Purpose of review
The current review provides an update on the recent research developments regarding amino acid bioavailability in conditions of both good health and gut disorders.
Recent findings
Determination of amino acid bioavailability is complex and invasive. Minimally invasive methods using stable isotopes have been developed for humans. Data were collected in different models – humans, pigs and rats with various procedures – leading to interstudy variability. They mainly focused on either plant protein or the effect of food processing on animal protein. Plant protein in their original food matrix (legumes, grains, nuts) are generally less digestible (about 80%) than animal protein (meat, egg, milk; about 93%). Food processing has a limited impact on animal protein but its effect might be higher on plant protein. Few studies have documented the effect of gut disorders on protein digestibility, except in gastric bypass where paradoxical effects were reported. Data are needed to identify the amplitude of protein malabsorption in diseases such as inflammatory bowel disease or environmental enteric dysfunction.
Summary
The past 5 years have seen a renewed interest in amino acid bioavailability in view of assessing protein quality to support current shifts in protein sourcing. Methodological developments have been performed and several studies have reported values in various models. The question of protein digestibility in gut disorders remains poorly addressed.
Keywords: animal models, digestibility, humans, plant and animal proteins, stable isotopes
INTRODUCTION
Amino acid bioavailability can be defined as the proportion of amino acids reaching systemic circulation and that can be incorporated into body protein synthesis. It mainly depends on the protein digestibility and amino acid absorption as well as the latter's metabolic fate in deamination pathways. Although metabolic losses are a component of amino acid bioavailability, most studies refer to the efficiency of protein digestion. The term ‘protein and amino acid digestibility’ generally refers to the amount of these undigested components recovered in the digesta. The term ‘amino acid bioavailability’ is related to absorbed amino acids. However, these terms are not fully consistent in the literature. In the context of designing strategies to ensure protein security in the future while responding to environmental issues [1], there has been a renewed interest in protein digestibility as a key factor of protein quality in the 5 past years. Many studies were conducted in healthy volunteers or their related animal models. Whereas digestive disorders can compromise protein security in some populations due to protein malabsorption, little objective data exist. This review aims to provide a state of the art of recent developments related to protein and amino acid digestibility in normal and abnormal gut function.
Box 1.
no caption available
METHODOLOGICAL CONSIDERATIONS FOR MEASURING AMINO ACID BIOAVAILABILITY
Methods for determining digestive efficiency, namely digestibility, have evolved in the context of the Food and Agriculture Organization of the United Nations-recommended Digestible Indispensable Amino Acid Score (DIAAS) values as the preferred criteria of protein quality [2]. In this context, digestibility of each indispensable amino acid (IAA) must be measured at the ileal level because absorption occurs in the small intestine. Amino acids reaching the large intestine are fermented, and molecules are partly transformed into either other amino acids or metabolites that can be absorbed at the colon level, such as ammonia or indoles for nitrogen molecules, or H2S, phenol and branched-chain fatty acids for nonnitrogenous compounds [3]. If the measurement of protein digestibility at the faecal level has been considered an overestimated but acceptable proxy of ileal digestibility, the measurement of faecal amino acid digestibility is generally not accepted, though already reported for pulse proteins, for instance [4].
It must be mentioned that the term ‘amino acid availability’ is sometimes used on the basis of the plasma amino acid area under the curve (AUC) after a test meal, but it is confusing because it does not reflect amino acid absorption alone [5,6]. Indeed, other phenomena such as the speed of digestion and the efficiency of amino acid uptake in tissues considerably influences the incremental plasma amino acid postprandially. As an illustration of the confusion that can occur, casein generally displays a moderate AUC compared with other protein sources, such as whey; a result that is not ascribed to a low amino acid bioavailability from casein since it is one of the most digestible proteins, but rather to a slow digestion rate. As the entry of amino acids into protein synthesis rapidly saturates the process, there are more circulating, remnant amino acids when proteins are digested rapidly than when amino acids arrive progressively in the blood. When dietary protein is intrinsically labelled with a stable isotope and a labelled amino acid is intravenously infused, it is possible to determine the amount of dietary amino acids that appear in circulation [7]. However, owing to splanchnic extraction, this value reflects the availability of dietary amino acids for peripheral organs but not digestibility.
