2020 will be remembered as the year that the COVID-19 pandemic spread throughout the world [1]. It became rapidly evident that this virus was very likely to infect older persons and was particularly lethal in the older population [2,3]. Persons with comorbidity and frailty were at very high risk of poor outcomes [4,5]. Not surprisingly, persons living in nursing homes were at especially high risk of contracting and dying from COVID-19 [6].
Recinella et al.[7] found that hospitalized older persons at high nutritional risk, as determined by the Geriatric Nutritional Risk Index, were at high risk for mortality. They also found that older persons with hypoalbuminemia and a low BMI had an increased death rate. Pironi et al.[8] applying the Global Initiative on Malnutrition (GLIM) criteria found that in Italy 50% of COVID-19 patients in hospital were malnourished. In this study, 70% of included patients were above age 65. Also using the GLIM criteria another study found that 42.1% of hospitalized COVID-19 patients were malnourished [9]. In a study of hospitalized patients above age 65 with COVID-19 infection in Wuhan, China, using the Mini Nutritional Assessment (MNA), 27.5% were at malnutrition risk and 52.7% had malnutrition [10]. In another study, that applied the MNA in COVID-19 patients, average age 55 years, after discharge from the ICU 65.9% were at risk and 14.5% of patients were malnourished [11]. Hypoalbuminemia was present in 19.5%.
Liu et al. compared the NRS 2002, the MUST, the MNA short from (MNA-SF) and the Nutritional Risk Index in hospital patients with COVID-19-infection above age 65. The identification of patients at nutritional risk varied widely between the four instruments with the highest rate of at risk patients identified by the NRS 2002 (85.8%) and the lowest rate identified by the MUST (41.1%). Patients at risk according to the MNA-SF, the NRS 2002 and the NRI had longer length of stay, higher hospital expenses, poor appetite, higher disease severity and higher weight loss when compared with patients with normal nutritional status. The authors concluded that NRS 2002, MNA-SF and NRI are suitable instrument for nutritional screening in older patients with COVID-19 infection [12]. In a recently published systematic review on the screening tools for malnutrition in COVID-19 patients Silva et al.[13] provided an overview on the current literature, but were not able to identify a superior tool for this purpose, but they stated that all tested tools – MNA, MNA-SF, NRS, MUST and Nutritional Risk Index – demonstrated high sensitivity.
It can be summarized that a high percentage of hospital patients with COVID-19 is at nutritional risk and its presence is associated with worse outcomes in this population. While nutritional risk screening must become a routine in this patient group, several screening tools may be recommended for this purpose.
In a review on nutrition support guidelines for recovering COVID-19 patients in the community the authors stressed the importance of screening for malnutrition, the installment of nutritional care plans and the continuity of nutritional care between settings [14]. In this context, it also has to be considered that nutritional care is also an essential component of rehabilitation in this population.
The COVID-19 pandemic may also have negative effects on the wider public beyond those that are directly affected by the infection. Physical activity and nutritional behavior during COVID-19 home confinement was explored with the help of a specifically designed electronic questionnaire which was answered by more than 1000 respondents mostly from Asia, Europe and Northern Africa [15]. The age of 80% of respondents was below 55 years. While there was a negative effect on all intensity levels of physical activity in this rather young group, food consumption and meal patterns were more unhealthy than before the confinement period. The latter was characterized by eating more often out of control, consuming more snacks and increasing the number of meals. In a large survey from the United States that included participants with an average age of 51 years and a wide age range between 19 and 94 years in one-third of participants an increase of household food insecurity since the begin of the pandemic was documented. The latter was associated with a coping strategy of eating less in a third of cases [16].
On the contrary, an online survey from Spain included 7514 respondents with 77.9% university-level education or higher and documented that participants followed a healthier diet during the confinement than before [17]. The latter included a higher adherence to the Mediterranean diet and a lower intake of fried foods, red meat, pastries and sweet beverages. The authors proposed that this change of diet might have a positive effect on COVID-19-related complications.
