Supplemental Digital Content is Available in the Text.
Abstract
BACKGROUND
SAKURA 3 was a Phase 3, open-label, repeat-dose safety study of DaxibotulinumtoxinA for Injection (DAXI); a component of the largest Phase 3 clinical development program of an aesthetic neuromodulator in glabellar lines.
OBJECTIVE
To evaluate the use of DAXI (40U) up to 3 treatments for moderate or severe glabellar lines.
METHODS
Eligible subjects rolled over from the placebo-controlled trials (n = 477) or were de novo (n = 2,214) and received 1 to 3 treatments over a maximum of 84 weeks. Safety and efficacy were evaluated at least every 4 weeks up to Week 36 (Treatments 1 and 2) and Week 12 (Treatment 3). Select subjects could be retreated after Week 12 if glabellar lines returned to baseline.
RESULTS
Safety results are reported for 2,691 subjects, of which 882 received a second treatment and 568 a third. Treatment-related adverse events (AEs) occurred in 17.8% of subjects, which were generally mild and resolved. No serious AEs were treatment-related. Eyelid ptosis occurred in 0.9% of treatments. Adverse events were consistent across treatments and no new safety signals were observed.
CONCLUSION
The safety of DAXI in this large open-label safety study confirms the findings from the pivotal Phase 3 trials, providing reassurance in its overall safety profile.
The commercially available Type A botulinum toxins (BoNTA), produced by the bacterium Clostridium botulinum, are highly purified and used to inhibit neurotransmitter release at the neuromuscular junction, which leads to muscle weakening. This provides an effective method to treat glabellar lines, a common aesthetic use of BoNTAs. Because the effect on muscle weakening is not permanent, currently available agents require repeat treatment, generally every 3 to 4 months to maintain glabellar smoothness. Glabellar frown lines can deepen with age, causing an appearance that is often interpreted as angry or unhappy1; therefore, many patients desire multiple treatments per year.
Although the cosmetic use of BoNTA has an overall favorable safety profile, given the mechanism of action, adverse events (AEs) relating to over-weakening of the injected muscles and spread of the toxin to neighboring muscles may occur.2 In addition, local injection-related AEs, such as bruising and erythema, are possible. As botulinum toxins are proteins and repeat treatments are required over time, there is a theoretical risk of antibody formation that may block their function and lead to treatment failure.3 Given the potential for AEs, multiple treatments per year, and several years of use for cosmetic purposes, it is important that the safety and tolerability profile of new BoNTAs are thoroughly evaluated in clinical trials that reflect actual use in clinical practice.
DaxibotulinumtoxinA for Injection (DAXI), a 150-kDa BoNTA formulated with a novel proprietary stabilizing peptide excipient (RTP004), has demonstrated a median of 27.1 weeks' duration of clinical response in the treatment of glabellar lines in 2 pivotal Phase 3, randomized, placebo-controlled, single-dose trials, SAKURA 1 and SAKURA 2.4 Because BoNTA is used as a repeat treatment over time, the final component of the SAKURA clinical development program was a large, open-label safety (OLS) study, which enrolled de novo patients and also allowed patients from the 2 Phase 3 trials to receive additional treatments. This enabled the assessment of the safety and efficacy of single and repeat administration of DAXI in a manner that mirrors clinical practice. Safety results from this trial are reported here, with efficacy results in an accompanying publication.5
Methods
Study Design
This Phase 3, open-label, multicenter study evaluated single and repeat treatment of DAXI 40U, with post-treatment evaluation of up to 84 weeks (ClinicalTrials.gov identifier NCT03004248). Specific targets for the number of subjects exposed to single versus repeat treatments were outlined in the protocol; therefore, a subset of enrolled subjects received more than one treatment. The study was conducted in 65 centers in the United States and Canada from December 2016 to October 2018. The protocol was approved by the institutional review boards and/or independent ethics committees. All subjects provided written informed consent, and the study was conducted in compliance with Good Clinical Practice and the Declaration of Helsinki.
