Abstract
Chronic low-grade inflammation contributes to the development of several diseases, including cardiovascular disease. Adequate strategies to target inflammation in cardiovascular disease are in their infancy and remain an avenue of great interest. The purinergic receptor P2X7 is a ubiquitously expressed receptor that predominately mediates inflammation and cellular death. P2X7 is a ligand-gated cation channel that is activated in response to high concentrations of extracellular ATP, triggering the assembly and activation of the NLRP3 (nuclear oligomerization domain like receptor family pyrin domain containing 3) inflammasome and subsequent release of proinflammatory cytokines IL (interleukin)-1β and IL-18. Increased P2X7 activation and IL-1β and IL-18 concentrations have been implicated in the development of many cardiovascular conditions including hypertension, atherosclerosis, ischemia/reperfusion injury, and heart failure. P2X7 receptor KO (knockout) mice exhibit a significant attenuation of the inflammatory response, which corresponds with reduced disease severity. P2X7 antagonism blunts blood pressure elevation in hypertension and progression of atherosclerosis in animal models. IL-1β and IL-18 inhibition has shown efficacy in clinical trials reducing major adverse cardiac events, including myocardial infarction, and heart failure. With several P2X7 antagonists available with proven safety margins, P2X7 antagonism could represent an untapped potential for therapeutic intervention in cardiovascular disorders.
Keywords: cardiovascular diseases, heart failure, infant, inflammation, interleukins
Highlights.
Accumulating evidence demonstrates that the purinergic receptor P2X7 plays a prominent role in chronic inflammatory conditions, including cardiovascular disease.
P2X7 activation contributes to the development of hypertension through promotion of renal and vascular dysfunction.
P2X7-mediated endothelial dysfunction and inflammation direct atherosclerotic plaque formation and rupture.
In ischemic injury in the heart, P2X7 activation promotes cardiomyocyte death and enhances inflammation, leading to cardiovascular dysfunction.
P2X7 inhibitors may provide a new avenue for treatment in cardiovascular disorders.
Cardiovascular disease is the leading cause of mortality worldwide, representing up to 31% of annual global deaths.1 Despite its widespread prevalence, there remain inadequate treatment options for a large proportion of patients, in part, due to the complex and varied pathophysiology involved in cardiovascular disease. In recent years, the role of inflammation in cardiovascular disease has been garnishing a lot of attention. Numerous studies have indicated a prominent role for low-grade inflammation in the development of hypertension, atherosclerosis, myocardial ischemic injury, and heart failure. Consequently, targeting the source of inflammation in these conditions remains a tantalizing yet elusive option. One such target that has shown promising results is the purinergic receptor P2X7.
P2RX7 (P2X7 receptor) belongs to a family of purinergic receptors divided into 2 classes: metabotropic G-protein–coupled P2Y receptors and ligand-gated ion channel P2X receptors. P2X receptors are primarily activated by extracellular ATP, with P2X7 being distinct from the other receptors due to its low affinity for ATP. P2X7 requires 100 to 1000× physiological concentrations of extracellular ATP for its activation with a reported EC50 (half maximal effective concentration) of ≈100 µmol/L.2 With transient stimulation, the P2X7 receptor acts as a nonspecific cation channel facilitating Na+ and Ca2+ influx and K+ efflux, resulting in the activation of numerous downstream signaling complexes in a cell type–dependent manner.3,4 The most prominent downstream effector of P2X7 activation is the NLRP3 (nuclear oligomerization domain like receptor family pyrin domain containing 3) inflammasome. The NLRP3 inflammasome cleaves and activates caspase-1, which subsequently cleaves the proinflammatory cytokines pro-IL (interleukin)-1β and pro-IL-18 into their mature, active forms. Prolonged stimulation of P2X7 with ATP promotes the formation of macropores in the cell membrane, resulting in an inflammatory cell death program termed pyroptosis.2,5–7 IL-1β and IL-18 are the primary mediators of P2X7-induced inflammation, which facilitate immune cell recruitment and inflammation, endothelial dysfunction, plaque formation, and cardiac dysfunction.8–10
Beyond the predominant role of P2X7 in triggering inflammation and cellular death, it has been implicated in numerous other functions including nociception, vascular function, glucose uptake, and paradoxically, promoting cellular survival (interested readers are directed to the following excellent articles11–15). The pleiotropic effect of P2X7 is in part cell type–dependent and in part dependent on the isoform of P2X7 expressed. Ten human splice variants have been identified, named P2X7A to P2X7J.16–19 Isoform A (P2X7A)—the full length receptor—responds in a biphasic manner, with tonic activation by low concentrations of ATP promoting cellular proliferation and high concentrations of ATP promoting the typical responses of P2X7 activation, such as inflammasome activation and pore formation.16,20,21 P2X7B has a truncated carboxy terminal, impairing its pore-forming ability.20 It has been demonstrated to have an antiapoptotic effect in numerous cell types.14,20–26 Isoforms P2X7C, P2X7E, P2X7G, and P2X7J also have a truncated carboxy terminal inhibiting their pore-forming ability, but their functional role is unclear, while the P2X7I isoform results in loss of function of the receptor.16,19,27,28 In rodent T cells, an additional variant, P2X7K, mediates T-cell responses to ATP and NAD+ (nicotinamide-adenine dinucleotide), facilitating T-cell class switching through CD62L (cluster of differentiation 62L) and CD27 cleavage and cellular death via externalization of phosphatidyl serine.17,29–31 To date, a human homologue of P2X7K has yet to be identified, and its relevance in human pathology is unclear. Finally, P2X7 variants may be preferentially expressed in different cell types and are known to have varying affinities to ATP (P2X7K>A>B), which may help account for the cell type–dependent P2X7 responses.17,18,21,32 Despite the work done to date to delineate the function of P2X7 variants, much remains unknown with regard to their role in disease progression, particularly in cardiovascular disease.
P2X7 receptor activation has been implicated in the progression of many chronic inflammatory diseases. In animal models, P2X7 inhibition has proven to be an effective treatment strategy for many chronic inflammatory disorders including arthritis, Duchenne muscular dystrophy, multiple sclerosis, Alzheimer disease, chronic pain, and cardiovascular disease.33 However, the functional role of P2X7 outside of inflammation remains largely uncharacterized, and the pleiotropic nature of P2X7 function raises the question of the feasibility of P2X7 as a therapeutic target.
This review will focus on the role of P2X7 in the cardiovascular system and postulate on its utility as a target for treatment and management of cardiovascular conditions.
Hypertension
Hypertension affects ≈1.13 billion people worldwide and is the largest cause of burden of disease worldwide and the most important risk factor for the development of cardiovascular disease.1 Current studies investigating the role of P2X7 in hypertension are limited. However, available studies point to a role of P2X7 in regulating inflammation, as well as vascular and renal function in response to hypertensive challenges. The single-nucleotide polymorphism (rs598174) for P2X7 was strongly associated with both systolic and diastolic ambulatory blood pressure (BP) in a white population.34 In a Chinese population of postmenopausal women, a hypomorphic single-nucleotide polymorphism (rs3751143) for P2X7 was associated with a decreased risk of primary (formerly called essential) hypertension.35 Increased inflammasome expression and circulating IL-1β in subjects over the age of 60 years was strongly associated with increased risk for hypertension and vascular dysfunction, as well as all-cause mortality.36 Furthermore, elevated plasma ATP levels have been observed in hypertensive patients in comparison to normotensive controls or patients with controlled hypertension, leading to heightened T-cell responses in a P2X7-dependent manner.37
P2X7, IL-1β, and Hypertension
There is accumulating evidence that P2X7 contributes to the connection between low-grade chronic inflammation and hypertension.8 Macrophages isolated from Dahl salt-sensitive rats produced more IL-1β in response to ATP than normotensive Lewis rats, highlighting heightened inflammasome responses in rats genetically predisposed to hypertension.38 NLRP3 inflammasome proteins in mice and IL-1β in humans have been reported to be elevated in hypertension, and directly antagonizing the NLRP3 inflammasome has modestly reduced BP in various animal models of hypertension.39–42 Targeting IL-1β using anakinra—an IL-1 receptor antagonist—significantly reduced BP in a 1-kidney deoxycorticosterone acetate–salt model of hypertension.43 However, the efficacy of targeting IL-1β in human hypertensive patients is unclear. In CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcome Study), patients given an anti-IL-1β monoclonal antibody (canakinumab) had no reduction in BP at 3, 6, or 12 months of follow-up, and there was no reduction in incident hypertension in the cohort.44,45 Despite a lack in reduction of BP, patients with elevated systolic BP (130–140 mm Hg) or hypertension (systolic BP, >140 mm Hg) treated with canakinumab had a significant reduction in composite end points (myocardial infarction [MI], stroke, or cardiovascular mortality).44,45 The CANTOS trial suggests that blocking IL-1β alone may be insufficient to reduce BP, and, therefore, targeting upstream of IL-1β, at the P2X7 receptor, may provide a more attractive target for BP management as it could antagonize additional downstream effects of P2X7 activation outside of IL-1β production (discussed below).
P2X7 and Kidney Function
Under nonpathological conditions, there is sparse P2X7 expression throughout the kidney. However, P2X7 expression is significantly increased in hypertensive states.46 Transgenic rats expressing the mouse Ren2 gene have an overactivated renin-angiotensin-aldosterone system and develop severe hypertension that can be attenuated with angiotensin-converting enzyme inhibitors.47,48 These transgenic rats have increased P2X7 expression in the glomeruli in comparison to normotensive rats.46 Other hypertensive models demonstrate similar results, with P2X7 expression significantly increased in the kidney in Ang II (angiotensin II) and deoxycorticosterone acetate–salt-induced hypertensive rodents, as well as in Dahl salt-sensitive rats.38,49–51 P2X7 receptor silencing decreased renin activity and angiotensin-converting enzyme 1 and 2 expression in the renal cortex, preventing renal dysfunction in a model of diabetic nephropathy.52 In addition, P2X7 antagonism may also reduce the prohypertensive effects of Ang II. Ang II acts as a potent vasoconstrictor of the renal vasculature, and it can alter renal sodium and water handling through increased aldosterone release.53 In rodent models, P2X7 antagonism reduced renal vascular resistance and increased medullary perfusion, resulting in enhanced pressure natriuresis.49,50,54 Menzies et al49 reported a 6-fold increase in sodium excretion with P2X7 antagonism, blunting Ang II–induced BP elevation in rats. In addition, ATP promotes transepithelial sodium transport through epithelial sodium channels, which can be attenuated by Brilliant Blue G—a P2X7 antagonist.55 This, along with increased pressure natriuresis, may account for the increased Na+ excretion associated with P2X7 antagonism.49,50 However, another study found that P2X7 antagonism had no effect on Ang II–induced BP elevation in rats, although the authors used a 10-fold higher dose of Ang II, which may account for the differences observed.50 Overall, these studies provide evidence for a role of P2X7 in the regulation of kidney responses to hypertensive stimuli and support P2X7 as a novel antihypertensive target.
Further supporting the beneficial effects of inhibiting P2X7, activation of the receptor itself exerts prohypertensive effects in the kidney. Ang II and aldosterone both increase renal ATP concentrations, with the concentration of renal interstitial ATP strongly correlated with BP increase.56,57 P2X7 activation on the renal vasculature, by elevated ATP, appears to exert a tonic vasoconstrictive effect.49 In addition, P2X7-mediated vasoconstriction of the medullary microcirculation has been shown to cause regional hypoxia promoting vascular hypertrophy and renal inflammation.49 Prolonged exposure to elevated extracellular ATP results in P2X7-mediated mesangial, fibroblast, endothelial, and renal tubular cell death, contributing to renal inflammation and fibrosis, as well as promoting endothelial dysfunction.58–62 P2X7 antagonism results in a partially NO-dependent vasodilation of the afferent, efferent, and renal arteries, increasing renal perfusion and reducing renal inflammation and fibrosis.49,50,52,54 P2X7 KO (knockout) or antagonism has also proved effective in preventing renal fibrosis, renal immune cell infiltration, and lowering BP and albuminuria in Dahl salt-sensitive rats and in a deoxycorticosterone acetate–salt model of hypertension.38,51 In summary, continuous P2X7 activation leads to microvascular dysfunction and regional hypoxia. This promotes renal inflammation and renal fibrosis, leading to a decline in renal function that contributes to hypertension.