In another online-survey from Lithuania with 2447 participants it was observed that 49.4% ate more than before and 45.1% increased snacking, while intake of fast food, sugary drinks and commercial pastries decreased. A third of respondents described a gain of weight, more often those with overweight and those that were older [18]. Summarizing the current literature on the effects of confinement and quarantine on nutritional intake and nutritional status during the COVID-19 pandemic it has to be acknowledged that most studies relied on convenience samples that were recruited online. Therefore, the participants have not been representative of the population as a whole as they were mostly belonging to younger age groups and a majority was highly educated. Different results may be expected in older populations and in those that have been less well educated. In addition, it is highly likely that the economic effects of the pandemic and the consequences with regard to food insecurity will differ significantly between countries.
Primary sarcopenia is the loss of muscle mass and function that occurs with aging [19]. A major cause of sarcopenia is the decline in physical activity and exercise. During the COVID-19 pandemic, in an attempt to protect older persons from contracting the virus, both social isolation and lockdown have led to a decrease in physical activity in older persons [20]. This has led to the loss of muscle coupled with a decline in function. When an older person becomes infected with COVID-19 and admitted to hospital, there is a further loss of muscle due to immobility and usually an increase in inflammatory cytokines. There is now evidence that exercise during hospitalization can prevent the functional loss that normally occurs in hospital [21]. To prevent the acceleration of sarcopenia that occurs during ‘lockdown’ it is important that older persons are encouraged to follow an exercise program such as the ‘Vivifrail’ [22]. In addition, an increase in high-quality protein intake is recommended [23].
Cachexia is ‘a complex metabolic syndrome associated with underlying illness and a severe loss of muscle mass’ [24]. The major cause of cachexia is an increase in inflammatory cytokines [25]. Persons infected with COVID-19 have an increase in inflammatory cytokines which can be excessive if a ‘cytokine storm’ occurs [26]. Corona virus can enter muscle cells directly through the ACE2 receptor on the spike protein, resulting in local inflammation [27]. Inflammatory cytokines not only lead to muscle loss but also result in decreased muscle function and myalgia [28] (Fig. 1). These effects are coupled with anorexia and hypoalbuminemia. COVID-19 produces loss of smell and taste leading to anorexia [29]. Persons who are mechanically ventilated are immobilized leading to further loss of muscle [30].
FIGURE 1.
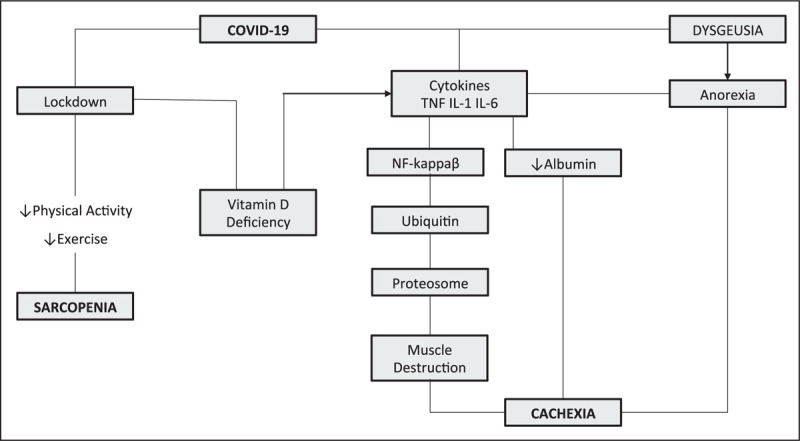
COVID-19 effects on nutrition.
A number of studies have suggested that low 25 (OH) vitamin D levels may be associated with worse outcomes in persons with COVID-19 [31–35]. These effects are best seen in persons with levels below 50 nmol/l (20 ng/ml) [36]. Biesalski [37] focuses on the available evidence that currently illustrates the relevance of vitamin D for the course of COVID-19 infections.
