% of the estimated energy requirement (EER); nevertheless, this percentage was significantly (P < .001) higher compared to 7% in younger children (6‐9 years). Carbohydrate was the main source of energy (70–72%) in their diets, closer to the upper limit of the recommendation of (55–75%). Energy intake from fats was suboptimal, that is, 14–15% compared with the requirement of 15–30% (WHO, 2003). The younger children (6–9 years) consumed a lower amount of most of the macronutrients (P < .01) and micronutrients (P < .01) except total fat and vitamin C, whereas they consumed a higher amount of vitamin B12 (P < .05), which corroborates with a higher egg intake (Tables S2 and S3). Irrespective of age group, the probability of inadequacy (Table 2) was very high for calcium (100%), dietary folate (96.2%), vitamin B12 (92.8%), vitamin A (91.8%), riboflavin (85.5%) and iron (82%).
The overall adequacy of micronutrients (MPA) was significantly highest (36%) in the youngest age group (6–9 years) compared with 29% and 15%, respectively, in older age categories (10–13 years and 14–17 years), despite significantly lower absolute intakes of most micronutrients by virtue of their lower requirements (Table 2). Though food diversity was good and energy intake was positively associated with MPA (R 2 = 0.40, P < .001), only 14% of the children (6–17 years) in the study had MPA ≥ 0.5. None of the children were sufficient for all 11 micronutrients, whereas 82% of them had intakes below EAR for 6–11 micronutrients, and significant differences were observed between age categories (χ 2 = 61.659, P < .001), indicating an imbalanced macronutrient and insufficient micronutrient intakes.
The cereals group was the major contributor (25–50%) for almost 9 out of the 11 micronutrients. The second largest contributor of thiamine, folate, iron and zinc was the pulse and legume group (10–17%). Despite low quantities of consumption, eggs, flesh foods and dairy contributed to 20–33% of the B‐complex micronutrients and significant amounts of iron, zinc and calcium. Though, animal source foods were the major contributors (44%) to calcium intake, the amount consumed was dismally low. The vegetable and fruit group contributed significant amounts of vitamin A (44%), vitamin C (42%) and folate (25%). Condiments and spices provided <7% of most micronutrients, whereas away‐from‐home foods contributed 3–19% of nine micronutrients except vitamins B6 and B12.
3.3. Biochemical status of haemoglobin, plasma ferritin, folic acid and vitamin B12
Plasma ferritin and B vitamin concentrations showed a decline in older (10–17 years) children compared with younger children (6–9 years), while haemoglobin improved with age (Table 3). The overall prevalence of anaemia, ID and IDA was 53%, 57% and 38%, respectively, whereas the prevalence of folic acid and vitamin B12 deficiency were 24% and 43%, respectively. The prevalence of IDA and folic acid deficiency differed significantly (P < .05) between age categories, with higher prevalence in the older children. The prevalence of anaemia, ID and vitamin B12 deficiency did not differ in the three age groups (Figure 1).
TABLE 3.
Blood haemoglobin, plasma ferritin, folic acid and vitamin B12 status among different age groups
| Biomarker | 6–9 years (n = 54) | 10–13 years (n = 174) | 14–17 years (n = 72) | Pooled (N = 300) | P value |
|---|---|---|---|---|---|
| Blood haemoglobin, g dL−1 | 11.16 ± 1.24a | 11.68 ± 1.24b | 11.61 ± 1.64ab | 11.57 ± 1.36 | 0.047 * |
| 11.37a (10.37, 12.04) | 11.77b (11.19, 12.54) | 11.63ab (10.64, 12.78) | 11.66 (10.83, 12.51) | 0.027 * | |
| Plasma ferritin, μg L−1† | 20.26 ± 14.32a | 17.05 ± 13.4a | 14.43 ± 10.99a | 16.99 ± 13.12 | 0.051 |
| 15.62a (9.60, 28.05) | 12.17ab (7.90, 22.31) | 9.16b (5.65, 19.8) | 12.20 (7.62, 23.18) | 0.023 * | |
| Plasma folic acid, nmol L−1 | 16.70 ± 5.95a | 14.21 ± 8.28b | 11.12 ± 3.56c | 13.81 ± 7.24 | <0.001 *** |
| 15.86a (11.17, 21.11) | 12.67b (10.20, 16.10) | 11.06c (8.42, 13.14) | 12.46 (9.90, 16.09) | <0.001 *** | |
| Plasma vitamin B12, pmol L−1 | 191.48 ± 121.62a | 188.57 ± 98.75a | 111.26 ± 80.44b | 169.91 ± 103.73 | <0.001 *** |
| 158.76a (100.84, 291.7) | 165.84a (118.7, 244.08) | 91.72b (42.45, 159.48) | 149.83 (98.84, 229.09) | <0.001 *** |
Note: Values are mean ± SD and median (IQR); IQR, (P25, P75); P25, 25th percentile; P75, 75th percentile. Mean values between age groups were compared by one‐way ANOVA with post‐hoc LSD test. Median values between age groups were compared using Kruskal–Wallis test. Significant differences in values between age groups are indicated by different superscript letters a, b and c.
†Plasma ferritin values of seven children, that is, two children (6–9 years), four children (10–13 years) and one child (14–17 years), were excluded from analysis as their C reactive protein concentration was > 5 mg L−1.
Abbreviations: ANOVA, analysis of variance; IQR, inter quartile range; LSD, least significant disfference; SD, standard deviation.
Significantly different at P ≤ .05.
Significantly different at P≤ .001.
FIGURE 1.
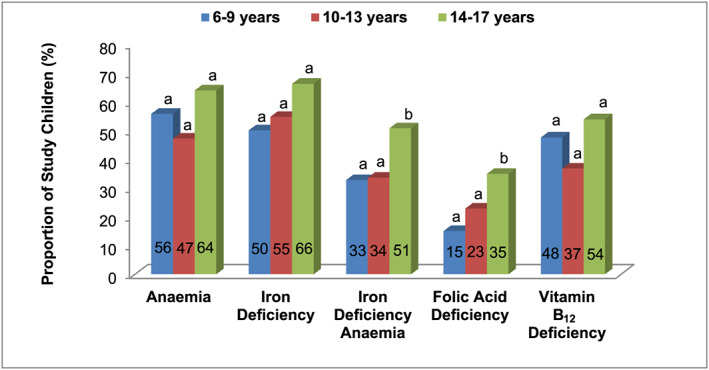
Prevalence of anaemia, iron deficiency, iron deficiency anaemia, folic acid deficiency and vitamin B12 deficiency among different age groups. The proportions between age groups were compared by Chi square test. The bars that do not share common superscript differ significantly (P < .05)
3.4. Factors associated with MPA
Logistic regression was used to assess the association of selected individual and household level factors with risk of dietary micronutrient inadequacy (MPA < 0.5) in unadjusted and adjusted models (Model 1, adjusted for age and gender and Model 2, adjusted for age, gender and energy intake). These factors were chosen based on recent literature (Pillay et al., 2018; Shalini et al., 2019). The odds of micronutrient inadequacy were two times higher in children belonging to backward and other communities (OR: 2.43; 95% CI: 1.22, 4.81; P = .011) when compared with scheduled caste and schedule tribe communities and lower in children belonging to other religions (OR: 0.35; 95% CI: 0.16, 0.73; P = .005) compared with Hindu religion. The associations remained significant when adjusted for age and gender but disappeared when further adjusted for dietary energy (Table S4).