P2X7 and Systemic Vasculature
P2X7 expression has been reported in the endothelium and the smooth muscle layer of most of the systemic arterial and venous circulation in human and animal tissues.63–66 In the microvasculature, P2X7 activation has been shown to promote vascular dysfunction through increased oxidative stress and increased endothelial cell permeability and apoptosis. In a rat model of type 1 diabetes, P2X7 expression was found to be elevated in the retinal microvasculature, contributing to increased microvasculature permeability, whereas in human retinal endothelial cells, P2X7 activation induced endothelial cell death.67,68 In both experiments, microvasculature dysfunction could be reversed by a P2X7 inhibitor. Further, it was demonstrated that P2X7 vasotoxicity was mediated through P2X7-dependent pore formation, as well as NADPH (reduced nicotinamide-adenine dinucleotide phosphate) oxidase–dependent ROS generation.69 In addition, surgical stretch of human saphenous veins prepared for coronary artery bypass grafts caused P2X7 activation inducing apoptosis resulting in vascular dysfunction.60 P2X7 activation can also induce constriction of the retinal and renal microvasculature, as well as of large veins, which could lead to increased systemic vascular resistance.49,50,63,70 In diabetic rats, P2X7 antagonism improved endothelium-dependent relaxation and decreased constrictor responses to phenylephrine in the aorta.71 A model investigating vascular surgical stretch injury demonstrated that P2X7 activation diminished endothelium-dependent relaxation through decreased NO production.62 The resulting vascular dysfunction and remodeling can contribute to increased systemic vascular resistance and the development of hypertension.72,73
However, conflicting results suggest P2X7 activation may also play a role in vasodilatation. P2X7 activation on murine mesenteric artery endothelial cells resulted in enhanced NO production.74 In addition, P2X7-mediated responses to lipopolysaccharides have been reported to cause hyporeactivity of the thoracic aorta in mice, leading to P2X7-mediated hypotension in an IL-1β–dependent and NO-dependent manner.75,76 These studies highlight critical differences in the role of P2X7 responses in different tissues and different disease states. It remains unclear whether, in a chronic disease setting such as hypertension, P2X7 antagonism could be beneficial or detrimental to vascular function and mechanics, and the area warrants further investigation.
Atherosclerosis
Atherosclerosis is a common comorbidity with hypertension and presents similar features, such as endothelial dysfunction and low-grade inflammation.77 Immune cell recruitment and activation at the site of plaques is required for the development of atherosclerotic lesions, and P2X7-directed inflammation could play a central role in plaque formation and promoting plaque rupture. In human carotid arteries presenting with atherosclerotic plaques, there is increased P2X7 expression in plaque-rich areas compared with regions devoid of plaques.66,78 In addition, the expression of P2X7 mRNA in circulating mononuclear cells significantly correlates with the degree of coronary artery stenosis.79 ATP accumulates in atherosclerotic vessels as compared with nonatherosclerotic ones, and elements of the inflammasome (NLRP3, caspase-1, and IL-1β) are increased in plaque-rich regions, providing an indication of P2X7 activation.66,80,81 Together, these studies provide support for an involvement of P2X7 in the development of atherosclerosis, and there are several potential mechanisms for P2X7 activation in atherosclerosis.
P2X7 activation in atherosclerosis may be initiated through alterations in blood flow (turbulent blood flow) or as a result of a secondary metabolic disorder. At sites with turbulent blood flow, there is a dramatic elevation in local extracellular ATP.82,83 The increase in ATP is driven through decreased ATPase (CD39) expression and an enhanced release of ATP from endothelial cells in regions rich with caveolin.84–86 These sites of turbulent blood flow have increased P2X7 expression, which can colocalize with caveolin-1, placing P2X7 receptors proximal to sites of ATP release.87–91 These P2X7 receptors expressed in caveolin-1–rich domains have been shown to be nonpore forming and instead facilitate intracellular Ca2+ accumulation, leading to p38 mitogen-activated protein kinase phosphorylation and subsequent upregulation of surface adhesion molecules in plaque prone regions.87,88,92 In addition, exposure of endothelial cells and human fibroblasts to high concentrations of glucose or palmitate, such as in diabetes, causes extracellular ATP release and the formation of P2X7 aggregates near the cell periphery.93–95 These P2X7 aggregates have a lowered threshold for activation to ATP and mediate endothelial dysfunction through elevated ROS generation, increased cell permeability, and expression of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1.78,93,94,96,97 Furthermore, oxidized low-density lipoproteins and cholesterol crystals, common elements in atherosclerosis, can activate the NLRP3 inflammasome resulting in the release of IL-1β and IL-18.98–101 Consequently, factors common to atherosclerosis development, hyperglycemia, hyperlipidemia, oxidized low-density lipoproteins, cholesterol crystals, and turbulent blood flow have been shown to influence P2X7 activation.
P2X7 activation on endothelial cells at sites prone to development of atherosclerosis promotes leukocyte recruitment, adhesion, and transmigration into the developing plaque through the production of proinflammatory cytokines, ROS generation, and increased cellular adhesion molecules on endothelial cells.78,80,87,93 The subsequent tissue damage amplifies extracellular ATP concentrations and facilitates P2X7-mediated IL-1β secretion from smooth muscle cells and infiltrating leukocytes.78,80,102 Secreted IL-β then triggers the release of matrix metalloprotease 9 from vascular smooth muscle cells and leukocytes, which destabilizes the plaque and renders it vulnerable and prone to rupture.80,102–104 Furthermore, P2X7 facilitates thrombosis at the site of the ruptured plaque. When exposed to elevated circulating ATP, myeloid and smooth muscle cells release tissue factor in a P2X7/ROS-dependent manner, which triggers thrombus formation, and can lead to coronary obstruction and sudden death.105,106
Strategies targeting P2X7 or its downstream effectors have proven efficacious in preventing atherosclerosis progression in several preclinical and clinical models. P2X7 KO mice present with lower blood cholesterol than wild-type mice and in atherosclerotic animal models have decreased plaque size.78,107 The reduction in lesion size appears to be the result of decreased leukocyte recruitment and macrophage infiltration in P2X7 KO animals or after P2X7 antagonism.78,87 The attenuated immune infiltration was associated with decreased adhesion molecule expression on endothelial cells, with decreased caspase-1 activation and proinflammatory cytokine release.78,87 Decreased cholesterol levels in P2X7 KO mice may also play a role in decreasing inflammation, as oxidized low-density lipoproteins and cholesterol crystals have been shown to induce inflammasome activation that promotes atherosclerosis.98,99,101,107 In addition, P2X7 receptor targeting or IL-1β blockade increased plaque stability through inhibition of matrix metalloprotease 9 release.80,104 In the CANTOS trial, IL-1β blockade resulted in a reduction in all cardiovascular events, including coronary revascularization and MI, without lowering systemic lipid levels.44 This reduction in adverse cardiovascular events was comparable to the effects of lipid lowering by proprotein convertase subtilisin-kexin type 9 inhibitors.44,108,109 Whether P2X7 antagonism rather than P2X7 KO also reduces blood cholesterol has yet to be determined. In summary, targeting downstream P2X7 effector molecules or P2X7 receptors prevents leukocyte recruitment and inflammation in plaques, prevents plaque rupture, and may have lipid-lowering and antithrombotic effects, making P2X7 a potential target in managing atherosclerosis.
Heart Disease
Heart disease encompasses a wide variety of conditions with inflammation being the primary driver of many noncongenital conditions.110 IL-1β and IL-18, downstream effectors of P2X7, have been repeatedly identified as mediators to this inflammatory response.111,112 A loss-of-function P2X7 variant rs3751143 was significantly associated with a decreased risk of ischemic heart disease and stroke, especially in individuals with hypertension.113 However, the contribution of P2X7 to heart disease has still yet to be fully elucidated.
Myocardial Ischemic Injury
During cardiac ischemia, there is an interruption of blood flow to coronary tissue that can disrupt cardiac function and damage surrounding tissues, resulting in a substantial release of ATP.114,115 The rise of ATP following ischemia/reperfusion (I/R) activates surrounding cardiac fibroblasts, stimulating P2X7-mediated release of IL-1β, IL-18, and ROS that can lead to the recruitment of leukocytes to the hypoxic region.116–118 The recruited leukocytes then contribute to amplify inflammation through P2X7-mediated activation and release of IL-1β and IL-18, thus promoting myocardial damage and cardiac fibrosis leading to declining cardiac function.118–120 Inhibition of IL-1β, IL-18, or caspase-1 significantly decreased infarct size and improved contractile function of the heart.118,119 However, whether P2X7 antagonism alone in I/R in the heart would also be protective is unclear.
Paradoxically, preconditioning cardiac tissue with short bouts of I/R has shown to protect from I/R injury through an ATP-driven mechanism.121 Cardiac protection was facilitated through the release of sphingosine-I-phosphate and adenosine via P2X7/pannexin-1 pores, occurring pre-ischemia and post-reperfusion.121–123 Inhibition of pannexin-1 or P2X7 abrogated the protective effect of I/R conditioning and resulted in increased infarct sizes.121 The difference between protection and harm associated with P2X7 activation may be the result of P2X7 splice variants. Splice variants of P2X7 are known to have varying affinities for ATP and can elicit different responses.17 Further strengthening this hypothesis, P2X7 functional coupling with pannexin-1 was found to be dependent on the P2X7 isoform expressed, specifically to a common allelic mutation resulting in a proline-to-leucine mutation at amino acid 451 in the P2X7A variant.17 This same mutation was found to result in a decreased sensitivity to ATP (≈10-fold).124 In addition, activation of P2X7A with low concentrations of ATP has been demonstrated to have growth-promoting effects.21 Since P2X7-mediated protection from I/R was dependent on pannexin-1 coupling, it is possible that the differing effects of P2X7 in I/R are dependent on the isoform of P2X7 expressed. Whether the protective effect of P2X7 activation during I/R is mediated through one of these splice variants has yet to be shown, but if this is the case, this could provide a selective target to protect the heart during I/R without the accompanying inflammation.
Angina Pectoris
Angina is a common symptom in many patients experiencing coronary ischemia, and P2X7 appears to play an important role in persistent angina symptoms post-MI. After acute MI, P2X7 mRNA and protein were upregulated in the superior cervical ganglia and in cardiac sympathetic afferents of rats.125–127 P2X7-dependent transmission of nociception down these cardiac afferents has been demonstrated, along with activated cardiac sympathetic efferent nerves, leading to increased BP, heart rate, and circulating proinflammatory cytokines (TNF-α [tumor necrosis factor alpha] and IL-6). P2X7 antagonism post-MI attenuates sympathetic stimulation of cardiac tissue, reducing tachycardia, BP, myocardial injury, and nociception signaling, ultimately alleviating symptoms of angina.