Subjects
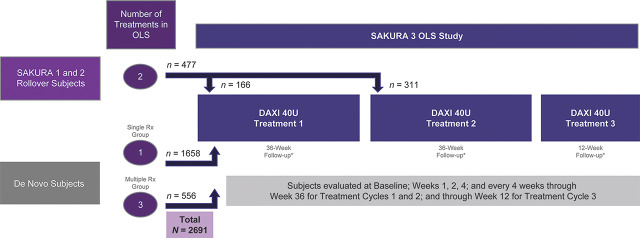
Study participants were either rollover subjects who had completed the SAKURA 1 or SAKURA 2 Phase 3 trials,6 or newly enrolled (Figure 1). Eligible subjects included those aged 18 years or older, in good health, with moderate or severe glabellar lines based on both the Investigator Global Assessment-Frown Wrinkle Severity (IGA-FWS) and Patient Frown Wrinkle Severity scales.6 Pregnant or nursing women were excluded from the study. During the study and evaluation period, all subjects were required to abstain from facial fillers, laser treatments, products that affect skin remodeling, or products that may cause an active dermal response in the treatment area. Key exclusion criteria included any neurological condition that may place the subject at increased risk with exposure to BoNTA; muscle weakness or paralysis in the area receiving treatment or a history of facial nerve palsy; facial treatment with BoNTA within 24 weeks before screening, plans to receive BoNTA anywhere in the face through the duration of the study, or treatment with ≥200 U BoNTA anywhere else in the body within the past 3 months before screening or during the study.
Figure 1:
Study design.
Treatments
The study was designed to enroll at least 2,100 subjects, of whom 400 subjects were eligible to receive up to 3 treatments, with the remaining subjects eligible to receive only a single treatment. For rollover subjects, all study personnel remained blinded to treatment assignment; therefore, they could receive only 2 additional treatments in SAKURA 3. Those who were treated with DAXI in the placebo-controlled trials received their second and third treatments in SAKURA 3, whereas the placebo rollover subjects received their first and second DAXI treatments.
Each DAXI vial was reconstituted with unpreserved saline, and all subjects received a total dose of 40U of DAXI administered in five 0.1-mL injections: 2 injections into each corrugator muscle and 1 injection into the procerus muscle. Subjects who received repeat treatments could be retreated between Week 12 and Week 36 after their first and second treatments if their IGA-FWS and Patient Frown Wrinkle Severity scores had returned to baseline and other eligibility criteria were met. Subjects were followed up to 12 weeks after their final treatment.
Outcome Measures
The primary outcome measure was safety, specifically the incidence, severity, and relationship to DAXI of treatment-emergent AEs and treatment-emergent serious AEs during each treatment and in the overall study. Investigators evaluated all AEs for their relationship to DAXI (definite, probable, possible, or unrelated) and their severity (mild, moderate, or severe). Injection sites were evaluated at the screening visit, immediately pretreatment and 30 minutes post-treatment, and at all follow-up visits. The injection site was evaluated for erythema and edema while the patients were queried for injection site burning, stinging, or itching. The assessment was a global evaluation of the 5 injection sites for the presence of erythema, edema, burning or stinging, itching, and bruising. Any reactions considered clinically significant were reported as an AE.
Nonfasting samples for assessment of hematology, serum chemistry, and urinalysis were collected at screening, Week 4, before retreatment (as applicable), and at the final evaluation visit. Vital signs were monitored and physical examinations were conducted. Blood samples for antibodies were collected at screening and at Weeks 2, 4, and 12. The presence of binding antibodies was assessed using validated enzyme-linked immunosorbent assays, and samples confirmed positive for binding antibodies were further tested for the presence of neutralizing antibodies via the mouse protection assay.
Statistical Methods
The safety evaluable population included all subjects who received at least one dose of DAXI and had post-treatment safety data. Adverse events were recorded and classified using Medical Dictionary for Regulatory Activities version 20.1, and were summarized by system organ class, preferred term, severity, relationship to treatment, and seriousness. Serious AEs were summarized by severity and relationship to DAXI. Data are reported using descriptive statistics.
A sample size of approximately 2,100 was considered adequate in assessing the safety based on several approaches. With approximately 2,100 treated subjects, it can be concluded with 95% confidence that the incidence of an AE would be no more than 0.15% if no such event occurred during the study. With N = 2,100, the precision (based on an exact 95% confidence interval) of estimating an event incidence would be at most ±2.22% when the true incidence was around 50%, or approximately ±1.0% when the true incidence was in the range of 5%. This size would also have an adequate power to evaluate whether the incidence of a certain event was equivalent to a fixed incidence; for example, there was a power of >95% to demonstrate that the incidence was equivalent to 3% under an equivalent margin of 1.5% and a significant level of 0.05 (based on two 1-sided tests).
