Supplemental Digital Content is available in the text.
Keywords: arrhythmias, cardiac; defibrillators, implantable; heart failure; primary prevention; sudden cardiac death; ventricular fibrillation; ventricular tachycardia
Abstract
Background:
The subcutaneous (S) implantable cardioverter-defibrillator (ICD) is safe and effective for sudden cardiac death prevention. However, patients in previous S-ICD studies had fewer comorbidities, had less left ventricular dysfunction, and received more inappropriate shocks (IAS) than in typical transvenous ICD trials. The UNTOUCHED trial (Understanding Outcomes With the S-ICD in Primary Prevention Patients With Low Ejection Fraction) was designed to evaluate the IAS rate in a more typical, contemporary ICD patient population implanted with the S-ICD using standardized programming and enhanced discrimination algorithms.
Methods:
Primary prevention patients with left ventricular ejection fraction ≤35% and no pacing indications were included. Generation 2 or 3 S-ICD devices were implanted and programmed with rate-based therapy delivery for rates ≥250 beats per minute and morphology discrimination for rates ≥200 and <250 beats per minute. Patients were followed for 18 months. The primary end point was the IAS-free rate compared with a 91.6% performance goal, derived from the results for the ICD-only patients in the MADIT-RIT study (Multicenter Automatic Defibrillator Implantation Trial–Reduce Inappropriate Therapy). Kaplan-Meier analyses were performed to evaluate event-free rates for IAS, all-cause shock, and complications. Multivariable proportional hazard analysis was performed to determine predictors of end points.
Results:
S-ICD implant was attempted in 1116 patients, and 1111 patients were included in postimplant follow-up analysis. The cohort had a mean age of 55.8±12.4 years, 25.6% were women, 23.4% were Black, 53.5% had ischemic heart disease, 87.7% had symptomatic heart failure, and the mean left ventricular ejection fraction was 26.4±5.8%. Eighteen-month freedom from IAS was 95.9% (lower confidence limit, 94.8%). Predictors of reduced incidence of IAS were implanting the most recent generation of device, using the 3-incision technique, no history of atrial fibrillation, and ischemic cause. The 18-month all-cause shock-free rate was 90.6% (lower confidence limit, 89.0%), meeting the prespecified performance goal of 85.8%. Conversion success rate for appropriate, discrete episodes was 98.4%. Complication-free rate at 18 months was 92.7%.
Conclusions:
This study demonstrates high efficacy and safety with contemporary S-ICD devices and programming despite the relatively high incidence of comorbidities in comparison with earlier S-ICD trials. The inappropriate shock rate (3.1% at 1 year) is the lowest reported for the S-ICD and lower than many transvenous ICD studies using contemporary programming to reduce IAS.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02433379.
Clinical Perspective.
What Is New?
The subcutaneous implantable cardioverter-defibrillator (ICD) is safe and effective in primary prevention patients with a reduced ejection fraction, as evidenced by low complication and inappropriate shock rates, as well as high efficacy for terminating ventricular tachyarrhythmias.
With the use of high rate cutoffs as well as current generation electrogram filtering and discrimination algorithms, the inappropriate shock rates in the subcutaneous ICD are lower than those reported for transvenous ICDs with contemporary programming.
What Are the Clinical Implications?
The subcutaneous ICD can be considered in all primary prevention patients without pacing indications, regardless of left ventricular function or underlying heart disease.
The device programming used in this study, with discrimination algorithms active from 200 to 250 bpm, should be adopted routinely in patients with subcutaneous ICD to avoid unnecessary shocks.
Editorial, see p 18
The implantable cardioverter-defibrillator (ICD) has become an important component of the prevention of sudden cardiac death. The ICD reduces all-cause mortality for both primary and secondary prevention cohorts.1–5 Despite these results, long-term complications, most commonly infections and lead malfunctions, as well as inappropriate shocks (IAS), have emerged as limitations of these devices. To help address these problems, standardized programming algorithms to reduce shocks were developed as well as improved arrhythmia discrimination algorithms.6–10
The subcutaneous (S) ICD was developed as an alternative to transvenous (TV) devices to help reduce lead-related complications and to improve rhythm discrimination.11,12 Although achieving those goals,13–15 the cohorts studied with this device were younger with fewer comorbidities and more preserved left ventricular function. Moreover, IAS resulting from cardiac oversensing are more frequent than with TV-ICDs.16 To address these limitations of the S-ICD, the UNTOUCHED trial (Understanding Outcomes With the S-ICD in Primary Prevention Patients With Low Ejection Fraction) was designed as a prospective, multinational study among primary prevention patients with a reduced ejection fraction using standardized programming and improved sensing algorithms.17
Methods
Study Design
The data and study protocol for this clinical trial may be made available to other researchers in accordance with Boston Scientific’s Data Sharing Policy (http://www.bostonscientific.com/en-US/data-sharing-requests.html).
The UNTOUCHED trial (Clinical Trial Registration URL: https://clinicaltrials.gov/ct2/show/NCT02433379; Unique identifier: NCT02433379) was a multinational, prospective, nonrandomized study of primary prevention patients undergoing de novo implant of an S-ICD who had a left ventricular ejection fraction (LVEF) ≤35%. The study design, population, and end points have been described previously.17,18 The study was approved at each site’s overseeing institutional review board/ethics committee and it was conducted in accordance with applicable postmarket guidelines and the Declaration of Helsinki, and followed applicable sections of International Standards Organization 14155:2011. Subjects were considered enrolled in the study at the time the consent form was signed.
The study enrolled subjects across 110 sites located in the United States, Canada, and Europe. Enrollment began in June 2015 and was completed in February 2018. Follow-up continued until the study was completed in December 2019, based on the final subject visit.
Following enrollment, subjects were implanted with an EMBLEM (Boston Scientific, Marlborough, MA) model A209 (Generation [Gen] 2) or A219 (Gen 3) S-ICD. The difference between these devices on discrimination is that Gen 3 devices have the SMART Pass filter, which is automatically enabled at device implant. In the older Gen 2 devices, this filter could be added as an upgrade once it was released—April 14, 2016, in Europe and August 8, 2016, in the United States. The optimal sensing vector was automatically selected at implantation, per standard device programming, but it could be manually overridden. Devices were programmed per protocol with a conditional zone, between 200 and 250 beats per minute (bpm), so that discrimination algorithms are used to avoid delivering IAS when the arrhythmia instantaneous rate is in this range, and arrhythmias are considered shockable based on rate alone when the instantaneous rate is >250 bpm.
Study Population
The study recruited primary prevention patients with an LVEF ≤35% (ischemic or nonischemic heart disease) eligible for S-ICD therapy (based on S-ICD screening requirements done per standard of care), who were intending to undergo a de novo S-ICD implant procedure. S-ICD screening during exercise was not required by the protocol. Patients were excluded from the study if they had a pacing indication for bradycardia or cardiac resynchronization therapy (CRT), as determined by the investigator; history of sustained ventricular tachycardia (VT) or ventricular fibrillation; New York Heart Association classification IV; or life expectancy shorter than 18 months, or met other exclusionary criteria.17,18
End Points
The primary end point for the study was the IAS-free rate at 540 days (18 months) compared with a performance goal of 91.6%. To compare across studies, results are also reported at times as the IAS rate—the complement of the IAS-free rate. Development of the performance goal, based on the results of patients with an ICD in treatment arms B and C of the MADIT-RIT (Multicenter Automatic Defibrillator Implantation Trial–Reduce Inappropriate Therapy) TV-ICD programming study, has been detailed previously.17 MADIT-RIT treatment arm A was the control group in that trial. Lower rates of inappropriate therapies were observed in arms B and C, which used higher rate cutoffs or longer detection duration. Such programming is now part of the standard of care for TV devices and more closely aligned with programming in UNTOUCHED (see Gold et al,17 Table IV). Of note, patients with CRT implants in MADIT-RIT arms B and C were excluded from the calculation of the performance goal, because CRT was an exclusion criterion for UNTOUCHED. It is also noteworthy that patients with CRT in MADIT-RIT had a lower risk of inappropriate therapy than those with an ICD.19
All episodes were required to be collected. A clinical events committee, made up of 3 physicians (independent from the study), reviewed shock appropriateness for each treated episode. The primary end point results are based on the clinical events committee’s adjudication. The more detailed subclassification for reason for IAS was completed by an independent clinical research organization consultant and the Boston Scientific Medical Safety Team.