MI and Heart Failure
MI is a life-threatening condition caused by obstruction of blood flow to cardiac tissue. Following an acute MI, there is a substantial increase in extracellular ATP released from damaged cells. This rising extracellular ATP promotes P2X7-mediated inflammasome formation and activation around the border of the infarct in surrounding fibroblasts, cardiomyocytes, and invading leukocytes, leading to elevated IL-1β and IL-18.128–130 P2X7 activation in cardiomyocytes promotes caspase-dependent apoptosis, which contributes to cardiac dysfunction.128,131,132 During acute MI, epicardium-derived cells are also directed to the infarct region.120 Epicardium-derived cells give rise to various cardiovascular cells and migrate to injured myocardium to initiate tissue repair.133–136 However, during ischemia, invading epicardium-derived cells can also promote further inflammation by secreting ATP, NAD, and tenascin-C.120 Tenascin-C can prime the NLRP3 inflammasome via toll-like receptor 4 activation and coupled with elevated ATP, can activate the inflammasome in infiltrating leukocytes, further amplifying inflammation.118,120 Elevated IL-1β and IL-18 contribute to cardiac enlargement, cardiac fibrosis, and a deterioration of heart function post-MI leading to heart failure.119,128,137,138 Additionally, the NAD released by epicardium-derived cells can cause P2X7-mediated phosphatidyl serine exposure on the outer leaflet of T regulatory cells (Tregs) leading to their death.29,30,139,140 Tregs normally increase in ischemic tissue 3 to 7 days after reperfusion and contribute to resolution of inflammation and promote tissue repair.141 P2X7 activation may lead to a decreased presence of anti-inflammatory Tregs in ischemic tissue, and indeed, P2X7 antagonism in a kidney I/R model resulted in a significant increase of infiltrating Tregs and improved tissue recovery.142
Antagonizing or knocking out P2X7 or its downstream effectors, caspase-1 or NLRP3, in animal models decreased infarct size, improved cardiac function, and enhanced survival post-MI via reduced IL-1β and IL-18 levels in the heart.128,130,137,143 Targeting IL-1β directly has also proven effective in reducing cardiac dysfunction and promoting survival post-MI in animal models and in clinical trials.44,138,144,145 The protective effect of P2X7 antagonism in ischemia and acute MI appears to be due to decreased inflammation through decreased proinflammatory cytokine production and increased anti-inflammatory Tregs. Therefore, targeting P2X7-mediated inflammation post-MI may provide a therapeutic avenue for improved cardiac function and survival in patients. Indeed, circulating P2X7 mRNA expression is predictive of prognosis in acute MI, with elevated P2X7 expression correlating with worse patient outcomes.79
Cerebral Ischemic Injury
P2X7 activation has also been implicated in cerebral ischemic injury (ischemic stroke). In a permanent focal cerebral ischemia model, P2X7 expression was upregulated on neuronal and glial cells post-ischemia and was particularly associated with apoptotic cells.146 P2X7 antagonism in rat transient focal cerebral ischemia models resulted in decreased infarct size and neuronal death and improved survival.147,148 Interestingly, a protective effect in P2X7 KO mice has not been demonstrated. Le Feuvre et al149 saw no improvement in infarct volume 24 hours after inducing temporary cerebral ischemia in P2X7 KO mice but did see an improvement using an IL-1 receptor antagonist. In an acute ischemic stroke model in mice, P2X7 KO led to larger edema size within the first 24 hours of reperfusion but not after 72 hours.150 It is possible that P2X7 activation on microglia by low concentrations of ATP after cerebral I/R provides neuroprotection,150–152 while prolonged stimulation of P2X7 on glial and neural cells results in cellular death and inflammation.147,148,153 Therefore, P2X7 appears to be a double-edged sword in cerebral I/R injury, with P2X7 activation initially providing a neuroprotective benefit, but with prolonged activation shifting to become a proinflammatory mediator exaggerating cerebral ischemic injury.
Therapeutic Potential of P2X7 Intervention
Downstream targets of P2X7 activation, mainly IL-1β, have been investigated in several clinical studies for efficacy in managing cardiovascular disease and have yielded promising results. The CANTOS trial using the IL-1β antagonist canakinumab was one of the first trials to demonstrate that the risk for recurrent cardiovascular disease could be decreased by lowering inflammation without lowering systemic lipid levels.44 However, patients on canakinumab had a significantly increased risk of fatal infection, although there was no difference in all-cause mortality between groups (median patient follow-up of ≈3.7 years). Targeting P2X7 rather than IL-1β could have several advantages. First, P2X7 activation is a major mediator of IL-1β production but not the only one, and whether prolonged use of a P2X7 antagonist would also increase the risk of fatal infection is unclear at this time, although clinical trials conducted thus far with P2X7 antagonists have had limited-to-no serious adverse advents reported for up to 6 months of treatment. Additionally, P2X7 antagonism has the added benefit of blocking other downstream effects of P2X7 activation that can be deleterious to health, such as cellular death. Finally, P2X7 antagonism may be especially beneficial in patients with cardiovascular disease and metabolic disorders such as hyperlipidemia or hyperglycemia. In preclinical models, P2X7 antagonism was able to diminish inflammasome activation by non-nucleotide agonists such as oxidized low-density lipoproteins, glucose, and palmitate, highlighting an additional benefit when treating disorders such as atherosclerosis.93,101
Although animal models targeting P2X7 in cardiovascular disease have shown favorable results, to date, there have been no clinical trials investigating P2X7 antagonism in cardiovascular disease. Over 70 patents for P2X7 antagonists have been filed, with several P2X7 antagonists having undergone clinical investigation for various inflammatory conditions with mixed results (Table).154 AstraZeneca P2X7 antagonist (AZD9056) had no effect on reduction of inflammatory biomarkers or disease index in patients with chronic obstructive pulmonary disease or rheumatoid arthritis but modestly improved the disease index in Crohn disease (specifically decreased nociception) despite no reduction in inflammatory biomarkers.155–157 Similarly, Pfizer P2X7 antagonist (CE-224535) was inefficacious in lowering disease activity or inflammatory biomarkers in rheumatoid arthritis patients inadequately controlled by methotrexate.158 Ex vivo analysis had demonstrated that AZD9056 was able to inhibit IL-1β ex vivo in human monocytes, and, therefore, it was postulated that in these pathologies, inhibiting the P2X7-IL-1β and IL-18 inflammatory axis was insufficient to control disease progression and that other inflammatory cytokines could potentially be major contributors.156 Due to the failure of these drugs to adequately suppress systemic inflammation, both companies abandoned their clinical trials after completion of phase II.155–158 Recently, a phase II clinical trial by Evotec and Second Genome investigating P2X7 antagonism in nonalcoholic steatohepatitis was also terminated due to an unfavorable risk-benefit profile with their P2X7 inhibitor.159
Table.
Current and Past Clinical Trials Investigating the Efficacy of P2X7 Antagonism for Disease Management

Despite underwhelming results from early clinical trials, the recent crystallization of P2X7 has further facilitated the development of more targeted P2X7 antagonist therapeutic strategies that could further enhance clinical efficacy.164 Janssen has designed new P2X7 agents for diagnosis and treatment of mood disorders that can penetrate the blood-brain barrier and have shown encouraging results in phase I clinical trials.162,163,165 Specific interest has begun to emerge at targeting P2X7 variants in disease settings. Biosceptre has developed a monoclonal antibody to an epitope termed E200, which is associated with nonfunctional variants of P2X7 and has demonstrated efficacy in a phase I clinical trial for the treatment of basal cell carcinoma.160 As P2X7 variants may also contribute to the pathogenesis of cardiovascular disease, such as I/R injury, it is an interesting avenue of research that merits more attention. Recently, a P2X7-specific nanobody, one-tenth the size of an antibody, was developed that was able to block P2X7-mediated IL-1β release with 1000× greater potency than Janssen or AstraZeneca small molecule inhibitors JNJ47965567 and AZ10606120.166,167 The enhanced specificity of P2X7 antagonists opens the door for potentially targeting other P2X7 variants in disease settings and will be an interesting avenue of research to follow over the coming years.
Despite the lack of efficacy for disease management of early P2X7 antagonists in human clinical trials, they provide evidence for the relative tolerability of P2X7 antagonists, as limited-to-no serious adverse advents were reported in the majority of clinical trials conducted to date. Therefore, since animal models have demonstrated a potential benefit for P2X7 antagonism in the context of hypertension, atherosclerosis, and heart disease and clinical trials have provided a precedent for safety of P2X7-directed inhibitors, P2X7 antagonists may represent a viable therapeutic option in the management of cardiovascular disease.
Conclusions
P2X7 is a key player in promoting inflammatory responses to tissue injury. In cardiovascular disease, P2X7 activation promotes endothelial dysfunction and inflammation that drives kidney and cardiac dysfunction, atherosclerosis, hypertension development, and the progression of heart failure. Preclinical models investigating P2X7 receptor KO or antagonism in cardiovascular disease have shown promising results in attenuating disease. Current clinical trials of P2X7 antagonists have shown P2X7 antagonists to be mostly well tolerated, and, therefore, P2X7 inhibition may represent an untapped resource for the management of cardiovascular disease.
Figure 1.
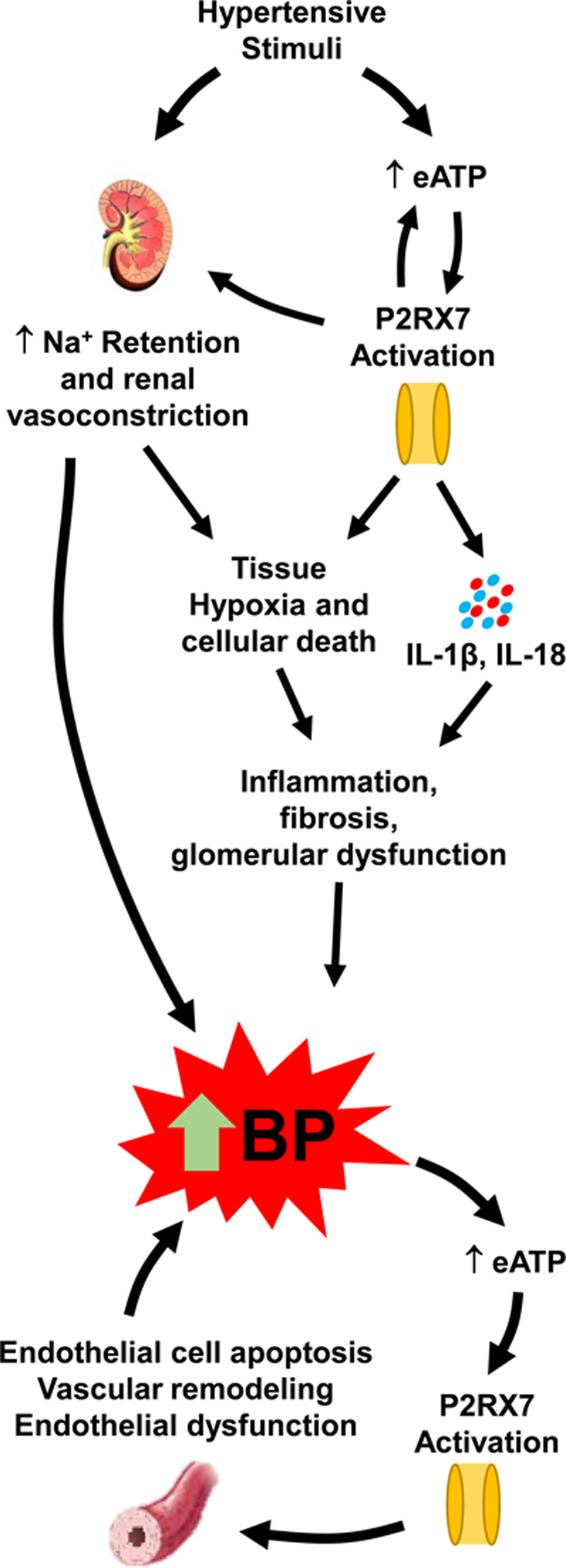
P2X7 and hypertension. Hypertensive stimuli induce an upregulation of P2RX7 (P2X7 receptor) surface expression,46,49,50 as well as directly and indirectly cause increases in extracellular ATP (eATP) in the renal interstitial fluid.56,57 Elevated ATP activates P2RX7 promoting cellular death, causing the release of proinflammatory cytokines, inducing renal vasoconstriction, and promoting sodium retention.49 P2X7-induced renal vasoconstriction causes tissue hypoxia, where along with inflammatory cytokines and reactive oxygen species, it causes inflammation, fibrosis, and glomerular dysfunction.49,58–62 Together, renal fibrosis, increased sodium retention, and renal vasoconstriction promote a rise in blood pressure (BP) that can increase systemic circulating ATP concentrations. The resulting P2X7 activation promotes endothelial cell apoptosis,67,68 vascular remodeling, and ultimately endothelial dysfunction,62,71 which further exacerbates the increase in BP. IL indicates interleukin.