3.5. Association of PA and MPA with anaemia and micronutrient deficiencies
The 11 micronutrients were analysed individually using logistic regression, considering PA of individual micronutrients as a continuous predictor variable and each of the biochemical deficiencies (anaemia, ID, IDA, folic acid and vitamin B12 deficiencies) as dichotomous categorical outcome variables separately. These associations were tested in all the unadjusted and adjusted models. For every unit increase in the PA of dietary iron and zinc, a 63% and 48% decrease in the odds of anaemia, and a 72% and 47% decrease in the odds of IDA, respectively, were noticed in the unadjusted model. These associations remained significant for anaemia even after adjusting for age, gender and energy intake. However, with respect to IDA, the association remained significant only for dietary iron (P < .01), in the adjusted models. In addition, a significant association (P < .05) of riboflavin adequacy with reduced risk of IDA was observed in the unadjusted model but disappeared when adjusted for covariates (P = .133). Dietary vitamin B12 adequacy was weakly associated with reduced odds of iron deficiency in all the unadjusted (P = .067) and adjusted (P = .056) models (Tables S5 and S6).
The MPA was significantly (P < .05) associated with anaemia and IDA. One unit increase in MPA led to a 69% and 78% decrease in risk of anaemia and IDA, respectively, in the unadjusted model and remained significant in the adjusted models as well. However, MPA was weakly associated (P = .08) with ID only in the unadjusted model (Tables S5 and S6).
3.6. Potential of fortified staple foods and IFA supplements in filling nutrient gaps and their contribution to excessive intake
The current impetus of the government of India to promote use of fortified foods in the public funded programmes (‘FFRC‐FSSAI,’ 2019) and intensification of the ABM programme to provide IFA supplements (Bhatia et al., 2018; MoHFW‐GoI, 2018) opens up the possibility for the school‐age children to enhanced nutrient intakes through multiple fortified foods and supplements. Such efforts may help to fill the nutrient gaps but might also increase the risk of excessive intake above the tolerable upper limit (TUL). Therefore, the predictive risk of inadequacy and excess intakes of iron, folic acid and vitamin B12 through fortified foods and IFA supplements was modelled either separately or in combinations (Table 4). The FSSAI suggests lower and upper ranges for the fortification of nutrients, and ABM programme suggests a weekly once regimen for IFA supplementation for school‐age children. Therefore, both the suggested ranges for fortified foods and the per day equivalent dose of iron and folic acid from IFA supplements were modelled to predict adequacy and risk of excessive nutrient intakes. The predicted risk of dietary inadequacy of iron from habitual diet was 82% that decreased to 13% with use of DFS, to 11% with the use of rice fortified at minimum fortification level (FR‐mFL) and to 3% with use of rice fortified at maximum fortification level (FR‐MFL) when considered separately, while there is virtually no risk of excess intake above TUL. But when both DFS and FR (either in combination of habitual diet + DFS + FR‐mFL or habitual diet + DFS + FR‐MFL) were considered simultaneously to deliver additional iron, the risk of inadequacy disappeared but concomitantly increased the risk of excess intake to 6% and 16%, respectively. When iron only from IFA supplement was considered in addition to habitual diets, a 7% risk of inadequacy with no concern of excess intake is observed. However, when IFA together with DFS or FR‐MFL was modelled into habitual diets, the risk of excess iron intake increased to 4% and 8%, respectively. When iron from all the three sources (DFS + FR‐mFL + IFA or DFS + FR‐MFL + IFA) in addition to iron from habitual diet was considered, the risk of consumption far exceeded the TUL to 22% and 40%, respectively. In contrast to iron, even with the additional folic acid from FR and IFA and additional B12 from FR, the risk of inadequacy of folate (25%) and B12 (91%) remained considerably high (Table 4).
TABLE 4.
Risk of inadequate and excess intake of iron, folate and vitamin B12 before and after inclusion of fortified staple foods and IFA supplements
| Source of intake | Iron | Dietary folate equivalent | Vitamin B12 a | ||
|---|---|---|---|---|---|
| Risk of inadequate intake | Risk of excess intake | Risk of inadequate intake | Risk of excess intake | Risk of inadequate intake | |
| Habitual diet | 82 | 0 | 96 | 0 | 93 |
| Habitual diet + DFS | 13 | 1 | 96 | 0 | 93 |
| Habitual diet + FR‐mFL | 11 | 1 | 91 | 0 | 92 |
| Habitual diet + FR‐MFL | 3 | 2 | 81 | 0 | 91 |
| Habitual diet + IFA | 7 | 0 | 62 | 0 | 93 |
| Habitual diet + DFS + FR‐mFL | 1 | 6 | 91 | 0 | 92 |
| Habitual diet + DFS + FR‐MFL | 0 | 16 | 81 | 0 | 91 |
| Habitual diet + DFS + IFA | 0 | 4 | 62 | 0 | 93 |
| Habitual diet + FR‐mFL + IFA | 0 | 2 | 37 | 0 | 92 |
| Habitual diet + FR‐MFL + IFA | 0 | 8 | 25 | 0 | 91 |
| Habitual diet + DFS + FR‐mFL + IFA | 0 | 22 | 37 | 0 | 92 |
| Habitual diet + DFS + FR‐MFL + IFA | 0 | 40 | 25 | 0 | 91 |
Note: Values are percentages.