Secondary end points tested against predefined performance goals determined in a similar manner as the primary performance goal included freedom from all-cause shock at 18 months, with the performance goal derived from ICD-only patients in MADIT-RIT arms B and C and freedom from system- and procedure-related complications at 30 days,18 with the performance goal derived from the complication rates observed in previous S-ICD studies.
Statistical Analysis
Basic characteristics were analyzed using descriptive statistics. Continuous variables were summarized by the number of patients and mean±SD. Categorical variables were summarized by frequencies and percentages of patients in each category. Variables were compared using a pooled t test, ANOVA, Kruskal-Wallis, Cochran-Armitage Trend, or χ2 test when appropriate.
As it was anticipated that the outcomes may differ for patients with permanent atrial fibrillation (AF),17 who were excluded from MADIT-RIT,10 a prespecified pooling analysis was performed using a likelihood ratio test from a logistic regression model. The logistic regression model included additional baseline covariates to attempt to adjust for any imbalances between baseline data. The following baseline covariates were considered: age, sex, race, LVEF, New York Heart Association classification, medications, arrhythmia history, associated diseases/risk factors, height, weight, and geography. The justification for pooling the data for permanent atrial fibrillation subjects was confirmed for all end points if the P value for the permanent AF subgroup remained nonsignificant (α=15%) after adjusting for significant baseline covariates.
The analyses for the primary and secondary effectiveness end points were conducted using 2 types of Kaplan-Meier analyses:
Intent-to-treat—Analysis of the time from implant until the occurrence of the event. Data from any subjects who were event-free were right-censored on the date of study exit. Main study results are reported as intent-to-treat analysis.
-
Per protocol—Additional subjects who were event-free were right-censored as follows:
○ At the time of reprogramming if device programming deviated from the protocol-required programming (conditional zone cutoff not set to 200 bpm, shock zone cutoff not set to 250 bpm, or device turned off for more than 24 consecutive hours), or
○ At the time when the initial implanted device was taken out of service, if the patients experienced a device replacement.
The 1-sided 95% lower confidence limit (LCL) was calculated using the log-log methodology for each end point. If the LCL did not exceed the performance goal for the primary and secondary end points, then the null hypothesis was rejected, and the end point was deemed successful. Cumulative incidence outcomes were compared using the log-rank test.
Univariable and multivariable proportional hazard analysis models were fit for the primary and secondary effectiveness end points, as well as complications over 18 months. For the multivariable modeling, backward model selection was used (α=20%). Compliance to the protocol-required programming was modeled as a time-varying covariate, to account for programming changes during the study. The proportional hazard assumption was verified graphically using Schoenfeld residuals. For the remaining covariates, the proportional hazard assumption was assessed both graphically and by the P value from the Kolmogorov-type supremum test based on a sample of 1000 simulated residual patterns. Frailty analysis was performed for study sites. When frailty analysis converged (IAS and complications), the proportional hazard analysis models were adjusted for study site as a random effect.
Tipping point analysis was performed for all trial end points to determine whether the results were sensitive to incomplete data from the subject population. The tipping points were found to identify the number of additional end point failures necessary to not meet the performance goal. The P value for the tipping point was calculated by determining the probability of being at the tipping point or beyond, given the rate from the nonmissing subjects. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Study Population and Procedural Characteristics
A device implant was attempted in 1116 patients, and 1111 patients were included in postimplant follow-up analysis, including 808 patients (72.4%) from the United States. Four patients were not implanted with the device because of conversion testing failure at implant as previously reported.18 One patient was co-enrolled in a conflicting study and was thus withdrawn from the UNTOUCHED study. Over the course of the study, 67 patients (6.0%) were lost to follow-up. Patient characteristics are shown in Table 1. The trial cohort was typical of a primary prevention population with the exception that patients were younger.18 A total of 142 patients had AF, with 83 patients having paroxysmal, 35 persistent, and 24 permanent AF. Baseline medical therapy included β-blockers in 95% of subjects and angiotensin converting enzyme inhibitors/angiotensin II receptor blockers in 75%.
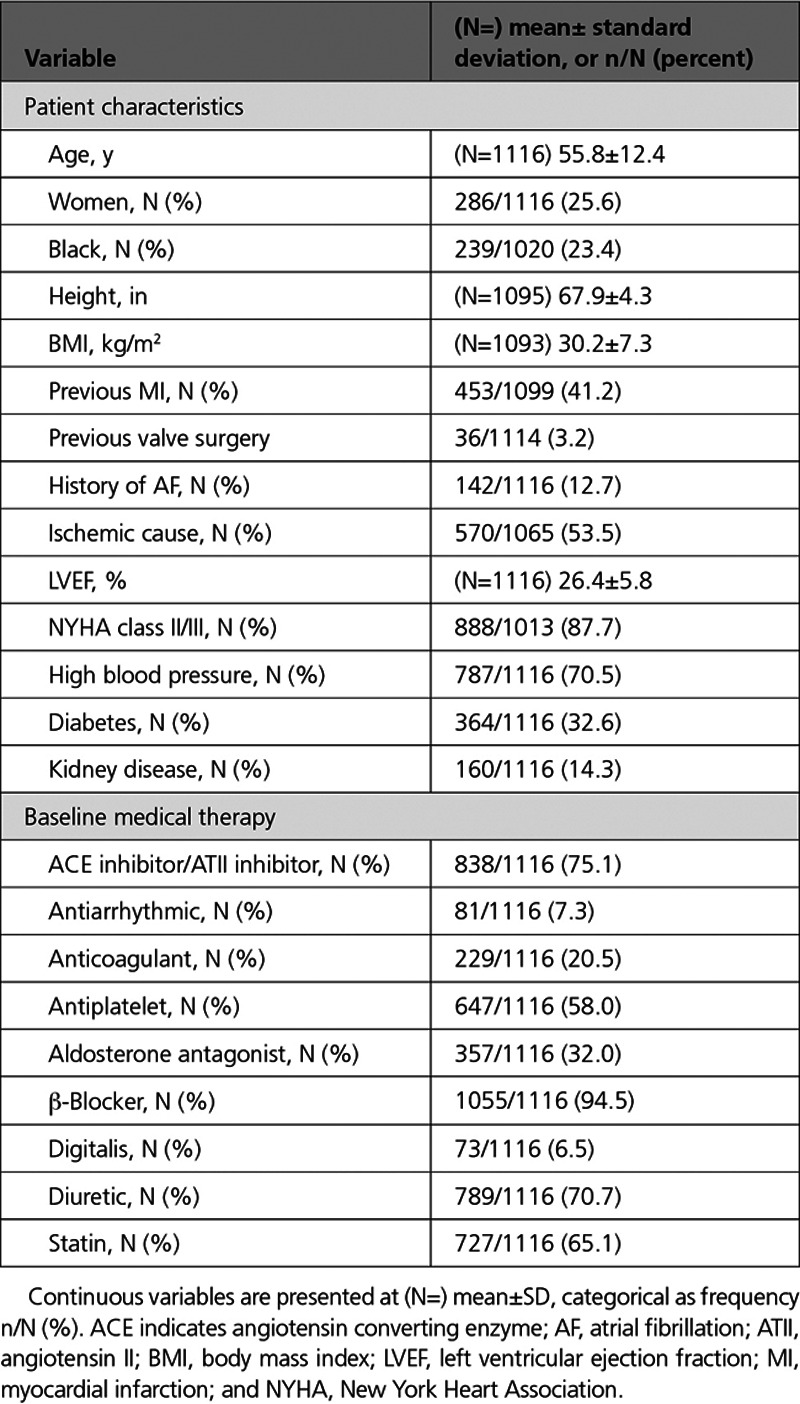
Table 1.