With at least 400 of the 2,100 enrolled subjects receiving up to 3 treatments, there would be an 86% power to detect an increase in incidence from Treatment 1 to Treatment 3 at a significant level of 0.05 (1-sided test) if the actual event incidence increased from 3% at Treatment 1% to 6% at Treatment 3. Also, with n = 400, the 95% exact confidence interval would be 1.6% to 5.2% for an observed incidence of 3% and 3.1% to 7.6% for an observed incidence of 5%.
Because the observation period after Treatment 3 was 12 weeks, to allow for a cross-treatment cycle comparison, a posthoc analysis was performed to evaluate the incidence of treatment-emergent and treatment-related AEs that occurred within the first 12 weeks of each treatment cycle.
Results
Subjects
Of 3,052 subjects screened, 2,691 enrolled and received at least one DAXI treatment in this study. Of these, 477 subjects rolled over from SAKURA 1 or SAKURA 2 and 2,214 de novo subjects were enrolled. Of the 477 rollover subjects, 311 had received active treatment in the previous trials and received their second DAXI dose in SAKURA 3, whereas 166 received placebo in the prior trials. A total of 2,380 subjects thus received their first dose of DAXI in the SAKURA 3 study (see Supplemental Digital Content 1, Figure S1, http://links.lww.com/DSS/A371). In SAKURA 3, there were 1892 subjects who received only a single DAXI treatment, 459 who received only 2 treatments, and 340 who received 3 treatments, for a total of 3,830 treatments.
Of the 2,691 subjects enrolled, 2,314 completed the study (86.0%) and 377 (14.0%) withdrew from the study. Reasons for discontinuation were withdrawal by subject (n = 196; 7.3%), lost to follow-up (n = 99; 3.7%), protocol deviation (n = 53; 2.0%), investigator discretion (n = 8; 0.3%), AE (n = 5; 0.2%), death (n = 1; <0.1%), and other (n = 15; 0.6%). The AEs leading to study discontinuation were basal cell carcinoma, bile duct cancer, “brow spocking,” fractured humerus, and optic neuritis; only the single case of unnatural lateral brow elevation was considered related to DAXI treatment.
Subject demographics and baseline characteristics are shown in Supplemental Digital Content 2, Table S1, http://links.lww.com/DSS/A372. Most of the subjects were white women, and nearly 40% had previously received BoNTA treatment, with a median washout period of 18.0 months.
Adverse Events
Of the 2,691 DAXI-treated subjects, 1,043 (38.8%) reported experiencing at least one AE (see Supplemental Digital Content 3, Table S2, http://links.lww.com/DSS/A373). Most AEs were mild in severity and unrelated to DAXI administration. A total of 480 (17.8%) subjects experienced AEs related to DAXI administration. There were 31 serious AEs, none of which were deemed treatment-related. The only serious AE appearing more than once was breast cancer, in 2 subjects. One death occurred during the study, which was secondary to homicide and not related to treatment. There was no evidence that any AEs were related to distant spread of DAXI.
The most common AEs (reported in ≥1% of subjects) regardless of causality were headache (5.9%), nasopharyngitis (4.4%), injection site pain (3.9%), and injection site erythema (3.3%) (Table 1). With the exception of nasopharyngitis, these were also the most common treatment-related AEs. The median duration of treatment-related AEs in ≥1% of subjects was: headache, 2 days; injection site pain, 1 day; and injection site erythema, 1 day.
TABLE 1.
