Supplemental Digital Content is available in the text
Keywords: kidney disease, muscle wasting, nutrition, physical activity, protein
Purpose of review
Poor nutritional status is prevalent among end-stage renal disease patients undergoing hemodialysis. Chronic hemodialysis patients show an accelerated decline in skeletal muscle mass and strength, which is associated with higher mortality rates and a reduced quality of life. The current review aims to summarize recent advances regarding underlying causes of muscle loss and interventions that support muscle mass maintenance in patients with chronic hemodialysis.
Recent findings
Muscle maintenance in chronic hemodialysis patients is compromised by low dietary protein intake levels, anabolic resistance of skeletal muscle tissue, sedentary behavior, and amino acid removal during hemodialysis. Studies assessing the effect of increased protein intake on nutritional status generally show beneficial results, especially in hypoalbuminemic chronic hemodialysis patients. The muscle protein synthetic response following protein ingestion in chronic hemodialysis patients may be enhanced through incorporation of structured physical activity and/or concurrent ketoacid ingestion.
Summary
A coordinated program that combines nutritional and physical activity interventions is likely required to attenuate the decline in muscle mass and strength of chronic hemodialysis patients. Nephrologists, dieticians, and exercise specialists should collaborate closely to establish guidelines regarding the appropriate quantity and timing of protein ingestion. In addition, they should provide tailored nutritional and physical activity interventions for chronic hemodialysis patients (see video, Supplemental Digital Content 1, Video abstract).
INTRODUCTION
Poor nutritional status is frequently observed in all stages of chronic kidney disease (CKD), but its prevalence increases with advanced stages [1]. In patients with end-stage renal disease (ESRD), the glomerular filtration rate is below 15 ml/min/1.73 m2 and metabolic waste products are insufficiently excreted from the body. Waste products can accumulate to lethal concentrations in ESRD and should be removed from the body through the use of renal replacement strategies.
Hemodialysis is globally the most applied chronic renal replacement strategy when kidney transplantation is not possible. However, after initiation of chronic hemodialysis (CHD) therapy, the rate of loss of skeletal muscle mass and strength is high and impairments in physical function become a common observation [2,3]. A recent meta-analysis by Carrero et al.[4▪] reported that protein-energy wasting, a syndrome characterized by the progressive loss of body protein and energy stores (i.e. muscle and fat mass), is present in 28–54% of CHD patients.
Skeletal muscle mass is regulated by a dynamic balance between the continuous synthesis and breakdown of muscle proteins. The skeletal muscle protein pool possesses a turnover rate of 1–2% per day, which results in quick adaptation of muscle tissue to circumstances (e.g. muscle atrophy following disuse) [5]. Loss of skeletal muscle mass can be attributed both to a decrease in muscle protein synthesis, as well as to an increase in muscle protein breakdown rates. It is suggested that catabolic pathways in patients with ESRD are upregulated because of disease-related complications, such as insulin resistance and accumulation of metabolic waste products [3]. To support muscle maintenance in those patients, anabolic interventions to induce an equivalent increase in muscle protein synthesis rates should be implemented in clinical care. Two key anabolic strategies essential for muscle maintenance are protein ingestion and physical activity.
Therefore, the purpose of the current review is to describe the impact of dietary protein interventions with and without physical activity on the nutritional status of ESRD patients undergoing hemodialysis as demonstrated in recent literature.
Box 1.
no caption available
DIETARY PROTEIN INTAKE
Protein ingestion is required to maintain skeletal muscle mass. Dietary protein is digested and amino acids are absorbed in the gut, with ∼50% of the dietary protein-derived amino acids being subsequently released into the circulation over a 5-h postprandial period [6]. Amino acids that become available in the circulation can serve as precursors for de-novo muscle protein synthesis [7]. However, amino acids are more than just building blocks for muscle protein synthesis, as they also function as signaling molecules. Essential amino acids, and leucine in particular, have the ability to stimulate the mammalian target of rapamycin complex 1 (mTORC1) activity, thereby increasing anabolic signaling [8]. As shown in Fig. 1A, ample protein ingestion and subsequent hyperaminoacidemia increases muscle protein synthesis rates and inhibits muscle protein breakdown rates, allowing net muscle protein accretion during the postprandial period [9].
FIGURE 1.