UNTOUCHED Patient Characteristics
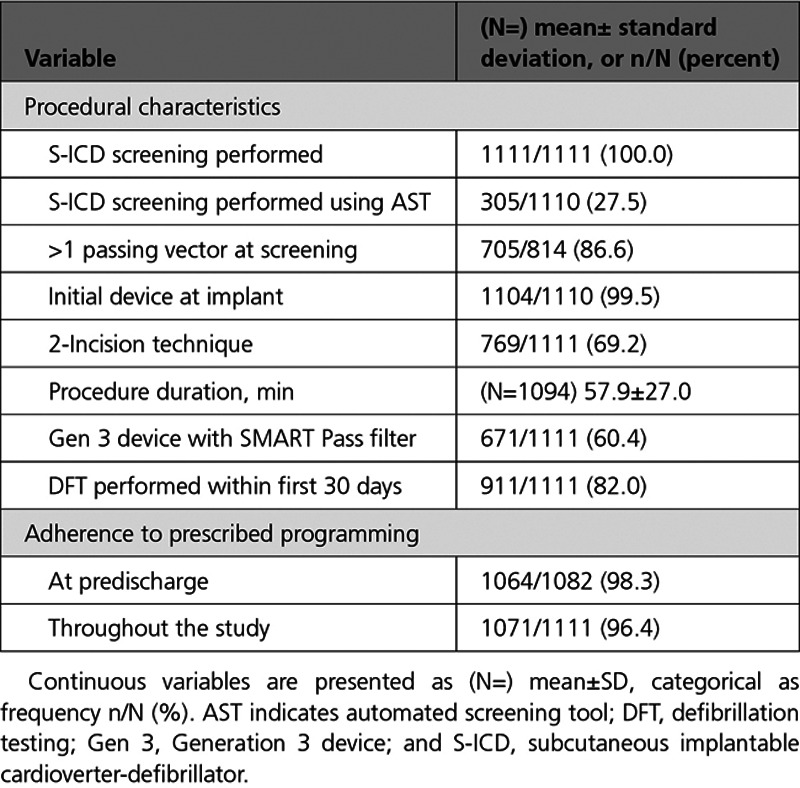
Procedural characteristics are provided in Table 2. Per protocol, all patients underwent S-ICD ECG screening and passed in at least 1 of 3 vectors before device implantation and enrollment, with 27.5% screened using the Automated Screening Tool as it became available. Most patients (86.6%) passed ECG screening in >1 sense vector. On device programming, 98.3% of patients were initially programmed to the prescribed 200 bpm/250 bpm conditional/shock zone programming, and 96.4% of the patient cohort adhered to the prescribed programming throughout the study. Aftershock pacing was activated after implant in 83.7% of subjects. The vector selection method was automatically chosen by the device algorithm in 891/931 (95.7%) patients. The sense vector was programmed to the primary, secondary, and alternate vector in 56.7%, 35.1%, and 8.3% of patients, respectively. Follow-up duration across the population was 17.3±4.3 months.
Table 2.
Procedural Characteristics
Pooling analysis results showed that permanent AF was not a significant predictor for any end point (Table I in the Data Supplement). Patients with permanent AF were pooled with the rest of the population for each end point analysis and included as part of the AF subgroup.
Primary End Point: IAS-Free Rate
The IAS-free time course is presented in Figure 1. At 18 months, the IAS-free rate is 95.9% with an LCL of 94.8%, meeting the performance goal of 91.6%. Univariable and multivariable predictors of IAS are shown in Figure 2. Patients with a history of AF (paroxysmal, persistent, or permanent) and nonischemic cause had a higher risk of IAS in the multivariable model (P=0.0003 and 0.019, respectively), as well as patients with lower LVEF (P=0.042; Figure 2B). Overall, 8.5% of patients with a history of AF received an IAS, with IAS rates higher for patients with paroxysmal (9.6%) or persistent (8.6%) AF compared with permanent AF (4.2%), although these differences were not statistically significant (P=0.67 and 0.89, respectively). Procedural factors that were multivariable predictors of reduced IAS were the use of the 3-incision technique (P=0.011) for device implant and the implantation of a third-generation S-ICD (Gen 3; P=0.031), which had IAS rates of 2.9% (95% upper confidence limit [UCL], 4.4%) for Gen 3 versus 6.0% (UCL, 8.5%) for Gen 2 devices (Figure I in the Data Supplement). Procedural factors, such as whether defibrillation testing was performed within the first 30 days, or if screening passed on >1 vector, were not significant predictors of IAS. Screening using the Automated Screening Tool was a significant univariable predictor (P=0.046) but not in the multivariable model. Adherence to prescribed programming trended toward predicting fewer IAS in the multivariate analysis (P=0.078).
Figure 1.
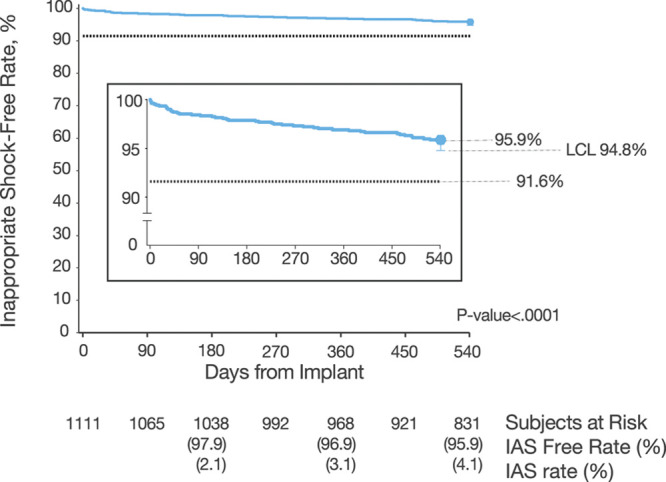
Inappropriate shock (IAS)-free rate. Kaplan-Meier curve illustrating primary end point result: freedom from IAS at 18 months, compared with a performance goal of 91.6%. Performance goal was derived from the ICD-only inappropriate shock free rate of 94.6% found in MADIT-RIT trial (Multicenter Automatic Defibrillator Implantation Trial–Reduce Inappropriate Therapy) arms B (high rate) and C (long duration).17 IAS-free rates as well as IAS rates are provided at 180, 360, and 540 days. ICD indicates implantable cardioverter-defibrillator; and LCL, lower confidence limit.
Figure 2.
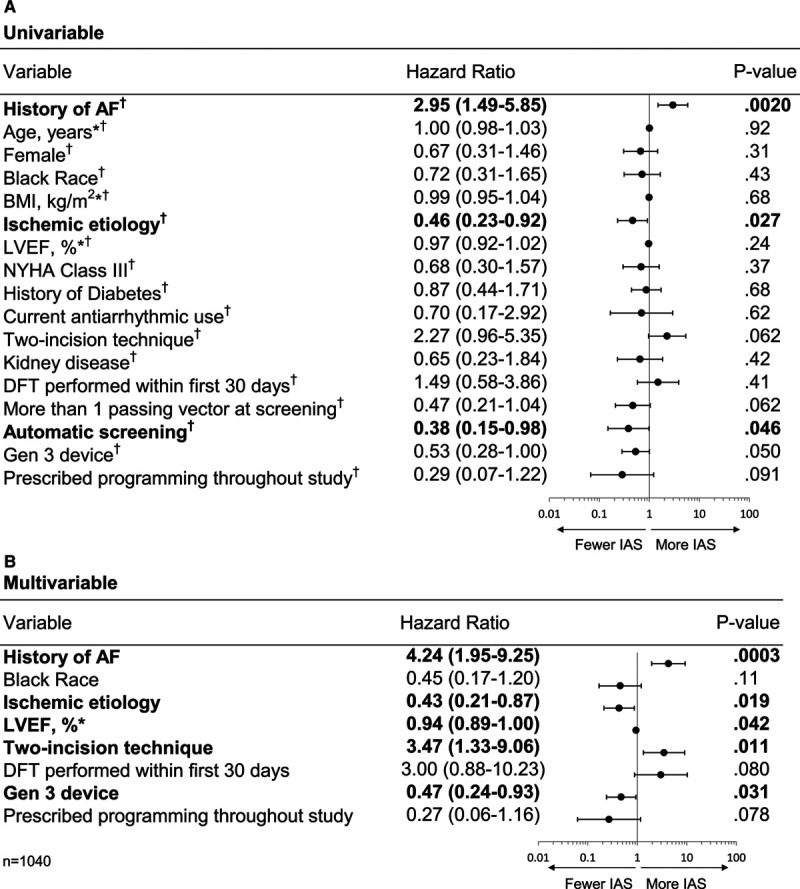
Predictors of inappropriate shocks (IAS) based on hazard analysis. A, Univariable model. All variables passed the test for proportional hazards. B, Multivariable model. AF indicates atrial fibrillation; BMI, body mass index; DFT, defibrillation testing; Gen 3, Generation 3 device; LVEF, left ventricular ejection fraction; and NYHA, New York Heart Association. *Continuous variable. †Satisfied the proportional hazard assumption. Entries in bold indicate predictors with P<0.05.