Measuring ileal digestibility is complex and requires invasive procedures, depending on the model. In humans, intestinal tubing allows for the continuous collection of effluents and adequate measurements of ileal amino acid losses [8▪]. However, this method is very invasive and ileostomized patients have therefore been used as convenient alternative due to the direct access to digesta. Nevertheless, there are currently few eligible patients, making recruitment difficult. Recently, a minimally invasive method has been developed in healthy volunteers. The dual isotope method is indirect as it is based on the use of a reference prote
in in the meal together with the test protein. The latter is labelled with one isotope, preferably 2H [9▪], or alternately 15N [10] and the reference protein (spirulina or free amino acids) with 13C. The amino acid absorption from the test protein is determined by analyzing the relative ratio of these two tracers in the meal and the plasma amino acids. The low invasiveness is a major strength of this method, but it is analytically complicated. Moreover, it involves indirect calculations and to date, it has not yet been validated against the direct method of ileal sampling.
An entirely noninvasive method has been developed from the indicator amino acid oxidation (IAAO) method that was primarily used to determine amino acid requirements. It was adapted to identify the metabolic availability (i.e. including digestive and metabolic losses) of the limiting amino acid [11▪]. The principle is that if one amino acid is limiting in the diet, the other amino acids are subjected to increased oxidation because their entry into protein synthesis is impaired. This is thus reflected by the increased 13CO2 excretion in breath air from the indicator amino acid, generally 13C phenylalanine. The method consists in giving either synthetic meals with increasing levels of the limiting amino acid in a free form (thus theoretically 100% digestible) or meals containing the test protein with the same levels of the limiting amino acid. The amino acids are given under the requirement level to obtain a linear regression between the amino acid level and the 13CO2 excretion. The slopes obtained with free amino acids and the protein meals are then compared, reflecting a lower bioavailability of the limiting amino acid in the test protein if the slope is weaker than with the free amino acid meal. As with the dual isotope method, it is an indirect method. It has the advantage of being analytically simple and entirely noninvasive, but it is cumbersome to formulate the meals and then adapt volunteers to different ones. Moreover, it ultimately only measures the bioavailability of the limiting amino acid.
Animal models are also used to provide amino acid digestibility data, the pig model equipped with an ileal or ileocaecal cannula that allows the continuous recovery of digesta, in particular [12]. However, ethical concerns can limit the use of this model. Rats offer an alternative model for digestion studies. They are much less expensive and easy to use, but a major drawback is the impossibility of continuously collecting the ileal digesta. To circumvent the problem, two strategies exist. One consists of collecting the ileal digesta at a single time postmeal, with a repeated meal protocol [13▪]. The second strategy is to collect all the digesta in the caecum 5–6 h after meal ingestion, a period of time that allows complete meal digestion but limits the fermentation processes [14▪]. Both methods present some limitations but allow discrimination of protein digestibility in different conditions. Lastly, another methodological factor of variation is the method of differentiation between dietary and endogenous protein losses by either using a protein-free diet to assess the endogenous losses or stable isotopes to trace dietary or endogenous proteins [3].
DETERMINANTS OF AMINO ACID BIOAVAILABILITY FROM INGESTED PROTEIN IN HEALTHY GUT CONDITIONS
In normal physiological conditions, when digestive capacities are not impaired, the main determinant of digestive bioavailability is the nature of the protein as well as the food matrix in which it is incorporated – protein isolates being generally more digestible than proteins in their crude matrices. Table 1 presents a representative set of the recent values of protein/amino acid digestibility, obtained in different models and with different methodologies described above. Both are major factors of variation that must be considered for any data comparison from the literature.
Table 1.