Figure 2.
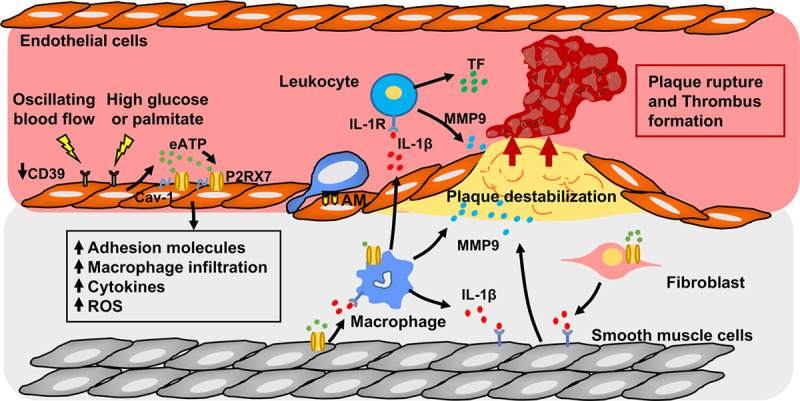
P2X7 and atherosclerosis. Oscillating flow or high glucose or palmitate promote P2RX7 (P2X7 receptor) surface expression,87 elevate extracellular ATP (eATP),82,83 and decrease CD39 (cluster of differentiation 39) expression84,85 in the endothelium at sites prone to develop atherosclerosis, creating an environment suitable for enhanced P2X7 activation. Endothelial P2X7 activation promotes leukocyte recruitment, adhesion, and transmigration into the developing plaque through production of inflammatory cytokines and increased adhesion molecule (AM) expression on endothelial cells.78,80,87,93 P2X7-dependent IL (interleukin)-1β production from vascular smooth muscle cells (VSMCs), macrophages, and fibroblasts promotes MMP9 (matrix metalloprotease 9) release from macrophages and VSMCs.80,102–104 MMP9 destabilizes the plaque, making it vulnerable to rupture,80,104 whereas P2X7 activation on myeloid cells induces the release of TF (tissue factor) promoting thrombus formation.105,106 Cav-1 indicates caveolin-1; IL-1R, interleukin-1 receptor; and ROS, reactive oxygen species.
Sources of Funding
This work was supported by the Canadian Institutes of Health Research (CIHR) First Pilot Foundation Grant 143348, a Canada Research Chair (CRC) on Hypertension and Vascular Research by the CRC Government of Canada/CIHR Program, and by the Canada Fund for Innovation, to E.L. Schiffrin and by the Fonds de recherche Santé Quebec bourse 289184, Lady Davis Institute/TD Bank Studentship award, and CIHR Canada Graduate Scholarship to B.G. Shokoples.
Disclosures
None.
Nonstandard Abbreviations and Acronyms
- Ang II
- angiotensin II
- BP
- blood pressure
- CANTOS
- Canakinumab Anti-Inflammatory Thrombosis Outcome Study
- I/R
- ischemia-reperfusion
- IL
- interleukin
- KO
- knockout
- MI
- myocardial infarction
- NLRP3
- nuclear oligomerization domain like receptor family pyrin domain containing 3
- TNF-α
- tumor necrosis factor alpha
- Treg
- T regulatory cell
For Sources of Funding and Disclosures, see page 194.
References
- 1.World Health Organization. Cardiovascular Disease. https://www.who.int/health-topics/cardiovascular-diseases/#tab=tab_1. Accessed May 28, 2020.
- 2.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735 [DOI] [PubMed] [Google Scholar]
- 3.Guerra Martinez C. P2X7 receptor in cardiovascular disease: the heart side. Clin Exp Pharmacol Physiol. 2019;46:513–526. doi: 10.1111/1440-1681.13079 [DOI] [PubMed] [Google Scholar]
- 4.Kopp R, Krautloher A, Ramírez-Fernández A, Nicke A. P2X7 interactions and signaling - making head or tail of it. Front Mol Neurosci. 2019;12:183 doi: 10.3389/fnmol.2019.00183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falzoni S, Munerati M, Ferrari D, Spisani S, Moretti S, Di Virgilio F. The purinergic P2Z receptor of human macrophage cells. Characterization and possible physiological role. J Clin Invest. 1995;95:1207–1216. doi: 10.1172/JCI117770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang D, He Y, Muñoz-Planillo R, Liu Q, Núñez G. Caspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity. 2015;43:923–932. doi: 10.1016/j.immuni.2015.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bidula S, Dhuna K, Helliwell R, Stokes L. Positive allosteric modulation of P2X7 promotes apoptotic cell death over lytic cell death responses in macrophages. Cell Death Dis. 2019;10:882 doi: 10.1038/s41419-019-2110-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishnan SM, Sobey CG, Latz E, Mansell A, Drummond GR. IL-1β and IL-18: inflammatory markers or mediators of hypertension? Br J Pharmacol. 2014;171:5589–5602. doi: 10.1111/bph.12876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savio LEB, de Andrade Mello P, da Silva CG, Coutinho-Silva R. The P2X7 receptor in inflammatory diseases: angel or demon? Front Pharmacol. 2018;9:52 doi: 10.3389/fphar.2018.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnstock G. Purinergic signaling in the cardiovascular system. Circ Res. 2017;120:207–228. doi: 10.1161/CIRCRESAHA.116.309726 [DOI] [PubMed] [Google Scholar]
- 11.Bernier LP, Ase AR, Séguéla P. P2X receptor channels in chronic pain pathways. Br J Pharmacol. 2018;175:2219–2230. doi: 10.1111/bph.13957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou R, Dang X, Sprague RS, Mustafa SJ, Zhou Z. Alteration of purinergic signaling in diabetes: focus on vascular function. J Mol Cell Cardiol. 2020;140:1–9. doi: 10.1016/j.yjmcc.2020.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourzac JF, L’Ériger K, Larrivée JF, Arguin G, Bilodeau MS, Stankova J, Gendron FP. Glucose transporter 2 expression is down regulated following P2X7 activation in enterocytes. J Cell Physiol. 2013;228:120–129. doi: 10.1002/jcp.24111 [DOI] [PubMed] [Google Scholar]
- 14.Thompson BA, Storm MP, Hewinson J, Hogg S, Welham MJ, MacKenzie AB. A novel role for P2X7 receptor signalling in the survival of mouse embryonic stem cells. Cell Signal. 2012;24:770–778. doi: 10.1016/j.cellsig.2011.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lara R, Adinolfi E, Harwood CA, Philpott M, Barden JA, Di Virgilio F, McNulty S. P2X7 in cancer: from molecular mechanisms to therapeutics. Front Pharmacol. 2020;11:793 doi: 10.3389/fphar.2020.00793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheewatrakoolpong B, Gilchrest H, Anthes JC, Greenfeder S. Identification and characterization of splice variants of the human P2X7 ATP channel. Biochem Biophys Res Commun. 2005;332:17–27. doi: 10.1016/j.bbrc.2005.04.087 [DOI] [PubMed] [Google Scholar]
- 17.Xu XJ, Boumechache M, Robinson LE, Marschall V, Gorecki DC, Masin M, Murrell-Lagnado RD. Splice variants of the P2X7 receptor reveal differential agonist dependence and functional coupling with pannexin-1. J Cell Sci. 2012;125:3776–3789. doi: 10.1242/jcs.099374 [DOI] [PubMed] [Google Scholar]
- 18.Masin M, Young C, Lim K, Barnes SJ, Xu XJ, Marschall V, Brutkowski W, Mooney ER, Gorecki DC, Murrell-Lagnado R. Expression, assembly and function of novel C-terminal truncated variants of the mouse P2X7 receptor: re-evaluation of P2X7 knockouts. Br J Pharmacol. 2012;165:978–993. doi: 10.1111/j.1476-5381.2011.01624.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sluyter R, Stokes L. Significance of P2X7 receptor variants to human health and disease. Recent Pat DNA Gene Seq. 2011;5:41–54. doi: 10.2174/187221511794839219 [DOI] [PubMed] [Google Scholar]
- 20.Giuliani AL, Colognesi D, Ricco T, Roncato C, Capece M, Amoroso F, Wang QG, De Marchi E, Gartland A, Di Virgilio F, et al. Trophic activity of human P2X7 receptor isoforms A and B in osteosarcoma. PLoS One. 2014;9:e107224 doi: 10.1371/journal.pone.0107224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adinolfi E, Cirillo M, Woltersdorf R, Falzoni S, Chiozzi P, Pellegatti P, Callegari MG, Sandonà D, Markwardt F, Schmalzing G, et al. Trophic activity of a naturally occurring truncated isoform of the P2X7 receptor. FASEB J. 2010;24:3393–3404. doi: 10.1096/fj.09-153601 [DOI] [PubMed] [Google Scholar]
- 22.Baricordi OR, Melchiorri L, Adinolfi E, Falzoni S, Chiozzi P, Buell G, Di Virgilio F. Increased proliferation rate of lymphoid cells transfected with the P2X(7) ATP receptor. J Biol Chem. 1999;274:33206–33208. doi: 10.1074/jbc.274.47.33206 [DOI] [PubMed] [Google Scholar]
- 23.Adinolfi E, Melchiorri L, Falzoni S, Chiozzi P, Morelli A, Tieghi A, Cuneo A, Castoldi G, Di Virgilio F, Baricordi OR. P2X7 receptor expression in evolutive and indolent forms of chronic B lymphocytic leukemia. Blood. 2002;99:706–708. doi: 10.1182/blood.v99.2.706 [DOI] [PubMed] [Google Scholar]
- 24.Adinolfi E, Callegari MG, Ferrari D, Bolognesi C, Minelli M, Wieckowski MR, Pinton P, Rizzuto R, Di Virgilio F. Basal activation of the P2X7 ATP receptor elevates mitochondrial calcium and potential, increases cellular ATP levels, and promotes serum-independent growth. Mol Biol Cell. 2005;16:3260–3272. doi: 10.1091/mbc.e04-11-1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adinolfi E, Callegari MG, Cirillo M, Pinton P, Giorgi C, Cavagna D, Rizzuto R, Di Virgilio F. Expression of the P2X7 receptor increases the Ca2+ content of the endoplasmic reticulum, activates NFATc1, and protects from apoptosis. J Biol Chem. 2009;284:10120–10128. doi: 10.1074/jbc.M805805200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gómez-Villafuertes R, García-Huerta P, Díaz-Hernández JI, Miras-Portugal MT. PI3K/Akt signaling pathway triggers P2X7 receptor expression as a pro-survival factor of neuroblastoma cells under limiting growth conditions. Sci Rep. 2015;5:18417 doi: 10.1038/srep18417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng YH, Li X, Wang L, Zhou L, Gorodeski GI. A truncated P2X7 receptor variant (P2X7-j) endogenously expressed in cervical cancer cells antagonizes the full-length P2X7 receptor through hetero-oligomerization. J Biol Chem. 2006;281:17228–17237. doi: 10.1074/jbc.M602999200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skarratt KK, Fuller SJ, Sluyter R, Dao-Ung LP, Gu BJ, Wiley JS. A 5’ intronic splice site polymorphism leads to a null allele of the P2X7 gene in 1-2% of the Caucasian population. FEBS Lett. 2005;579:2675–2678. doi: 10.1016/j.febslet.2005.03.091 [DOI] [PubMed] [Google Scholar]
- 29.Seman M, Adriouch S, Scheuplein F, Krebs C, Freese D, Glowacki G, Deterre P, Haag F, Koch-Nolte F. NAD-induced T cell death: ADP-ribosylation of cell surface proteins by ART2 activates the cytolytic P2X7 purinoceptor. Immunity. 2003;19:571–582. doi: 10.1016/s1074-7613(03)00266-8 [DOI] [PubMed] [Google Scholar]
