Objectives:
When assessing arterial stiffness, heart rate (HR) and blood pressure (BP) are potential confounders. It appears that the HR/BP dependences of pulse wave velocity (PWV) and distensibility are different, even though both assess arterial stiffness. This study aims to compare aortic PWV as measured using pulse transit time (PWVTT) and as calculated from distensibility (PWVdist) at the same measurement site and propose a solution to the disparity in dependences of PWVTT and PWVdist.
Methods:
Adult anaesthetized rats (n = 24) were randomly paced at HRs 300–500 bpm, at 50 bpm steps. At each step, aortic PWVTT (two pressure-tip catheters) and PWVdist (pressure-tip catheter and ultrasound wall-tracking; abdominal aorta) were measured simultaneously while BP was varied pharmacologically.
Results:
HR dependence of PWVdist paradoxically decreased at higher levels of BP. In addition, BP dependence of PWVdist was much larger than that of PWVTT. These discrepancies are explained in that standard PWVdist uses an approximate derivative of pressure to diameter, which overestimates PWV with increasing pulse pressure (PP). In vivo, PP decreases as HR increases, potentially causing a PWVdist decrease with HR. Estimating the full pressure-diameter curve for each HR corrected for this effect by enabling calculation of the true derivative at diastolic BP. This correction yielded a PWVdist that shows HR and BP dependences similar to those of PWVTT. As expected, BP dependence of all PWV metrics was much larger than HR dependence.
Conclusion:
Measured and calculated PWV have different dependences on HR and BP. These differences are, at least in part, because of approximations made in using systolic and diastolic values to calculate distensibility.
Keywords: arterial stiffness, blood pressure, distensibility, heart rate, pulse wave analysis, pulse wave velocity
INTRODUCTION
Stiffness of the large arteries is an independent risk factor of all-cause mortality [1]. The two main methods for measuring arterial stiffness in vivo are pulse wave velocity (PWV) and distensibility. PWV is calculated by measuring the time difference of the passage of the blood pressure or flow wave between two points (e.g. carotid--femoral PWV). Distensibility is calculated from pressure and diameter measured at one arterial location. Pressure can be measured using cuff-based techniques in the case of the brachial artery, invasive pressure catheters in accessible vessels, or using filters or transfer functions to calculate pressure at one site (e.g. the aorta) by using measurement at another site (e.g. the brachial or radial artery) [2]. The required diameter measurements are commonly obtained using ultrasound wall tracking [3].
The American Heart Association recommendations for standardizing arterial stiffness research include that the intrinsic dependence of arterial stiffness on acute blood pressure and heart rate (HR) should be taken into consideration [4]. The acute effect of HR on carotid--femoral PWV, as a measure of large artery stiffness, has been studied in humans [5–12] with disparate results, largely because of the interaction between HR and blood pressure, which in turn affects arterial stiffness. Accounting for blood pressure reveals that an increase in HR causes a small increase in PWV (measured using the transit-time method) in humans [10], with controlled animal studies showing up to a 6% increase in aortic PWV over a 150 bpm increase in HR [13]. The acute effect of HR on carotid artery distensibility has been studied in rats [14,15] and showed a 15--43% decrease in distensibility with a 120 bpm increase in HR. No study has investigated if the disparity in distensibility and PWV changes with HR are because of anatomical location or the method of measurement.
In correcting arterial stiffness for blood pressure, controversy exists whether to use mean arterial pressure (MAP) [4], or DBP [16]; for a review, see reference [17]. It is also unclear whether this choice for MAP or DBP influences the obtained HR dependence.
The present study aims to quantify and explain the effect of HR and blood pressure changes on PWV and distensibility as measured at the same arterial location to investigate their disparity in HR dependence.
METHODS
Animal preparation
Adult Sprague--Dawley rats (n = 24, five female/19 male, obtained from the Animal Resource Centre, Perth, Australia) aged 12--17 weeks and weighing 446 ± 99 g (mean ± standard deviation, SD) were studied. Anaesthesia was induced through intraperitoneal injection of urethane (1.3 g/kg in 10% w/w Ringer's solution) and was maintained by intravenous injections of the same solution as required. Electrocardiogram electrodes were connected to the rat's front paws (signal) and the right back paw (reference). Body temperature was maintained at 37 ± 1 oC using a heat mat and rectal temperature probe. Polyethylene tubes were placed into the left and right femoral veins for drug delivery. Atrial pacing was accomplished using a custom-made 2.7F bipolar catheter introduced into the right atrium through the right external jugular vein [13]. Pressure tip-catheters (Scisense 1.2F; Transonic Systems Inc., Ithaca, New York, USA or Millar 1.4F Mikro-Tip; Millar Inc., Houston, Texas, USA) were placed in the upper thoracic (n = 12) and abdominal aorta (n = 24) via the left carotid and femoral arteries to continuously measure blood pressure and determine transit-time PWVTT (n = 12, including data from n = 7 described previously [13]). An ultrasound probe (13 MHz LA523 probe; MyLab70; Esaote Europe, Maastricht, The Netherlands) was placed laterally on the shaved skin (n = 17) and held in place with an XYZ-micromanipulator. Probe location and rotation were manipulated to obtain a clear longitudinal fast B-mode image of the abdominal aorta immediately proximal to the pressure catheter most distal in the abdominal aorta.
All procedures were conducted in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, and the experimental protocol was approved by the Macquarie University Animal Ethics Committee.
Data acquisition
Blood pressures, electrocardiogram, and temperature were recorded continuously using a Power1401 data acquisition system interfaced through Spike2 software (Cambridge Electronic Design, Cambridge, UK) at a sampling rate of 2000 Hz. Pulse trains were generated using Spike2 and were used to trigger an isolated pulse stimulator (Model 2100; A-M Systems Inc., Sequim, Washington, USA; pulse duration and amplitude of 2 ms and 0.6–1.0 V, respectively) that was connected to the atrial pacing catheter. The distance between the two pressure recording sites was measured postmortem after dissection (while the catheters were still in place) by aligning a small suture along the artery and subsequently measuring the length of suture between the catheter sites.
Abdominal aortic blood pressure was additionally recorded on a separate computer (ART.LAB, Esaote) on which the ultrasound radiofrequency signal as output by the echo scanner was also recorded. Recording of abdominal blood pressure on both systems enabled post hoc beat-to-beat synchronization of the acquisitions on those systems.
Experimental protocol
To enable stable pacing even at low HRs, intrinsic HR was reduced through intravenous injection of a specific bradycardic agent [zatebradine (Sigma-Aldrich, Castle Hill, New South Wales, Australia; n = 22) or ivabradine (Servier Laboratories, Hawthorn, Victoria, Australia; n = 2); 1 mg/kg in 1 mg/ml of 0.9% w/w saline)] to <300 bpm (n = 20) and, in four animals greater than 300 bpm but less than 350 bpm. Rats were then paced in a random sequence of HRs of 300, 350, 400, 450, and 500 bpm (n = 20, 24, 24, 24, and 18, respectively). At each rate, blood pressure was raised with phenylephrine (30 μg/kg/per min intravenously) until MAP was greater than 130 mmHg and lowered with sodium nitroprusside (SNP, 30 μg/kg/min intravenously) until MAP was less than 70 mmHg. During the return of blood pressure to baseline, when MAP crossed 70, 100, or 130 mmHg, ultrasound radiofrequency data in memory (∼5 s) was saved for subsequent wall tracking. At these pressure points, a square wave zero signal was inserted into the abdominal blood pressure signal by means of an electronic switch, serving as a fiducial point for post hoc synchronization of ultrasound and blood pressure signals.