Treatment-Emergent (Regardless of Causality) and Treatment-Related AEs Occurring in ≥1% of Subjects (Safety-Evaluable Population)
| Preferred Term | Sakura 3 (N = 2691), n (%) |
| Treatment-emergent AEs regardless of causality | |
| Headache | 158 (5.9) |
| Nasopharyngitis | 119 (4.4) |
| Injection site pain | 105 (3.9) |
| Injection site erythema | 90 (3.3) |
| Injection site edema | 76 (2.8) |
| Erythema | 56 (2.1) |
| Upper respiratory tract infection | 55 (2.0) |
| Sinusitis | 49 (1.8) |
| Edema | 47 (1.7) |
| Urinary tract infection | 42 (1.6) |
| Influenza | 37 (1.4) |
| Eyelid ptosis | 35 (1.3) |
| Injection site bruising | 32 (1.2) |
| Injection site pruritus | 29 (1.1) |
| Treatment-related AEs | |
| Headache | 124 (4.6) |
| Injection site pain | 98 (3.6) |
| Injection site erythema | 81 (3.0) |
| Injection site edema | 76 (2.8) |
| Erythema | 48 (1.8) |
| Edema | 47 (1.6) |
| Eyelid ptosis | 34 (1.3) |
AE, adverse event.
Eyelid ptosis occurred in 35 (1.3%) subjects; however, one case, with onset at day 57, was deemed unrelated to treatment (infection of upper eyelid) by the investigator. Eyelid ptosis lasted a median of 43 days. Treatment-related eyelid ptosis occurred in 34 of 3,830 (0.9%) treatments. Of these 34 cases, 28 (82.4%) were mild in severity and 6 (17.6%) were moderate in severity. The incidence of treatment-related eyelid ptosis was 23/2,380 (1.0%) in Cycle 1, 7/882 (0.8%) in Cycle 2, and 4/568 (0.7%) in Cycle 3. No subject had eyelid ptosis in more than one treatment cycle. Brow ptosis occurred in 11 (0.4%) subjects.
Adverse events, both those regardless of causality and treatment-related, were similar across treatment cycles, with most decreasing with each treatment cycle (see Supplemental Digital Content 4 and 5, Tables S3 and S4, http://links.lww.com/DSS/A374 and http://links.lww.com/DSS/A375, respectively). Most were reported in the first 12 weeks after injection, and a cross-cycle comparison focused on the first 12 weeks of each treatment cycle confirmed the decrease with each treatment cycle. Headache was the most commonly reported AE for all subjects after Treatment 1 (5.5%) and 3 (2.5%); however, the most commonly reported treatment-related/treatment-emergent AE for all subjects after Treatment 2 was injection site erythema (2.5%).
The most common injection site reactions classified as AEs by investigators are included in Table 1 and Table S4, Supplemental Digital Content 5, http://links.lww.com/DSS/A375, respectively. Most injection site reactions were mild in severity (99% of erythema, 100% of edema, and 82% of pain). Similar to the other AEs, the incidence of injection site reactions decreased with the number of DAXI treatment cycles.
Laboratory Tests and Vital Signs
ptMost hematology, chemistry, and urinalysis values were within normal range, with minor changes from baseline at Week 4 and final evaluation. A total of 29 laboratory abnormality AEs were reported in 26 (1.0%) subjects. Most were of mild severity and unrelated to treatment. Clinically significant abnormalities in urinalysis occurred at a rate of <1.0%. Changes in vital signs from screening to the final evaluation were minimal and not clinically significant.
Immunogenicity
A total of 19 (0.7%) subjects developed binding antibodies against daxibotulinumtoxinA during the course of the study. None of these subjects tested positive for neutralizing antibodies, and all subjects with binding antibodies exhibited clinical efficacy based on investigator assessment. Furthermore, no clinically meaningful immune-related AEs were observed in any subject in any treatment cycle.
Discussion
This OLS study contributes to the largest Phase 3 clinical development program of an aesthetic neuromodulator globally. Although the placebo-controlled trials enrolled 609 subjects at 30 clinical sites, the present study significantly increased the number of trials exposed to DAXI and broadened the number of sites from 30 to 65, thus expanding the pool of injectors treating with DAXI and encompassing a total of 3,830 treatments. This facilitated a more accurate estimate of the anticipated AEs when treating the glabella with DAXI 40U. In addition, with some subjects receiving repeat treatments, there was no indication of worsening of the AE profile with subsequent treatments. In fact, there was a trend for lower incidence. Moreover, the findings from the pivotal trials, which demonstrated that DAXI treatment for glabellar lines was well tolerated with no new safety signals,4 were confirmed as the safety profiles were similar. Although an OLS study can be limited by the potential for bias, the similarity in the results to the placebo-controlled trials suggests that this was not the case in SAKURA 3.