Abbreviations: †DFS, double fortified salt, that is, iodized salt fortified with iron at 1 mg g−1; †FR, rice fortified with iron, folic acid and vitamin B12; †mFL, minimum fortification level (iron—28 mg kg−1; folic acid—75 μg kg−1; vitamin B12—0.75 μg kg−1); †MFL, maximum fortification level (iron—42.5 mg kg−1; folic acid—125 μg kg−1; vitamin B12—1.25 μg kg−1); †Source: FSSAI, 2018
‡IFA, iron folic acid supplements (6‐ to 9‐year‐old children, 6.4‐mg iron and 57.1‐μg folic acid per day; 10‐ to 19‐year‐old adolescents, 8.6‐mg iron and 71.4‐μg folic acid per day); ‡Source: MoHFW‐GoI, 2018.
Risk of excess intake is not determinable as there is no established tolerable upper limit for vitamin B12.
4. DISCUSSION
4.1. Magnitude and factors associated with dietary micronutrient adequacy in study children
The children in the study had suboptimal diets, with an imbalanced macronutrient intake and a low MPA of 11 micronutrients (27%). The severity of inadequacy was more for vitamin A, dietary folate, vitamin B12, iron and riboflavin. A lower MPA (18%) is reported among rural adolescent girls (10‐19 years) from 10 states in India (Radhika et al., 2018). Another recent study (Shalini et al., 2019) among urban adults (20–60 years) and elderly (>60 years) from the district sharing geographic boundaries with the current study, reported a higher MPA (38%) and PA for iron (82% vs. 18%) and inequalities in other micronutrient intakes except folate and vitamin B12. It is likely that higher pulse, vegetable, fruit and dairy intakes in adult population might have contributed to the higher micronutrient adequacies, particularly for iron. In addition, the requirement of iron is higher for adolescents compared with adults thereby increasing their risk for greater diet inadequacies. The inequalities could also be due to differences in food availability, access and affordability between rural, peri‐urban and urban areas.
Despite a high food diversity and a significant positive association of mean dietary energy intakes with MPA, only 14% of the study children had an MPA > 0.5. These children mainly subsisted on rice as the major staple, which contributed to more than 50% of the energy, whereas the relative contribution of other food groups was low. Although a high proportion of children consumed pulses, leafy vegetables and eggs, their median intake was grossly low. The predominance of rice as the major source of dietary energy in South Asia has been associated with inadequate intakes of many micronutrients (Arsenault et al., 2013). This indicates that, though the children were living in a food environment with higher food access and consuming foods from different food groups, the quantities of the micronutrient‐rich foods consumed are less. Similar consumption patterns are also reported in adolescents from other parts of India (Ganesan, Chacko, & Muhammad, 2019; Rathi, Riddell, & Worsley, 2017; Shaikh, Patil, Halli, Ramakrishnan, & Cunningham, 2016) as well as the neighbouring country, Bangladesh (Ahmed, Rahman, Noor, Akhtaruzzaman, & Hughes, 2006; Leroy, Ruel, Sununtnasuk, & Ahmed, 2018; Nguyen et al., 2018). However, it should be noted that EARs in the current study are adopted from IOM, and may not necessarily reflect actual requirements, and serve only to assess the relative risk. During the analysis of this data, EARs of iron for Indian children and adolescents were reported (Ghosh, Sinha, Thomas, Sachdev, & Kurpad, 2019; Swaminathan et al., 2019), which are higher compared with IOM recommendations. As a consequence, the risk of iron inadequacy increased further (93% vs. 82%) when these EARs were considered (Table S7).
Rising prices of micronutrient‐rich foods such as pulses, legumes, fresh fruits, vegetables and animal source foods may have limited the access to these foods to complement the staples adequately (Levay, Mumtaz, Faiz Rashid, & Willows, 2013). More recent qualitative studies (Rathi, Riddell, & Worsley, 2016) report some important undesirable influencers on food habits of adolescents in urban India that include authoritative/authoritarian parenting styles, peer influences, availability and access to ready to eat energy dense, nutrient poor convenience foods at homes, schools and urban neighbourhoods, rising women employment and eye‐catching repeated commercials on undesirable foods in mass media. Though we did not measure most of these factors, they are likely to exist as the study schools are situated in the peri‐urban area and may contribute to children developing unhealthy food habits leading to MNDs. Further, it was noticed that most households of the study children had migrated, for livelihood, to areas near the study sites. Migration to urban and peri‐urban areas may have improved food access but more so towards unhealthy foods and processed foods that are available at a much lower price compared with fruits and vegetables (Hawkes, Harris, & Gillespie, 2017). It is also possible that the households in the study were spending more on non‐food items as a coping mechanism due to pressures of urbanization, thereby limiting access to adequate micronutrient rich foods.
More often, a significant association between maternal illiteracy, a lower household wealth status, lower household food security and access to a diet with low nutrient adequacy is reported (Pasricha & Biggs, 2010; Syahrul et al., 2016; Wolde, Berhan, & Chala, 2015). The non‐availability of data on some of the individual and household level variables due to logistic hurdles related to time and transport precluded us from analysing the association of the potential non‐dietary mediators of micronutrient adequacy. Moreover, the sample size could have been inadequate to evaluate the association of the potential non‐dietary mediators. The exact nature in which religion and caste affects individual characteristics and impact micronutrient inadequacy is less studied and requires more research (Mahadevan & Suardi, 2013).
4.2. Association of PA and MPA with subclinical micronutrient status in study children
The prevalence of anaemia and deficiencies of plasma ferritin, folic acid and vitamin B12 was found to be high in children, consistent with dietary inadequacies. A high prevalence of these deficiencies has been reported in Indian children, adolescents and adults (CNNS, 2019; Nair et al., 2016; Shalini et al., 2019). In the present study, inadequacy of dietary iron adjusted for potential covariates is significantly associated with anaemia, ID and IDA. Further, the prevalence of IDA was found to be lower than that of anaemia and ID. It is now increasingly recognized that only half of anaemia is attributable to ID, while other concurrent nutrient deficiencies and infections might affect the erythropoiesis leading to anaemia, independent of iron status (Khatib et al., 2006; Pasricha et al., 2018; Shalini et al., 2019; Thoradeniya, Wickremasinghe, Ramanayake, & Atukorala, 2006). Apart from iron, multiple nutrients influence haemoglobin synthesis (Koury & Ponka, 2004). MPA is independently associated with increased odds of anaemia and IDA, after adjustment for covariates, implying the role of other nutrients in anaemia. Interestingly, dietary inadequacy of zinc was also associated with higher odds of anaemia. It has been reported that zinc is positively associated with haemoglobin status in human subjects and animal models (Kondaiah, Yaduvanshi, Sharp, & Pullakhandam, 2019). MPA has been reported to be associated with IDA and folic acid deficiency in adult and elderly population (Shalini et al., 2019), but a few studies showed no association between clinical deficiencies and dietary intakes (Henjum et al., 2014; Seshadri, 2001; Swaminathan et al., 2019).