Amino acid digestibility of various protein sources in recent studies using different models and methodologies
| Protein source | Parameter | Bioavailability (%) | Model | Methoda | Reference |
| WheyZein | Mean AAProteinMean AAProtein | 92 ± 691 ± 663 ± 1360 ± 13 | Humans | Nasoileal tube/protein-free group | Calvez et al.[8▪] |
| Chickpea | Mean IAA | 74.5 ± 0.8 | Humans | Dual isotope method/repeated meals | Kashyap et al.[16] |
| Mungo beans | Mean IAA | 63 ± 1.5 | |||
| Yellow pea | Mean IAA | 71.5 ± 1.5 | |||
| Egg | Mean IAA | 89.5 ± 4.5 | Humans | Dual isotope method/repeated meals | Kashyap et al.[17] |
| Meat | Mean IAA | 92 ± 3 | |||
| Chickpea | Methionine | 63 | Humans | IAAO | Rafii et al.[11▪] |
| Rice | Methionine | 100 | |||
| Corn meal | Tryptophan | 80 | Humans | IAAO | Rafii et al.[19] |
| Lys | 71 | ||||
| Meat | Mean AA | 96.5 (grilled) to 98.5 (boiled) | Pigs | Single ileal sample/repeated meals/endogenous losses from the literature | Hodgkinson et al.[21] |
| Pork products | Mean AA | 95 (Noncured ham) to 100 (raw belly) ± 2.5 | Pigs | Ileal cannula/protein-free diet | Bailey et al.[23▪] |
| Pistachio | Mean AA | 85 (Roasted) to 95 (raw) ± 7 | Pigs | Ileal cannula/protein-free diet | Bailey and Stein [24] |
| Sunflower isolateGoat whey | Mean AA | 96 ± 0.597.5 ± 0.5 | Rats | Postprandial caecal-faecal losses/intrinsic 15N labelling | Tessier et al.[14▪] |
| Pea isolateGluten | Mean AA | 94.5 ± 494.5 ± 3.5 | Rats | Postprandial caecal-faecal losses/protein-free group | Guillin et al.[15] |
| Spirulina whole cells | Mean AA | 83.5 ± 4.5 | Rats | Postprandial caecal-faecal losses/intrinsic 15N labelling | Tessier et al.[20] |
| Brown rice | Mean AA | 85 ± 2.3 | Rats | Single ileal sample/repeated meals/endogenous losses from the literature | Han et al.[13▪] |
| Polished rice | 77 ± 8.5 | ||||
| Buckwheat | 88 ± 6 | ||||
| Whole wheat | 93 ± 2 |
Bioavailability values are presented as mean ± SD. AA, amino acid; IAA, indispensable amino acid; IAAO, indicator amino acid oxidation.
Type of method, sample collection, specific feeding procedure, procedure to determine true digestibility (assessment of endogenous intestinal losses) or real digestibility (stable isotopes to trace dietary protein).
Notably, the bioavailability of amino acids from plant sources has been substantially reported owing to the increasing interest in evaluating the quality of nonconventional protein sources. Data obtained in humans with the dual isotope method indicate a mean IAA bioavailability in three different legumes of 63–74% [16], whereas the value is around 90% for meat and eggs [17]. However, the amino acid digestibility of whole eggs can be severely decreased, down to 75%, when ingested with black tea [18], a result that can be ascribed to the polyphenols. Using ileal tubes to measure the true ileal digestibility (endogenous protein being evaluated in a protein-free group), mean amino acid bioavailability from whey was close to that of egg and meat, whereas that of zein was very low [8▪], a phenomenon that can be partly ascribed to the isolate's low solubility. Using the IAAO method, Rafii et al.[19] determined the metabolic availability of the limiting amino acids in corn meal, lysine and tryptophan, and found higher values for these individual amino acids than those found for zein by Calvez et al.[8▪]. It must be pointed out that in the latter study, the digestibility of lysine and tryptophan could not be determined because zein was almost completely deficient in both amino acids. Rafii et al.[11▪] also reported that the most limiting amino acid in chickpea, methionine, was only bioavailable at 63% against 100% in steamed rice. In rats, our team found a high digestibility of a sunflower isolate, almost as high as that observed for whey in the same model, whereas the digestibility of spirulina cell amino acids was moderate [20]. In rats, the amino acid digestibility from different cereals was shown to be higher in wheat than in rice, and especially in polished rice [13▪].
The treatments applied to food are also a determinant of bioavailability. The effect of bovine meat cooking processes such as boiling and grilling were reported to be modest in pigs, with differences of 2.5% between grilled and boiled beef [21]. This is in accordance with a previous result in rats, reporting the same amplitude of variation between roasting, grilling, barbecuing and boiling, except that the lowest digestibility was observed for boiled meat [22], in contrast to the study cited above. In a pig model, it was also observed that the cooking temperature of pork products, varying from 63 to 72 °C, did not modify amino acid digestibility; neither did the smoking process applied to bacon, however, curing improved the amino acid digestibility of ham from 95 to 99% [23▪]. In contrast, heat treatment applied to nuts had an inverse effect with a decrease of pistachio protein digestibility by 10% after roasting, as measured in pigs [24].