- 30.Scheuplein F, Schwarz N, Adriouch S, Krebs C, Bannas P, Rissiek B, Seman M, Haag F, Koch-Nolte F. NAD+ and ATP released from injured cells induce P2X7-dependent shedding of CD62L and externalization of phosphatidylserine by murine T cells. J Immunol. 2009;182:2898–2908. doi: 10.4049/jimmunol.0801711 [DOI] [PubMed] [Google Scholar]
- 31.Frascoli M, Marcandalli J, Schenk U, Grassi F. Purinergic P2X7 receptor drives T cell lineage choice and shapes peripheral γδ cells. J Immunol. 2012;189:174–180. doi: 10.4049/jimmunol.1101582 [DOI] [PubMed] [Google Scholar]
- 32.Nicke A, Kuan YH, Masin M, Rettinger J, Marquez-Klaka B, Bender O, Górecki DC, Murrell-Lagnado RD, Soto F. A functional P2X7 splice variant with an alternative transmembrane domain 1 escapes gene inactivation in P2X7 knock-out mice. J Biol Chem. 2009;284:25813–25822. doi: 10.1074/jbc.M109.033134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. The P2X7 receptor in infection and inflammation. Immunity. 2017;47:15–31. doi: 10.1016/j.immuni.2017.06.020 [DOI] [PubMed] [Google Scholar]
- 34.Palomino-Doza J, Rahman TJ, Avery PJ, Mayosi BM, Farrall M, Watkins H, Edwards CR, Keavney B. Ambulatory blood pressure is associated with polymorphic variation in P2X receptor genes. Hypertension. 2008;52:980–985. doi: 10.1161/HYPERTENSIONAHA.108.113282 [DOI] [PubMed] [Google Scholar]
- 35.Gong C, Liu X, Ding L, Liu Y, Li T, Wang S, Zhao J, Rao S, Xiong C, Yang Y, et al. A non-synonymous polymorphism in purinergic P2X7 receptor gene confers reduced susceptibility to essential hypertension in Chinese postmenopausal women. Clin Exp Hypertens. 2019;41:558–563. doi: 10.1080/10641963.2018.1523914 [DOI] [PubMed] [Google Scholar]
- 36.Furman D, Chang J, Lartigue L, Bolen CR, Haddad F, Gaudilliere B, Ganio EA, Fragiadakis GK, Spitzer MH, Douchet I, et al. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat Med. 2017;23:174–184. doi: 10.1038/nm.4267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao TV, Li Y, Liu X, Xia S, Shi P, Li L, Chen Z, Yin C, Eriguchi M, Chen Y, Bernstein EA, Giani JF, Bernstein KE, Shen XZ. ATP release drives heightened immune responses associated with hypertension. Sci Immunol. 2019;4:eaau6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji X, Naito Y, Hirokawa G, Weng H, Hiura Y, Takahashi R, Iwai N. P2X(7) receptor antagonism attenuates the hypertension and renal injury in Dahl salt-sensitive rats. Hypertens Res. 2012;35:173–179. doi: 10.1038/hr.2011.153 [DOI] [PubMed] [Google Scholar]
- 39.Dalekos GN, Elisaf M, Bairaktari E, Tsolas O, Siamopoulos KC. Increased serum levels of interleukin-1beta in the systemic circulation of patients with essential hypertension: additional risk factor for atherogenesis in hypertensive patients? J Lab Clin Med. 1997;129:300–308. doi: 10.1016/s0022-2143(97)90178-5 [DOI] [PubMed] [Google Scholar]
- 40.Shirasuna K, Karasawa T, Usui F, Kobayashi M, Komada T, Kimura H, Kawashima A, Ohkuchi A, Taniguchi S, Takahashi M. NLRP3 deficiency improves angiotensin II-induced hypertension but not fetal growth restriction during pregnancy. Endocrinology. 2015;156:4281–4292. doi: 10.1210/en.2015-1408 [DOI] [PubMed] [Google Scholar]
- 41.Krishnan SM, Dowling JK, Ling YH, Diep H, Chan CT, Ferens D, Kett MM, Pinar A, Samuel CS, Vinh A, et al. Inflammasome activity is essential for one kidney/deoxycorticosterone acetate/salt-induced hypertension in mice. Br J Pharmacol. 2016;173:752–765. doi: 10.1111/bph.13230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krishnan SM, Ling YH, Huuskes BM, Ferens DM, Saini N, Chan CT, Diep H, Kett MM, Samuel CS, Kemp-Harper BK, et al. Pharmacological inhibition of the NLRP3 inflammasome reduces blood pressure, renal damage, and dysfunction in salt-sensitive hypertension. Cardiovasc Res. 2019;115:776–787. doi: 10.1093/cvr/cvy252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ling YH, Krishnan SM, Chan CT, Diep H, Ferens D, Chin-Dusting J, Kemp-Harper BK, Samuel CS, Hewitson TD, Latz E, et al. Anakinra reduces blood pressure and renal fibrosis in one kidney/DOCA/salt-induced hypertension. Pharmacol Res. 2017;116:77–86. doi: 10.1016/j.phrs.2016.12.015 [DOI] [PubMed] [Google Scholar]
- 44.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. ; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 45.Rothman AM, MacFadyen J, Thuren T, Webb A, Harrison DG, Guzik TJ, Libby P, Glynn RJ, Ridker PM. Effects of interleukin-1β inhibition on blood pressure, incident hypertension, and residual inflammatory risk: a secondary analysis of CANTOS. Hypertension. 2020;75:477–482. doi: 10.1161/HYPERTENSIONAHA.119.13642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vonend O, Turner CM, Chan CM, Loesch A, Dell’Anna GC, Srai KS, Burnstock G, Unwin RJ. Glomerular expression of the ATP-sensitive P2X receptor in diabetic and hypertensive rat models. Kidney Int. 2004;66:157–166. doi: 10.1111/j.1523-1755.2004.00717.x [DOI] [PubMed] [Google Scholar]
- 47.Mullins JJ, Peters J, Ganten D. Fulminant hypertension in transgenic rats harbouring the mouse Ren-2 gene. Nature. 1990;344:541–544. doi: 10.1038/344541a0 [DOI] [PubMed] [Google Scholar]
- 48.Moriguchi A, Brosnihan KB, Kumagai H, Ganten D, Ferrario CM. Mechanisms of hypertension in transgenic rats expressing the mouse Ren-2 gene. Am J Physiol. 1994;2664 pt 2R1273–R1279. doi: 10.1152/ajpregu.1994.266.4.R1273 [DOI] [PubMed] [Google Scholar]
- 49.Menzies RI, Howarth AR, Unwin RJ, Tam FW, Mullins JJ, Bailey MA. Inhibition of the purinergic P2X7 receptor improves renal perfusion in angiotensin-II-infused rats. Kidney Int. 2015;88:1079–1087. doi: 10.1038/ki.2015.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franco M, Bautista-Pérez R, Cano-Martínez A, Pacheco U, Santamaría J, Del Valle Mondragón L, Pérez-Méndez O, Navar LG. Physiopathological implications of P2X1 and P2X7 receptors in regulation of glomerular hemodynamics in angiotensin II-induced hypertension. Am J Physiol Renal Physiol. 2017;313:F9–F19. doi: 10.1152/ajprenal.00663.2016 [DOI] [PubMed] [Google Scholar]
- 51.Ji X, Naito Y, Weng H, Endo K, Ma X, Iwai N. P2X7 deficiency attenuates hypertension and renal injury in deoxycorticosterone acetate-salt hypertension. Am J Physiol Renal Physiol. 2012;303:F1207–F1215. doi: 10.1152/ajprenal.00051.2012 [DOI] [PubMed] [Google Scholar]
- 52.Nascimento M, Punaro GR, Serralha RS, Lima DY, Mouro MG, Oliveira LCG, Casarini DE, Rodrigues AM, Higa EMS. Inhibition of the P2X7 receptor improves renal function via renin-angiotensin system and nitric oxide on diabetic nephropathy in rats. Life Sci. 2020;251:117640 doi: 10.1016/j.lfs.2020.117640 [DOI] [PubMed] [Google Scholar]
- 53.Lemarié CA, Paradis P, Schiffrin EL. New insights on signaling cascades induced by cross-talk between angiotensin II and aldosterone. J Mol Med (Berl). 2008;86:673–678. doi: 10.1007/s00109-008-0323-5 [DOI] [PubMed] [Google Scholar]
- 54.Menzies RI, Unwin RJ, Dash RK, Beard DA, Cowley AW, Jr, Carlson BE, Mullins JJ, Bailey MA. Effect of P2X4 and P2X7 receptor antagonism on the pressure diuresis relationship in rats. Front Physiol. 2013;4:305 doi: 10.3389/fphys.2013.00305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Sanchez D, Gorelik J, Klenerman D, Lab M, Edwards C, Korchev Y. Basolateral P2X4-like receptors regulate the extracellular ATP-stimulated epithelial Na+ channel activity in renal epithelia. Am J Physiol Renal Physiol. 2007;292:F1734–F1740. doi: 10.1152/ajprenal.00382.2006 [DOI] [PubMed] [Google Scholar]
- 56.Gorelik J, Zhang Y, Sánchez D, Shevchuk A, Frolenkov G, Lab M, Klenerman D, Edwards C, Korchev Y. Aldosterone acts via an ATP autocrine/paracrine system: the Edelman ATP hypothesis revisited. Proc Natl Acad Sci USA. 2005;102:15000–15005. doi: 10.1073/pnas.0507008102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Graciano ML, Nishiyama A, Jackson K, Seth DM, Ortiz RM, Prieto-Carrasquero MC, Kobori H, Navar LG. Purinergic receptors contribute to early mesangial cell transformation and renal vessel hypertrophy during angiotensin II-induced hypertension. Am J Physiol Renal Physiol. 2008;294:F161–F169. doi: 10.1152/ajprenal.00281.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schulze-Lohoff E, Hugo C, Rost S, Arnold S, Gruber A, Brüne B, Sterzel RB. Extracellular ATP causes apoptosis and necrosis of cultured mesangial cells via P2Z/P2X7 receptors. Am J Physiol. 1998;275:F962–F971. doi: 10.1152/ajprenal.1998.275.6.F962 [DOI] [PubMed] [Google Scholar]
- 59.Ponnusamy M, Ma L, Gong R, Pang M, Chin YE, Zhuang S. P2X7 receptors mediate deleterious renal epithelial-fibroblast cross talk. Am J Physiol Renal Physiol. 2011;300:F62–F70. doi: 10.1152/ajprenal.00473.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luo W, Feldman D, McCallister R, Brophy C, Cheung-Flynn J. P2X7R antagonism after subfailure overstretch injury of blood vessels reverses vasomotor dysfunction and prevents apoptosis. Purinergic Signal. 2017;13:579–590. doi: 10.1007/s11302-017-9585-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pereira JMS, Barreira AL, Gomes CR, Ornellas FM, Ornellas DS, Miranda LC, Cardoso LR, Coutinho-Silva R, Schanaider A, Morales MM, et al. Brilliant blue G, a P2X7 receptor antagonist, attenuates early phase of renal inflammation, interstitial fibrosis and is associated with renal cell proliferation in ureteral obstruction in rats. BMC Nephrol. 2020;21:206 doi: 10.1186/s12882-020-01861-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Komalavilas P, Luo W, Guth CM, Jolayemi O, Bartelson RI, Cheung-Flynn J, Brophy CM. Vascular surgical stretch injury leads to activation of P2X7 receptors and impaired endothelial function. PLoS One. 2017;12:e0188069 doi: 10.1371/journal.pone.0188069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cario-Toumaniantz C, Loirand G, Ladoux A, Pacaud P. P2X7 receptor activation-induced contraction and lysis in human saphenous vein smooth muscle. Circ Res. 1998;83:196–203. doi: 10.1161/01.res.83.2.196 [DOI] [PubMed] [Google Scholar]