4.3. Potential contribution of staple food fortification and nutrient supplementation to micronutrient adequacy in study children
The above results indicate high prevalence of dietary inadequacies and subclinical micronutrient deficiencies among school‐age children and thus warrant the need for effective intervention strategies for their prevention and control. Indeed, fortified foods with iron have been reported to reduce anaemia and improve iron status among different physiological groups (Andersson et al., 2008; Moretti et al., 2006; Radhika et al., 2011; Sivakumar et al., 2006). Keeping in view the deficits in dietary intakes and high prevalence of biochemical deficiencies, concerted efforts are being made by the national government to promote fortified staple foods through national safety net programs and intensification of IFA supplement uptake (‘FFRC‐FSSAI,’ 2019; Bhatia et al., 2018; MoHFW‐GoI, 2018). Nevertheless, simultaneous ingestion of nutrients through multiple fortified foods and/or IFA supplements might lead to excessive intake. Excessive iron intake has been implicated to increase oxidative stress and intestinal inflammation (Casanueva & Viteri, 2003; Jaeggi et al., 2015). Further, unabsorbed iron from fortified foods was reported to induce undesirable growth in pathogenic gut microbiota and weaken gut immune function (Ma et al., 2016; Zimmermann et al., 2010). As demonstrated through the modelling analysis, the potential risk of excess iron intake in school‐age children rises to 6–16%, if two fortified foods are simultaneously ingested, and increases further to 22–40%, if IFA supplement is also consumed in addition to two fortified foods in habitual diets. Similar findings, with variations among different states in India, have been reported recently, when predicted iron intakes from diet data extracted from national database and that likely to be obtained from fortification and supplementation programs were modelled (Ghosh et al., 2019; Swaminathan et al., 2019). Together, these results suggest a potential contribution of multiple fortified foods and IFA supplementation towards excess intake of iron when parallely implemented in the country. Therefore, a more systematic analysis of population level dietary inadequacies and adjustments in nutrient levels in fortified foods and supplements is required to inform policy. The fact that substantial amount of anaemia is independent of iron status and other micronutreint inadequacies are associated with anaemia, more systematic approach such as screening for ID followed by tailored treatment could be considered.
4.4. Strengths and limitations of the study
An important strength of the study is that the usual dietary intakes from multiple day diet recalls were estimated by using robust methods to adjust daily intakes with a variance estimate derived from the same study group. The more robust probability approach was used in assessing the dietary micronutrient adequacies. The IOM (IOM, 2000b) suggests diet measurements in a moderately large number of subjects (30–40 subjects per stratum/group) on two non‐consecutive days or on three consecutive days to assess intra‐individual variance and probability of nutient adequacy. In this study, the single day 24HR was conducted in 300 children, and the multiple day 24HR was conducted on three non‐consecutive days in a subsample of 105 children, that is, in 30 (6–9 years), 45 (10–13 years) and 30 (14–17 years) children, respectively. In addition, we also related the dietary intakes to that of subclinical nutrient levels, that is, blood haemoglobin, plasma ferritin, folic acid and vitamin B12.
However, there are several limitations: (1) There could be underreporting of food consumed away‐from‐home by the target child; (2) the EARs were adopted from IOM, USA and may not reflect the actual requirement for our study population, which might influence the results; (3) the single time point data is specific to school‐age group of poorer socio‐economic strata, restricted to a geographic region and thus may not be representative and cannot be generalized to the entire population; (4) the study highlights the need for more research at the population level, to consider adjustment of the nutrient levels in fortified foods and supplements, at least of iron in the context of multiple interventions; (5) as it was not logistically feasible to collect data on some of the individual and household level facilities, we were not able to assess the potential effects, if any, of the unmeasured non‐dietary factors on dietary inadequacies and micronutrient status in the study children.
In conclusion, these results indicate a high risk of multiple micronutrient dietary inadequacies, high prevalence of anaemia, ID, IDA, subclinical deficiencies of folic acid and vitamin B12 in the study children and suggest need for fortification and/or supplementation. However, dosage of nutrient levels, in fortified foods and/or nutrient supplements, needs to be adjusted to avoid excessive intakes, particularly of iron.
CONTRIBUTIONS
MSR, RP and LT designed the study. MSR and BS coordinated data and sample collection from study sites and assisted in preparation of the dietary data. RP, PR and YWJ carried out biochemical analysis. NKB performed the statistical analysis. RSM, AN and BK assisted in collection and supervision of health‐related data. MSR, RP and LT supervised the entire study. MSR has written the first draft of the paper, and all the authors reviewed it, provided critical inputs and approved the manuscript in the current form.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
Supporting information
Table S1: Estimated average requirements and Tolerable upper limits of micronutrients for children and adolescents.
Table S2: Median Intake of food groups among different age groups.
Table S3: Median Intake of dietary energy, macronutrients and micronutrients – By age group.
Table S4: Multivariate logistic regression to identify factors associated with risk of micronutrient deficiencies
Table S5: Effect of probability of adequacy of individual micronutrients and mean probability of adequacy of multiple micronutrients on risk of anaemia and biochemical deficiencies of iron, folic acid and vitamin B12 – Unadjusted Model.
Table S6: Effect of probability of adequacy (PA) of individual micronutrients and mean probability of adequacy (MPA) of multiple micronutrients on risk of anaemia and biochemical deficiencies of iron, folic acid and vitamin B12 – Adjusted Models.
Table S7: Risk of inadequate and excess intake of iron before and after fortification of staple foods and IFA supplementation ‐ Comparison of estimates derived using the EAR suggested by IOM with EAR suggested for Indian children and adolescents.
ACKNOWLEDGMENTS
The financial grant provided by Suvarnabhoomi Enterprises Private Limited, Namakkal District, Tamil Nadu, India and the research fellowships provided to SB and YWJ by the University Grants Commission, Government of India are greatly acknowledged. The funders had no role in study design, data analysis or preparation of the manuscript. The authors are grateful to the research, technical, field and support staffs who were involved in the study. They are also grateful to the district and school authorities, children and their parents for consenting to participate in the study.
Madhari RS, Boddula S, Ravindranadh P, et al. High dietary micronutrient inadequacy in peri‐urban school children from a district in South India: Potential for staple food fortification and nutrient supplementation. Matern Child Nutr. 2020;16(S3):e13065 10.1111/mcn.13065
Contributor Information
Radhika S. Madhari, Email: radhika.madhari@gmail.com.
Raghu Pullakhandam, Email: raghu_nin2000@yahoo.com.