In post hoc analysis, patients with both prescribed programming and Gen 3 devices (n=652) had a remarkably low IAS rate of 2.2% (UCL, 3.2%) at 18 months. Furthermore, the 1-year IAS rate for the entire UNTOUCHED patient population was 3.1% (Figure 1), with a rate of 2.4% for patients with Gen 3 devices and 4.1% with Gen 2 devices (Figure I in the Data Supplement).
The etiologies of IAS are summarized in Table 3. Patients receiving a shock for cardiac oversensing included 18 (1.6%) because of T-waves, 4 (0.4%) because of oversensing during a VT/ventricular fibrillation arrhythmia below the rate zone (2 because of double-counting QRS and 2 because of oversensed T-waves), and 10 (0.9%) because of other sources of cardiac oversensing. Sixteen patients (1.4%) received shocks for noncardiac oversensing: 2 patients (0.2%) were shocked because of sensing myopotentials, and 14 patients (1.3%) were shocked from other sources of noncardiac oversensing, including electromagnetic interference. No patients received IAS because of discrimination errors or supraventricular tachycardias with rates above the discrimination zone. For patients with Gen 3 devices, despite the low rate of IAS overall, T-wave oversensing remains the most common cause (1.6%).
Table 3.
Cause of Inappropriate Shock
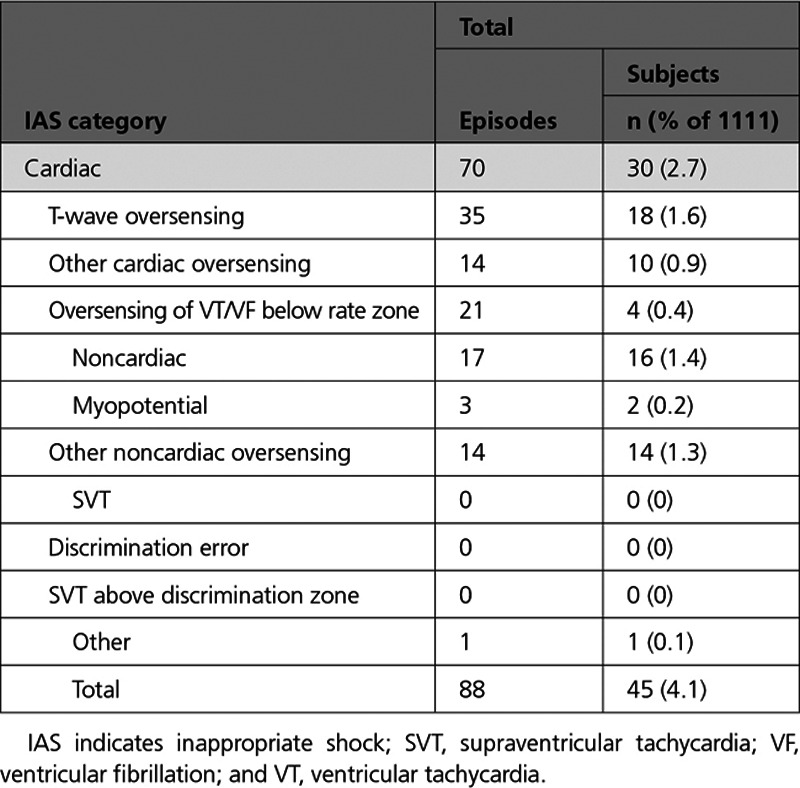
Post hoc analysis showed that neither the overall IAS rates nor the rates of IAS because of T-wave oversensing differed significantly between sense vectors (P=0.81 and P=0.64, respectively). Further post hoc analysis showed that the IAS rates were 4.4%, 1.1%, and 1.2% for patients who passed 1, 2, and 3 vectors, respectively, and that this difference was not statistically significant (P=0.07). Kaplan-Meier IAS rates at 18 months for patients implanted with the 2- versus 3-incision technique were 4.9% (UCL, 6.6%) versus 2.5% (UCL, 4.4%), respectively (Figure II in the Data Supplement). Of the 35 patients with the 2-incision technique, 28 (80%) had IAS later than 30 days after implant. The only cases of IAS caused by myopotential were experienced by 2 patients implanted using the 2-incision technique (Table 3).
Of the 45 patients who received an IAS, device reprogramming was the corrective action in 26 patients (57.7%), whereas 3 patients (6.7%; Table 4) underwent a device explant with a subsequent TV-ICD. Four patients experienced 3 or more IAS episodes within 24 hours: 1 because of oversensing of VT below the rate zone, 2 because of T-wave oversensing, and 1 because of other cardiac oversensing.
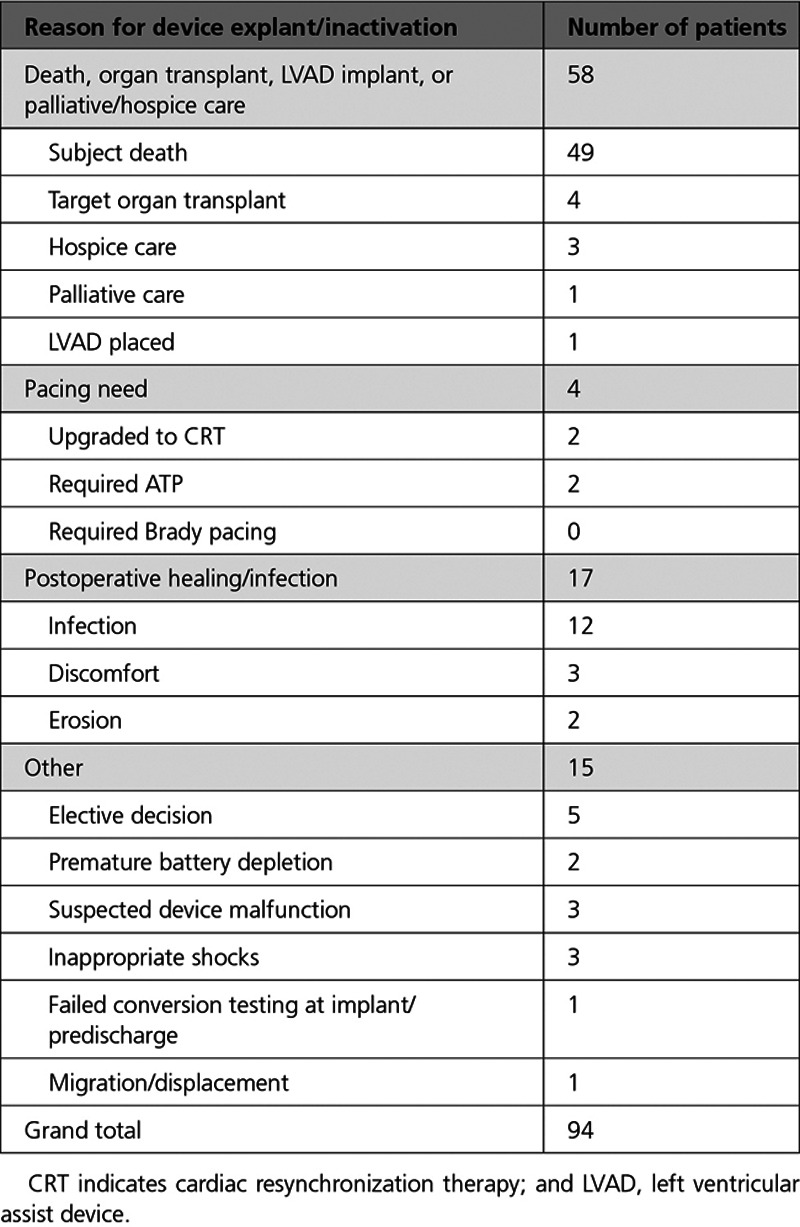
Table 4.