Data processing
Offline radiofreqency wall tracking was performed using ART.LAB software (Esaote). Diameter waveforms for all 14 recorded echo lines were exported together with the abdominal pressure signal. These files were imported in a custom MATLAB-based program (MATLAB R2018a; MathWorks, Natick, Massachusetts, USA) where they were synchronized to the Spike-recorded pressure signals, after which blood pressure first and second derivatives were computed, and wave separation analysis was performed [details in Supplemental Digital Content (SDC) 1,]. Beat detection and computation of minimum (diastolic), maximum (systolic), and mean values were performed separately for diameter and pressure signals. In the pressure signals, the diastolic foot was identified as the second derivative peak. As pressure and diameter were synchronized, data in detected beats in those signals could be easily matched. Pressure and diameter waveforms were all visually inspected; heart beats where the diameter waveforms showed artefacts because of ultrasound probe or body movement (e.g. in some respiratory cycles) were removed from further analysis.
Pulse transit time (t) was calculated as the time difference between the diastolic feet of the thoracic and abdominal pressure signals; PWVTT was subsequently calculated as
| (1) |
with d the distance between thoracic and abdominal pressure measurement sites as measured post mortem.
Abdominal aortic distensibility was calculated using SBP and DBP, and peak (As) and minimum (Ad) abdominal aortic cross-sectional area (calculated from systolic and diastolic diameters as ):
| (2) |
Distensibility was converted to (PWVdist) using the Bramwell--Hill equation (Equation 3) for means of comparison to PWVTT:
| (3) |
with ρ the blood mass density, taken to be 1050 kg/m3. In order to obtain PWVdist in metres per second, all preceding equations were evaluated using values in SI units of metres (squared), seconds, and Pascals.
SBP, MAP, DBP, PWVTT, PWVdist, reflection index (RI), reflection magnitude (RM), and cardiac contractility (dp/dtmax) were obtained for each heartbeat. MAP was obtained by arithmetic averaging of the blood pressure waveform. Beat-to-beat data (i.e. one data point per heartbeat) were subsequently statistically analysed.
Statistical analyses
A detailed description of the statistical methods is provided in SDC 1. Briefly, data were analysed on a beat-to-beat basis (total dataset of n = 9142 heartbeats) by means of multilevel linear modelling with blood pressure (either SBP, MAP, or DBP) or PWV (either PWVTT or PWVdist) as a function of HR (as a categorical variable), blood pressure (either MAP or DBP; quadratically, as a continuous variable), HR × blood pressure (interaction); and age. Similar models with HR as a continuous instead of a categorical predictor were also created to assess linear trends of blood pressure or PWV with HR. All reported blood pressure values are those that were measured abdominally. Note that in our statistical modelling approach, no blood pressure windows were used (contrary to our previous study [13]). Rather, PWVs were computed for each heart beat individually. Then, a quadratic relation was fit through these pressure–PWV points by means of multilevel modelling. This (analytical) relation can then be used to estimate PWV for any given pressure.
Calculation of pulse wave velocity from distensibility using analytical derivatives
The regular Bramwell--Hill equation as often used in clinical studies (Equation 3) uses an approximated diastolic-to-systolic compliance term (; Equation 2). However, this compliance depends on the pulse pressure at the time of measurement (Fig. 1). As PWVTT is based on the arrival time difference of the diastolic foot between two sites, PWVTT depends on DBP. To obtain an equivalent PWV from a distensibility measurement, the Bramwell--Hill equation should be used based on the arterial compliance at diastole. In the present, experimental study, arterial diameter waveforms were measured at different pressures, enabling construction of a pressure--area curve from the systolic and diastolic pressure and cross-sectional area points acquired at different MAP levels (Supplemental Methods in SDC 1). These curves can then be used to obtain analytical derivatives from pressure to area at diastole, from which PWV can be calculated as
| (4) |
FIGURE 1.
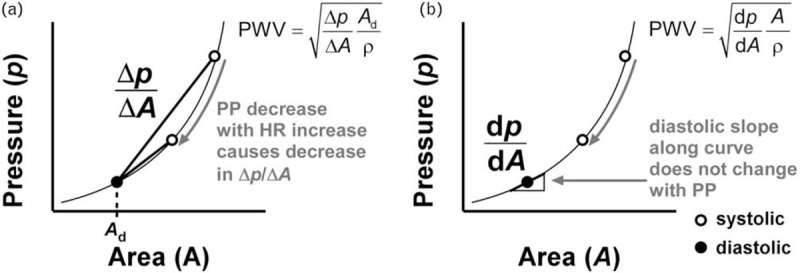
Two ways of calculating local pulse wave velocity (PWV) using the Bramwell--Hill equation from pressure and cross-sectional area measurements. (a) The standard Bramwell--Hill equation as often employed uses an approximated derivative of pressure (p) to area (A), (). Due to the nonlinearity of the p--A curve, varies with pressure and is an overestimation of in the diastolic point. As pulse pressure (PP) is prone to decrease with a heart rate (HR) increase, a relative decrease in local pulse wave velocity with an increase in HR could result. This paradoxical effect can be overcome by estimating the pressure--area curve for each HR, and calculating the true derivative along this curve, at DBP (b). ρ, blood mass density.
Note that pressure--area curves were fitted though beat-to-beat pressure-diameter points – we did not fit a pressure--area curve for each individual heartbeat.
RESULTS
Heart rate and blood pressure dependence of pulse wave velocity as a function of mean blood pressure
Table 1 shows the dependence of SBP, DBP, PWVTT, and PWVdist on HR as a function of MAP. Figure 2a illustrates the PWV dependences on HR. PWVTT's HR dependence increases with MAP (P < 0.001, 70 vs. 100 mmHg; P = 0.005, 100 vs. 130 mmHg; Table S1 in SDC 1). In contrast, PWVdist's dependence does not show a statistically significant increase, but does show a trend to increase from MAP = 70--100 mmHg (P = 0.070; Table S1). Figure 2b illustrates the PWV dependences on MAP, as a function of HR. MAP dependence of PWVTT increases with HR (Table S2). PWVdist's dependence does not show a statistically significant increase.
TABLE 1.