Significantly, an inverse relationship between digestibility and interindividual variability has consistently been observed. In others words, when a protein is highly digestible, all individuals display a great capacity to digest the protein, but when the protein is less digestible some of them have a great capacity to digest it while others have a low capacity. This is illustrated in Fig. 1, which depicts the individual data of amino acid bioavailability in rats for 15N goat whey, sunflower isolate and spirulina. These three data sets are directly comparable because they are methodologically uniform. It appears that for spirulina, which has a digestibility 12–15% lower than that of the other two proteins, the data dispersion is dramatically expanded. We observed the same in humans, for instance, concerning zein digestibility, which was the lowest we observed among our intestinal tube studies [8▪]. The SD was 13% (for a mean of 60%), with the lowest individual value being 39% and the highest 82%. This is consistent with a previous observation of rapeseed isolate that contains resistant albumin (napin), with a mean value of 84 ± 8.8% and extreme values of 65 and 90%, as reviewed recently by Walther et al.[25]. In this review, the authors proposed variations in protease activity and polymorphism of amino acid transporters as putative causes of interindividual variability, but data are lacking to support these hypotheses. The microbiome may also play a role as indirectly suggested by an intervention study in humans where the administration of vancomycin increased faecal energy losses [26]. However, the rationale for a putative role of the large intestine microbiota in amino acid bioavailability is unclear since there is no significant absorption of amino acids in the colon.
FIGURE 1.
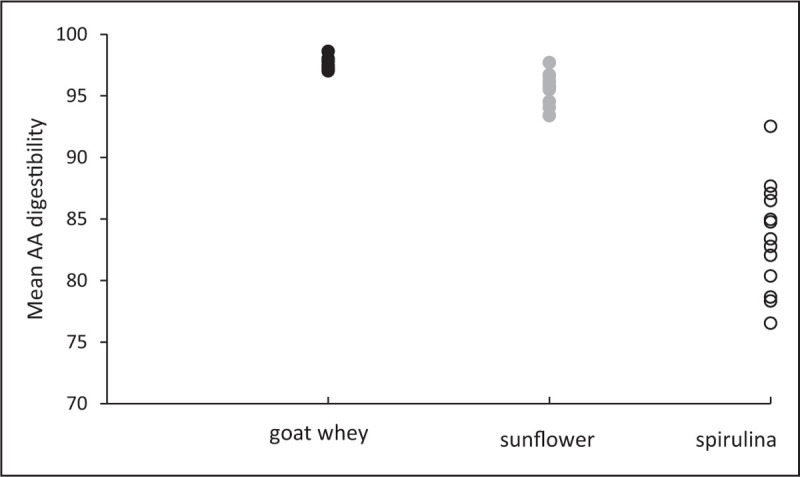
Interindividual dispersion of amino acid digestibility in rats depending on the protein source. Rats were given a 15N test meal and 15N amino acid losses were determined in the caecum 6 h after the ingestion to determine the orocaeal amino acid digestibility. 15N goat whey: n = 8; 15N sunflower isolate: n = 15; 15N spirulina (whole cell): n = 14. Adapted from [14▪,20].
PROTEIN DIGESTIBILITY IN DIGESTIVE DISORDERS
In digestive disorders there are little data on protein and amino acid bioavailability, especially in recent years. Protein malabsorption has been observed or supposed in different situations such as bariatric surgery, short bowel syndrome (SBS), pancreatic insufficiency, inflammatory bowel disease (IBD) and environmental enteric dysfunctions (EED).
In the 3 last years, the most documented unhealthy gut condition is related to bariatric surgery. Indeed, after a gastric bypass, which drastically modifies the gut anatomy, amino acid bioavailability may be altered in a paradoxical manner. Significantly, the blood appearance of amino acids from 13C labelled casein was shown to occur faster after gastric bypass than in normal volunteers [27▪▪]. This result was also associated with a faster glucose handling by the intestine. It is thus likely that bioavailability of amino acids from ingested protein is not impaired by a gastric bypass. Our team confirmed this hypothesis in a gastric bypass rat model in which we observed that 15N labelled milk protein was paradoxically more digestible in operated rats than in sham rats [28]. This better digestive efficiency was slight (+2%) but significant. In this study, we also evaluated the metabolic bioavailability of dietary nitrogen (N) by measuring the 15N retention in tissues. We found a lower dietary N retention in organs, especially liver and muscle, a paradox that can be explained by a higher retention of dietary N in intestinal mucosa. Indeed, as previously observed, the mucosa was hypertrophied and the 15N retention/g of tissue was as high in gastric bypass as in sham rats. Gastric bypass but not sleeve gastrectomy slightly improves digestive amino acid bioavailability but lowers metabolic bioavailability which could thus contribute to protein deficiency [29]. Energy absorption was evaluated in children with three types of SBS, differing by the length of the bowel and the presence or not of the colon and ileocaecal valve [30▪▪]. The energy absorption was only 60–68% and was linearly linked to the length of the small bowel, with a better efficiency in the presence of a remnant colon. The patient characteristic data indicated that apparent protein digestibility was 57–65%, without any effect of the type of SBS, suggesting a protein malabsorption of about 20–30% in this syndrome.