Reasons for Device Explant/Inactivation
All-Cause Shocks and Appropriate Shocks
The freedom from all-cause shock rate was 90.6%, with a LCL of 89.0%, meeting the performance goal set to 85.8% (P<0.0001; Figure 3). Significant predictors of all-cause shock were a history of AF and lower LVEF (P=0.009 and P=0.008, respectively; both variables satisfied the proportional hazards assumption; n=1110). The appropriate shock-free rate over 18 months was 94.3%, with a LCL of 93.0%.
Figure 3.
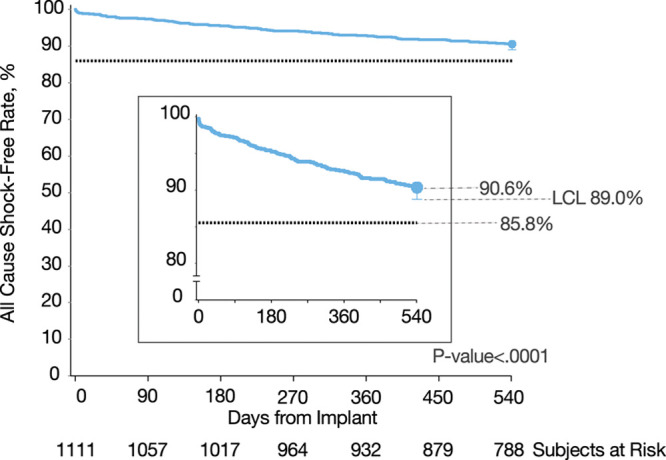
All-cause shock-free rate. Kaplan-Meier curve illustrating secondary end point result: freedom from all-cause shock at 18 months, compared with a performance goal of 85.8%. Performance goal was derived from the implantable cardioverter-defibrillator–only all-cause shock-free rate found in MADIT-RIT trial (Multicenter Automatic Defibrillator Implantation Trial–Reduce Inappropriate Therapy) arms B (high rate) and C (long duration). LCL indicates lower confidence limit.
A total of 58 patients experienced 64 discrete appropriate shock episodes. The first shock success rate for these episodes was 93.8%, and the final shock success rate was 98.4% (63/64). The 1 conversion failure was a monomorphic VT (MVT) at a rate of about 200 bpm whose rate decreased below the programmed zone (200 bpm) after delivery of 1 shock, which inhibited further therapy. The episode resolved spontaneously. This episode took place while the patient was exercising; she experienced light-headedness but no syncope, and the patient was managed by medication adjustment alone. A total of 36 subjects experienced 42 discrete MVTs: 1 patient had an MVT of 170 bpm, 20 patients had 25 MVTs 200 to 230 bpm, and 15 patients had 16 MVTs >230 bpm.
Seven subjects experienced 58 episodes that took place in 9 storm events. The final conversation rate for VT storms was 100%. None of these patients experienced a pause greater than 2 s before arrhythmia onset.
Complications and Survival
All 1116 patients were included in complications end point analysis. Figure 4 shows the Kaplan-Meier complication-free rate out to 18 months (540 days), as well as the UNTOUCHED study end point results related to complications. The complication-free rate at 18 months was 92.7% (LCL, 91.2%). The only significant multivariable predictor of increased complications was implant procedure time (P=0.0051; Figure III in the Data Supplement). Post hoc analysis indicated that complications other than infection were related to longer procedure times (69.3±38.8, n=69 versus 47.5±22.4, n=12; P=0.04), but procedure times for patients with infection did not differ significantly from procedure times for patients with no complications (P=0.14).
Figure 4.
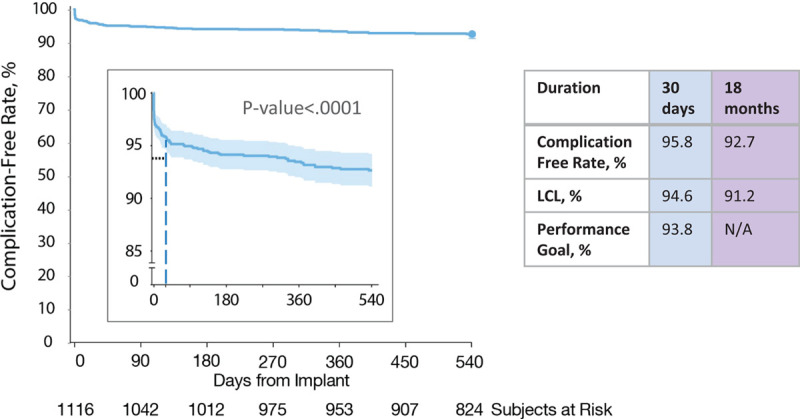
Complication-free rate. Kaplan-Meier curve illustrating freedom from complications at 30 days (secondary end point; previously reported18) and at 18 months. LCL indicates lower confidence limit; and N/A, not applicable.
A summary of all 83 complications reported in the study is provided in Table II in the Data Supplement. Infection leading to device explant occurred in 12 patients (1.1%), with 8 of these patients experiencing an infection in the first 30 days. No patient had systemic infection with bacteremia. There were 10 patients (0.9%) who experienced syncope during the trial. Only 1 of these patients experienced syncope associated with a tachyarrhythmia. This patient had a MVT arrhythmia at a rate of ≈160 bpm that spontaneously terminated, with no change in patient management subsequent to the event.
A total of 94 patients had their S-ICD explanted or inactivated over the course of the study (Table 4). A majority of the devices were explanted or inactivated (58) because of death, organ transplant, left ventricular assist device implant, or palliative/hospice care. Explants for the purpose of providing pacing therapy with a TV-ICD included 2 for the need for ATP, 2 for the need for CRT pacing, and none for bradycardia pacing.
The overall survival rate of the UNTOUCHED study was 94.9%, LCL 93.7% at 18 months, with 57 deaths reported in the study. The 1-year survival rate was 96.7%. The cause of death for these patients is summarized in Table III in the Data Supplement. Two deaths were identified as arrhythmic in their cause, with the underlying arrhythmias identified as asystole in both cases. No tachycardia events were recorded or treated for either episode.
Sensitivity and Per-Protocol Analyses
Tipping point analysis results are shown in Table IV in the Data Supplement. For all end points, the probability that the end point would not be met with complete data from all patients, given the event-free rates among subjects with complete data, is <1 in 10 000. Results are presented throughout this article using intent-to-treat analysis. Per-protocol analysis also met performance goals with P values <0.0001.
Discussion
The UNTOUCHED study was a large, multinational, prospective trial designed to assess the performance of the S-ICD in primary prevention patients with a low LVEF and New York Heart Association II/III heart failure or coronary artery disease. This is the most common indication for ICD implantation in North America and Europe. This study includes the “sickest” S-ICD population studied to date, and it serves as a counterpoint to the noted limitation that previous S-ICD studies included relatively healthier patients. Specifically, previous S-ICD studies included younger patients with fewer comorbidities and better cardiovascular function compared with cohorts receiving TV devices. In addition, this is the first S-ICD trial to evaluate standardized programming to evaluate rhythm discrimination to high rates (250 bpm) using contemporary electrogram filtering and algorithms.