Persons infected with COVID-19 are at increased risk for developing hypocalcemia and hypomagnesemia [37]. Magnesium has antioxidant and inhibits release of inflammatory cytokines [38]. Magnesium also inhibits bronchial muscle contraction. In a study by Tan et al.[39], a combination of vitamin D, magnesium and vitamin B12 reduced the need for oxygen support in persons with COVID-19.
Overall, persons with COVID-19 are at increased risk for developing protein energy undernutrition, sarcopenia and cachexia. The development of low calcium and magnesium need to be monitored for in persons with COVID-19. ‘Lockdown’ increases the propensity for persons to become vitamin D deficient.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
References
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Morley JE, Vellas B. Editorial: COVID-19 and older adults. J Nutr Health Aging 2020; 24:364–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbatecola AM, Antonelli-Incalzi R. Editorial: COVID-19 spiraling of frailty in older Italian patients. J Nutr Health Aging 2020; 24:453–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cesari M, Proietti M. Editorial: Geriatric medicine in Italy in the time of COVID-19. J Nutr Health Aging 2020; 24:459–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagg S, Jylhava J, Wang Y, et al. Age, frailty, and comorbidity as prognostic factors for short-term outcomes in patients with coronavirus disease 2019 in geriatric care. J Am Med Dir Assoc 2020; 21:1555–1559.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma Y, Lou L, Yang X, et al. The association between frailty and severe disease among COVID-19 patients aged over 60 years in China: a prospective cohort study. BMC Med 2020; 18:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rolland Y, Benetos A, Villars H, et al. Editorial: COVID-19 support platform for long term care facilities. J Nutr Health Aging 2020; 24:461–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Recinella G, Marasco G, Serafini G, et al. Prognostic role of nutritional status in elderly patients hospitalized for COVID-19: a monocentric study. Aging Clin Exp Res 2020; doi.10.1007/s40520-020-01727-5. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pironi L, Sasdelli AS, Ravaioli F, et al. Malnutrition and nutritional therapy in patients with SARS-CoV-2 disease. Clin Nutr 2020; doi.10.1016/j.clnu.2020.08.021. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bedock D, Bel Lassen P, Mathian A, et al. Prevalence and severity of malnutrition in hospitalized COVID-19 patients. Clin Nutr ESPEN 2020; 40:214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li T, Zhang Y, Gong C, et al. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan. China Eur J Clin Nutr 2020; 74:871–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haraj NE, El Aziz S, Chadli A, et al. Nutritional status assessment in patients with Covid-19 after discharge from the intensive care unit. Clin Nutr ESPEN 2020; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu G, Zhang S, Mao Z, et al. Clinical significance of nutritional risk screening for older adult patients with COVID-19. Eur J Clin Nutr 2020; 74:876–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva DFO, Lima SCVC, Sena-Evangelista KCM, et al. Nutritional risk screening tools for older adults with COVID-19: a systematic review. Nutrients 2020; 12:E2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cawood AL, Walters ER, Smith TR, et al. A review of nutrition support guidelines for individuals with or recovering from COVID-19 in the community. Nutrients 2020; 12:E3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ammar A, Brach M, Trabelsi K, et al. Effects of COVID-19 home confinement on eating behaviour and physical activity: results of the ECLB-COVID19 international online survey. Nutrients 2020; 12:1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niles MT, Bertmann F, Belarmino EH, et al. The early food insecurity impacts of COVID-19. Nutrients 2020; 12:2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodríguez-Pérez C, Molina-Montes E, Verardo V, et al. Changes in dietary behaviours during the COVID-19 outbreak confinement in the Spanish COVIDiet Study. Nutrients 2020; 12:1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kriaucioniene V, Bagdonaviciene L, Rodríguez-Pérez