REFERENCES
- Ahluwalia, N. (2002). Intervention strategies for improving iron status of young children and adolescents in India. Nutrition Reviews, 60(Suppl. 5), S115–S117. 10.1301/00296640260130858 [DOI] [PubMed] [Google Scholar]
- Ahmed, F. , Rahman, A. , Noor, A. N. , Akhtaruzzaman, M. , & Hughes, R. (2006). Anaemia and vitamin A status among adolescent schoolboys in Dhaka City, Bangladesh. Public Health Nutrition, 9(3), 345–350. 10.1079/PHN2006858 [DOI] [PubMed] [Google Scholar]
- Andersson, M. , Thankachan, P. , Muthayya, S. , Goud, R. B. , Kurpad, A. V. , Hurrell, R. F. , & Zimmermann, M. B. (2008). Dual fortification of salt with iodine and iron: A randomized, double‐blind, controlled trial of micronized ferric pyrophosphate and encapsulated ferrous fumarate in southern India. The American Journal of Clinical Nutrition, 88(5), 1378–1387. 10.3945/ajcn.2008.26149 [DOI] [PubMed] [Google Scholar]
- Arsenault, J. E. , Yakes, E. A. , Islam, M. M. , Hossain, M. B. , Ahmed, T. , Hotz, C. , … Brown, K. H. (2013). Very low adequacy of micronutrient intakes by young children and women in rural Bangladesh is primarily explained by low food intake and limited diversity. The Journal of Nutrition, 143(2), 197–203. 10.3945/jn.112.169524 [DOI] [PubMed] [Google Scholar]
- Bailey, R. L. , West, K. P. Jr. , & Black, R. E. (2015). The epidemiology of global micronutrient deficiencies. Annals of Nutrition and Metabolism, 66(Suppl. 2), 22–33. 10.1159/000371618 [DOI] [PubMed] [Google Scholar]
- Becquey, E. , & Martin‐Prevel, Y. (2010). Micronutrient adequacy of women's diet in urban Burkina Faso is low. The Journal of Nutrition, 140(11), 2079S–2085S. 10.3945/jn.110.123356 [DOI] [PubMed] [Google Scholar]
- Bhatia, V. , Sahoo, D. P. , & Parida, S. P. (2018). India steps ahead to curb anemia: Anemia Mukt Bharat. Indian Journal of Community Health, 30(4), 312–316. https://www.iapsmupuk.org/journal/index.php/IJCH/article/view/942 [Google Scholar]
- Burchi, F. , Fanzo, J. , & Frison, E. (2011). The role of food and nutrition system approaches in tackling hidden hunger. International Journal of Environmental Research and Public Health, 8(2), 358–373. 10.3390/ijerph8020358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanueva, E. , & Viteri, F. E. (2003). Iron and oxidative stress in pregnancy. The Journal of Nutrition, 133(5), 1700S–1708S. 10.1093/jn/133.5.1700S [DOI] [PubMed] [Google Scholar]
- CNNS (Comprehensive National Nutrition Survey) . (2019). Comprehensive National Nutrition Survey National Report. Ministry of Health and Family Welfare (MoHFW), Government of India. New Delhi, India: Retrieved from: https://nhm.gov.in/showfile.php?lid=712 [Google Scholar]
- FAO/WHO/UNU. (Food and Agriculture Organization/World Health Organization/United Nations University) . (2004). Human energy requirements: Report of a joint FAO/WHO/UNU expert consultation. Food & Agriculture Organization. Rome, Italy. Retrieved from http://www.fao.org/publications/card/en/c/e1faed04-3a4c-558d-8ec4-76a1a7323dcc/
- FFRC‐FSSAI. (Food Fortification Resource Centre ‐ Food Safety Standards Authority of India) . (2019). Safety Net Programs (2011‐19). Ministry of Health and Family Welfare (MoHFW), Government of India. New Delhi, India. Retrieved from https://ffrc.fssai.gov.in/snp
- FSSAI. (Food Safety Standards Authority of India) . (2018). Food safety and standards (fortification of foods) regulations, 2018. In: The Gazette of India: Extraordinarry (PART 111, Section 4). Ministry of Health and Family Welfare (MoHFW), Government of India. New Delhi, India. Retrieved from https://fssai.gov.in/upload/uploadfiles/files/Gazette_Notification_Food_Fortification_10_08_2018.pdf
- Ganesan, S. , Chacko, T. V. , & Muhammad, G. M. (2019). Are our rural adolescents eating healthy?: Implications for redesigning school health interventions—A cross sectional study in rural Coimbatore. Indian Journal of Public Health, 63(4), 293–297. 10.4103/ijph.IJPH_420_18 [DOI] [PubMed] [Google Scholar]
- Ghosh, S. , Sinha, S. , Thomas, T. , Sachdev, H. S. , & Kurpad, A. V. (2019). Revisiting dietary iron requirement and deficiency in indian women: Implications for food iron fortification and supplementation. The Journal of Nutrition, 149(3), 366–371. 10.1093/jn/nxy283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, R. S. (2011). Strategies for preventing multi‐micronutrient deficiencies: A review of experiences with food based approaches in developing countries In Thompson B. & Amoroso L. (Eds.), Combating micronutrient deficiencies: food‐based approaches (pp. 7–27). Rome, Italy and Wallingford, UK: Food and Agricultural Organization (FAO) and CAB International; http://www.fao.org/3/a-am027e.pdf [Google Scholar]
- Gibson, R. S. , Bailey, K. B. , Gibbs, M. , & Ferguson, E. L. (2010). A review of phytate, iron, zinc, and calcium concentrations in plant‐based complementary foods used in low‐income countries and implications for bioavailability. Food and Nutrition Bulletin, 31(Suppl. 2), S134–S146. 10.1177/15648265100312S206 [DOI] [PubMed] [Google Scholar]
- Girard, A. W. , Self, J. L. , McAuliffe, C. , & Olude, O. (2012). The effects of household food production strategies on the health and nutrition outcomes of women and young children: a systematic review. Paediatric and Perinatal Epidemiology, 26(Suppl. 1), 205–222. 10.1111/j.1365-3016.2012.01282.x [DOI] [PubMed] [Google Scholar]
- Gopalan, C. , Rama Sastri, B. V. , Balasubramanian, S. C. , Narasinga Rao, B. S. , Deosthale, Y. G. , & Pant, K. C. (1989). Nutritive Value of Indian Foods, Revised Edition (pp. 47–94). Hyderabad, India: Indian Council of Medical Research‐National Institute of Nutrition. [Google Scholar]
- Guamuch, M. , Dary, O. , Rambelson, Z. , de la Cruz, V. , Villalpando, S. , Tom, C. , … Makhumula, P. (2014). Model for estimating nutrient addition contents to staple foods fortified simultaneously: Mexico and Kampala data. Annals of the New York Academy of Sciences, 1312(1), 76–90. 10.1111/nyas.12350 [DOI] [PubMed] [Google Scholar]