We report 5 major findings. First, the IAS rate was only 4.1% at 18 months, meeting the performance goal of 8.4%, which was based on the IAS rate of 5.4% observed in the ICD cohort of MADIT-RIT arms B and C17 (Figure 1). The IAS rate was lower than in any previously reported prospective, multicenter trial of the S-ICD, despite a cohort with more left ventricular dysfunction and heart failure.13,15,20 Second, the appropriate shock rate was low, with a high defibrillation success similar to that reported with TV-ICD trials.1,21 This was noted for both discrete episodes and for episodes of VT storm. Third, complication rates remain low, despite enrolling a sicker population than previously studied. No lead failures occurred, and only 12 infections were observed (1.1%). None of these infections resulted in bacteremia, which reinforces a key advantage of the S-ICD over TV-ICDs in this high-risk population. Fourth, replacement of the S-ICD with a TV system for pacing indications (bradycardia, tachycardia, or resynchronization therapy) occurred in <0.5% in a cohort with almost 90% having symptomatic heart failure and more than half having coronary artery disease. This indicates that the need to include standard pacing options in appropriately selected primary prevention patients is low, although serial ECGs to screen for CRT indications or bradycardia were not mandated in this study. Fifth, syncope was infrequent (0.9% of patients) and occurred in only 1 patient experiencing a concurrent tachycardia episode, despite high rate cutoffs and the longer time to therapy of this device. However, the S-ICD does not record bradyarrhythmic episodes and does not provide backup bradycardia pacing. Consequently, we cannot fully exclude that none of the 9 syncopal episodes unrelated to a recorded tachyarrhythmia were related to bradycardia, although none of these patients subsequently received a device with pacing therapy.
Early studies of the S-ICD in Europe and the United States, such as the EFFORTLESS (Evaluation of Factors Impacting Clinical Outcome and Cost Effectiveness of the S-ICD)13,14 and IDE (investigational device exemption)15 studies, showed the effectiveness of the first-generation S-ICD, but the populations enrolled were atypical of most contemporary ICD cohorts with younger age, better preserved left ventricular function, more frequent primary electric disease, and fewer comorbidities. IAS rates were relatively high (1-year rates were 13.1% for IDE15 and 8.1% for EFFORTLESS13), which was largely because of oversensing rather than rhythm discrimination errors. The IAS rate was found to be significantly lower in the primary vector in the EFFORTLESS registry,22 whereas the programmed sensing vector did not impact IAS in UNTOUCHED. This observation may be a result of the much lower rate of IAS in UNTOUCHED. Subsequently, the US postapproval study was the largest prospective study of the S-ICD to date and enrolled a cohort more typical of TV-ICD studies. Implant success rate was high and complication rates remain low in that cohort.23 The 1-year IAS rate in this trial was 6.8%. The UNTOUCHED study enrolled a population with a still lower ejection fraction and with a higher incidence of heart failure than the postapproval study, yet IAS rates in UNTOUCHED were the lowest of any of the previous multicenter S-ICD trials.
Improvements in S-ICD device technology have reduced the IAS rate over time.15,24,25 The latest improvement, added to the Gen 3 device, was the SMART Pass filter and has been shown to reduce 1-year IAS rates by 50% in a real-world population.25 This is similar to the present study, which showed a 53% reduction of IAS with Gen 3 devices.
Both compliance to protocol programming and implanting Gen 3 devices with the SMART Pass technology were associated with lower rates of IAS. This strongly supports the concept that both programming high rate cutoffs for discrimination and improved filtering and discrimination algorithms contributed to the low incidence of IAS. Indeed, the IAS rate observed in UNTOUCHED is lower than the rate previously reported with SMART Pass technology in a study that did not prescribe programming.25 The present study suggests similar or lower rates of IAS compared with studies of TV-ICDs with contemporary programming and discrimination algorithms. A meta-analysis reported an annualized IAS rate of 6.4% for single-chamber and S-ICD devices.26 Furthermore, in a TV-ICD cohort, ADVANCE III (Avoid Delivering Therapies for Non-Sustained Arrhythmias in ICD Patients III) reported 5.8% and 4.8% 1-year rates of IAS in the standard and long detection arms, respectively,7 and MADIT-RIT reported a 5.0% 1-year rate across all study arms.19 In UNTOUCHED, the 1-year rate of IAS was 3.1%, which was further reduced to 2.4% with the Gen 3 devices. In ADVANCE III, MADIT-RIT, and UNTOUCHED, there also appears to be a reduction of appropriate shocks with programing high detection rates and prolonged detection, supporting the importance of preventing unnecessary appropriate shocks as well.
The all-cause mortality in this study was 3.3% at 12 months. This is a low mortality rate for a primary prevention study of subjects with a reduced LVEF.1–4 It is noteworthy that the 2 episodes of arrhythmia death were secondary to asystole. No electrograms are available to characterize the rhythm or heart rate preceding asystole. However, this is an arrhythmia that is often observed with sudden cardiac death in patients with heart failure with a reduced ejection fraction. It appears unlikely that this is preventable with pacing therapy,27 although we cannot exclude that backup pacing may have been helpful.
One unexpected finding from this study was the association of the 2-incision technique with a higher rate of IAS. This approach shortens procedural time, does not increase implantation complications, and is rapidly growing in use.18,23,28 The IAS rate using the 2-incision technique was still low enough to meet the study’s performance requirement. Although early postimplant IAS has been reported with the 2-incision technique, presumably because of air along the electrode tract,29 other mechanisms to explain the higher rate of IAS associated with eliminating the sternal incision are likely involved. Theoretically, the 2-incision technique may result in less than ideal lead placement or higher risks of lead displacement or migration over time. Although chest radiographs were not included in this study’s data collection to evaluate lead displacement or migration directly, the only cases of IAS caused by myopotentials were from patients implanted using the 2-incision technique, suggesting distal electrode migration toward the pectoral muscle. Further confirmation from other studies is needed to determine if this finding is reproducible and, if so, to help provide insights on the mechanism of effect. At this time, implanters should take this finding into consideration when planning an implant strategy.
This study should be interpreted in light of certain methodologic limitations. First, this was a nonrandomized trial, so direct comparisons with TV devices should be made cautiously. The PRAETORIAN study (A Prospective, Randomized Comparison of Subcutaneous and Transvenous Implantable Cardioverter-Defibrillator Therapy) is the first randomized trial of these 2 ICD device types, although mostly older generation devices were used in that study.30 The ATLAS study (Avoid Transvenous Leads in Appropriate Subjects) is a more recently conducted randomized trial, primarily evaluating early complications.31 Second, the duration of the study was 18 months, so the longer-term trends in IAS cannot be ascertained. More long-term rates of IAS will be assessed further in the planned 5-year follow-up of the EFFORTLESS and postapproval study trials.13,14,23 Third, the pulse generator implant location was not used as a variable in the predictor models, as there was not consensus on the definitions of location (eg, subcutaneous, intermuscular). Fourth, the study included only primary prevention patients, so these results should not be extrapolated to secondary prevention patients without further confirmation.
In summary, the results of the UNTOUCHED study showed low complications rates, high success rates for termination of ventricular arrhythmias, and low IAS rates without compromising patient safety. These data indicate that the S-ICD can be considered in all primary prevention patients without pacing indications regardless of underlying heart disease or left ventricular function. Moreover, the device programming used in this study should be adopted routinely to avoid unnecessary shocks.
Acknowledgments
The authors thank all investigators and institutions participating in the UNTOUCHED study (listed in Table V in the Data Supplement) for their large contribution, Ursula Appl for study and article preparation support, and Timothy Stivland for article preparation support.
Sources of Funding
The UNTOUCHED study was funded entirely by Boston Scientific Corp.