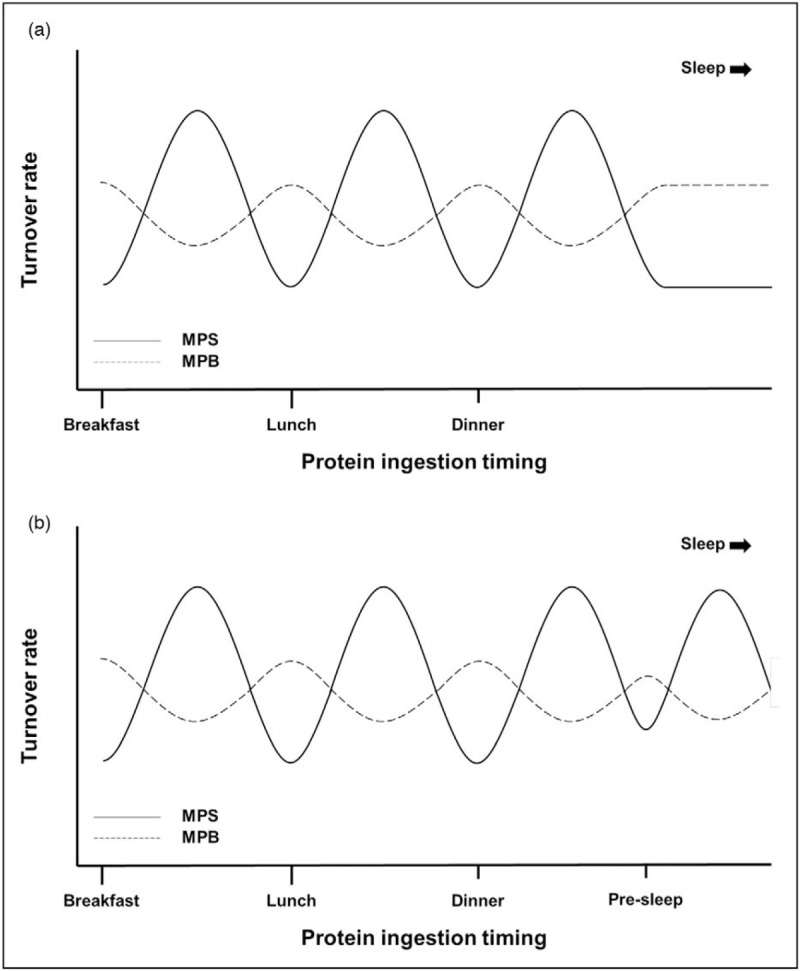
Effect of ingesting protein at (a) three moments and (b) four moments including presleep on muscle protein synthesis and breakdown rates throughout the day. In the postabsorptive state, muscle protein breakdown rates exceed synthesis rates. Following protein ingestion, muscle protein synthesis rates increase while breakdown rates decrease, allowing net muscle accretion during the postprandial period. Ingestion of ample protein prior to sleep can increase overnight muscle protein synthesis rates and lower proteolysis. MPB, muscle protein breakdown; MPS, muscle protein synthesis.
Habitual dietary protein intake
In healthy young adults, ingestion of 20 g high-quality protein has been shown to maximize postprandial muscle protein synthesis rates [10]. In older adults, the muscle protein synthetic response following such an amount of protein ingestion is attenuated when compared with younger adults, a phenomenon that has been coined anabolic resistance [11]. Recently, van Vliet et al.[12▪▪] reported that in CHD patients, ingestion of a mixed-meal containing 20 g intrinsically labeled protein on a nondialysis day failed to increase skeletal muscle protein synthesis rates. In addition, less protein-derived amino acids were released into the circulation following meal ingestion (41 ± 5% in CHD patients vs. 61 ± 4% in control subjects). Together, this suggests that CHD patients show anabolic resistance to protein ingestion.
In healthy young adults, a daily protein intake of 0.8 g/kg body weight is generally considered sufficient to achieve a net balance between muscle protein synthesis and breakdown rates. Because of anabolic resistance of skeletal muscle tissue and the upregulation of catabolic pathways in CHD patients, a higher protein intake level may be required to maintain muscle mass. Current clinical guidelines recommend CHD patients to ingest 1.2 g protein/kg body weight/day [13]. CHD patients often struggle to follow such dietary recommendations because of time constraints on dialysis days, altered taste, and early satiety [14–16]. Consequently, many CHD patients ingest less protein than recommended, with recent studies reporting a protein intake below 1.0 g/kg body weight/day in ∼50% of ESRD patients undergoing hemodialysis [17–20]. As low protein intake is associated with loss of muscle mass in patients with advanced stage CKD [21,22], dietary (protein) intake of CHD patients, especially those at high risk of malnutrition, should be monitored closely.
Protein and/or amino acid supplementation
Nutritional interventions should be started when CHD patients have a habitual protein intake below 1.0 g/kg body weight/day or energy intake below 30 kcal/kg body weight/day [13]. Ideally, a renal dietician should provide nutritional counseling after initiation of CHD therapy [23]. According to current guidelines, nutritional counseling should aim to achieve a dietary intake of 1.2 g protein and 30–35 kcal/kg body weight/day in patients at risk of malnutrition [13]. This can be achieved through increased intake of normal foods, food fortification, and/or the provision of oral nutritional supplements. Furthermore, each meal should contain ample protein to induce a postprandial increase in skeletal muscle protein synthesis and inhibit muscle protein breakdown. Considering the anabolic resistance of skeletal muscle tissue in CHD patients [12▪▪], patients likely need to ingest well above 20 g high-quality protein to allow a proper muscle protein synthetic response.
In healthy older adults, it has been shown that due to anabolic resistance ingestion of ∼35 g protein is required to maximize postprandial muscle protein synthesis rates [11]. Yet, in CHD patients, the ingestion of such large amounts of protein would also represent a high phosphate intake. This is undesirable in patients with ESRD because their renal excretion of phosphate is compromised and hemodialysis usually is ineffective to normalize serum phosphate concentrations [18]. Dietary phosphate intake is, therefore, an important contributor to hyperphosphatemia in CHD patients, which is associated with a greater prevalence of cardiovascular disease [24]. Because phosphate absorption from plant-based proteins is lower than from animal-based proteins, it has been suggested that increasing the percentage of dietary protein derived from plant-based protein sources may reduce hyperphosphatemia in CHD patients [24,25,26▪]. However, plant-based protein sources generally have lower (essential) amino acid contents and a specific lack of lysine and/or methionine compared to animal-based (high-quality) protein sources [27,28▪]. The latter may be compensated for by ingesting a combination of several plant-based protein sources. Nonetheless, the lower essential amino acid contents, and the low leucine content in particular, of plant-based protein sources may further increase the amount of protein required to induce a proper muscle protein synthetic response in CHD patients [28▪].