Blood pressure and pulse wave velocity parameters corrected for mean arterial pressure
| Heart rate (HR) | HR dependence | ||||||
| 300 bpm | 350 bpm | 400 bpm | 450 bpm | 500 bpm | Slope | P | |
| Mean arterial pressure (MAP) | |||||||
| 70 mmHg | |||||||
| SBP (mmHg) | 96 ± 2 | 93 ± 2 | 92 ± 2 | 91 ± 2 | 89 ± 2 | −3.6 ± 0.4 | <0.001 |
| DBP (mmHg) | 53 ± 1 | 54 ± 1 | 56 ± 1 | 56 ± 1 | 57 ± 1 | 1.8 ± 0.2 | <0.001 |
| PWVTT (m/s) | 3.46 ± 0.15 | 3.46 ± 0.15 | 3.47 ± 0.15 | 3.47 ± 0.15 | 3.46 ± 0.15 | 0.01 ± 0.02 | 0.708 |
| PWVdist (m/s) | 3.39 ± 0.14 | 3.36 ± 0.13 | 3.51 ± 0.13 | 3.60 ± 0.13 | 3.63 ± 0.13 | 0.15 ± 0.04 | <0.001 |
| 100 mmHg | |||||||
| SBP (mmHg) | 136 ± 1 | 132 ± 1 | 130 ± 1 | 126 ± 1 | 124 ± 1 | −5.7 ± 0.3 | <0.001 |
| DBP (mmHg) | 77 ± 1 | 80 ± 1 | 81 ± 1 | 83 ± 1 | 84 ± 1 | 3.3 ± 0.2 | <0.001 |
| PWVTT (m/s) | 3.65 ± 0.14 | 3.70 ± 0.14 | 3.74 ± 0.14 | 3.84 ± 0.14 | 3.86 ± 0.14 | 0.12 ± 0.01 | <0.001 |
| PWVdist (m/s) | 3.92 ± 0.11 | 4.12 ± 0.11 | 4.31 ± 0.11 | 4.30 ± 0.11 | 4.44 ± 0.11 | 0.24 ± 0.03 | <0.001 |
| 130 mmHg | |||||||
| SBP (mmHg) | 172 ± 2 | 169 ± 2 | 167 ± 2 | 164 ± 2 | 161 ± 2 | −5.4 ± 0.3 | <0.001 |
| DBP (mmHg) | 103 ± 1 | 105 ± 1 | 107 ± 1 | 108 ± 1 | 110 ± 1 | 3.4 ± 0.2 | <0.001 |
| PWVTT (m/s) | 4.22 ± 0.15 | 4.28 ± 0.15 | 4.36 ± 0.15 | 4.46 ± 0.15 | 4.53 ± 0.15 | 0.17 ± 0.02 | <0.001 |
| PWVdist (m/s) | 4.98 ± 0.13 | 5.00 ± 0.13 | 5.21 ± 0.13 | 5.23 ± 0.13 | 5.26 ± 0.13 | 0.16 ± 0.04 | <0.001 |
| MAP dependence | |||||||
| Slope | |||||||
| SBP (mmHg) | 127 ± 2 | 126 ± 2 | 124 ± 2 | 122 ± 2 | 121 ± 2 | ||
| DBP (mmHg) | 83 ± 1 | 85 ± 1 | 85 ± 1 | 87 ± 1 | 88 ± 1 | ||
| PWVTT (m/s) | 1.3 ± 0.2 | 1.4 ± 0.2 | 1.5 ± 0.2 | 1.6 ± 0.2 | 1.8 ± 0.2 | ||
| PWVdist (m/s) | 2.6 ± 0.3 | 2.7 ± 0.3 | 2.8 ± 0.3 | 2.7 ± 0.3 | 2.7 ± 0.3 | ||
Values denote mean ± standard error. SBP, DBP, transit time pulse wave velocity (PWVTT), and distensibility-based pulse wave velocity (PWVdist) as a function of mean arterial pressure (MAP, vertically) and heart rate (HR, horizontally). Note that values in each row are corrected to a fixed mean arterial pressure of 70, 100, or 130 mmHg. Hence, as expected, the decreasing pulse pressure with increasing heart rate leads to a decrease in SBP and an increase in DBP with heart rate. HR and MAP dependences are given in units of measurement per 100 bpm or per 100 mmHg, respectively. MAP dependences were calculated at 100 mmHg. All values are corrected for age. P denotes P-value for slope. For MAP dependence, all P < 0.001.
FIGURE 2.
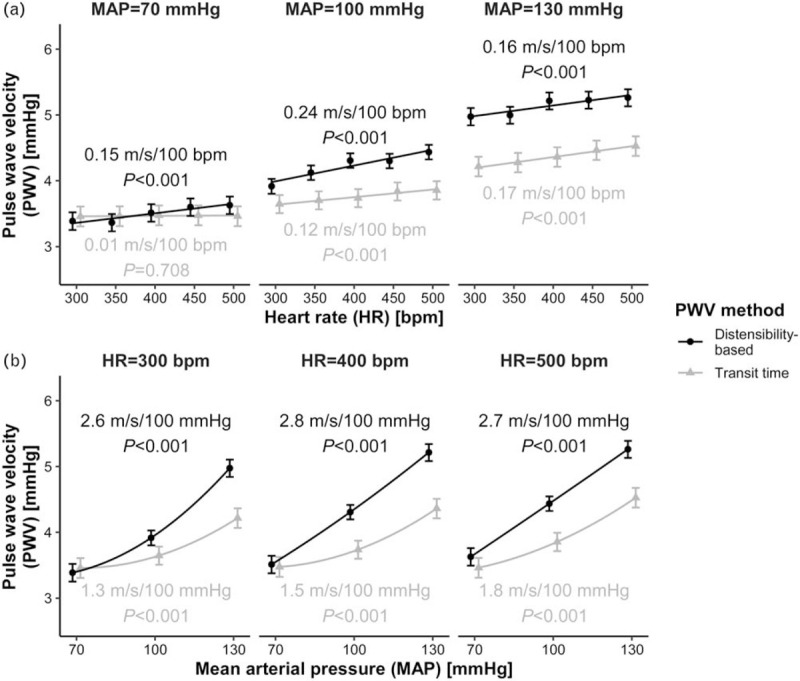
Dependence of transit time-based and distensibility-based pulse wave velocities (PWVs) on heart rate (HR, a) and mean arterial pressure (MAP, b). (a) PWV as a function of HR, for three values of MAP. (b) PWV as a function of MAP, for three values of HR. Blood pressure dependences are calculated for MAP = 100 mmHg. Points indicate mean ± standard error.
Heart rate and blood pressure dependence of pulse wave velocity as a function of DBP
Table 2 shows the dependence of SBP, MAP, PWVTT, and PWVdist on HR as a function of DBP. Figure 3 illustrates the PWV dependences. Overall, the HR dependence of PWV is much lower in this case (i.e. as compared with MAP correction). PWVTT's HR dependence increases from DBP = 60 to 85 mmHg (P = 0.011; Table S3). PWVdist's dependence is equal for DBPs of 60 and 85 mmHg but decreases to loss of statistical significance (P = 0.686) at 110 mmHg (P = 0.023 for change from 85 to 110 mmHg; Table S2). Figure 3B illustrates the PWV dependences on DBP, as a function of HR. DBP dependence of PWVTT did not change with HR. Dependence of PWVdist on DBP, however, decreased with HR, though not significantly (Table S4).
TABLE 2.