In gut disorders involving pancreatitis insufficiency, protein malabsorption has been little documented in contrast to lipids, of which absorption is majorly impaired. After ligation of the pancreatic duct in minipigs, we observed a drastic alteration of real ileal protein digestibility that was 29 ± 11 vs. 89 ± 6% in control pigs [31]. The administration of pancreatic enzymes partially restored protein absorption in a dose-dependent manner. Significantly, the ligation of the canal duct did not completely suppress protein digestion as about 30% of dietary protein was digested. This indicates that nonpancreatic enzymes, such as pepsin and/or brush border enzymes, can partially compensate for the experiment-induced pancreatitis insufficiency. Cystic fibrosis (CF) is also associated with pancreatic insufficiency. Using the principle of the dual isotope method described above, Engelen et al.[32] reported that the digestibility of phenylalanine from spirulina was 80% in healthy volunteers but less than half in CF patients. This is a dysfunction that could be counteracted by enzyme substitution, as reported for chronic pancreatitis.
In inflammatory gut disorders, such as EED and IBD, there are almost no data on protein digestibility. Protein malabsorption can be suspected due to diarrhoea, intestinal mucosal abrasion and alteration of permeability, leading to undernutrition, especially in children [33]. Devi et al.[34▪] indirectly addressed this question by assessing amino acid bioavailability in moderately stunted Indian children. They used the ratio kynurenine/tryptophan as a probable biomarker for intestinal inflammation. This marker was negatively associated with height-for-age Z score. The authors observed a positive association between the kynurenine/tryptophan ratio and proline digestibility, which they interpreted as a reflection of the specific role of metalloproteinases from the brush border membrane that both respond to inflammatory stress and activate proline-rich peptide cleavage. However, they did not find any correlation with the digestibility of other amino acids. In addition, the mean amino acid bioavailability of chickpea (87 ± 10%) and yellow pea (86 ± 10%) were higher that what was found in adults, as reported in the former paragraph [16]. It is noteworthy, though, that the interindividual variability is greater and thus suggests an impairment of amino acid bioavailability. However, the experimental conditions, without any direct evaluation of gut dysfunction or inflammation, do not permit drawing conclusions regarding the absence of any protein absorption alteration in EED.
CONCLUSION
The past few years have seen a renewed interest in protein and amino acid bioavailability in view of documenting the quality of plant and alternative proteins to animal proteins and, furthermore, to enrich the DIAAS database. As protein and amino acid digestibility are methodologically complex and invasive, many developments have been conducted to reduce the invasiveness or to improve the accuracy of data. In conditions of healthy gut functioning, the true digestibility depends on the protein source, animal more than plant protein, especially in the context of the food matrix where several values are below 75% for plants. Important interindividual variability is observed for low digestible protein but the reasons remain unknown. In abnormal gut functions, protein digestibility has been little-addressed and dedicated studies to determine protein malabsorption and the subsequent risk of protein malnutrition are required.