The use of essential amino acid fortification may provide an option to induce a muscle protein synthetic response in CHD patients with less phosphate intake. Our laboratory has shown in healthy older adults that ingestion of 15 g milk protein together with 1.5 g free leucine induces a greater postprandial muscle protein synthetic response than the ingestion of 15 g milk protein only [8]. More recently, we showed that the ingestion of 6 g branched-chain ketoacids can induce a postprandial anabolic response in healthy older adults [29▪]. Ketoacids are precursors of corresponding amino acids and can be utilized for de-novo muscle protein synthesis without providing any nitrogen, and, as such, would not add to uremic toxin accumulation [30]. However, it should be noted that Li et al.[19] recently reported no benefits of 6 months ketoacid supplementation on the nutritional status of Chinese CHD patients. Future studies that assess the muscle anabolic response following ketoacid ingestion in CHD patients should be conducted to assess whether it could help to support muscle maintenance.
Oral nutritional supplements
Specific oral nutritional supplements have been developed for CHD patients. These products typically have a higher energy and protein density and lower phosphate, potassium, and sodium contents when compared to other nutritional supplements for clinically compromised patient groups [13]. CHD patients receiving such supplements twice daily for a period of 3 months showed improvements in postabsorptive plasma amino acid profiles and serum (pre)albumin concentrations, a surrogate measurement often used to estimate nutritional status of dialysis patients [31]. However, no changes in lean body mass were observed over the study period. This indicates that the provided supplements (containing 9.4 g protein) were not able to induce net muscle protein accretion, which is at least partly attributed because of the anabolic resistance in the CHD population. A recent meta-analysis showed that though short term (≤3 months) oral nutritional supplementation increases body mass index and serum albumin concentrations in CHD patients, prolonged (>3 months) supplementation does not necessarily show such benefits [32▪]. These contradictory results may be due the low compliance and adherence to oral nutritional supplementation often observed in CHD patients. To achieve long-term compliance in CHD patients, protein supplements should be easy to prepare, convenient to consume, and have an acceptable taste. Consequently, the use of more protein dense meals and healthy food products may represent a more practical means to increase long-term daily protein consumption. The use of nonprotein supplements, such as creatine and omega-3 fatty acids, to support muscle maintenance has recently been described in detail [33].
Timing of oral nutritional supplement ingestion may also influence its efficiency to stimulate muscle protein synthesis and, as such, to support muscle maintenance. Ingestion of high-protein liquids induce satiety and may reduce energy intake during a subsequent meal [34]. Recently, protein ingestion before sleep has been introduced as an additional (meal) moment to augment daily protein consumption in populations with low protein intake [35]. Presleep ingestion of 40 g casein has been shown to have no effect on appetite and energy intake the next morning in older adults [36]. Furthermore, protein ingested before sleep has been shown to be properly digested and absorbed, thereby increasing overnight muscle protein synthesis rates (Fig. 1B). However, the impact of ingesting a protein-rich supplement/snack prior to sleep on dietary protein intake and nutritional status in CHD patients remains to be determined.
PHYSICAL ACTIVITY TO AUGMENT POST-PRANDIAL MUSCLE PROTEIN SYNTHESIS
In addition to protein ingestion, physical activity is essential for muscle maintenance. Current guidelines recommend CHD patients without contraindications to perform at least 150 min of moderate-intensity physical activity per week [37]. Regrettably, CHD patients typically have a sedentary lifestyle and often fail to meet these guidelines [38]. This low level of habitual physical activity represents the key factor responsible for the development of anabolic resistance in CHD patients. Physical inactivity has been shown to induce anabolic resistance of skeletal muscle tissue [39▪] and is related to lower physical functioning in CHD patients [40]. Therefore, ample physical activity is likely required to achieve the full anabolic potential of dietary protein. A single bout of physical activity can increase the sensitivity of skeletal muscle tissue to the anabolic effects of protein ingestion for a period up to 24 h [41]. Although aerobic exercise has been shown to increase aerobic capacity in CHD patients [42], resistance-type exercise is considered to be the most potent form of physical activity to increase muscle mass and strength [43]. Nevertheless, a recent study by Sheshadri et al.[44▪] reported that low-intensity exercise (i.e. walking) already resulted in improved muscle mass maintenance in CHD patients. Structured and sustained physical activity interventions can be applied to alleviate anabolic resistance and, as such, will likely further complement and augment the effects of dietary protein intervention. Therefore, CHD patients should be counseled to incorporate more habitual physical activity and structured exercise training in their daily living routines. However, because of the high prevalence of exercise intolerance and low adherence and compliance to physical activity interventions in the CHD population [45], more effective exercise programs will need to be developed.
INTERVENTIONS DURING HEMODIALYSIS SESSIONS
ESRD patients typically undergo two to three hemodialysis sessions per week, each lasting ∼4 h. These hemodialysis sessions represent a unique situation of compromised food intake and physical activity. During hemodialysis, patients sit in a lounge chair or lie in bed and typically stay sedentary for the entire procedure. In dialysis departments not providing in-center meals, food intake during hemodialysis is generally low because of restrictive eating policies, disrupted meal schedules, and/or inconvenience of bringing foods from home [46]. Furthermore, hemodialysis activates an inflammatory cascade and removes small-sized nutrients, such as amino acids, along with metabolic waste products through the semipermeable dialysis membrane. Recently, we have shown that during a single hemodialysis session approximately 12 g amino acids are removed from the circulation, which would be equivalent to the amount of amino acids that is released into the circulation following the ingestion of a typical meal containing ∼25 g protein [47▪]. As a consequence, plasma amino acids decline by ∼20% despite the average consumption of ∼20 g protein during hemodialysis. Pupim et al.[48] previously showed that the decline in circulating amino acid concentrations during hemodialysis induces a negative net forearm amino acid balance, indicative of increased skeletal muscle proteolysis. As depicted in Fig. 2A, such a catabolic state may endure for at least 2 h after the end of a hemodialysis session in fasted patients [48]. To support muscle maintenance during hemodialysis, amino acid removal should be (at least) compensated for through protein administration.