Blood pressure and pulse wave velocity parameters corrected for DBP
| Heart rate (HR) | HR dependence | ||||||
| 300 bpm | 350 bpm | 400 bpm | 450 bpm | 500 bpm | Slope | P | |
| DBP | |||||||
| 60 mmHg | |||||||
| SBP (mmHg) | 109 ± 2 | 102 ± 2 | 100 ± 2 | 96 ± 2 | 93 ± 3 | −7.7 ± 0.5 | <0.001 |
| MAP (mmHg) | 80 ± 1 | 77 ± 1 | 76 ± 1 | 74 ± 1 | 74 ± 1 | −2.6 ± 0.2 | <0.001 |
| PWVTT, m/s | 3.49 ± 0.13 | 3.51 ± 0.13 | 3.53 ± 0.13 | 3.54 ± 0.13 | 3.54 ± 0.14 | 0.03 ± 0.01 | 0.023 |
| PWVdist, m/s | 3.60 ± 0.14 | 3.62 ± 0.14 | 3.72 ± 0.14 | 3.77 ± 0.14 | 3.80 ± 0.14 | 0.11 ± 0.04 | 0.003 |
| 85 mmHg | |||||||
| SBP (mmHg) | 148 ± 2 | 140 ± 2 | 136 ± 2 | 130 ± 2 | 126 ± 2 | −10.6 ± 0.5 | <0.001 |
| MAP (mmHg) | 109 ± 1 | 106 ± 1 | 105 ± 1 | 103 ± 1 | 101 ± 1 | −3.9 ± 0.2 | <0.001 |
| PWVTT, m/s | 3.79 ± 0.13 | 3.83 ± 0.13 | 3.85 ± 0.13 | 3.90 ± 0.13 | 3.91 ± 0.13 | 0.06 ± 0.01 | <0.001 |
| PWVdist, m/s | 4.32 ± 0.13 | 4.39 ± 0.12 | 4.54 ± 0.12 | 4.47 ± 0.12 | 4.56 ± 0.12 | 0.11 ± 0.03 | 0.001 |
| 110 mmHg | |||||||
| SBP (mmHg) | 181 ± 3 | 175 ± 3 | 172 ± 3 | 167 ± 3 | 162 ± 3 | −9.2 ± 0.7 | <0.001 |
| MAP (mmHg) | 137 ± 1 | 135 ± 1 | 134 ± 1 | 132 ± 1 | 130 ± 1 | −3.7 ± 0.2 | <0.001 |
| PWVTT, m/s | 4.49 ± 0.14 | 4.47 ± 0.14 | 4.51 ± 0.14 | 4.55 ± 0.14 | 4.59 ± 0.14 | 0.06 ± 0.02 | 0.001 |
| PWVdist, m/s | 5.51 ± 0.16 | 5.29 ± 0.16 | 5.45 ± 0.15 | 5.42 ± 0.15 | 5.38 ± 0.15 | −0.02 ± 0.05 | 0.686 |
| DBP dependence | |||||||
| Slope | |||||||
| SBP (mmHg) | 144 ± 4 | 146 ± 4 | 144 ± 4 | 141 ± 4 | 139 ± 4 | ||
| MAP (mmHg) | 117 ± 1 | 117 ± 1 | 116 ± 1 | 114 ± 1 | 112 ± 1 | ||
| PWVTT, m/s | 2.0 ± 0.2 | 1.9 ± 0.2 | 2.0 ± 0.2 | 2.0 ± 0.2 | 2.1 ± 0.2 | ||
| PWVdist, m/s | 3.8 ± 0.4 | 3.3 ± 0.4 | 3.5 ± 0.4 | 3.3 ± 0.4 | 3.2 ± 0.4 | ||
Values denote mean ± standard error. SBP, mean arterial pressure (MAP), transit time pulse wave velocity (PWVTT), and distensibility-based pulse wave velocity (PWVdist) as a function of DBP (vertically) and heart rate (HR) (horizontally). Note that values in each row are corrected to a fixed DBP of 60, 85, or 110 mmHg. Hence, as expected, the decreasing pulse pressure with increasing heart rate leads to a decrease in both SBP and MAP with heart rate. HR and DBP dependences are given in units of measurement per 100 bpm or per 100 mmHg, respectively. DBP dependences were calculated at 85 mmHg. All values are corrected for age. P denotes P-value for slope. For DBP dependence, all P < 0.001.
FIGURE 3.
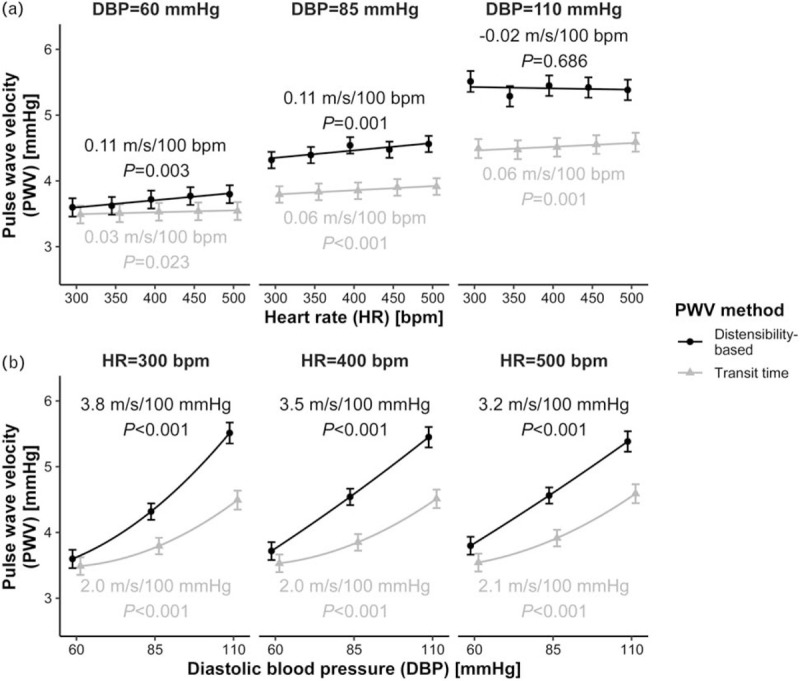
Dependence of transit time-based and distensibility-based pulse wave velocities (PWVs) on heart rate (HR, a) and DBP, b). (a) PWV as a function of HR, for three values of DBP. (b) PWV as a function of DBP, for three values of HR. Blood pressure dependences are calculated for DBP = 85 mmHg. Points indicate mean ± standard error.
Using an analytical derivative to calculate local pulse wave velocity
Figure 4 shows the effect of using an analytical derivative to calculate distensibility-based PWV (PWVdist,ana, Eq. 4), which is facilitated by the availability of full pressure--area curves in this study (average curves as a function of HR are shown in Supplemental Figure S1 in SDC 1). Results using this method do not show the paradoxical decrease in HR dependence of PWV at high DBP as seen with the ordinary PWVdist method. Instead, PWVdist,ana's blood pressure and HR dependences are very similar to those of PWVTT.
FIGURE 4.