- Hawkes, C. , Harris, J. , & Gillespie, S. (2017). Changing diets: Urbanization and the nutrition transition. In: 2017 Global Food Policy Report. International Food Policy Research Institute (IFPRI). Washington, DC. Chapter 4, 34‐41. Retreived from 10.2499/9780896292529_04 [DOI]
- Henjum, S. , Manger, M. , Skeie, E. , Ulak, M. , Thorne‐Lyman, A. L. , Chandyo, R. , … Strand, T. A. (2014). Iron deficiency is uncommon among lactating women in urban Nepal, despite a high risk of inadequate dietary iron intake. British Journal of Nutrition, 112(1), 132–141. 10.1017/S0007114514000592 [DOI] [PubMed] [Google Scholar]
- Hosmer, D. W. Jr. , Lemeshow, S. , & Sturdivant, R. X. (2013). Applied Logistic Regression (3rd ed.). New Jersey, USA: John Wiley & Sons Inc; https://onlinelibrary.wiley.com/doi/book/10.1002/9781118548387 [Google Scholar]
- IOM (US). (Institute of Medicine, United States) . (1998). Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. National Academies Press (US). Washington (DC). Available from: https://www.ncbi.nlm.nih.gov/books/NBK114310/doi:10.17226/6015 [PubMed]
- IOM (US). (Institute of Medicine, United States) . (2000a). Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. National Academies Press (US). Washington (DC). Available from: https://www.ncbi.nlm.nih.gov/books/NBK225483/doi:10.17226/9810 [PubMed]
- IOM (US). (Institute of Medicine, United States) . (2000b). Subcommittee on Interpretation and Uses of Dietary Reference Intakes; Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. DRI Dietary Reference Intakes: Applications in Dietary Assessment. National Academies Press (US). Washington (DC). Available from: https://www.ncbi.nlm.nih.gov/books/NBK222890/doi:10.17226/9956 [PubMed]
- IOM (US). (Institute of Medicine, United States) . (2001). Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academies Press (US). Washington (DC). Available from: https://www.ncbi.nlm.nih.gov/books/NBK222310/doi: 10.17226/10026. [PubMed]
- IOM (US). (Institute of Medicine, United States) . (2011). Committee to review dietary reference intakes for vitamin D and calcium In Ross A. C., Taylor C. L., Yaktine A. L., & Del Valle H. B. (Eds.), Dietary Reference Intakes for Calcium and Vitamin D. Washington (DC): National Academies Press (US) Available from: https://www.ncbi.nlm.nih.gov/books/NBK56070/doi: 10.17226/13050 [DOI] [PubMed] [Google Scholar]
- IZiNCG (International Zinc Nutrition Consultative Group) , Brown, K. H. , Rivera, J. A. , Bhutta, Z. , Gibson, R. S. , King, J. C. , … Hotz, C. (2004). International zinc nutrition consultative group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food and Nutrition Bulletin, 25(1 Suppl. 2), S99–S203. Available from: https://www.izincg.org/technical-documents [PubMed] [Google Scholar]
- Jaeggi, T. , Kortman, G. A. M. , Moretti, D. , Chassard, C. , Holding, P. , Dostal, A. , … Zimmermann, M. B. (2015). Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut, 64(5), 731–742. 10.1136/gutjnl-2014-307720 [DOI] [PubMed] [Google Scholar]
- Joseph, M. L. , & Carriquiry, A. (2010). A measurement error approach to assess the association between dietary diversity, nutrient intake, and mean probability of adequacy. The Journal of Nutrition, 140(11), 2094S–2101S. 10.3945/jn.110.123588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe, S. H. , Krishnaveni, G. V. , Veena, S. R. , Guntupalli, A. M. , Margetts, B. M. , Fall, C. H. , & Robinson, S. M. (2014). Diet patterns are associated with demographic factors and nutritional status in South Indian children. Maternal & Child Nutrition, 10(1), 145–158. 10.1111/mcn.12046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatib, L. A. , Obeid, O. , Sibai, A.‐M. , Batal, M. , Adra, N. , & Hwalla, N. (2006). Folate deficiency is associated with nutritional anaemia in Lebanese women of childbearing age. Public Health Nutrition, 9(7), 921–927. 10.1017/phn2005921 [DOI] [PubMed] [Google Scholar]
- Kondaiah, P. , Yaduvanshi, P. S. , Sharp, P. A. , & Pullakhandam, R. (2019). Iron and zinc homeostasis and interactions: Does enteric zinc excretion cross‐talk with intestinal iron absorption? Nutrients, 11(8), 1885 10.3390/nu11081885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koury, M. J. , & Ponka, P. (2004). New insights into erythropoiesis: The roles of folate, vitamin B12, and iron. Annual Review of Nutrition, 24, 105–131. 10.1146/annurev.nutr.24.012003.132306 [DOI] [PubMed] [Google Scholar]
- Leroy, J. L. , Ruel, M. , Sununtnasuk, C. , & Ahmed, A. (2018). Understanding the determinants of adolescent nutrition in Bangladesh. Annals of the New York Academy of Sciences, 1416(1), 18–30. 10.1111/nyas.13530 [DOI] [PubMed] [Google Scholar]
- Levay, A. V. , Mumtaz, Z. , Faiz Rashid, S. , & Willows, N. (2013). Influence of gender roles and rising food prices on poor, pregnant women's eating and food provisioning practices in Dhaka, Bangladesh. Reproductive Health, 10(1), 53 10.1186/1742-4755-10-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longvah, T. , Ananthan, R. , Bhaskarachary, K. , & Venkaiah, K. (2017). Indian Food Composition Tables. Hyderabad, India: Indian Council of Medical Research‐National Institute of Nutrition (ICMR‐NIN). [Google Scholar]
- Ma, J. , Sun, Q. , Liu, J. , Hu, Y. , Liu, S. , Zhang, J. , … Hambidge, K. M. (2016). The effect of iron fortification on iron (Fe) status and inflammation: A randomized controlled trial. PLoS ONE, 11(12), e0167458 10.1371/journal.pone.0167458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan, R. , & Suardi, S. (2013). Is there a role for caste and religion in food security policy? A look at rural India. Economic Modelling, 31, 58–69. 10.1016/j.econmod.2012.11.060 [DOI] [Google Scholar]