FIGURE 2.
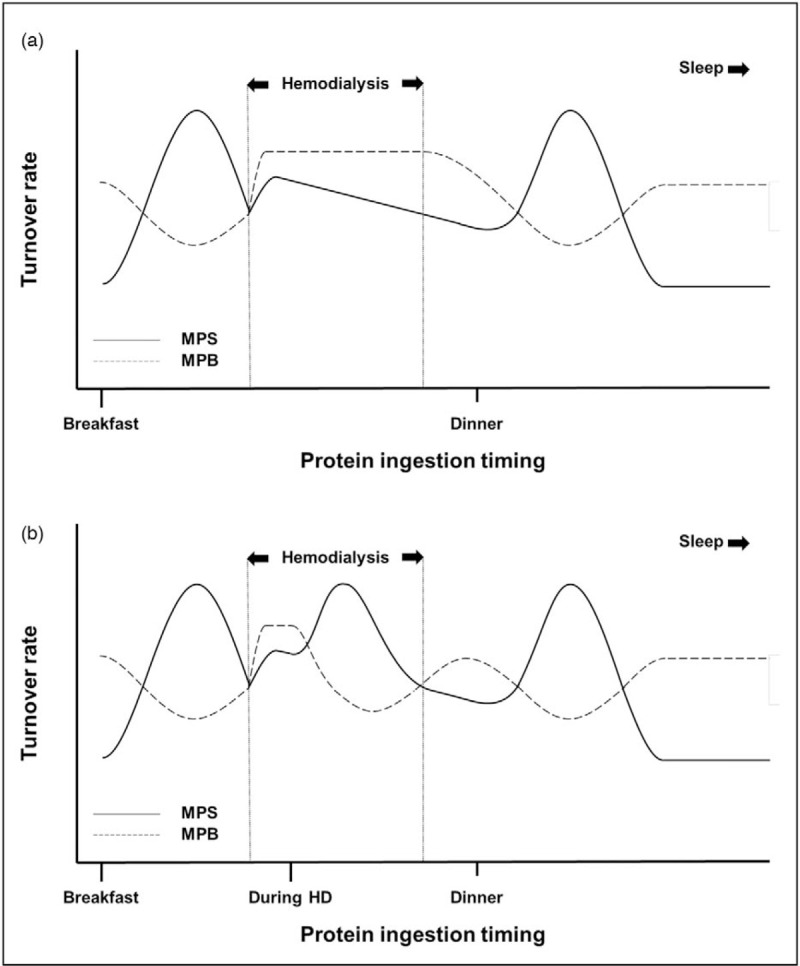
Effect of (a) no protein ingestion and (b) protein ingestion during hemodialysis on muscle protein synthesis and breakdown rates throughout the day. Because of amino acid removal during hemodialysis, muscle protein breakdown rates increase and exceed synthesis rates. In fasted patients, this catabolic period continues even after the end of the hemodialysis session. Ingestion of ample protein during hemodialysis stimulates muscle protein synthesis rates and inhibits breakdown rates, allowing net muscle accretion. MPB, muscle protein breakdown; MPS, muscle protein synthesis.
Protein intake during hemodialysis
Protein-rich meals, snacks, or oral nutritional supplements during hemodialysis are suggested to increase dietary protein intake on dialysis days, and can be used to improve nutritional status and survival rates of CHD patients [49▪]. Muscle catabolism during hemodialysis may be prevented, or even reverted into anabolism, through ingestion of ample high-quality protein (Fig. 2B). Ingestion of ∼60 g protein during hemodialysis has been shown to prevent the decline in circulating amino acid concentrations and induce a positive forearm amino acid balance throughout hemodialysis [48,50]. However, protein ingestion during hemodialysis will also be accompanied by greater amino acid removal [47▪]. Also considering the presence of anabolic resistance, CHD patients likely need to ingest well above 30 g protein during hemodialysis to allow increased muscle protein synthesis rates. Ingestion of a lower amount of protein may not increase muscle protein synthesis rates but could attenuate muscle proteolysis and, as such, improve net protein balance during hemodialysis [50]. Yet, the optimal amount of protein that should be ingested during hemodialysis to support muscle maintenance remains to be determined.
Many studies have reported benefits of providing various amounts of protein-rich nutrition during hemodialysis. Benner et al.[51] performed a retrospective evaluation of a pilot program and found that oral nutritional supplementation during hemodialysis, providing 16 or 22 g protein, reduced mortality rates in hypoalbuminemic CHD patients. More recently, it has been reported that hypoalbuminemic CHD patients who ingested a small meal (200 mL milk with 2 egg whites; providing 14 g protein) every hemodialysis session showed a greater increase in serum albumin levels after 3 months than control subjects receiving nutritional counseling [52]. In support of these positive findings, it has been reported that providing oral nutritional supplements, containing 14–20 g protein, during hemodialysis after hospitalization of hypoalbuminemic CHD patients reduced readmission rates [53]. Thus, it seems likely that malnourished/hypoalbuminemic CHD patients benefit from protein ingestion during hemodialysis. However, the impact of protein ingestion during hemodialysis in better nourished patients is still a matter of debate, probably because the benefits are less distinct. Jeong et al.[54▪▪] recently performed a large randomized controlled trial to assess the effect of protein supplementation during hemodialysis on physical functioning in CHD patients with normal (∼4 g/dl) serum albumin levels. Though gait speed was increased after 6 and 12 months of supplementation, no effects on body composition were shown. This suggests that simply providing a protein supplement during hemodialysis is not sufficient to improve muscle mass in well nourished CHD patients. Thus, more research to identify effective nutritional strategies during hemodialysis to prevent muscle loss in CHD patients is still required.