- 64.Lewis CJ, Evans RJ. P2X receptor immunoreactivity in different arteries from the femoral, pulmonary, cerebral, coronary and renal circulations. J Vasc Res. 2001;38:332–340. doi: 10.1159/000051064 [DOI] [PubMed] [Google Scholar]
- 65.Ramirez AN, Kunze DL. P2X purinergic receptor channel expression and function in bovine aortic endothelium. Am J Physiol Heart Circ Physiol. 2002;282:H2106–H2116. doi: 10.1152/ajpheart.00892.2001 [DOI] [PubMed] [Google Scholar]
- 66.Piscopiello M, Sessa M, Anzalone N, Castellano R, Maisano F, Ferrero E, Chiesa R, Alfieri O, Comi G, Ferrero ME, et al. P2X7 receptor is expressed in human vessels and might play a role in atherosclerosis. Int J Cardiol. 2013;168:2863–2866. doi: 10.1016/j.ijcard.2013.03.084 [DOI] [PubMed] [Google Scholar]
- 67.Clapp C, Diaz-Lezama N, Adan-Castro E, Ramirez-Hernandez G, Moreno-Carranza B, Sarti AC, Falzoni S, Solini A, Di Virgilio F. Pharmacological blockade of the P2X7 receptor reverses retinal damage in a rat model of type 1 diabetes. Acta Diabetol. 2019;56:1031–1036. doi: 10.1007/s00592-019-01343-4 [DOI] [PubMed] [Google Scholar]
- 68.Portillo JC, Lopez Corcino Y, Dubyak GR, Kern TS, Matsuyama S, Subauste CS. Ligation of CD40 in human müller cells induces P2X7 receptor-dependent death of retinal endothelial cells. Invest Ophthalmol Vis Sci. 2016;57:6278–6286. doi: 10.1167/iovs.16-20301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shibata M, Ishizaki E, Zhang T, Fukumoto M, Barajas-Espinosa A, Li T, Puro DG. Purinergic vasotoxicity: role of the pore/oxidant/KATP Channel/Ca(2+) pathway in P2X7-induced cell death in retinal capillaries. Vision (Basel). 2018;2:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kawamura H, Sugiyama T, Wu DM, Kobayashi M, Yamanishi S, Katsumura K, Puro DG. ATP: a vasoactive signal in the pericyte-containing microvasculature of the rat retina. J Physiol. 2003;551pt 3787–799. doi: 10.1113/jphysiol.2003.047977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mahdi A, Jiao T, Tratsiakovich Y, Yang J, Ostenson CG, Pernow J, Zhou Z. Altered purinergic receptor sensitivity in Type 2 diabetes-associated endothelial dysfunction and Up(4)A-mediated vascular contraction. Int J Mol Sci. 2018;19:3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34:575–584. doi: 10.1016/j.cjca.2017.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schiffrin EL. How structure, mechanics, and function of the vasculature contribute to blood pressure elevation in hypertension. Can J Cardiol. 2020;36:648–658. doi: 10.1016/j.cjca.2020.02.003 [DOI] [PubMed] [Google Scholar]
- 74.Oliveira SD, Coutinho-Silva R, Silva CL. Endothelial P2X7 receptors’ expression is reduced by schistosomiasis. Purinergic Signal. 2013;9:81–89. doi: 10.1007/s11302-012-9332-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chiao CW, Tostes RC, Webb RC. P2X7 receptor activation amplifies lipopolysaccharide-induced vascular hyporeactivity via interleukin-1 beta release. J Pharmacol Exp Ther. 2008;326:864–870. doi: 10.1124/jpet.107.135350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chiao CW, da Silva-Santos JE, Giachini FR, Tostes RC, Su MJ, Webb RC. P2X7 receptor activation contributes to an initial upstream mechanism of lipopolysaccharide-induced vascular dysfunction. Clin Sci (Lond). 2013;125:131–141. doi: 10.1042/CS20120479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gimbrone MA, Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118:620–636. doi: 10.1161/CIRCRESAHA.115.306301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stachon P, Heidenreich A, Merz J, Hilgendorf I, Wolf D, Willecke F, von Garlen S, Albrecht P, Härdtner C, Ehrat N, et al. P2X7 deficiency blocks lesional inflammasome activity and ameliorates atherosclerosis in mice. Circulation. 2017;135:2524–2533. doi: 10.1161/CIRCULATIONAHA.117.027400 [DOI] [PubMed] [Google Scholar]
- 79.Shi X, Zheng K, Shan P, Zhang L, Wu S, Huang Z. Elevated circulating level of P2X7 receptor is related to severity of coronary artery stenosis and prognosis of acute myocardial infarction. Cardiol J. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lombardi M, Mantione ME, Baccellieri D, Ferrara D, Castellano R, Chiesa R, Alfieri O, Foglieni C. P2X7 receptor antagonism modulates IL-1β and MMP9 in human atherosclerotic vessels. Sci Rep. 2017;7:4872 doi: 10.1038/s41598-017-05137-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paramel Varghese G, Folkersen L, Strawbridge RJ, Halvorsen B, Yndestad A, Ranheim T, Krohg-Sorensen K, Skjelland M, Espevik T, Aukrust P, Lengquist M, Hedin U, Jansson JH, Fransen K, Hansson GK, Eriksson P, Sirsjo A. NLRP3 inflammasome expression and activation in human atherosclerosis. J Am Heart Assoc. 2016;5:e003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Milner P, Bodin P, Loesch A, Burnstock G. Rapid release of endothelin and ATP from isolated aortic endothelial cells exposed to increased flow. Biochem Biophys Res Commun. 1990;170:649–656. doi: 10.1016/0006-291x(90)92141-l [DOI] [PubMed] [Google Scholar]
- 83.Burnstock G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat. 1999;194 (pt 3):335–342. doi: 10.1046/j.1469-7580.1999.19430335.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Robson SC, Kaczmarek E, Siegel JB, Candinas D, Koziak K, Millan M, Hancock WW, Bach FH. Loss of ATP diphosphohydrolase activity with endothelial cell activation. J Exp Med. 1997;185:153–163. doi: 10.1084/jem.185.1.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kanthi Y, Hyman MC, Liao H, Baek AE, Visovatti SH, Sutton NR, Goonewardena SN, Neral MK, Jo H, Pinsky DJ. Flow-dependent expression of ectonucleotide tri(di)phosphohydrolase-1 and suppression of atherosclerosis. J Clin Invest. 2015;125:3027–3036. doi: 10.1172/JCI79514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yamamoto K, Furuya K, Nakamura M, Kobatake E, Sokabe M, Ando J. Visualization of flow-induced ATP release and triggering of Ca2+ waves at caveolae in vascular endothelial cells. J Cell Sci. 2011;124:3477–3483. doi: 10.1242/jcs.087221 [DOI] [PubMed] [Google Scholar]
- 87.Green JP, Souilhol C, Xanthis I, Martinez-Campesino L, Bowden NP, Evans PC, Wilson HL. Atheroprone flow activates inflammation via endothelial ATP-dependent P2X7-p38 signalling. Cardiovasc Res. 2018;114:324–335. doi: 10.1093/cvr/cvx213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Garcia-Marcos M, Pérez-Andrés E, Tandel S, Fontanils U, Kumps A, Kabré E, Gómez-Muñoz A, Marino A, Dehaye JP, Pochet S. Coupling of two pools of P2X7 receptors to distinct intracellular signaling pathways in rat submandibular gland. J Lipid Res. 2006;47:705–714. doi: 10.1194/jlr.M500408-JLR200 [DOI] [PubMed] [Google Scholar]
- 89.Barth K, Weinhold K, Guenther A, Young MT, Schnittler H, Kasper M. Caveolin-1 influences P2X7 receptor expression and localization in mouse lung alveolar epithelial cells. FEBS J. 2007;274:3021–3033. doi: 10.1111/j.1742-4658.2007.05830.x [DOI] [PubMed] [Google Scholar]
- 90.Barth K, Weinhold K, Guenther A, Linge A, Gereke M, Kasper M. Characterization of the molecular interaction between caveolin-1 and the P2X receptors 4 and 7 in E10 mouse lung alveolar epithelial cells. Int J Biochem Cell Biol. 2008;40:2230–2239. doi: 10.1016/j.biocel.2008.03.001 [DOI] [PubMed] [Google Scholar]
- 91.Barth K, Pfleger C, Linge A, Sim JA, Surprenant A, Steinbronn N, Strasser RH, Kasper M. Increased P2X7R expression in atrial cardiomyocytes of caveolin-1 deficient mice. Histochem Cell Biol. 2010;134:31–38. doi: 10.1007/s00418-010-0716-8 [DOI] [PubMed] [Google Scholar]
- 92.Karasawa A, Michalski K, Mikhelzon P, Kawate T. The P2X7 receptor forms a dye-permeable pore independent of its intracellular domain but dependent on membrane lipid composition. Elife. 2017;6:e31186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sathanoori R, Swärd K, Olde B, Erlinge D. The ATP receptors P2X7 and P2X4 modulate high glucose and palmitate-induced inflammatory responses in endothelial cells. PLoS One. 2015;10:e0125111 doi: 10.1371/journal.pone.0125111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Solini A, Chiozzi P, Falzoni S, Morelli A, Fellin R, Di Virgilio F. High glucose modulates P2X7 receptor-mediated function in human primary fibroblasts. Diabetologia. 2000;43:1248–1256. doi: 10.1007/s001250051520 [DOI] [PubMed] [Google Scholar]
- 95.Gonnord P, Delarasse C, Auger R, Benihoud K, Prigent M, Cuif MH, Lamaze C, Kanellopoulos JM. Palmitoylation of the P2X7 receptor, an ATP-gated channel, controls its expression and association with lipid rafts. FASEB J. 2009;23:795–805. doi: 10.1096/fj.08-114637 [DOI] [PubMed] [Google Scholar]
- 96.Hung SC, Choi CH, Said-Sadier N, Johnson L, Atanasova KR, Sellami H, Yilmaz Ö, Ojcius DM. P2X4 assembles with P2X7 and pannexin-1 in gingival epithelial cells and modulates ATP-induced reactive oxygen species production and inflammasome activation. PLoS One. 2013;8:e70210 doi: 10.1371/journal.pone.0070210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bartlett R, Yerbury JJ, Sluyter R. P2X7 receptor activation induces reactive oxygen species formation and cell death in murine EOC13 microglia. Mediators Inflamm. 2013;2013:271813 doi: 10.1155/2013/271813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Westerterp M, Fotakis P, Ouimet M, Bochem AE, Zhang H, Molusky MM, Wang W, Abramowicz S, la Bastide-van Gemert S, Wang N, et al. Cholesterol efflux pathways suppress inflammasome activation, NETosis, and atherogenesis. Circulation. 2018;138:898–912. doi: 10.1161/CIRCULATIONAHA.117.032636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang S, Xie X, Lei T, Zhang K, Lai B, Zhang Z, Guan Y, Mao G, Xiao L, Wang N. Statins attenuate activation of the NLRP3 inflammasome by oxidized LDL or TNFα in vascular endothelial cells through a PXR-dependent mechanism. Mol Pharmacol. 2017;92:256–264. doi: 10.1124/mol.116.108100 [DOI] [PubMed] [Google Scholar]