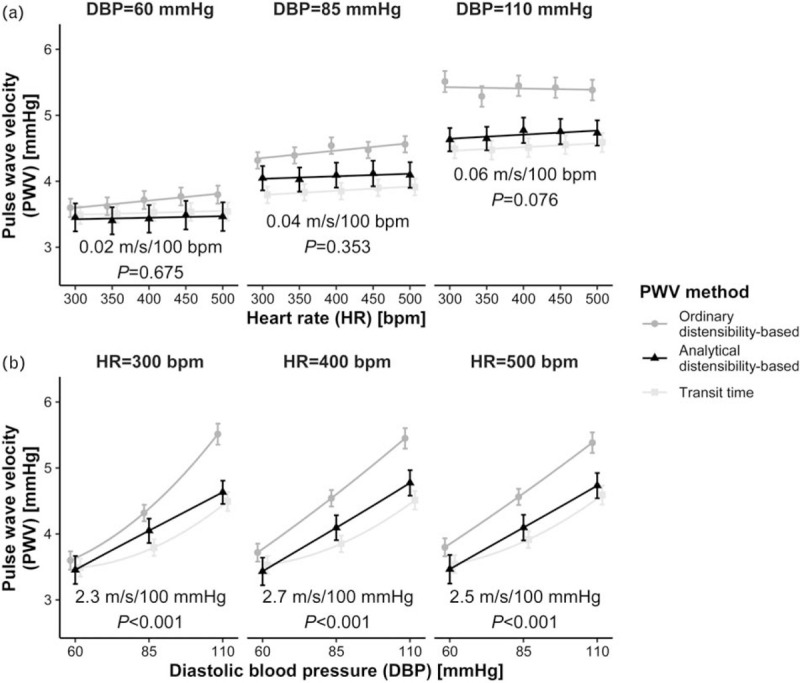
The effect of using an analytical derivative in calculating distensibility-based pulse wave velocity (PWV). Figure shows ordinary distensibility-based (PWVdist) and transit time data in the background (dots and squares, as in Fig. 3), with analytical PWVdist data (triangles) and P-values superimposed, as a function of heart rate (HR, a) and DBP (b). Note the similarity between analytical PWVdist and transit time PWV, both in absolute and relative (HR and blood pressure dependence) terms. Blood pressure dependences are calculated for DBP = 85 mmHg. Points indicate mean ± standard error.
Potential confounders
Five of the studied rats were females, whereas the other 19 were males. A separate statistical model was constructed to check for a potential influence of sex. The addition of sex as a predictor, with or without HR interaction, did not improve the statistical models (increased Bayesian information criterion, BIC). Rat weight may be another confounder. However, its addition as a predictor also did not improve the models.
Age was found to significantly correlate with PWVTT and PWVdist. No age--HR or age--blood pressure interactions were identified. Therefore, in our models, age only shifts the PWV–HR) relationship as a whole but does not influence the slope of the HR/blood pressure dependence of PWV.
Wave reflection and contractility
Figure S2, shows RI and RM as function of HR and blood pressure. Two aspects can be noted: first, with increasing blood pressure (i.e. progressively shifting from SNP infusion, to no infusion, to phenylephrine infusion), we observed a clear increase in RM and RI (Fig. S2b). Second, for a given DBP, RI/RM decreased with increasing HR (Fig. S2a).
Cardiac contractility, estimated as the maximum first derivative of the aortic arch blood pressure signal (dp/dtmax), showed a modest negative HR dependence at low blood pressure, gradually changing to a positive dependence at higher blood pressure (Fig. S3a). Blood pressure dependence of contractility (Fig. S3b) was negative at low HR (300 bpm), but the dependence gradually changes with increasing HR and does not reach significance at high HR (500 bpm).
Methodological aspects
PWVTT computed using the intersecting tangent method showed an HR/blood pressure dependence very similar to that obtained using the second derivative method (Fig. S4). A sensitivity analysis of the effect of the finite impulse response (FIR) filter width (k) showed that enlarging k from 1.5 ms (as used in all analyses presented herein) to 4.5 ms modestly changed the HR and DBP dependence (Fig. S5; green curves do not overlap with blue curves). This discrepancy became slightly larger with increasing DBP (i.e. with a progressive shift from SNP infusion, to no infusion, to phenylephrine infusion). Decreasing k from 1.5 to 0.5 ms, however, had a negligible effect (Fig S5; red and blue curves overlap).
DISCUSSION
Principal findings
In this article, we present the largest animal study to date on the effect of HR and blood pressure on PWVTT and the first animal study on the effect of HR on PWVdist allowing comparison of arterial stiffness measurements using transit time and distensibility approaches. We showed that both PWVTT and PWVdist show an HR dependence, whether corrected for MAP or DBP. PWVdist as commonly calculated differed in its relationship with HR compared with PWVTT, which is explained by the method of calculation (Fig. 1).
Implications of heart rate dependence and mean arterial pressure vs. DBP correction
The HR dependence of PWVTT and PWVdist as obtained using MAP correction is of larger magnitude than when obtained using DBP correction. This can be explained as follows. PWVTT, being estimated from the diastolic foot of the pulse wave [16,18–21], is determined by DBP. This is of particular relevance with respect to the HR dependence of PWV for the following reason (Fig. 5): assuming that cardiac output remains approximately equal among pacing settings, stroke volume will be inversely related to HR. Therefore, with an increase in HR, pulse pressure will decrease. Due to baroreflex regulation maintaining MAP at a steady level, this decreased pulse pressure will result in an increased DBP with increased HR. Therefore, PWVTT's dependence on DBP causes PWVTT to increase with HR, an effect which is not corrected for if MAP is used for correction.
FIGURE 5.
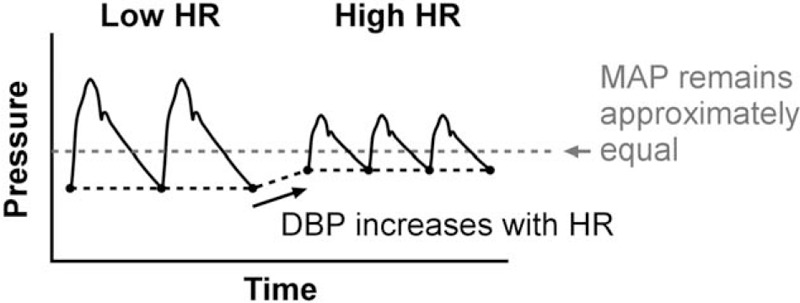
The significance of properly correcting transit time pulse wave velocity using DBP instead of mean arterial pressure (MAP). Changes in heart rate (HR) may induce substantial changes in DBP as DBP typically increases (and SBP decreases) because of the reduced stroke volume and resulting reduced pulse pressure with higher HR. If an MAP-based correction is employed, the heart beats in the figure and their PWVs are directly compared with each other. However, the beats at high HR have an elevated DBP, and therefore, an elevated transit time PWV, because of blood pressure alone. This leads to an increased, apparent HR dependence of transit time PWV when an MAP correction is employed.
Implications of distensibility findings
We showed that, when using distensibility as calculated from SBP and DBP and cross-sectional area values to obtain PWV, the resulting measure (PWVdist) shows a strong, nonlinear HR dependence. PWVdist shows the highest dependence at low DBP, which vanishes at high DBP. This paradoxical effect is to a large extent explained by blood pressure changes that lead to artefactual changes in the (linearly approximated) PWVdist (Fig. 1).
The linear approximation used in PWVdist also explains why PWVdist progressively diverges from PWVTT with increasing DBP: With increasing DBP, pulse pressure also increases (as can be seen from the progressively bigger difference between SBP and DBP in Table 2). This increases the error that is made when estimating (diastolic) distensibility using the linearized diastolic-to-systolic slope (Fig. 1).