Dietary protein intake during hemodialysis is presently not implemented in clinical guidelines, as some nephrologists are concerned about patient safety and increased staff burden [55]. Dialysis departments in Europe and Asia are more likely to allow food ingestion during hemodialysis compared to dialysis departments in North America [56]. One of the most frequently cited concerns regarding food intake during hemodialysis is the postprandial decrease in systemic blood pressure, which occurs because of increased perfusion of the splanchnic region following food ingestion [55,57▪]. Though the postprandial drop in blood pressure is amplified during hemodialysis [57▪], Choi et al.[58] reported no effect of high-protein meals on the frequency of symptomatic hypotensive events. Patients’ hemodynamic stability, meal composition, and meal timing may influence the postprandial drop in blood pressure. In patients who do not tolerate food intake during hemodialysis , intradialytic parenteral nutrition may be used to prevent hemodialysis-related catabolism [59]. In addition, Deleaval et al.[60] recently showed that branched-chain amino acids can be provided during hemodialysis by adding them to the dialysate. We would advocate dialysis departments not to restrict eating per se, but to apply patient-tailored nutritional care during hemodialysis combined with nutritional counseling to improve habitual dietary intake between hemodialysis sessions.
Exercise during hemodialysis
In addition to nutritional interventions, physical activity interventions can also be implemented during hemodialysis sessions to prevent the loss of muscle mass and strength (Fig. 3). Exercise during hemodialysis can induce (moderate) improvements in physical functioning, muscle strength, and quality of life [43,54▪▪,61]. It also represents an opportunity to counsel patients on physical activity in their daily living. In patients who are not able to exercise during hemodialysis, neuromuscular electrical stimulation could be employed to activate skeletal muscles, thereby increasing muscle strength and physical functioning [62]. However, studies that apply (resistance-type) exercise training prior to or during hemodialysis generally fail to show increases in muscle mass [43]. Exercise during hemodialysis is performed in a period of reduced circulating amino acid availability. This may prevent an anabolic response following exercise and, as such, prevent net muscle accretion over time. In addition, a greater blood flow to exercising muscles during this period of reduced amino acid availability may actually lead to increased muscle proteolysis. Therefore, protein ingestion, and the subsequent postprandial hyperaminoacidemia, may be required throughout and after exercise during hemodialysis to achieve a positive net muscle protein balance. We advocate that exercise during hemodialysis should be combined with sufficient protein ingestion to allow a postexercise increase in muscle protein synthesis rates and to inhibit proteolysis. This strategy may also lower the amount of protein required to induce a muscle protein synthetic response, as the performance of exercise prior to hemodialysis has been shown to make the muscle more sensitive to the anabolic properties of subsequent protein ingestion [63]. Though the combination of exercise and nutrition during hemodialysis is likely preferred, future research is needed to assess their synergy and establish nutritional and exercise guidelines for effective implementation during hemodialysis.
FIGURE 3.
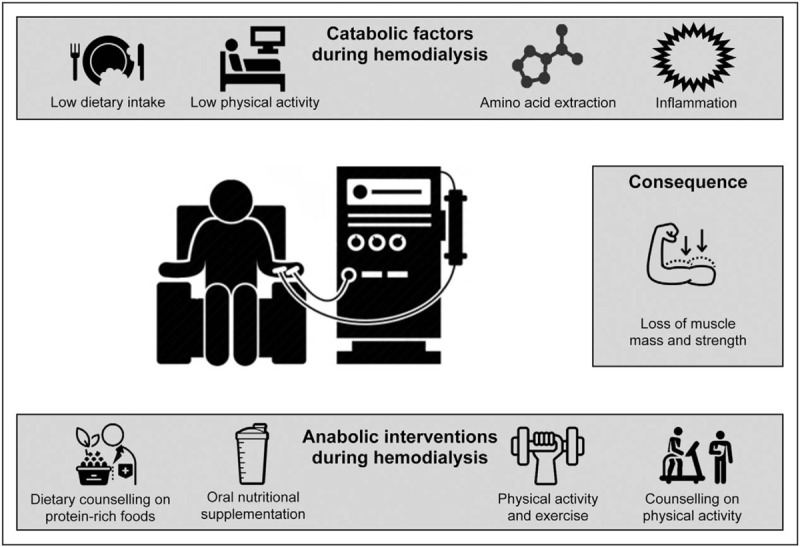
Overview of catabolic factors during hemodialysis and anabolic interventions that may be applied during this period. During a hemodialysis session, patients typically have low levels of dietary intake and physical activity, while the procedure removes amino acids from their circulation and activates an inflammatory cascade. This results in a catabolic period during hemodialysis, which promotes the loss of muscle mass and strength. Interventions to support muscle maintenance that can be applied during hemodialysis include dietary counseling to improve habitual dietary intake, providing protein-rich foods, prescribing physical activity, and counseling to increase habitual physical activity levels.
CONCLUSION
Poor nutritional status is common among CHD patients because of low protein intake and physical inactivity, anabolic resistance of skeletal muscle tissue, and amino acid removal during hemodialysis. Patient-tailored dietary interventions should aim to increase habitual protein intake levels and induce a muscle protein synthetic response with every meal through ingestion of ample high-quality protein with or without essential amino acid/ketoacid fortification. Implementing structured supervised exercise in the daily routine of CHD patients will enhance the muscle protein synthetic response to protein ingestion. A combination of more physical activity or exercise training with nutritional interventions during hemodialysis will further augment the capacity to maintain or even increase muscle mass. Nephrologists, dieticians, and exercise specialists should collaborate to develop patient-specific lifestyle programs to prevent the loss of skeletal muscle mass in CHD patients.