- 101.Peng K, Liu L, Wei D, Lv Y, Wang G, Xiong W, Wang X, Altaf A, Wang L, He D, et al. P2X7R is involved in the progression of atherosclerosis by promoting NLRP3 inflammasome activation. Int J Mol Med. 2015;35:1179–1188. doi: 10.3892/ijmm.2015.2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mantione ME, Lombardi M, Baccellieri D, Ferrara D, Castellano R, Chiesa R, Alfieri O, Foglieni C. IL-1β/MMP9 activation in primary human vascular smooth muscle-like cells: exploring the role of TNFα and P2X7. Int J Cardiol. 2019;278:202–209. doi: 10.1016/j.ijcard.2018.12.047 [DOI] [PubMed] [Google Scholar]
- 103.Gu BJ, Wiley JS. Rapid ATP-induced release of matrix metalloproteinase 9 is mediated by the P2X7 receptor. Blood. 2006;107:4946–4953. doi: 10.1182/blood-2005-07-2994 [DOI] [PubMed] [Google Scholar]
- 104.Bhaskar V, Yin J, Mirza AM, Phan D, Vanegas S, Issafras H, Michelson K, Hunter JJ, Kantak SS. Monoclonal antibodies targeting IL-1 beta reduce biomarkers of atherosclerosis in vitro and inhibit atherosclerotic plaque formation in Apolipoprotein E-deficient mice. Atherosclerosis. 2011;216:313–320. doi: 10.1016/j.atherosclerosis.2011.02.026 [DOI] [PubMed] [Google Scholar]
- 105.Furlan-Freguia C, Marchese P, Gruber A, Ruggeri ZM, Ruf W. P2X7 receptor signaling contributes to tissue factor-dependent thrombosis in mice. J Clin Invest. 2011;121:2932–2944. doi: 10.1172/JCI46129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ming Y, Xin G, Ji B, Ji C, Wei Z, Zhang B, Zhang J, Yu K, Zhang X, Li S, et al. Entecavir as a P2X7R antagonist ameliorates platelet activation and thrombus formation. J Pharmacol Sci. 2020;144:43–51. doi: 10.1016/j.jphs.2020.07.002 [DOI] [PubMed] [Google Scholar]
- 107.Beaucage KL, Xiao A, Pollmann SI, Grol MW, Beach RJ, Holdsworth DW, Sims SM, Darling MR, Dixon SJ. Loss of P2X7 nucleotide receptor function leads to abnormal fat distribution in mice. Purinergic Signal. 2014;10:291–304. doi: 10.1007/s11302-013-9388-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ridker PM, Revkin J, Amarenco P, Brunell R, Curto M, Civeira F, Flather M, Glynn RJ, Gregoire J, Jukema JW, et al. ; SPIRE Cardiovascular Outcome Investigators. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med. 2017;376:1527–1539. doi: 10.1056/NEJMoa1701488 [DOI] [PubMed] [Google Scholar]
- 109.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, et al. ; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664 [DOI] [PubMed] [Google Scholar]
- 110.Kalogeropoulos AP, Georgiopoulou VV, Butler J. From risk factors to structural heart disease: the role of inflammation. Heart Fail Clin. 2012;8:113–123. doi: 10.1016/j.hfc.2011.08.002 [DOI] [PubMed] [Google Scholar]
- 111.Szekely Y, Arbel Y. A review of interleukin-1 in heart disease: where do we stand today? Cardiol Ther. 2018;7:25–44. doi: 10.1007/s40119-018-0104-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Akar FG. Starve a fever to heal a heart? Interleukin-18 gives new meaning to an old adage. Am J Physiol Heart Circ Physiol. 2016;311:H311–H312. doi: 10.1152/ajpheart.00445.2016 [DOI] [PubMed] [Google Scholar]
- 113.Gidlöf O, Smith JG, Melander O, Lövkvist H, Hedblad B, Engström G, Nilsson P, Carlson J, Berglund G, Olsson S, et al. A common missense variant in the ATP receptor P2X7 is associated with reduced risk of cardiovascular events. PLoS One. 2012;7:e37491 doi: 10.1371/journal.pone.0037491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Born GV, Kratzer MA. Source and concentration of extracellular adenosine triphosphate during haemostasis in rats, rabbits and man. J Physiol. 1984;354:419–429. doi: 10.1113/jphysiol.1984.sp015385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vial C, Owen P, Opie LH, Posel D. Significance of release of adenosine triphosphate and adenosine induced by hypoxia or adrenaline in perfused rat heart. J Mol Cell Cardiol. 1987;19:187–197. doi: 10.1016/s0022-2828(87)80561-8 [DOI] [PubMed] [Google Scholar]
- 116.Dolmatova E, Spagnol G, Boassa D, Baum JR, Keith K, Ambrosi C, Kontaridis MI, Sorgen PL, Sosinsky GE, Duffy HS. Cardiomyocyte ATP release through pannexin 1 aids in early fibroblast activation. Am J Physiol Heart Circ Physiol. 2012;303:H1208–H1218. doi: 10.1152/ajpheart.00251.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sandanger Ø, Ranheim T, Vinge LE, Bliksøen M, Alfsnes K, Finsen AV, Dahl CP, Askevold ET, Florholmen G, Christensen G, et al. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Cardiovasc Res. 2013;99:164–174. doi: 10.1093/cvr/cvt091 [DOI] [PubMed] [Google Scholar]
- 118.Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H, Izawa A, Takahashi Y, Masumoto J, Koyama J, et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123:594–604. doi: 10.1161/CIRCULATIONAHA.110.982777 [DOI] [PubMed] [Google Scholar]
- 119.Pomerantz BJ, Reznikov LL, Harken AH, Dinarello CA. Inhibition of caspase 1 reduces human myocardial ischemic dysfunction via inhibition of IL-18 and IL-1beta. Proc Natl Acad Sci USA. 2001;98:2871–2876. doi: 10.1073/pnas.041611398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hesse J, Leberling S, Boden E, Friebe D, Schmidt T, Ding Z, Dieterich P, Deussen A, Roderigo C, Rose CR, et al. CD73-derived adenosine and tenascin-C control cytokine production by epicardium-derived cells formed after myocardial infarction. FASEB J. 2017;31:3040–3053. doi: 10.1096/fj.201601307R [DOI] [PubMed] [Google Scholar]
- 121.Vessey DA, Li L, Kelley M. Pannexin-I/P2X 7 purinergic receptor channels mediate the release of cardioprotectants induced by ischemic pre- and postconditioning. J Cardiovasc Pharmacol Ther. 2010;15:190–195. doi: 10.1177/1074248409360356 [DOI] [PubMed] [Google Scholar]
- 122.Vessey DA, Li L, Kelley M. P2X7 receptor agonists pre- and postcondition the heart against ischemia-reperfusion injury by opening pannexin-1/P2X7 channels. Am J Physiol Heart Circ Physiol. 2011;301:H881–H887. doi: 10.1152/ajpheart.00305.2011 [DOI] [PubMed] [Google Scholar]
- 123.Vessey DA, Li L, Kelley M. Ischemic preconditioning requires opening of pannexin-1/P2X(7) channels not only during preconditioning but again after index ischemia at full reperfusion. Mol Cell Biochem. 2011;351:77–84. doi: 10.1007/s11010-011-0713-9 [DOI] [PubMed] [Google Scholar]
- 124.Adriouch S, Dox C, Welge V, Seman M, Koch-Nolte F, Haag F. Cutting edge: a natural P451L mutation in the cytoplasmic domain impairs the function of the mouse P2X7 receptor. J Immunol. 2002;169:4108–4112. doi: 10.4049/jimmunol.169.8.4108 [DOI] [PubMed] [Google Scholar]
- 125.Liu J, Li G, Peng H, Tu G, Kong F, Liu S, Gao Y, Xu H, Qiu S, Fan B, et al. Sensory-sympathetic coupling in superior cervical ganglia after myocardial ischemic injury facilitates sympathoexcitatory action via P2X7 receptor. Purinergic Signal. 2013;9:463–479. doi: 10.1007/s11302-013-9367-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tu G, Li G, Peng H, Hu J, Liu J, Kong F, Liu S, Gao Y, Xu C, Xu X, et al. P2X(7) inhibition in stellate ganglia prevents the increased sympathoexcitatory reflex via sensory-sympathetic coupling induced by myocardial ischemic injury. Brain Res Bull. 2013;96:71–85. doi: 10.1016/j.brainresbull.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 127.Kong F, Liu S, Xu C, Liu J, Li G, Li G, Gao Y, Lin H, Tu G, Peng H, et al. Electrophysiological studies of upregulated P2X7 receptors in rat superior cervical ganglia after myocardial ischemic injury. Neurochem Int. 2013;63:230–237. doi: 10.1016/j.neuint.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 128.Mezzaroma E, Toldo S, Farkas D, Seropian IM, Van Tassell BW, Salloum FN, Kannan HR, Menna AC, Voelkel NF, Abbate A. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci USA. 2011;108:19725–19730. doi: 10.1073/pnas.1108586108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yin J, Wang Y, Hu H, Li X, Xue M, Cheng W, Wang Y, Li X, Yang N, Shi Y, et al. P2X7 receptor inhibition attenuated sympathetic nerve sprouting after myocardial infarction via the NLRP3/IL-1β pathway. J Cell Mol Med. 2017;21:2695–2710. doi: 10.1111/jcmm.13185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gao H, Yin J, Shi Y, Hu H, Li X, Xue M, Cheng W, Wang Y, Li X, Li Y, et al. Targeted P2X7 R shRNA delivery attenuates sympathetic nerve sprouting and ameliorates cardiac dysfunction in rats with myocardial infarction. Cardiovasc Ther. 2017;35:e12245 doi: 10.1111/1755-5922.12245 [DOI] [PubMed] [Google Scholar]
- 131.Syed FM, Hahn HS, Odley A, Guo Y, Vallejo JG, Lynch RA, Mann DL, Bolli R, Dorn GW., 2nd. Proapoptotic effects of caspase-1/interleukin-converting enzyme dominate in myocardial ischemia. Circ Res. 2005;96:1103–1109. doi: 10.1161/01.RES.0000166925.45995.ed [DOI] [PubMed] [Google Scholar]
- 132.Merkle S, Frantz S, Schön MP, Bauersachs J, Buitrago M, Frost RJ, Schmitteckert EM, Lohse MJ, Engelhardt S. A role for caspase-1 in heart failure. Circ Res. 2007;100:645–653. doi: 10.1161/01.RES.0000260203.55077.61 [DOI] [PubMed] [Google Scholar]