Disclosures
Dr Gold receives honoraria and research grants from Boston Scientific and Medtronic. Dr Lambiase receives research grants and speaker fees from Boston Scientific, Abbott and Medtronic; and is supported by University College of London/University College of London Hospitals Biomedicine National Institute for Health Research and Barts Biomedical Research Centre. Dr El-Chami is a consultant for Medtronic and Boston Scientific. Dr Knops receives honoraria for consulting and speaking and research grants from Boston Scientific, Abbott, and Medtronic. J. Aasbo reports Boston Scientific and Biotronik consulting fees. Dr Grazia Bongiorni receives honoraria from Abbott Medical Italia Società Per Azioni, Biotronik, Boston Scientific, and Medtronic. Dr Russo serves on the steering committee (no honoraria accepted) for Boston Scientific and Apple and receives research support from Boston Scientific and Medilynx. Dr Deharo receives research grants, travel grants, and honoraria for lectures/consulting from Medtronic, Boston Scientific, Abbott, Microport, and Biotronik. M.C. Burke receives honoraria from Boston Scientific as a consultant, receives research grants from Boston Scientific, and has equity in AtaCor Medical. Dr Dinerman receives speaking honoraria from Boston Scientific and consulting honoraria from Abbott. Dr Shaik receives honoraria for consulting, research, and speaking from Boston Scientific, Abbott, Biotronik, and Biosense Webster. N. Carter, T. Stoltz, Dr Stein, and Dr Brisben are employees and shareholders of Boston Scientific. Dr Boersma serves as a consultant and receives research grants from Boston Scientific and Medtronic. Dr Barr reports no conflicts.
Supplemental Materials
Data Supplement Tables I–V
Data Supplement Figures I–III
Supplementary Material
Appendix
The UNTOUCHED Investigators
Johan Aasbo, Timothy Phelan, Hazim Al-Ameri, Abdulhay Albirini, Rizwan Alimohammad, Miguel Arias, Nicolas Badenco, Craig Barr, Geraldine Bertaux, Deepak Bhakta, Sanjay Bindra, Hugues Blangy, Lucas Boersma, Serge Boveda, Johansen Brock, Martin Burke, Mathias Busch, Naiara Calvo, Christopher Cassidy, Michel Chauvin, Halim Marzak, Jason Chinitz, Allen Ciuffo, Jude Clancy, Karl Crossen, Paolo De Filippo, Jean-Claude Deharo, Fausto Devecchi, Sreekanth Karanam, Jay Dinerman, Rahul Doshi, Lars Eckardt, Matthew Fedor, Roger Freedman, Anil Gehi, Peter Goethals, Nils Gosau, Charles Gottlieb, Gregory Granrud, Radmira Greenstein, Firas Hamdan, Sam Hanon, Alborz Hassankhani, Rick Henderson, Stefan Hohnloser, David Huang, Didier Irles, Gautham Kalahasty, Pedram Kazemian, Farhat Khairallah, Brian Kim, Edward Kim, Christoph Klein, Bradley Knight, Reinoud Knops, Niuton Koide, Richard Kuk, Pier Lambiase, Christophe Leclercq, Michael Lee, Shang-Chiun Lee, Corinna Lenz, Nigel Lewis, Robert Lewis, George Mark, Christelle Marquie, Kelly McDonnell, John Mckenzie, Faisal Merchant, Sameh Mobarek, Tiziano Moccetti, Franck Molin, Francois Philliopon, Giovanni Morani, Daniel Morin, G. Ng, Emmanuel Nsah, Manoj Panday, Jean-Luc Pasquie, Nicasio Castellano Perez, Francisco Perez-Gil, Pawel Ptaszynski, Anil Rajendra, Troy Rhodes, Paul Roberts, Steven Rowe, Andrea Russo, Samir Saba, Venkata Sagi, Brian Sarter, John Schoenhard, John Schutzman, Luis Scott, Nathan Segerson, Naushad Shaik, Ali Shakir, Matthew Smelley, Jan Steffel, J. Lacy Sturdivant, Ghiyath Tabbal, Drory Tendler, Dominic Theuns, Liesbeth Timmers, Matthew Trojan, Shane Tsai, Gaurav Upadhyay, Santosh Varkey, Stefano Viani, Stanislav Weiner, Raul Weiss, Sherman Wiggins, David Wright, Andrew Zadeh, Edgar Zitron
Footnotes
A complete list of the investigators in the UNTOUCHED trial is provided in the Data Supplement.
Sources of Funding, see page 16
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
The Data Supplement, podcast, and transcript are available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.120.048728.
Contributor Information
Michael R. Gold, Email: goldmr@musc.edu.
Pier D. Lambiase, Email: p.lambiase@ucl.ac.uk.
Mikhael F. El-Chami, Email: melcham@emory.edu.
Reinoud E. Knops, Email: r.e.knops@amsterdamumc.nl.
Johan D. Aasbo, Email: jaasbo@mel.com.
Maria Grazia Bongiorni, Email: m.g.bongiorni@med.unipi.it.
Andrea M. Russo, Email: russo-andrea@cooperhealth.edu.
Jean-Claude Deharo, Email: jean-claude.deharo@ap-hm.fr.
Martin C. Burke, Email: mburke@corvitahealth.org.
Jay Dinerman, Email: jdinerman@comcast.net.
Craig S. Barr, Email: craigbarr@nhs.net.
Naushad Shaik, Email: NShaik@cvadrs.com.
Nathan Carter, Email: Nathan.Carter@bsci.com.
Thomas Stoltz, Email: thomas.stoltz@bsci.com.
Kenneth M. Stein, Email: kenneth.stein@bsci.com.
Amy J. Brisben, Email: amy.brisben@bsci.com.
Lucas V.A. Boersma, Email: l.boersma@antoniusziekenhuis.nl.
References
- 1.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, et al. Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399 [DOI] [PubMed] [Google Scholar]
- 2.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882–1890. doi: 10.1056/NEJM199912163412503 [DOI] [PubMed] [Google Scholar]
- 3.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601 [DOI] [PubMed] [Google Scholar]
- 4.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474 [DOI] [PubMed] [Google Scholar]
- 5.Zipes DP, Wyse DG, Friedman PL, Epstein AE, Hallstrom AP, Greene HL, Schron EB, Domanski M. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. N Engl J Med. 1997;337:1576–1583. doi: 10.1056/NEJM199711273372202 [DOI] [PubMed] [Google Scholar]
- 6.Compton SJ, Merrill JJ, Dorian P, Cao J, Zhou D, Gillberg JM. on behalf of the “Marquis VR” US/Canada Investigators. Continuous template collection and updating for electrogram morphology discrimination in implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 2006;29:244–254. doi: 10.1111/j.1540-8159.2006.00330.x [DOI] [PubMed] [Google Scholar]
- 7.Gasparini M, Lunati MG, Proclemer A, Arenal A, Kloppe A, Martínez Ferrer JB, Hersi AS, Gulaj M, Wijffels MCE, Santi E, et al. Long d etection p rogramming in s ingle-c hamber d efibrillators r educes u nnecessary t herapies and m ortality: t he ADVANCE III Trial. JACC Clin Electrophysiol. 2017;3:1275–1282. doi: 10.1016/j.jacep.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 8.Gold MR, Shorofsky SR, Thompson JA, Kim J, Schwartz M, Bocek J, Lovett EG, Hsu W, Morris MM, Lang DJ. Advanced rhythm discrimination for implantable cardioverter defibrillators using electrogram vector timing and correlation. J Cardiovasc Electrophysiol. 2002;13:1092–1097. doi: 10.1046/j.1540-8167.2002.01092.x [DOI] [PubMed] [Google Scholar]
- 9.Lüthje L, Vollmann D, Rosenfeld M, Unterberg-Buchwald C. Electrogram configuration and detection of supraventricular tachycardias by a morphology discrimination algorithm in single chamber ICDs. Pacing Clin Electrophysiol. 2005;28:555–560. doi: 10.1111/j.1540-8159.2005.50011.x [DOI] [PubMed] [Google Scholar]