The method of calculation of distensibility and PWVdist has important implications in comparison of clinical studies. For example, different trends in changes in aortic stiffness have been shown in cross-sectional studies, with PWV measurement (transit time) showing greatest changes (increases) in stiffness with age in the abdominal aorta [22] but distensibility measurement showing greatest changes (decreases) with age in the ascending aorta [23]. One of the contributors to this disparity may be the method of calculation of distensibility. In particular, distensibility and PWVdist as commonly calculated (Eqs. 2–3; as also employed in [23]) shows a greater blood pressure dependence than PWVTT. This, together with an increase in blood pressure with age hence potentially explains why distensibility/PWVdist show greater changes with age than PWVTT. Correction according to Fig. 1 brings these two metrics closer in line.
In the present study, we were able to construct full pressure--area relationships, as measurements were taken at multiple levels of MAP. From these relationships, local PWVs based on the analytical pressure--area derivative could be computed. A similar approach has been adopted by Mangoni and Mircoli [14,15], and by Albaladejo et al.[24], who derived carotid distensibility from such curves by making use of true curve derivatives, permitting a true blood pressure-independent analysis of the dependence of distensibility on HR. Whereas Mangoni et al.[14] found a marked reduction in distensibility with HR, Albaladejo et al.[24] did not observe a change.
In routine clinical practice, it is not feasible to measure full pressure-area curves, as this requires sweeping a patient's blood pressure over a wide range. However, instead, an exponential pressure--area relationship could be assumed [25–27], which would enable calculation of an analytical derivative [28].
Diastolic foot detection method
In line with our previous study [13], PWVTT was calculated by determining the time delay between the second derivative peaks of the proximal and distal pressure waveforms. This method has demonstrated accuracy similar to the intersecting tangent method [29], and yields nearly equal values [30]. Furthermore, in a previous study in patients, the foot detection method did not influence the finding of an HR dependence [5]. To confirm these findings, we re-estimated PWVTT also using the intersecting tangent method; the observed differences between PWVTT as estimated using both methods were indeed small (Fig. S4).
Possible explanations of the heart rate dependence of pulse wave velocity
The intrinsic mechanism that causes PWV to depend on HR remains to be accurately established. Hypothesized causes include: 1) confounding because of the foot detection algorithm, 2) pacing-induced changes in sympathetic tone, influencing vascular tone, 3) changes in wave reflection, or 4) visco-elasticity of the arterial wall. We will address these individually below. The reader is also referred to our previous works for discussions on this subject [10,13,31].
First, in the present study, we observed a very similar trend in HR dependence of transit-time PWV and distensibility-based PWV when calculated using an analytical derivative. Furthermore, we determined that using a different foot detection method (intersecting tangent instead of maximum second derivative) only minimally changed our results. This, together with the argumentation in the previous paragraph, excludes the influence of the foot detection algorithm as a cause for the observed HR dependence, which would only be applicable for PWVTT; leaving hypotheses 2–4 intact.
Second, sympathetic tone has been shown to have no influence on stiffness in the large arteries following sympathectomy in rats [15,32].
Third, in the present study, we used SNP and phenylephrine to modulate blood pressure. The peripheral vasodilatory effect of SNP has previously been shown to decrease wave reflections [33], while phenylephrine-induced peripheral vasoconstrictions may increase wave reflections [34]. In addition, broad correlations have been demonstrated between wave reflection and PWVTT[35] and PWVdist[36], albeit showing different patterns of change with ageing [37]. To study the effects of wave reflections, hence, we estimated RI/RM and their relations to HR and blood pressure. As expected from the modulating effects of SNP/phenylephrine, with an increase in blood pressure (i.e. when shifting from SNP infusion, to no infusion, to phenylephrine infusion), we observed an increase in wave reflection. At given (fixed) blood pressures, however, wave reflection decreased with increasing HR, corroborating earlier simulation studies [38]. This effect became stronger with increasing blood pressure. PWVdist (using diastolic as well as systolic points to linearly estimate dp/dA) strongly relies on determination of a systolic p--A point, which in turn will be influenced by wave reflections. Hence, the observed, paradoxical decrease in HR dependence of PWVdist at higher DBP (Fig. 3a) could well be explained by changes in wave reflection (Fig. S2a).
Fourth, given that the previous three hypothesized causes seem unlikely, visco-elasticity is our primary hypothesized cause of the HR dependence of PWV. To further investigate, we estimated arterial dp/dtmax as a measure of contractility, noting that arterial dp/dtmax correlates reasonably with cardiac dp/dtmax[39,40]. With an increase in blood pressure, dp/dtmax showed an increase in HR dependence (from negative, to nonsignificant, to positive, Fig S3a). This aligns with our observed pattern of contractility changes with HR, which also shows an increase in HR dependence with blood pressure. This increasing dependence concords with the observed increasing HR dependence of PWV. Indeed, dp/dtmax was also a significant predictor of PWVTT. The observed pattern of contractility changes with blood pressure (Fig. S3b) can be explained as follows: At a low DBP, afterload is relatively low as arterial compliance is high (little recruitment of collagen). Combined with a low HR (which requires a large stroke volume), this results in a large pulse pressure and large dp/dtmax. As DBP increases, afterload will increase, contraction becomes more difficult, and dp/dtmax will decrease. As HR increases towards 500 bpm (Fig. S3b, middle and right panels), this relationship flattens, indicating a saturation effect. Overall, changes in dp/dtmax with HR were subtle (−10% and +16% with HR increases from 300 to 500 bpm at DBPs of 60 and 110 mmHg, respectively). The reader is reminded that we are modifying HR through pacing and not pharmacologically, the latter of which would be expected to have a more pronounced effect on dp/dtmax[39]. The effects of pacing on dp/dtmax, however, are inconclusive [41,42]. Note, also, that cross-sectionally, dp/dtmax has been shown not to correlate with HR [40].
Clinical significance of heart rate dependence
The HR dependence of PWVTT when corrected for MAP is similar to what we observed previously (present study: 0.01--0.17 m/s/100 bpm; previously: no change to 0.17 m/s/100 bpm) [13]. The HR dependence of PWV when corrected for DBP is less pressure-dependent and generally smaller than when corrected for MAP. As explained above, DBP correction is most appropriate (considering the diastolic foot-detection transit time method), implying a (PWVTT) dependence of 0.02--0.06 m/s/100 bpm.
When expressed as a relative change (%-change in PWV per %-change in bpm), this amounts to 0.003--0.005 %/%. In a previous study in humans, where we also employed DBP-based correction, larger relative changes of 0.07--0.18 %/% were measured [10], though over the carotid--femoral trajectory and not over an ‘isolated’ abdominal aortic segment as in the present study. In both rats and humans, the absolute HR dependence is small compared with clinically attainable reproducibility levels. However, in studies involving large HR changes in many individuals (e.g. exercise studies), statistical differences between low-HR and high-HR conditions may be observed that can be (partially) attributed to the intrinsic HR dependence of PWV. Notably, the magnitude (and hence the clinical significance) of the blood pressure dependence of PWV is much larger than that of the HR dependence. Indeed, blood pressure correction is now commonplace in all major studies involving PWV [26,43]. Because of the magnitude of this dependence, blood pressure-corrected indices, such as cardio-ankle vascular index (CAVI) and CAVI0 are becoming increasingly popular [28,44].