Acknowledgements
We would like to thank Drs. Joey S.J. Smeets and Dr. Frank M. van der Sande for their contribution to our project.
Financial support and sponsorship
This work was supported by a grant from the NUTRIM NWO Graduate Programme.
Conflicts of interest
F.K. Hendriks, J.P. Kooman, and L.J.C. van Loon have no conflicts of interest.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
References
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Hyun YY, Lee KB, Han SH, et al. Nutritional status in adults with predialysis chronic kidney disease: KNOW-CKD Study. J Korean Med Sci 2017; 32:257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsuzawa R, Kamitani T, Roshanravan B, et al. Decline in the functional status and mortality in patients on hemodialysis: results from the Japan Dialysis Outcome and Practice Patterns Study. J Ren Nutr 2019; 29:504–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrero JJ, Stenvinkel P, Cuppari L, et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J Ren Nutr 2013; 23:77–90. [DOI] [PubMed] [Google Scholar]
- 4▪.Carrero JJ, Thomas F, Nagy K, et al. Global prevalence of protein-energy wasting in kidney disease: a meta-analysis of contemporary observational studies from the international society of renal nutrition and metabolism. J Ren Nutr 2018; 28:380–392. [DOI] [PubMed] [Google Scholar]; This meta-analysis shows that protein-energy wasting syndrome in end-stage renal disease patients is prevalent worldwide.
- 5.Kilroe SP, Fulford J, Holwerda AM, et al. Short-term muscle disuse induces a rapid and sustained decline in daily myofibrillar protein synthesis rates. Am J Physiol Endocrinol Metab 2020; 318:E117–E130. [DOI] [PubMed] [Google Scholar]
- 6.Gorissen SHM, Trommelen J, Kouw IWK, et al. Protein type, protein dose, and age modulate dietary protein digestion and phenylalanine absorption kinetics and plasma phenylalanine availability in humans. J Nutr 2020; 150:2041–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Vliet S, Beals JW, Holwerda AM, et al. Time-dependent regulation of postprandial muscle protein synthesis rates after milk protein ingestion in young men. J Appl Physiol 2019; 127:1792–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holwerda AM, Paulussen KJM, Overkamp M, et al. Leucine coingestion augments the muscle protein synthetic response to the ingestion of 15 g of protein following resistance exercise in older men. Am J Physiol Endocrinol Metab 2019; 317:E473–E482. [DOI] [PubMed] [Google Scholar]
- 9.Borack MS, Reidy PT, Husaini SH, et al. Soy-dairy protein blend or whey protein isolate ingestion induces similar postexercise muscle mechanistic target of rapamycin complex 1 signaling and protein synthesis responses in older men. J Nutr 2016; 146:2468–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witard OC, Jackman SR, Breen L, et al. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am J Clin Nutr 2014; 99:86–95. [DOI] [PubMed] [Google Scholar]
- 11.Olaniyan ET, O’Halloran F, McCarthy AL. Dietary protein considerations for muscle protein synthesis and muscle mass preservation in older adults. Nutr Res Rev 2020; 1–26. [DOI] [PubMed] [Google Scholar]
- 12▪▪.van Vliet S, Skinner SK, Beals JW, et al. Dysregulated handling of dietary protein and muscle protein synthesis after mixed-meal ingestion in maintenance hemodialysis patients. Kidney Int Rep 2018; 3:1403–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first clinical trial to report a reduced muscle protein synthetic response following protein ingestion in CHD patients. Furthermore, the dietary protein digestion and absorption kinetics in this population are measured for the first time using intrinsically labeled egg protein. This study suggests that skeletal muscle tissue of CHD patients demonstrates anabolic resistance to protein ingestion.
- 13.Sabatino A, Piotti G, Cosola C, et al. Dietary protein and nutritional supplements in conventional hemodialysis. Semin Dial 2018; 31:583–591. [DOI] [PubMed] [Google Scholar]
- 14.Clark-Cutaia MN, Sevick MA, Thurheimer-Cacciotti J, et al. Perceived barriers to adherence to hemodialysis dietary recommendations. Clin Nurs Res 2019; 28:1009–1029. [DOI] [PubMed] [Google Scholar]
- 15.Costa-Moreira P, Vilas-Boas F, Teixeira Fraga A, Macedo G. Particular aspects of gastroenterological disorders in chronic kidney disease and end-stage renal disease patients: a clinically focused review. Scand J Gastroenterol 2020; 55:129–138. [DOI] [PubMed] [Google Scholar]
- 16.Marquez-Herrera RM, Nunez-Murillo GK, Ruiz-Gurrola CG, et al. Clinical taste perception test for patients with end-stage kidney disease on dialysis. J Ren Nutr 2020; 30:79–84. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Qin X, Li Y, et al. The association between dietary energy intake and the risk of mortality in maintenance haemodialysis patients: a multicentre prospective cohort study. Br J Nutr 2020; 123:437–445. [DOI] [PubMed] [Google Scholar]
- 18.Tao X, Zhang H, Yang Y, et al. Daily dietary phosphorus intake variability and hemodialysis patient adherence to phosphate binder therapy. Hemodial Int 2019; 23:458–465. [DOI] [PubMed] [Google Scholar]
- 19.Li H-L, Li H, Cao Y-F, et al. Effects of keto acid supplements on Chinese patients receiving maintenance hemodialysis. Chin Med J (Engl) 2020; 133:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasegawa J, Kimachi M, Kurita N, et al. The normalized protein catabolic rate and mortality risk of patients on hemodialysis by frailty status: the Japanese Dialysis Outcomes and Practice Pattern Study. J Ren Nutr 2020; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 21.Barril G, Nogueira A, Ruperto López M, et al. Influence of dietary protein intake on body composition in chronic kidney disease patients in stages 3–5: a cross-sectional study. Nefrología (English Edition) 2018; 38:647–654. [DOI] [PubMed] [Google Scholar]
- 22.Hanafusa N, Kamei D, Tsukada M, et al. Association between increases in normalized protein catabolic rate and increases in creatinine generation rate in dialysis patients. Contrib Nephrol 2018; 195:51–61. [DOI] [PubMed] [Google Scholar]
- 23.Vijaya KL, Aruna M, Narayana Rao SVL, Mohan PR. Dietary counseling by renal dietician improves the nutritional status of hemodialysis patients. Indian J Nephrol 2019; 29:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cases A, Cigarran-Guldris S, Mas S, Gonzalez-Parra E. Vegetable-based diets for chronic kidney disease? It is time to reconsider. Nutrients 2019; 11:1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joshi S, Hashmi S, Shah S, Kalantar-Zadeh K. Plant-based diets for prevention and management of chronic kidney disease. Curr Opin Nephrol Hypertens 2020; 29:16–21. [DOI] [PubMed] [Google Scholar]
- 26▪.Carrero JJ, Gonzalez-Ortiz A, Avesani CM, et al. Plant-based diets to manage the risks and complications of chronic kidney disease. Nat Rev Nephrol 2020; 16:525–542. [DOI] [PubMed] [Google Scholar]; An extensive review on potential benefits of plant-based diets for patients with CKD.