- 10.Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP, Estes NA, 3rd, Greenberg H, Hall WJ, Huang DT, et al. MADIT-RIT Trial Investigators. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012;367:2275–2283. doi: 10.1056/NEJMoa1211107 [DOI] [PubMed] [Google Scholar]
- 11.Bardy GH, Smith WM, Hood MA, Crozier IG, Melton IC, Jordaens L, Theuns D, Park RE, Wright DJ, Connelly DT, et al. An entirely subcutaneous implantable cardioverter-defibrillator. N Engl J Med. 2010;363:36–44. doi: 10.1056/NEJMoa0909545 [DOI] [PubMed] [Google Scholar]
- 12.Gold MR, Theuns DA, Knight BP, Sturdivant JL, Sanghera R, Ellenbogen KA, Wood MA, Burke MC. Head-to-head comparison of arrhythmia discrimination performance of subcutaneous and transvenous ICD arrhythmia detection algorithms: the START study. J Cardiovasc Electrophysiol. 2012;23:359–366. doi: 10.1111/j.1540-8167.2011.02199.x [DOI] [PubMed] [Google Scholar]
- 13.Boersma L, Barr C, Knops R, Theuns D, Eckardt L, Neuzil P, Scholten M, Hood M, Kuschyk J, Jones P, et al. EFFORTLESS Investigator Group. Implant and m idterm o utcomes of the Subcutaneous Implantable Cardioverter-Defibrillator Registry: The EFFORTLESS Study. J Am Coll Cardiol. 2017;70:830–841. doi: 10.1016/j.jacc.2017.06.040 [DOI] [PubMed] [Google Scholar]
- 14.Lambiase PD, Barr C, Theuns DA, Knops R, Neuzil P, Johansen JB, Hood M, Pedersen S, Kääb S, Murgatroyd F, et al. EFFORTLESS Investigators. Worldwide experience with a totally subcutaneous implantable defibrillator: early results from the EFFORTLESS S-ICD Registry. Eur Heart J. 2014;35:1657–1665. doi: 10.1093/eurheartj/ehu112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss R, Knight BP, Gold MR, Leon AR, Herre JM, Hood M, Rashtian M, Kremers M, Crozier I, Lee KL, et al. Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation. 2013;128:944–953. doi: 10.1161/CIRCULATIONAHA.113.003042 [DOI] [PubMed] [Google Scholar]
- 16.Basu-Ray I, Liu J, Jia X, Gold M, Ellenbogen K, DiNicolantonio J, Komócsi A, Vorobcsuk A, Kim J, Afshar H, et al. Subcutaneous v ersus t ransvenous i mplantable d efibrillator t herapy: a m eta-a nalysis of c ase-c ontrol s tudies. JACC Clin Electrophysiol. 2017;3:1475–1483. doi: 10.1016/j.jacep.2017.07.017 [DOI] [PubMed] [Google Scholar]
- 17.Gold MR, Knops R, Burke MC, Lambiase PD, Russo AM, Bongiorni MG, Deharo JC, Aasbo J, El Chami MF, Husby M, et al. The d esign of the Understanding Outcomes with the S-ICD in Primary Prevention Patients with Low EF Study (UNTOUCHED). Pacing Clin Electrophysiol. 2017;40:1–8. doi: 10.1111/pace.12994 [DOI] [PubMed] [Google Scholar]
- 18.Boersma LV, El-Chami MF, Bongiorni MG, Burke MC, Knops RE, Aasbo JD, Lambiase PD, Deharo JC, Russo AM, Dinerman J, et al. Understanding Outcomes with the EMBLEM S-ICD in Primary Prevention Patients with Low EF Study (UNTOUCHED): c linical characteristics and perioperative results. Heart Rhythm. 2019;16:1636–1644. doi: 10.1016/j.hrthm.2019.04.048 [DOI] [PubMed] [Google Scholar]
- 19.Kutyifa V, Daubert JP, Schuger C, Goldenberg I, Klein H, Aktas MK, McNitt S, Stockburger M, Merkely B, Zareba W, et al. Novel ICD p rogramming and i nappropriate ICD t herapy in CRT-D v ersus ICD p atients: a MADIT-RIT Sub-Study. Circ Arrhythm Electrophysiol. 2016;9:e001965 doi: 10.1161/CIRCEP.114.001965 [DOI] [PubMed] [Google Scholar]
- 20.Burke MC, Aasbo JD, El-Chami MF, Weiss R, Dinerman J, Hanon S, Kalahasty G, Bass E, Gold MR. 1-Year p rospective e valuation of c linical o utcomes and s hocks. JACC Clin Electrophysiol. 2020;6:1537–1550. doi: 10.1016/j.jacep.2020.05.03 [DOI] [PubMed] [Google Scholar]
- 21.Gold MR, Higgins S, Klein R, Gilliam FR, Kopelman H, Hessen S, Payne J, Strickberger SA, Breiter D, Hahn S. Efficacy and temporal stability of reduced safety margins for ventricular defibrillation: primary results from the Low Energy Safety Study (LESS). Circulation. 2002;105:2043–2048. doi: 10.1161/01.cir.0000015508.59749.f5 [DOI] [PubMed] [Google Scholar]
- 22.Olde Nordkamp LR, Brouwer TF, Barr C, Theuns DA, Boersma LV, Johansen JB, Neuzil P, Wilde AA, Carter N, Husby M, et al. Inappropriate shocks in the subcutaneous ICD: i ncidence, predictors and management. Int J Cardiol. 2015;195:126–133. doi: 10.1016/j.ijcard.2015.05.135 [DOI] [PubMed] [Google Scholar]
- 23.Gold MR, Aasbo JD, El-Chami MF, Niebauer M, Herre J, Prutkin JM, Knight BP, Kutalek S, Hsu K, Weiss R, et al. Subcutaneous implantable cardioverter-defibrillator p ost-a pproval s tudy: c linical characteristics and perioperative results. Heart Rhythm. 2017;14:1456–1463. doi: 10.1016/j.hrthm.2017.05.016 [DOI] [PubMed] [Google Scholar]
- 24.Brisben AJ, Burke MC, Knight BP, Hahn SJ, Herrmann KL, Allavatam V, Mahajan D, Sanghera R, Gold MR. A new algorithm to reduce inappropriate therapy in the S-ICD system. J Cardiovasc Electrophysiol. 2015;26:417–423. doi: 10.1111/jce.12612 [DOI] [PubMed] [Google Scholar]
- 25.Theuns DAMJ, Brouwer TF, Jones PW, Allavatam V, Donnelley S, Auricchio A, Knops RE, Burke MC. Prospective blinded evaluation of a novel sensing methodology designed to reduce inappropriate shocks by the subcutaneous implantable cardioverter-defibrillator. Heart Rhythm. 2018;15:1515–1522. doi: 10.1016/j.hrthm.2018.05.011 [DOI] [PubMed] [Google Scholar]
- 26.Auricchio A, Hudnall JH, Schloss EJ, Sterns LD, Kurita T, Meijer A, Fagan DH, Rogers T. Inappropriate shocks in single-chamber and subcutaneous implantable cardioverter-defibrillators: a systematic review and meta-analysis. Europace. 2017;19:1973–1980. doi: 10.1093/europace/euw415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Packer M. What causes sudden death in patients with chronic heart failure and a reduced ejection fraction? Eur Heart J. 2020;41:1757–1763. doi: 10.1093/eurheartj/ehz553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Migliore F, Mattesi G, De Franceschi P, Allocca G, Crosato M, Calzolari V, Fantinel M, Ortis B, Facchin D, Daleffe E, et al. Multicentre experience with the second-generation subcutaneous implantable cardioverter defibrillator and the intermuscular two-incision implantation technique. J Cardiovasc Electrophysiol. 2019;30:854–864. doi: 10.1111/jce.13894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chinitz J. Inappropriate s hocks within 24 hours after i mplantation of a s ubcutaneous d efibrillator with a t wo-incision t echnique. J Innov Card Rhythm Manag. 2016;7:2295–2298. doi: 10.19102/icrm.2016.070302 [Google Scholar]
- 30.Knops RE, Olde Nordkamp LRA, Delnoy PHM, Boersma LVA, Kuschyk J, El-Chami MF, Bonnemeier H, Behr ER, Brouwer TF, Kääb S, et al. PRAETORIAN Investigators. Subcutaneous or t ransvenous d efibrillator t herapy. N Engl J Med. 2020;383:526–536. doi: 10.1056/NEJMoa1915932 [DOI] [PubMed] [Google Scholar]
- 31.Mondésert B, Bashir J, Philippon F, Dubuc M, Amit G, Exner D, Joza J, Birnie DH, Lane C, Tsang B, et al. Rationale and design of the randomized prospective ATLAS study: Avoid Transvenous Leads in Appropriate Subjects. Am Heart J. 2019;207:1–9. doi: 10.1016/j.ahj.2018.09.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