Limitations
In our study, urethane anaesthesia was used, which has been shown to potentially alter vascular tone [45]. However, randomization of the order of pacing HRs among rats ensured that any changes in level of anaesthesia over the time course of the experiments would not confound the result. The potential influence of urethane on blood pressure [46] was accounted for by actively controlling blood pressure using phenylephrine and SNP to ensure acquisition of data at distinct blood pressure values.
Furthermore, bradycardic agents (ivabradine and zatebradine) were used to lower HR before commencement of measurements. Both have been shown to elicit a ‘pure’ HR decrease, without modifying atrioventricular conduction, intraventricular conduction, or contractility; and without modifying blood pressure or showing vasoactive effects [47].
Vasoactive drugs were used to increase (phenylephrine) and reduce (SNP) blood pressure. A previous study [48] has shown that at the doses administered, phenylephrine altered rat aortic PWVTT by 5% and SNP did not alter PWVTT despite the same estimated concentrations of the vasoactive drugs altering aortic smooth muscle tension ex vivo. Although kinetics of drug absorption were not measured in that study, the kinetics are likely to be substantially different for an isolated aortic segment bathed in SNP or phenylephrine for a period of time to that of the in vivo aorta exposed to the same concentration of SNP or phenylephrine in blood for a short period of time. Regardless of the effect, the main comparison of the present study is between PWVTT and PWVdist. The arterial stiffness conditions under which those parameters are compared were inherently identical.
Perspectives
In the present study, we showed that in rats, transit time PWV as well as distensibility-derived PWV show a small but statistically significant HR dependence of about 0.1 m/s/100 bpm; and PWV derived from arterial distensibility, when based on commonly used methods to approximate distensibility, shows an artefactually inflated blood pressure dependence as well as reduction in HR dependence with blood pressure. When a true derivative is used to calculate distensibility -- as possible in this experimental setting -- the distensibility-derived PWV shows a blood pressure-- and HR dependence similar to transit time PWV.
ACKNOWLEDGEMENTS
This study was supported by Endeavour Research Fellowship ERF_PDR_142613_2015 awarded by the Australian Government to B.S.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Abbreviations: A, cross-sectional area; Ad, minimum (diastolic) cross-sectional area; As, peak (systolic) cross-sectional area; BIC, Bayesian information criterion; BP, blood pressure; d, distance between thoracic and abdominal pressure measurement sites; dp/dtmax, cardiac contractility, estimated as the maximum first derivative of the aortic arch blood pressure signal; FIR, finite impulse response; HR, heart rate; k, finite impulse response filter width; MAP, mean arterial pressure; p, pressure; P, statistical P-value; pb(t), backward pressure wave used in wave separation analysis; pf(t), forward pressure wave used in wave separation analysis; PP, pulse pressure; PWV, pulse wave velocity; PWVdist, distensibility-based pulse wave velocity based on linearized distensibility; PWVdist, distensibility-based pulse wave velocity based on analytical pressure–cross-sectional area curve; PWVTT, transit-time pulse wave velocity; Q(t), triangular, estimated blood flow pattern used in wave separation analysis; RI, reflection index; RM, reflection magnitude; SD, standard deviation; SDC, supplemental digital content; SNP, sodium nitroprusside; t, pulse transit time; Zc, characteristic impedance used in wave separation analysis; ρ, blood mass density
REFERENCES
- 1.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 2.Miyashita H. Clinical assessment of central blood pressure. Curr Hypertens Rev 2012; 8:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reneman RS, Meinders JM, Hoeks AP. Noninvasive ultrasound in arterial wall dynamics in humans: what have we learned and what remains to be solved. Eur Heart J 2005; 26:960–966. [DOI] [PubMed] [Google Scholar]
- 4.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, et al. American Heart Association Council on Hypertension, Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension 2015; 66:698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Millasseau SC, Stewart AD, Patel SJ, Redwood SR, Chowienczyk PJ. Evaluation of carotid-femoral pulse wave velocity: influence of timing algorithm and heart rate. Hypertension 2005; 45:222–226. [DOI] [PubMed] [Google Scholar]
- 6.Haesler E, Lyon X, Pruvot E, Kappenberger L, Hayoz D. Confounding effects of heart rate on pulse wave velocity in paced patients with a low degree of atherosclerosis. J Hypertens 2004; 22:1317–1322. [DOI] [PubMed] [Google Scholar]
- 7.Lantelme P, Mestre C, Lievre M, Gressard A, Milon H. Heart rate: an important confounder of pulse wave velocity assessment. Hypertension 2002; 39:1083–1087. [DOI] [PubMed] [Google Scholar]
- 8.Albaladejo P, Copie X, Boutouyrie P, Laloux B, Declere AD, Smulyan H, Benetos A. Heart rate, arterial stiffness, and wave reflections in paced patients. Hypertension 2001; 38:949–952. [DOI] [PubMed] [Google Scholar]
- 9.Liang YL, Gatzka CD, Du XJ, Cameron JD, Kingwell BA, Dart AM. Effects of heart rate on arterial compliance in men. Clin Exp Pharmacol Physiol 1999; 26:342–346. [DOI] [PubMed] [Google Scholar]
- 10.Tan I, Spronck B, Kiat H, Barin E, Reesink KD, Delhaas T, et al. Heart rate dependency of large artery stiffness. Hypertension 2016; 68:236–242. [DOI] [PubMed] [Google Scholar]
- 11.Giannattasio C, Vincenti A, Failla M, Capra A, Ciro A, De Ceglia S, et al. Effects of heart rate changes on arterial distensibility in humans. Hypertension 2003; 42:253–256. [DOI] [PubMed] [Google Scholar]
- 12.Sa Cunha R, Pannier B, Benetos A, Siche JP, London GM, Mallion JM, Safar ME. Association between high heart rate and high arterial rigidity in normotensive and hypertensive subjects. J Hypertens 1997; 15:1423–1430. [DOI] [PubMed] [Google Scholar]
- 13.Tan I, Butlin M, Liu YY, Ng K, Avolio AP. Heart rate dependence of aortic pulse wave velocity at different arterial pressures in rats. Hypertension 2012; 60:528–533. [DOI] [PubMed] [Google Scholar]
- 14.Mangoni AA, Mircoli L, Giannattasio C, Ferrari AU, Mancia G. Heart rate-dependence of arterial distensibility in vivo. J Hypertens 1996; 14:897–901. [DOI] [PubMed] [Google Scholar]
- 15.Mircoli L, Mangoni AA, Giannattasio C, Mancia G, Ferrari AU. Heart rate-dependent stiffening of large arteries in intact and sympathectomized rats. Hypertension 1999; 34 (4 Pt 1):598–602. [DOI] [PubMed] [Google Scholar]