- 133.Smart N, Bollini S, Dubé KN, Vieira JM, Zhou B, Davidson S, Yellon D, Riegler J, Price AN, Lythgoe MF, et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474:640–644. doi: 10.1038/nature10188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhou B, Honor LB, He H, Ma Q, Oh JH, Butterfield C, Lin RZ, Melero-Martin JM, Dolmatova E, Duffy HS, et al. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J Clin Invest. 2011;121:1894–1904. doi: 10.1172/JCI45529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.van Wijk B, Gunst QD, Moorman AF, van den Hoff MJ. Cardiac regeneration from activated epicardium. PLoS One. 2012;7:e44692 doi: 10.1371/journal.pone.0044692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ruiz-Villalba A, Simón AM, Pogontke C, Castillo MI, Abizanda G, Pelacho B, Sánchez-Domínguez R, Segovia JC, Prósper F, Pérez-Pomares JM. Interacting resident epicardium-derived fibroblasts and recruited bone marrow cells form myocardial infarction scar. J Am Coll Cardiol. 2015;65:2057–2066. doi: 10.1016/j.jacc.2015.03.520 [DOI] [PubMed] [Google Scholar]
- 137.Bracey NA, Beck PL, Muruve DA, Hirota SA, Guo J, Jabagi H, Wright JR, Jr, Macdonald JA, Lees-Miller JP, Roach D, et al. The Nlrp3 inflammasome promotes myocardial dysfunction in structural cardiomyopathy through interleukin-1β. Exp Physiol. 2013;98:462–472. doi: 10.1113/expphysiol.2012.068338 [DOI] [PubMed] [Google Scholar]
- 138.Toldo S, Mezzaroma E, Bressi E, Marchetti C, Carbone S, Sonnino C, Van Tassell BW, Abbate A. Interleukin-1β blockade improves left ventricular systolic/diastolic function and restores contractility reserve in severe ischemic cardiomyopathy in the mouse. J Cardiovasc Pharmacol. 2014;64:1–6. doi: 10.1097/FJC.0000000000000106 [DOI] [PubMed] [Google Scholar]
- 139.Adriouch S, Bannas P, Schwarz N, Fliegert R, Guse AH, Seman M, Haag F, Koch-Nolte F. ADP-ribosylation at R125 gates the P2X7 ion channel by presenting a covalent ligand to its nucleotide binding site. FASEB J. 2008;22:861–869. doi: 10.1096/fj.07-9294com [DOI] [PubMed] [Google Scholar]
- 140.Aswad F, Kawamura H, Dennert G. High sensitivity of CD4+CD25+ regulatory T cells to extracellular metabolites nicotinamide adenine dinucleotide and ATP: a role for P2X7 receptors. J Immunol. 2005;175:3075–3083. doi: 10.4049/jimmunol.175.5.3075 [DOI] [PubMed] [Google Scholar]
- 141.Weirather J, Hofmann UD, Beyersdorf N, Ramos GC, Vogel B, Frey A, Ertl G, Kerkau T, Frantz S. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res. 2014;115:55–67. doi: 10.1161/CIRCRESAHA.115.303895 [DOI] [PubMed] [Google Scholar]
- 142.Koo TY, Lee JG, Yan JJ, Jang JY, Ju KD, Han M, Oh KH, Ahn C, Yang J. The P2X7 receptor antagonist, oxidized adenosine triphosphate, ameliorates renal ischemia-reperfusion injury by expansion of regulatory T cells. Kidney Int. 2017;92:415–431. doi: 10.1016/j.kint.2017.01.031 [DOI] [PubMed] [Google Scholar]
- 143.Frantz S, Ducharme A, Sawyer D, Rohde LE, Kobzik L, Fukazawa R, Tracey D, Allen H, Lee RT, Kelly RA. Targeted deletion of caspase-1 reduces early mortality and left ventricular dilatation following myocardial infarction. J Mol Cell Cardiol. 2003;35:685–694. doi: 10.1016/s0022-2828(03)00113-5 [DOI] [PubMed] [Google Scholar]
- 144.Abbate A, Van Tassell BW, Biondi-Zoccai G, Kontos MC, Grizzard JD, Spillman DW, Oddi C, Roberts CS, Melchior RD, Mueller GH, et al. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study]. Am J Cardiol. 2013;111:1394–1400. doi: 10.1016/j.amjcard.2013.01.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, Libby P, Glynn RJ, Ridker PM. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation. 2019;139:1289–1299. doi: 10.1161/CIRCULATIONAHA.118.038010 [DOI] [PubMed] [Google Scholar]
- 146.Franke H, Günther A, Grosche J, Schmidt R, Rossner S, Reinhardt R, Faber-Zuschratter H, Schneider D, Illes P. P2X7 receptor expression after ischemia in the cerebral cortex of rats. J Neuropathol Exp Neurol. 2004;63:686–699. doi: 10.1093/jnen/63.7.686 [DOI] [PubMed] [Google Scholar]
- 147.Arbeloa J, Pérez-Samartín A, Gottlieb M, Matute C. P2X7 receptor blockade prevents ATP excitotoxicity in neurons and reduces brain damage after ischemia. Neurobiol Dis. 2012;45:954–961. doi: 10.1016/j.nbd.2011.12.014 [DOI] [PubMed] [Google Scholar]
- 148.Chu K, Yin B, Wang J, Peng G, Liang H, Xu Z, Du Y, Fang M, Xia Q, Luo B. Inhibition of P2X7 receptor ameliorates transient global cerebral ischemia/reperfusion injury via modulating inflammatory responses in the rat hippocampus. J Neuroinflammation. 2012;9:69 doi: 10.1186/1742-2094-9-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Le Feuvre RA, Brough D, Touzani O, Rothwell NJ. Role of P2X7 receptors in ischemic and excitotoxic brain injury in vivo. J Cereb Blood Flow Metab. 2003;23:381–384. doi: 10.1097/01.WCB.0000048519.34839.97 [DOI] [PubMed] [Google Scholar]
- 150.Kaiser M, Penk A, Franke H, Krügel U, Nörenberg W, Huster D, Schaefer M. Lack of functional P2X7 receptor aggravates brain edema development after middle cerebral artery occlusion. Purinergic Signal. 2016;12:453–463. doi: 10.1007/s11302-016-9511-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yanagisawa D, Kitamura Y, Takata K, Hide I, Nakata Y, Taniguchi T. Possible involvement of P2X7 receptor activation in microglial neuroprotection against focal cerebral ischemia in rats. Biol Pharm Bull. 2008;31:1121–1130. doi: 10.1248/bpb.31.1121 [DOI] [PubMed] [Google Scholar]
- 152.Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci. 2004;24:1–7. doi: 10.1523/JNEUROSCI.3792-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lu YM, Tao RR, Huang JY, Li LT, Liao MH, Li XM, Fukunaga K, Hong ZH, Han F. P2X7 signaling promotes microsphere embolism-triggered microglia activation by maintaining elevation of Fas ligand. J Neuroinflammation. 2012;9:172 doi: 10.1186/1742-2094-9-172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Park JH, Kim YC. P2X7 receptor antagonists: a patent review (2010-2015). Expert Opin Ther Pat. 2017;27:257–267. doi: 10.1080/13543776.2017.1246538 [DOI] [PubMed] [Google Scholar]
- 155.ClinicalTrialsRegister.eu. A Randomised, Double-blind, Placebo-controlled, Parallel Group, Multicentre, Phase II Study to Assess The Efficacy of AZD9056 (single oral 400 mg dose) when Administered for 4 Weeks in Patients with Moderate to Severe COPD. https://www.clinicaltrialsregister.eu/ctr-search/trial/2005-004110-32/results. Accessed July 23, 2020.
- 156.Keystone EC, Wang MM, Layton M, Hollis S, McInnes IB; D1520C00001 Study Team. Clinical evaluation of the efficacy of the P2X7 purinergic receptor antagonist AZD9056 on the signs and symptoms of rheumatoid arthritis in patients with active disease despite treatment with methotrexate or sulphasalazine. Ann Rheum Dis. 2012;71:1630–1635. doi: 10.1136/annrheumdis-2011-143578 [DOI] [PubMed] [Google Scholar]
- 157.Eser A, Colombel JF, Rutgeerts P, Vermeire S, Vogelsang H, Braddock M, Persson T, Reinisch W. Safety and efficacy of an oral inhibitor of the purinergic receptor P2X7 in adult patients with moderately to severely active crohn’s disease: a randomized placebo-controlled, double-blind, phase IIa study. Inflamm Bowel Dis. 2015;21:2247–2253. doi: 10.1097/MIB.0000000000000514 [DOI] [PubMed] [Google Scholar]
- 158.Stock TC, Bloom BJ, Wei N, Ishaq S, Park W, Wang X, Gupta P, Mebus CA. Efficacy and safety of CE-224,535, an antagonist of P2X7 receptor, in treatment of patients with rheumatoid arthritis inadequately controlled by methotrexate. J Rheumatol. 2012;39:720–727. doi: 10.3899/jrheum.110874 [DOI] [PubMed] [Google Scholar]
- 159.ClinicalTrials.gov. Study of SGM-1019 in Patients With Nonalcoholic Steatohepatitis (NASH). https://clinicaltrials.gov/ct2/show/study/NCT03676231. Accessed July 23, 2020.
- 160.Gilbert SM, Gidley Baird A, Glazer S, Barden JA, Glazer A, Teh LC, King J. A phase I clinical trial demonstrates that nfP2X7 -targeted antibodies provide a novel, safe and tolerable topical therapy for basal cell carcinoma. Br J Dermatol. 2017;177:117–124. doi: 10.1111/bjd.15364 [DOI] [PubMed] [Google Scholar]
- 161.Ali Z, Laurijssens B, Ostenfeld T, McHugh S, Stylianou A, Scott-Stevens P, Hosking L, Dewit O, Richardson JC, Chen C. Pharmacokinetic and pharmacodynamic profiling of a P2X7 receptor allosteric modulator GSK1482160 in healthy human subjects. Br J Clin Pharmacol. 2013;75:197–207. doi: 10.1111/j.1365-2125.2012.04320.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.ClinicalTrials.gov. Antidepressant Trial With P2X7 Antagonist JNJ-54175446 (ATP). https://clinicaltrials.gov/ct2/show/record/NCT04116606. Accessed July 23, 2020.
- 163.Timmers M, Ravenstijn P, Xi L, Triana-Baltzer G, Furey M, Van Hemelryck S, Biewenga J, Ceusters M, Bhattacharya A, van den Boer M, et al. Clinical pharmacokinetics, pharmacodynamics, safety, and tolerability of JNJ-54175446, a brain permeable P2X7 antagonist, in a randomised single-ascending dose study in healthy participants. J Psychopharmacol. 2018;32:1341–1350. doi: 10.1177/0269881118800067 [DOI] [PubMed] [Google Scholar]
- 164.Karasawa A, Kawate T. Structural basis for subtype-specific inhibition of the P2X7 receptor. Elife. 2016;5:e22153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bhattacharya A, Lord B, Grigoleit JS, He Y, Fraser I, Campbell SN, Taylor N, Aluisio L, O’Connor JC, Papp M, et al. Neuropsychopharmacology of JNJ-55308942: evaluation of a clinical candidate targeting P2X7 ion channels in animal models of neuroinflammation and anhedonia. Neuropsychopharmacology. 2018;43:2586–2596. doi: 10.1038/s41386-018-0141-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Danquah W, Meyer-Schwesinger C, Rissiek B, Pinto C, Serracant-Prat A, Amadi M, Iacenda D, Knop JH, Hammel A, Bergmann P, et al. Nanobodies that block gating of the P2X7 ion channel ameliorate inflammation. Sci Transl Med. 2016;8:366ra162 doi: 10.1126/scitranslmed.aaf8463 [DOI] [PubMed] [Google Scholar]
- 167.Koch-Nolte F, Eichhoff A, Pinto-Espinoza C, Schwarz N, Schäfer T, Menzel S, Haag F, Demeules M, Gondé H, Adriouch S. Novel biologics targeting the P2X7 ion channel. Curr Opin Pharmacol. 2019;47:110–118. doi: 10.1016/j.coph.2019.03.001 [DOI] [PubMed] [Google Scholar]