Adverse events reported in this study are comparable with those in trials of other BoNTA products for treatment of glabellar lines. Headache was the most frequently reported AE in this study (5.9%), similar to other trials of BoNTA for cosmetic use (5%–12%).7–10 In the DAXI Phase 2 study, the incidence of headache was 7.5% with 40U dose and 13.0% with onabotulinumtoxinA 20U.11 Likewise, the incidence of other important AEs such as eyelid ptosis, occurring in 1.3% of patients in this study, compares favorably with earlier studies of other toxins for treatment of glabellar lines (0.2%–3%)7–10 and is lower than reported in the SAKURA 1 and SAKURA 2 trials (2.2%).4
Because BoNTA products require repeat treatment to retain glabellar smoothness, the duration of response should factor into considerations for risk of AEs. The typical duration of benefit of current US Food and Drug Administration–approved BoNTA products in glabellar lines is about 3 to 4 months.7,10,12,13 In contrast, DAXI has a median time to return to moderate or severe glabellar lines of 24.0 weeks,4 which will result in the need for fewer treatments per year. Consequently, fewer treatments compared with other BoNTA products will potentially lower the overall annual risk for AEs. It is important to note that the commercial BoNTA products, including unit dosages, are not interchangeable due to differences in formulation and the lack of an international reference standard defining the activity of a unit.14
The efficacy of DAXI for the correction of glabellar lines in SAKURA 3 is discussed in detail in the companion article.5 It is important to note that the safety profile of DAXI in SAKURA 3, which was similar to that of the pivotal Phase 3 trials and registration trials of other BoNTAs, was accompanied by a high rate of efficacy. More than 90% of subjects achieved a score of none or mild on the IGA-FWS as early as Week 1, a peak response across treatment cycles at 2 to 4 weeks (97%–98%) and, at Week 24, the response was maintained in 34.0% and 38.5% of subjects for Treatments 1 and 2, respectively.5
Limitations of this study include its population of mainly white women, so the generalizability of the findings may be affected, although this is also the case in other trials with BoNTA for glabellar lines.12,13,15–19 However, unlike most clinical trials of a BoNTA for facial wrinkles, subjects who had previously received a BoNTA were eligible to participate in SAKURA 3 and comprised 40% of the population. This is reflective of the type of patients seen in clinical practice.
SAKURA 3 results demonstrate that the high rate of efficacy and prolonged duration of response with DAXI is not at the expense of the safety profile. The consistent results of the type and incidence of AEs in this OLS compared with the placebo-controlled pivotal trials, and the size of the Phase 3 clinical development program, which included a total of 2,823 subjects who received 4,444 treatments, gives clinicians confidence in the safety of DAXI for the treatment of glabellar lines.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.dermatologicsurgery.org).
The SAKURA 3 study was supported by Revance Therapeutics, Inc., Newark, CA. Writing and editorial assistance was provided to the authors by Evidence Scientific Solutions, Philadelphia, PA, and funded by Revance Therapeutics, Inc. All authors met the ICMJE authorship criteria. Neither honoraria nor payments were made for authorship.
J.B. Green, K. Mariwalla, K. Coleman, and G. Ablon have served as clinical trial investigators for Revance Therapeutics, Inc. S.H. Weinkle has served as a clinical trial investigator and consultant for Revance Therapeutics, Inc. D. Vitarella, C.J. Gallagher, and R.G. Rubio are employees of, and hold stock/stock options in Revance Therapeutics, Inc.