- Ministry of Health and Family Welfare, Government of India (MoHFW‐GoI) (2018). Interventions Anemia Mukt Bharat. Intensified National Iron Plus Initiative (I‐NIPI). Operational Guidelines for Program Managers. New Delhi, India: Ministry of Health and Family Welfare, Government of India; https://anemiamuktbharat.info/resources/#operational-guideline [Google Scholar]
- Moretti, D. , Zimmermann, M. B. , Muthayya, S. , Thankachan, P. , Lee, T‐C. , Kurpad, A. V. , & Hurrell, R. F. (2006). Extruded rice fortified with micronized ground ferric pyrophosphate reduces iron deficiency in Indian schoolchildren: a double‐blind randomized controlled trial. The American Journal of Clinical Nutrition, 84(4), 822–829. 10.1093/ajcn/84.4.822 [DOI] [PubMed] [Google Scholar]
- Muthayya, S. , Rah, J. H. , Sugimoto, J. D. , Roos, F. F. , Kraemer, K. , & Black, R. E. (2013). The global hidden hunger indices and maps: an advocacy tool for action. PLoS ONE, 8(6), e67860 10.1371/journal.pone.0067860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair, K. M. , Brahmam, G. N. , Radhika, M. S. , Dripta, R. C. , Ravinder, P. , Balakrishna, N. , … Abrams, S. A. (2013). Inclusion of guava enhances non‐heme iron bioavailability but not fractional zinc absorption from a rice‐based meal in adolescents. The Journal of Nutrition, 143(6), 852–858. 10.3945/jn.112.171702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair, K. M. , Fernandez‐Rao, S. , Nagalla, B. , Kankipati, R. V. , Punjal, R. , Augustine, L. F. , … Black, M. M. (2016). Characterisation of anaemia and associated factors among infants and pre‐schoolers from rural India. Public Health Nutrition, 19(5), 861–871. 10.1017/S1368980015002050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, P. H. , Huybregts, L. , Sanghvi, T. G. , Tran, L. M. , Frongillo, E. A. , Menon, P. , & Ruel, M. T. (2018). Dietary diversity predicts the adequacy of micronutrient intake in pregnant adolescent girls and women in Bangladesh, but use of the 5‐group cutoff poorly identifies individuals with inadequate intake. The Journal of Nutrition, 148(5), 790–797. 10.1093/jn/nxy045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasricha, S‐R. , Armitage, A. E. , Prentice, A. M. , & Drakesmith, H. (2018). Reducing anaemia in low income countries: Control of infection is essential. BMJ, 362, k3165 10.1136/bmj.k3165 [DOI] [PubMed] [Google Scholar]
- Pasricha, S. R. , & Biggs, B. A. (2010). Undernutrition among children in South and South‐East Asia. Journal of Paediatrics and Child Health, 46(9), 497–503. 10.1111/j.1440-1754.2010.01839.x [DOI] [PubMed] [Google Scholar]
- Pfeiffer, C. M. , Sternberg, M. R. , Schleicher, R. L. , Haynes, B. M. , Rybak, M. E. , & Pirkle, J. L. (2013). The CDC's second national report on biochemical indicators of diet and nutrition in the US population is a valuable tool for researchers and policy makers. The Journal of Nutrition, 143(6), 938S–947S. 10.3945/jn.112.172858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay, D. , Wham, C. , Moyes, S. , Muru‐Lanning, M. , Teh, R. , & Kerse, N. (2018). Intakes, adequacy, and biomarker status of iron, folate, and vitamin B12 in Māori and non‐Māori Octogenarians: Life and living in advanced age: A cohort study in New Zealand (LiLACS NZ). Nutrients, 10(8), 1090 10.3390/nu10081090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhika, M. S. , Nair, K. M. , Kumar, R. H. , Rao, M. V. , Ravinder, P. , Reddy, C. G. , & Brahmam, G. N. V. (2011). Micronized ferric pyrophosphate supplied through extruded rice kernels improves body iron stores in children: A double‐blind, randomized, placebo‐controlled midday meal feeding trial in Indian schoolchildren. The American Journal of Clinical Nutrition, 94(5), 1202–1210. 10.3945/ajcn.110.007179 [DOI] [PubMed] [Google Scholar]
- Radhika, M. S. , Swetha, B. , Kumar, B. N. , Krishna, N. B. , & Laxmaiah, A. (2018). Dietary and nondietary determinants of nutritional status among adolescent girls and adult women in India. Annals of the New York Academy of Sciences, 1416(1), 5–17. 10.1111/nyas.13599 [DOI] [Google Scholar]
- Rathi, N. , Riddell, L. , & Worsley, A. (2016). What influences urban Indian secondary school students' food consumption?—A qualitative study. Appetite, 105, 790–797. 10.1016/j.appet.2016.07.018 [DOI] [PubMed] [Google Scholar]
- Rathi, N. , Riddell, L. , & Worsley, A. (2017). Food consumption patterns of adolescents aged 14–16 years in Kolkata, India. Nutrition Journal, 16(1), 50 10.1186/s12937-017-0272-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri, S. (2001). Prevalence of micronutrient deficiency particularly of iron, zinc and folic acid in pregnant women in South East Asia. British Journal of Nutrition, 85(S2), S87–S92. 10.1049/BJN2000299 [DOI] [PubMed] [Google Scholar]
- Shaikh, N. I. , Patil, S. S. , Halli, S. , Ramakrishnan, U. , & Cunningham, S. A. (2016). Going global: Indian adolescents' eating patterns. Public Health Nutrition, 19(15), 2799–2807. 10.1017/s1368980016001087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalini, T. , Sivaprasad, M. , Balakrishna, N. , Madhavi, G. , Radhika, M. S. , Kumar, B. N. , … Reddy, G. B. (2019). Micronutrient intakes and status assessed by probability approach among the urban adult population of Hyderabad city in South India. European Journal of Nutrition, 58(8), 3147–3159. 10.1007/s00394-018-1859-y [DOI] [PubMed] [Google Scholar]
- Shergill‐Bonner, R. (2017). Micronutrients. Paediatrics and Child Health, 27(8), 357–362. 10.1016/j.paed.2017.04.002 [DOI] [Google Scholar]
- Sivakumar, B. , Nair, K. M. , Sreeramulu, D. , Suryanarayana, P. , Ravinder, P. , Shatrugna, V. , … Raghuramulu, N. (2006). Effect of micronutrient supplement on health and nutritional status of school children: Biochemical status. Nutrition, 22(1 Suppl), S15–S25. 10.1016/j.nut.2005.07.012 [DOI] [PubMed] [Google Scholar]
- Stomfai, S. , Ahrens, W. , Bammann, K. , Kovács, E. , Mårild, S. , Michels, N. , … Molnár, D. (2011). Intra‐ and inter‐observer reliability in anthropometric measurements in children. International Journal of Obesity, 35(Suppl 1), S45–S51. 10.1038/ijo.2011.34 [DOI] [PubMed] [Google Scholar]