- 27.Gorissen SHM, Crombag JJR, Senden JMG, et al. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018; 50:1685–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28▪.St-Jules DE, Goldfarb DS, Popp CJ, et al. Managing protein-energy wasting in hemodialysis patients: a comparison of animal- and plant-based protein foods. Semin Dial 2019; 32:41–46. [DOI] [PubMed] [Google Scholar]; An excellent review comparing the use of animal- and plant-based protein sources to support muscle maintenance in patients with hemodialysis.
- 29▪.Fuchs CJ, Hermans WJH, Holwerda AM, et al. Branched-chain amino acid and branched-chain ketoacid ingestion increases muscle protein synthesis rates in vivo in older adults: a double-blind, randomized trial. Am J Clin Nutr 2019; 110:862–872. [DOI] [PMC free article] [PubMed] [Google Scholar]; A state-of-the art study which compares the muscle protein synthetic response following ingestion of milk protein and ketoacids in elderly individuals.
- 30.Koppe L, Cassani de Oliveira M, Fouque D. Ketoacid analogues supplementation in chronic kidney disease and future perspectives. Nutrients 2019; 11:2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malgorzewicz S, Galezowska G, Cieszynska-Semenowicz M, et al. Amino acid profile after oral nutritional supplementation in hemodialysis patients with protein-energy wasting. Nutrition 2019; 57:231–236. [DOI] [PubMed] [Google Scholar]
- 32▪.Liu PJ, Ma F, Wang QY, He SL. The effects of oral nutritional supplements in patients with maintenance dialysis therapy: A systematic review and meta-analysis of randomized clinical trials. PLoS One 2018; 13:e0203706. [DOI] [PMC free article] [PubMed] [Google Scholar]; This meta-analysis reports on the prescription of oral nutritional supplements for CHD patients to improve nutritional status.
- 33.Marshall RN, Smeuninx B, Morgan PT, Breen L. Nutritional strategies to offset disuse-induced skeletal muscle atrophy and anabolic resistance in older adults: from whole-foods to isolated ingredients. Nutrients 2020; 12:1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melson CE, Nepocatych S, Madzima TA. The effects of whey and soy liquid breakfast on appetite response, energy metabolism, and subsequent energy intake. Nutrition 2019; 61:179–186. [DOI] [PubMed] [Google Scholar]
- 35.Snijders T, Trommelen J, Kouw IWK, et al. The impact of presleep protein ingestion on the skeletal muscle adaptive response to exercise in humans: an update. Front Nutr 2019; 6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morehen S, Smeuninx B, Perkins M, et al. Pre-sleep casein protein ingestion does not impact next-day appetite, energy intake and metabolism in older individuals. Nutrients 2019; 12:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA 2018; 320:2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hornik B, Dulawa J. Frailty, quality of life, anxiety, and other factors affecting adherence to physical activity recommendations by hemodialysis patients. Int J Environ Res Public Health 2019; 16:1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39▪.Shad BJ, Thompson JL, Holwerda AM, et al. One week of step reduction lowers myofibrillar protein synthesis rates in young men. Med Sci Sports Exerc 2019; 51:2125–2134. [DOI] [PubMed] [Google Scholar]; This paper highlights the importance of habitual physical activity for the maintenance of skeletal muscle mass.
- 40.Tarca BD, Wycherley TP, Bennett P, et al. Modifiable physical factors associated with physical functioning for patients receiving dialysis: a systematic review. J Phys Act Health 2020; 17:475–489. [DOI] [PubMed] [Google Scholar]
- 41.Hendriks FK, Smeets JSJ, van der Sande FM, et al. Dietary protein and physical activity interventions to support muscle maintenance in end-stage renal disease patients on hemodialysis. Nutrients 2019; 11:2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Regolisti G, Sabatino A, Fiaccadori E. Exercise in patients on chronic hemodialysis: current evidence, knowledge gaps and future perspectives. Curr Opin Clin Nutr Metab Care 2020; 23:181–189. [DOI] [PubMed] [Google Scholar]
- 43.Molsted S, Bjorkman ASD, Lundstrom LH. Effects of strength training to patients undergoing dialysis: a systematic review. Dan Med J 2019; 66:A5526. [PubMed] [Google Scholar]
- 44▪.Sheshadri A, Kittiskulnam P, Lai JC, Johansen KL. Effect of a pedometer-based walking intervention on body composition in patients with ESRD: a randomized controlled trial. BMC Nephrol 2020; 21:100. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study assessed the effect of a higher physical activity level (i.e. more steps per day) on the maintenance of muscle mass in CHD patients.