References
- 1.Finn JC, Cox SE, Earl ML. Social implications of hyperfunctional facial lines. Dermatol Surg 2003;29:450–5. [DOI] [PubMed] [Google Scholar]
- 2.Jia Z, Lu H, Yang X, Jin X, et al. Adverse events of botulinum toxin type A in facial rejuvenation: a systematic review and meta-analysis. Aesthet Plast Surg 2016;40:769–77. [DOI] [PubMed] [Google Scholar]
- 3.Naumann M, Boo LM, Ackerman AH, Gallagher CJ. Immunogenicity of botulinum toxins. J Neural Transm 2013;120:275–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertucci V, Solish N, Kaufman-Janette J, Yoelin S, et al. DaxibotulinumtoxinA for Injection has a prolonged duration of response in the treatment of glabellar lines: pooled data from two multicenter, randomized, double-blind, placebo-controlled, phase 3 studies (SAKURA 1 and SAKURA 2). J Am Acad Dermatol 2020;82:838–45. [DOI] [PubMed] [Google Scholar]
- 5.Fabi SG, Cohen JL, Green LJ, Dhawan S, et al. DaxibotulinumtoxinA for injection for the treatment of glabellar lines: efficacy results from SAKURA 3, a large, open-label, phase 3 safety study. Dermatol Surg 2021;47:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carruthers JD, Fagien S, Joseph JH, Humphrey S, et al. DaxibotulinumtoxinA in the treatment of glabellar lines: results from each of two multicenter, randomized, double-blind, placebo-controlled phase 3 studies (SAKURA 1 and SAKURA 2). Plast Reconstr Surg 2020;145:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.BOTOX Cosmetic® (OnabotulinumtoxinA) [Prescribing Information]. Irvine, CA: Allergan Pharmaceuticals Ireland, a subsidiary of Allergan, Inc.; 2018. [Google Scholar]
- 8.DYSPORT® (AbobotulinumtoxinA) [Prescribing Information]. Basking Ridge, NJ: Ipsen Biopharmaceuticals, Inc.; 2018. [Google Scholar]
- 9.JEUVEAU (PrabotulinumtoxinA-Xvfs) [Prescribing Information]. Santa Barbara, CA: Evolus Inc.; 2019. [Google Scholar]
- 10.XEOMIN® (IncobotulinumtoxinA) [Prescribing Information]. Raleigh, NC: Merz Pharmaceuticals, LLC; 2019. [Google Scholar]
- 11.Carruthers J, Solish N, Humphrey S, Rosen N, et al. Injectable daxibotulinumtoxinA for the treatment of glabellar lines: a phase 2, randomized, dose-ranging, double-blind, multicenter comparison with onabotulinumtoxinA and placebo. Dermatol Surg 2017;43:1321–31. [DOI] [PubMed] [Google Scholar]
- 12.Kane MA, Brandt F, Rohrich RJ, Narins RS, et al. Evaluation of variable-dose treatment with a new U.S. Botulinum Toxin Type A (Dysport) for correction of moderate to severe glabellar lines: results from a phase III, randomized, double-blind, placebo-controlled study. Plast Reconstr Surg 2009;124:1619–29. [DOI] [PubMed] [Google Scholar]
- 13.Brandt F, Swanson N, Baumann L, Huber B. Randomized, placebo-controlled study of a new botulinum toxin type a for treatment of glabellar lines: efficacy and safety. Dermatol Surg 2009;35:1893–901. [DOI] [PubMed] [Google Scholar]
- 14.Brin MF, James C, Maltman J. Botulinum toxin type A products are not interchangeable: a review of the evidence. Biologics 2014;8:227–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beer KR, Shamban AT, Avelar RL, Gross JE, et al. Efficacy and safety of prabotulinumtoxinA for the treatment of glabellar lines in adult subjects: results from 2 identical phase III studies. Dermatol Surg 2019;45:1381–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carruthers JA, Lowe NJ, Menter MA, Gibson J, et al. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol 2002;46:840–9. [DOI] [PubMed] [Google Scholar]
- 17.Carruthers JD, Lowe NJ, Menter MA, Gibson J, et al. Double-blind, placebo-controlled study of the safety and efficacy of botulinum toxin type A for patients with glabellar lines. Plast Reconstr Surg 2003;112:1089–98. [DOI] [PubMed] [Google Scholar]
- 18.Carruthers A, Carruthers J, Coleman WP, III, Donofrio L, et al. Multicenter, randomized, phase III study of a single dose of incobotulinumtoxinA, free from complexing proteins, in the treatment of glabellar frown lines. Dermatol Surg 2013;39:551–8. [DOI] [PubMed] [Google Scholar]
- 19.Hanke CW, Narins RS, Brandt F, Cohen JL, et al. A randomized, placebo-controlled, double-blind phase III trial investigating the efficacy and safety of incobotulinumtoxinA in the treatment of glabellar frown lines using a stringent composite endpoint. Dermatol Surg 2013;39:891–9. [DOI] [PubMed] [Google Scholar]