- Swaminathan, S. , Ghosh, S. , Varghese, J. S. , Sachdev, H. S. , Kurpad, A. V. , & Thomas, T. (2019). Dietary iron intake and anemia are weakly associated, limiting effective iron fortification strategies in India. The Journal of Nutrition, 149(5), 831–839. 10.1093/jn/nxz009 [DOI] [PubMed] [Google Scholar]
- Syahrul, S. , Kimura, R. , Tsuda, A. , Susanto, T. , Saito, R. , & Ahmad, F. (2016). Prevalence of underweight and overweight among school‐aged children and it's association with children's sociodemographic and lifestyle in Indonesia. International Journal of Nursing Sciences, 3(2), 169–177. 10.1016/j.ijnss.2016.04.004 [DOI] [Google Scholar]
- Thoradeniya, T. , Wickremasinghe, R. , Ramanayake, R. , & Atukorala, S. (2006). Low folic acid status and its association with anaemia in urban adolescent girls and women of childbearing age in Sri Lanka. British Journal of Nutrition, 95(3), 511–516. 10.1079/bjn20051590 [DOI] [PubMed] [Google Scholar]
- USDA . (2015). (United States Department of Agriculture, Agricultural Research Service, Nutrient Data Laboratory) . USDA National Nutrient Database for Standard Reference, Release 28. Version Current: September 2015, Slightly revised May 2016. Available from: https://www.ars.usda.gov/Services/docs.htm?docid=8964
- Walker, S. P. , Wachs, T. D. , Gardner, J. M. , Lozoff, B. , Wasserman, G. A. , Pollitt, E. , … International Child Development Steering Group . (2007). Child development: risk factors for adverse outcomes in developing countries. Lancet, 369(9556), 145–157. 10.1016/S0140-6736(07)60076-2 [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) (1995). Physical Status: The Use and Interpretation of Anthropometry—A Report of a WHO Expert Committee. Geneva, Switzerland: World Health Organization; https://apps.who.int/iris/bitstream/handle/10665/37003/WHO_TRS_854.pdf?sequence=1…isAllowed=y [PubMed] [Google Scholar]
- World Health Organization (WHO) (2003). Diet, nutrition, and the prevention of chronic diseases: Report of a joint WHO/FAO expert consultation, WHO Technical report series 916 (pp. 54–60). Geneva, Switzerland: World Health Organization; Available from: https://www.who.int/dietphysicalactivity/publications/trs916/en/gsfao_overall.pdf?ua=1 [PubMed] [Google Scholar]
- World Health Organization (WHO) (2005a). Vitamin and mineral requirements in human nutrition (2nd ed.). Geneva, Switzerland: World Health Organization; Available from: https://apps.who.int/iris/handle/10665/42716 [Google Scholar]
- World Health Organization (WHO) (2005b). WHO STEPS Surveillance Manual: The WHO STEPwise approach to chronic disease risk factor surveillance. Geneva, Switzerland: World Health Organization; https://apps.who.int/iris/bitstream/handle/10665/43376/9241593830_eng.pdf?sequence=1…isAllowed=y; Updated 2017 available at https://www.who.int/ncds/surveillance/steps/STEPS_Manual.pdf?ua=1 [Google Scholar]
- World Health Organization (WHO) (2009). WHO AnthroPlus for Personal Computers Manual: Software for Assessing Growth of the World's Children. Geneva, Switzerland: World Health Organization; Available from: https://www.who.int/growthref/tools/en/ [Google Scholar]
- World Health Oganization Multicentre Growth Reference Study Group (WHO MGRS Group) (2006). Reliability of anthropometric measurements in the WHO Multicentre Growth Reference Study. Acta paediatrica (Oslo, Norway: 1992), Supplement, 450, 38–46. 10.1111/j.1651-2227.2006.tb02374.x [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) . (2017). Nutritional anaemias: tools for effective prevention and control. Geneva, Switzerland: World Health Organization; Available from: https://apps.who.int/iris/handle/10665/259425 [Google Scholar]
- World Health Organization (WHO) & Food and Agricultural Organization (FAO) of the United Nations (2006). Zinc, folate, vitamin B12 and other B vitamins, vitamin C, vitamin D, calcium, selenium and fluoride In , Allen L., de Benoist B. & Dary O. (Eds.), Guidelines on food fortification with micronutrients (pp. 61–67). Geneva, Switzerland: World Health Organization; https://www.who.int/nutrition/publications/guide_food_fortification_micronutrients.pdf [Google Scholar]
- Wolde, M. , Berhan, Y. , & Chala, A. (2015). Determinants of underweight, stunting and wasting among schoolchildren. BMC Public Health, 15(1), 8 10.1186/s12889-014-1337-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann, M. B. , Chassard, C. , Rohner, F. , N'Goran, E. K. , Nindjin, C. , Dostal, A. , … Hurrell, R. F. (2010). The effects of iron fortification on the gut microbiota in African children: a randomized controlled trial in Cote d'Ivoire. The American Journal of Clinical Nutrition, 92(6), 1406–1415. 10.3945/ajcn.110.004564 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Estimated average requirements and Tolerable upper limits of micronutrients for children and adolescents.
Table S2: Median Intake of food groups among different age groups.
Table S3: Median Intake of dietary energy, macronutrients and micronutrients – By age group.
Table S4: Multivariate logistic regression to identify factors associated with risk of micronutrient deficiencies
Table S5: Effect of probability of adequacy of individual micronutrients and mean probability of adequacy of multiple micronutrients on risk of anaemia and biochemical deficiencies of iron, folic acid and vitamin B12 – Unadjusted Model.
Table S6: Effect of probability of adequacy (PA) of individual micronutrients and mean probability of adequacy (MPA) of multiple micronutrients on risk of anaemia and biochemical deficiencies of iron, folic acid and vitamin B12 – Adjusted Models.
Table S7: Risk of inadequate and excess intake of iron before and after fortification of staple foods and IFA supplementation ‐ Comparison of estimates derived using the EAR suggested by IOM with EAR suggested for Indian children and adolescents.