Acknowledgements
We thank Romain Tessier and Florence Guillin for their valuable work on AA digestibility assays in rats in the Unit PNCA.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
References
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Henchion M, Hayes M, Mullen A, et al. Future protein supply and demand: strategies and factors influencing a sustainable equilibrium. Foods 2017; 6:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Food and Agriculture Organization of the United Nations Dietary protein quality evaluation in human nutrition: report of an FAO expert consultation, 31 March–2 April, 2011, Auckland, New Zealand. 2013; Rome: Food and Agriculture Organization of the United Nations, 66. FAO food and nutrition paper. [Google Scholar]
- 3.Moughan PJ, Wolfe RR. Determination of dietary amino acid digestibility in humans. J Nutr 2019; 149:2101–2109. [DOI] [PubMed] [Google Scholar]
- 4.Nosworthy MG, Neufeld J, Frohlich P, et al. Determination of the protein quality of cooked Canadian pulses. Food Sci Nutr 2017; 5:896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J, Klebach M, Visser M, et al. Amino acid availability of a dairy and vegetable protein blend compared to single casein, whey, soy, and pea proteins: a double-blind, cross-over trial. Nutrients 2019; 11:2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vangsoe M, Thogersen R, Bertram H, et al. Ingestion of insect protein isolate enhances blood amino acid concentrations similar to soy protein in a human trial. Nutrients 2018; 10:1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorissen SHM, Trommelen J, Kouw IWK, et al. Protein type, protein dose, and age modulate dietary protein digestion and phenylalanine absorption kinetics and plasma phenylalanine availability in humans. J Nutr 2020; 150:2041–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8▪.Calvez J, Benoit S, Piedcoq J, et al. Very low ileal nitrogen and amino acid digestibility of zein compared to whey protein isolate in healthy volunteers. Am J Clin Nutr 2020; nqaa274; Online ahead of print. [DOI] [PubMed] [Google Scholar]; Unique data on ileal amino acid digestibility of two leucine-rich protein isolates in healthy humans equipped with an intestinal tube.
- 9▪.Devi S, Varkey A, Sheshshayee MS, et al. Measurement of protein digestibility in humans by a dual-tracer method. Am J Clin Nutr 2018; 107:984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]; Princeps study on amino acid bioavailability using the dual isotope method in healthy humans.
- 10.van der Wielen N, Khodorova NV, Gerrits WJJ, et al. Blood 15N:13C enrichment ratios are proportional to the ingested quantity of protein with the dual-tracer approach for determining amino acid bioavailability in humans. J Nutr 2020; 150:2346–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11▪.Rafii M, Pencharz PB, Ball RO, et al. Bioavailable methionine assessed using the indicator amino acid oxidation method is greater when cooked chickpeas and steamed rice are combined in healthy young men. J Nutr 2020; 150:1834–1844. [DOI] [PubMed] [Google Scholar]; Data on metabolic availability of the limiting amino acid in grain and pulse in healthy humans, using the entirely noninvasive indicator amino acid oxidation method.
- 12.Oliveira MSF, Htoo JK, Stein HH. The direct and difference procedures result in similar estimates for amino acid digestibility in feed ingredients fed to growing pigs. J Anim Sci 2020; 98:skaa225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13▪.Han F, Han F, Wang Y, et al. Digestible indispensable amino acid scores of nine cooked cereal grains. Br J Nutr 2019; 121:30–41. [DOI] [PubMed] [Google Scholar]; Data on ileal amino acid digestibility in rats of several cereals, using the single ileal sampling method.
- 14▪.Tessier R, Khodorova N, Calvez J, et al. 15N and 2H intrinsic labeling demonstrate that real digestibility in rats of proteins and amino acids from sunflower protein isolate is almost as high as that of goat whey. J Nutr 2020; 150:450–457. [DOI] [PubMed] [Google Scholar]; Data on caecal amino acid digestibility of sunflower isolate in rats, using a doubled intrinsically labelled protein.
- 15.Guillin FM, Gaudichon C, Guérin-Deremaux L, et al. Multicriteria assessment of pea protein quality in rats: a comparison between casein, gluten and pea protein alone or supplemented with methionine. Br J Nutr 2020; 27:1–9. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Kashyap S, Varkey A, Shivakumar N, et al. True ileal digestibility of legumes determined by dual-isotope tracer method in Indian adults. Am J Clin Nutr 2019; 110:873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kashyap S, Shivakumar N, Varkey A, et al. Ileal digestibility of intrinsically labeled hen's egg and meat protein determined with the dual stable isotope tracer method in Indian adults. Am J Clin Nutr 2018; 108:980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kashyap S, Shivakumar N, Varkey A, et al. Co-ingestion of black tea reduces the indispensable amino acid digestibility of hens’ egg in Indian adults. J Nutr 2019; 149:1363–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rafii M, Elango R, Ball RO, et al. Metabolic availability of the limiting amino acids lysine and tryptophan in cooked white African cornmeal assessed in healthy young men using the indicator amino acid oxidation technique. J Nutr 2018; 148:917–924. [DOI] [PubMed] [Google Scholar]
- 20.Tessier R, Calvez J, Khodorova N, et al. Protein and amino acid digestibility of (15)N Spirulina in rats. Eur J Nutr 2020; (In press). [DOI] [PubMed] [Google Scholar]
- 21.Hodgkinson SM, Montoya CA, Scholten PT, et al. Cooking conditions affect the true ileal digestible amino acid content and digestible indispensable amino acid score (DIAAS) of bovine meat as determined in pigs. J Nutr 2018; 148:1564–1569. [DOI] [PubMed] [Google Scholar]
- 22.Oberli M, Lan A, Khodorova N, et al. Compared with raw bovine meat, boiling but not grilling, barbecuing, or roasting decreases protein digestibility without any major consequences for intestinal mucosa in rats, although the daily ingestion of bovine meat induces histologic modifications in the colon. J Nutr 2016; 146:1506–1513. [DOI] [PubMed] [Google Scholar]
- 23▪.Bailey HM, Mathai JK, Berg EP, et al. Pork products have digestible indispensable amino acid scores (DIAAS) that are greater than 100 when determined in pigs, but processing does not always increase DIAAS. J Nutr 2020; 150:475–482. [DOI] [PubMed] [Google Scholar]; Data on ileal amino acid digestibility of pork products with various treatments in cannulated pigs.