- 45.McKenna CF, Salvador AF, Hendriks FK, et al. Exercising to offset muscle mass loss in hemodialysis patients: the disconnect between intention and intervention. Semin Dial 2019; 32:379–385. [DOI] [PubMed] [Google Scholar]
- 46.Martins AM, Dias Rodrigues JC, de Oliveira Santin FG, et al. Food intake assessment of elderly patients on hemodialysis. J Ren Nutr 2015; 25:321–326. [DOI] [PubMed] [Google Scholar]
- 47▪.Hendriks FK, Smeets JSJ, Broers NJH, et al. End-stage renal disease patients lose a substantial amount of amino acids during hemodialysis. J Nutr 2020; 150:1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study assessed the amount of amino acids that are removed from the circulation during a hemodialysis session. It emphasizes that tailored nutritional care should be provided throughout dialysis days.
- 48.Pupim LB, Majchrzak KM, Flakoll PJ, Ikizler TA. Intradialytic oral nutrition improves protein homeostasis in chronic hemodialysis patients with deranged nutritional status. J Am Soc Nephrol 2006; 17:3149–3157. [DOI] [PubMed] [Google Scholar]
- 49▪.Kistler BM, Benner D, Burrowes JD, et al. Eating during hemodialysis treatment: a consensus statement from the international society of renal nutrition and metabolism. J Ren Nutr 2018; 28:4–12. [DOI] [PubMed] [Google Scholar]; Clear overview of the benefits and challenges of providing foods/nutritional supplements during hemodialysis.
- 50.Sundell MB, Cavanaugh KL, Wu P, et al. Oral protein supplementation alone improves anabolism in a dose-dependent manner in chronic hemodialysis patients. J Ren Nutr 2009; 19:412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benner D, Brunelli SM, Brosch B, et al. Effects of oral nutritional supplements on mortality, missed dialysis treatments, and nutritional markers in hemodialysis patients. J Ren Nutr 2018; 28:191–196. [DOI] [PubMed] [Google Scholar]
- 52.Li J, Hou G, Sun X, et al. A low-cost, intradialytic, protein-rich meal improves the nutritional status in chinese hemodialysis patients. J Ren Nutr 2020; 30:e27–e34. [DOI] [PubMed] [Google Scholar]
- 53.Leonberg-Yoo AK, Wang W, Weiner DE, Lacson E., Jr Oral nutritional supplements and 30-day readmission rate in hypoalbuminemic maintenance hemodialysis patients. Hemodial Int 2019; 23:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54▪▪.Jeong JH, Biruete A, Tomayko EJ, et al. Results from the randomized controlled IHOPE trial suggest no effects of oral protein supplementation and exercise training on physical function in hemodialysis patients. Kidney Int 2019; 96:777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]; This randomized controlled trial assessed the effects of aerobic exercise and/or whey protein supplementation during hemodialysis for 12 months on physical function, nutritional status, and muscle strength. Despite the relative large sample size (138 patients), no significant differences in physical function or nutritional status were observed between groups.
- 55.Agarwal R, Georgianos P. Feeding during dialysis-risks and uncertainties. Nephrol Dial Transplant 2018; 33:917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kistler B, Benner D, Burgess M, et al. To eat or not to eat—international experiences with eating during hemodialysis treatment. J Ren Nutri 2014; 24:349–352. [DOI] [PubMed] [Google Scholar]
- 57▪.Svinth-Johansen C, Reinhard M, Ivarsen P. Hemodynamic response to glucose-insulin infusion and meals during hemodialysis. Kidney Blood Press Res 2020; 45:249–262. [DOI] [PubMed] [Google Scholar]; Excellent study that assessed changes in blood pressure following meals and during hemodialysis separately and combined.
- 58.Choi MS, Kistler B, Wiese GN, et al. Pilot study of the effects of high-protein meals during hemodialysis on intradialytic hypotension in patients undergoing maintenance hemodialysis. J Ren Nutr 2019; 29:102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anderson J, Peterson K, Bourne D, Boundy E. Effectiveness of intradialytic parenteral nutrition in treating protein-energy wasting in hemodialysis: a rapid systematic review. J Ren Nutr 2019; 29:361–369. [DOI] [PubMed] [Google Scholar]
- 60.Deleaval P, Luaire B, Laffay P, et al. Short-term effects of branched-chain amino acids-enriched dialysis fluid on branched-chain amino acids plasma level and mass balance: a randomized cross-over study. J Ren Nutr 2020; 30:61–68. [DOI] [PubMed] [Google Scholar]
- 61.Salhab N, Karavetian M, Kooman J, et al. Effects of intradialytic aerobic exercise on hemodialysis patients: a systematic review and meta-analysis. J Nephrol 2019; 32:549–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suzuki T, Ikeda M, Minami M, et al. Beneficial effect of intradialytic electrical muscle stimulation in hemodialysis patients: a randomized controlled trial. Artif Organs 2018; 42:899–910. [DOI] [PubMed] [Google Scholar]
- 63.Majchrzak KM, Pupim LB, Flakoll PJ, Ikizler TA. Resistance exercise augments the acute anabolic effects of intradialytic oral nutritional supplementation. Nephrol Dial Transplant 2008; 23:1362–1369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