C, Petkeviciene J. Associations between changes in health behaviours and body weight during the COVID-19 quarantine in Lithuania: the Lithuanian COVIDiet Study. Nutrients 2020; 12:E3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauer J, Morley JE, Schols AMWJ, et al. Sarcopenia: a time for action. An SCWD position paper. J Cachexia Sarcopenia Muscle 2019; 10:956–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berg-Weger M, Morley JE. Editorial: Loneliness and social isolation in older adults during the COVID-19 pandemic: implications for gerontological social work. J Nutr Health Aging 2020; 24:456–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valenzuela PL, Morales JS, Castillo-Garcia A, et al. Effects of exercise interventions on the functional status of acutely hospitalized older adults: a systematic review and meta-analysis. Ageing Res Rev 2020; 61:101076. [DOI] [PubMed] [Google Scholar]
- 22.Casas-Herrero A, Anton-Rodrigo I, Zambom-Ferraresi F, et al. Effect of multicomponent exercise programme (VIVIFRAIL) on functional capacity in frail community elders with cognitive decline: study protocol for a randomized multicentre control trial. Trials 2019; 20:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azzolino D, Saporiti E, Proietti M, Cesari M. Nutritional considerations in frail older patients with COVID-19. J Nutr Health Aging 2020; 24:696–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans WJ, Morley JE, Argiles J, et al. Cachexia: a new definition. Clin Nutr 2008; 27:793–799. [DOI] [PubMed] [Google Scholar]
- 25.Ezeoke CC, Morley JE. Pathophysiology of anorexia in the cancer cachexia syndrome. J Cachexia Sarcopenia Muscle 2015; 6:287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morley JE, Kalantar-Zadeh K, Anker SD. COVID-19: a major cause of cachexia and sarcopenia? J Cachexia Sarcopenia Muscle 2020; 11:863–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bahat G. COVID-19 and the renin angiotensin system: Implications for the older adults. J Nutr Health Aging 2020; 24:699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morley JE, Thomas DR, Wilson MMG. Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr 2006; 83:735–743. [DOI] [PubMed] [Google Scholar]
- 29.Gamba P, Zaniboni A. Smell and taste in CoViD-19 patients: the forgotten sense. Reenti Prog Med 2020; 111:614–618. [DOI] [PubMed] [Google Scholar]
- 30.Hermans G, Van den Bergh G. Clinical review: intensive care unit acquired weakness. Crit Care 2015; 19:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res 2020; 32:1195–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allegra A, Tonacci A, Pioggia G, et al. Vitamin deficiency as risk factor for SARS-CoV-2 infection: correlation with susceptibility and prognosis. Eur Rev Med Pharmacol Sci 2020; 24:9721–9738. [DOI] [PubMed] [Google Scholar]
- 33.Cereda E, Bogliolo L, de Stefano L, Caccialanza R. A brief discussion of the benefit and mechanism of vitamin D supplementation on coronavirus disease 2019. Curr Opin Clin Nutr Metab Care 2020; doi.10.1097/C0.0000000000000701. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 34.Maghbooli Z, Sahraian MA, Ebrahimi M, et al. Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. PLoS One 2020; 19:e0239799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Rhodes JM, Subramanian S, Laird E, et al. Perspective: vitamin D deficiency and COVID-19 severity – plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2 and thrombosis. J Intern Med 2020; doi.10.1111/joim.13149. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martineau AR, Forouhi NG. Vitamin D for COVID-19: a case to answer? Lancet Diabetes Endocrinol 2020; 8:735–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biesalski HK. Obesity, vitamin D and old age – a serious combination with respect to Covid-19 severity and outcome. Curr Opin Clin Nutr Metab Care 2021. [DOI] [PubMed] [Google Scholar]
- 38.Wallace TC. Combating COVID-19 and building immune resilience: a potential role for magnesium nutrition? J Am Coll Nutr 2020; 10:1–9. [DOI] [PubMed] [Google Scholar]
- 39.Tan CW, Ho LP, Kalimuddin S, et al. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B12 in combination on progression to severe outcomes in older patients with coronavirus (COVID-19). Nutrition 2020; 79–80:111017. [DOI] [PMC free article] [PubMed] [Google Scholar]