- 24.Bailey HM, Stein HH. Raw and roasted pistachio nuts (Pistacia vera L.) are ‘good’ sources of protein based on their digestible indispensable amino acid score as determined in pigs. J Sci Food Agric 2020; 100:3878–3885. [DOI] [PubMed] [Google Scholar]
- 25.Walther B, Lett AM, Bordoni A, et al. GutSelf: interindividual variability in the processing of dietary compounds by the human gastrointestinal tract. Mol Nutr Food Res 2019; 63:1900677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basolo A, Hohenadel M, Ang QY, et al. Effects of underfeeding and oral vancomycin on gut microbiome and nutrient absorption in humans. Nat Med 2020; 26:589–598. [DOI] [PubMed] [Google Scholar]
- 27▪▪.Svane MS, Bojsen-Møller KN, Martinussen C, et al. Postprandial nutrient handling and gastrointestinal hormone secretion after Roux-en-Y gastric bypass vs sleeve gastrectomy. Gastroenterology 2019; 156:1627–1641.e1. [DOI] [PubMed] [Google Scholar]; Unique data on the postprandial metabolic fate of nutrients in gastric bypass patients using multitracer approaches.
- 28.Tessier R, Ribeiro-Parenti L, Bruneau O, et al. Effect of different bariatric surgeries on dietary protein bioavailability in rats. Am J Physiol Gastrointest Liver Physiol 2019; 317:G592–G601. [DOI] [PubMed] [Google Scholar]
- 29.Mahawar KK, Sharples AJ. Contribution of malabsorption to weight loss after Roux-en-Y gastric bypass: a systematic review. Obes Surg 2017; 27:2194–2206. [DOI] [PubMed] [Google Scholar]
- 30▪▪.Norsa L, Lambe C, Abboud SA, et al. The colon as an energy salvage organ for children with short bowel syndrome. Am J Clin Nutr 2019; 109:1112–1118. [DOI] [PubMed] [Google Scholar]; Unique data on energy and nutrient (including protein) faecal losses in short bowel syndrome children with a tentative of correlation with plasma citrulline as a marker of intestinal mucosa mass.
- 31.Mary F, Moesseler A, Khodorova N, et al. Metabolic markers of protein maldigestion after a 15N test meal in minipigs with pancreatic exocrine insufficiency. Am J Physiol Gastrointest Liver Physiol 2018; 314:G223–G230. [DOI] [PubMed] [Google Scholar]
- 32.Engelen MPKJ, Com G, Anderson PJ, et al. New stable isotope method to measure protein digestibility and response to pancreatic enzyme intake in cystic fibrosis. Clin Nutr 2014; 33:1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salameh E, Morel FB, Zeilani M, et al. Animal models of undernutrition and enteropathy as tools for assessment of nutritional intervention. Nutrients 2019; 11:2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34▪.Devi S, Varkey A, Dharmar M, et al. Amino acid digestibility of extruded chickpea and yellow pea protein is high and comparable in moderately stunted South Indian children with use of a dual stable isotope tracer method. J Nutr 2020; 150:1178–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]; Data on amino acid digestibility using the dual isotope method in stunted children with a correlation attempt with the ratio kynurenine/tryptophan as a marker of intestinal inflammatory marker.