- 16.Nye ER. The effect of blood pressure alteration on the pulse wave velocity. Br Heart J 1964; 26:261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spronck B, Delhaas T, Butlin M, Reesink KD, Avolio AP. Options for dealing with pressure dependence of pulse wave velocity as a measure of arterial stiffness: an update of cardio-ankle vascular index (CAVI) and CAVI0. Pulse (Basel) 2018; 5:106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bramwell J, McDowall R, McSwiney B. The variation of arterial elasticity with blood pressure in man (part I). Proc R Soc Lond B 1923; 94:450–454. [Google Scholar]
- 19.Gao M, Cheng H-M, Sung S-H, Chen C-H, Olivier NB, Mukkamala R. Estimation of pulse transit time as a function of blood pressure using a nonlinear arterial tube-load model. IEEE Trans Biomed Eng 2017; 64:1524–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nichols W, O’Rourke M, Vlachopoulos C. McDonald's blood flow in arteries: theoretical, experimental and clinical principles. London, United Kingdom: CRC press; 2011. [Google Scholar]
- 21.Willemet M, Chowienczyk P, Alastruey J. A database of virtual healthy subjects to assess the accuracy of foot-to-foot pulse wave velocities for estimation of aortic stiffness. Am J Physiol Heart Circ Physiol 2015; 309:H663–H675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hickson SS, Butlin M, Graves M, Taviani V, Avolio AP, McEniery CM, Wilkinson IB. The relationship of age with regional aortic stiffness and diameter. JACC Cardiovasc Imaging 2010; 3:1247–1255. [DOI] [PubMed] [Google Scholar]
- 23.Redheuil A, Yu WC, Wu CO, Mousseaux E, de Cesare A, Yan R, et al. Reduced ascending aortic strain and distensibility: earliest manifestations of vascular aging in humans. Hypertension 2010; 55:319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albaladejo P, Challande P, Kakou A, Benetos A, Labat C, Louis H, et al. Selective reduction of heart rate by ivabradine: effect on the visco-elastic arterial properties in rats. J Hypertens 2004; 22:1739–1745. [DOI] [PubMed] [Google Scholar]
- 25.Spronck B, Delhaas T, De Lepper AG, Giroux J, Goldwasser F, Boutouyrie P, et al. Patient-specific blood pressure correction technique for arterial stiffness: evaluation in a cohort on antiangiogenic medication. Hypertens Res 2017; 40:752–757. [DOI] [PubMed] [Google Scholar]
- 26.Spronck B, Heusinkveld MH, Vanmolkot FH, Roodt JO, Hermeling E, Delhaas T, et al. Pressure-dependence of arterial stiffness: potential clinical implications. J Hypertens 2015; 33:330–338. [DOI] [PubMed] [Google Scholar]
- 27.Spronck B. Stiff vessels approached in a flexible way: advancing quantification and interpretation of arterial stiffness. Artery Res 2018; 21:63–68. [Google Scholar]
- 28.Spronck B, Avolio AP, Tan I, Butlin M, Reesink KD, Delhaas T. Arterial stiffness index beta and cardio-ankle vascular index inherently depend on blood pressure but can be readily corrected. J Hypertens 2017; 35:98–104. [DOI] [PubMed] [Google Scholar]
- 29.Chiu YC, Arand PW, Shroff SG, Feldman T, Carroll JD. Determination of pulse wave velocities with computerized algorithms. Am Heart J 1991; 121:1460–1470. [DOI] [PubMed] [Google Scholar]
- 30.Vardoulis O, Papaioannou TG, Stergiopulos N. Validation of a novel and existing algorithms for the estimation of pulse transit time: advancing the accuracy in pulse wave velocity measurement. Am J Physiol Heart Circ Physiol 2013; 304:H1558–H1567. [DOI] [PubMed] [Google Scholar]
- 31.Tan I, Butlin M, Spronck B, Xiao H, Avolio A. Effect of heart rate on arterial stiffness as assessed by pulse wave velocity. Curr Hypertens Rev 2018; 14:107–122. [DOI] [PubMed] [Google Scholar]
- 32.Mangoni AA, Mircoli L, Giannattasio C, Mancia G, Ferrari AU. Effect of sympathectomy on mechanical properties of common carotid and femoral arteries. Hypertension 1997; 30:1085–1088. [DOI] [PubMed] [Google Scholar]
- 33.Brin KP, Yin FC. Effect of nitroprusside on wave reflections in patients with heart failure. Ann Biomed Eng 1984; 12:135–150. [DOI] [PubMed] [Google Scholar]
- 34.Zuckerman BD, Weisman HF, Yin FC. Arterial hemodynamics in a rabbit model of atherosclerosis. Am J Physiol 1989; 257:H891–H897. [DOI] [PubMed] [Google Scholar]
- 35.Filipovsky J, Ticha M, Cifkova R, Lanska V, Stastna V, Roucka P. Large artery stiffness and pulse wave reflection: results of a population-based study. Blood Press 2005; 14:45–52. [DOI] [PubMed] [Google Scholar]
- 36.Segers P, Verdonck P, Deryck Y, Brimioulle S, Naeije R, Carlier S, Stergiopulos N. Pulse pressure method and the area method for the estimation of total arterial compliance in dogs: sensitivity to wave reflection intensity. Ann Biomed Eng 1999; 27:480–485. [DOI] [PubMed] [Google Scholar]
- 37.McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR. ACCT Investigators, Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol 2005; 46:1753–1760. [DOI] [PubMed] [Google Scholar]
- 38.Xiao H, Tan I, Butlin M, Li D, Avolio AP. Mechanism underlying the heart rate dependency of wave reflection in the aorta: a numerical simulation. Am J Physiol Heart Circ Physiol 2018; 314:H443–H451. [DOI] [PubMed] [Google Scholar]
- 39.Monge Garcia MI, Jian Z, Settels JJ, Hunley C, Cecconi M, Hatib F, Pinsky MR. Performance comparison of ventricular and arterial dP/dtmax for assessing left ventricular systolic function during different experimental loading and contractile conditions. Crit Care 2018; 22:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ostadal P, Vondrakova D, Kruger A, Janotka M, Naar J. Continual measurement of arterial dP/dtmax enables minimally invasive monitoring of left ventricular contractility in patients with acute heart failure. Crit Care 2019; 23:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neumann T, Ravens U, Heusch G. Characterization of excitation-contraction coupling in conscious dogs with pacing-induced heart failure. Cardiovasc Res 1998; 37:456–466. [DOI] [PubMed] [Google Scholar]
- 42.Schaefer S, Taylor AL, Lee HR, Niggemann EH, Levine BD, Popma JJ, et al. Effect of increasing heart rate on left ventricular performance in patients with normal cardiac function. Am J Cardiol 1988; 61:617–620. [DOI] [PubMed] [Google Scholar]
- 43.Reference Values for Arterial Stiffness’ Collaboration, Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ’establishing normal and reference values’. Eur Heart J 2010; 31:2338–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J Atheroscler Thromb 2006; 13:101–107. [DOI] [PubMed] [Google Scholar]
- 45.Altura BM, Weinberg J. Urethane and contraction of vascular smooth muscle. Br J Pharmacol 1979; 67:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kohn DF, Wixson SK, White WJ, Benson GJ. Anesthesia and analgesia in laboratory animals. San Diego, CA: Academic Press; 1997. [Google Scholar]
- 47.Mulder P, Thuillez C. Heart rate slowing for myocardial dysfunction/heart failure. Adv Cardiol 2006; 43:97–105. [DOI] [PubMed] [Google Scholar]
- 48.Butlin M, Lindesay G, Viegas KD, Avolio AP. Pressure dependency of aortic pulse wave velocity in vivo is not affected by vasoactive substances that alter aortic wall tension ex vivo. Am J Physiol Heart Circ Physiol 2015; 308:H1221–H1228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


