Purpose of review
The last decades, anesthesia has become safer, partly due to developments in monitoring. Advanced monitoring of children under anesthesia is challenging, due to lack of evidence, validity and size constraints. Most measured parameters are proxies for end organ function, in which an anesthesiologist is actually interested. Ideally, monitoring should be continuous, noninvasive and accurate. This present review summarizes the current literature on noninvasive monitoring in noncardiac pediatric anesthesia.
Recent findings
For cardiac output (CO) monitoring, bolus thermodilution is still considered the gold standard. New noninvasive techniques based on bioimpedance and pulse contour analysis are promising, but require more refining in accuracy of CO values in children. Near-infrared spectroscopy is most commonly used in cardiac surgery despite there being no consensus on safety margins. Its place in noncardiac anesthesia has yet to be determined. Transcutaneous measurements of blood gases are used mainly in the neonatal intensive care unit, and is finding its way to the pediatric operation theatre. Especially CO2 measurements are accurate and useful.
Summary
New techniques are available to assess a child's hemodynamic and respiratory status while under anesthesia. These new monitors can be used as complementary tools together with standard monitoring in children, to further improve perioperative safety.
Keywords: bioimpedance, near-infrared spectroscopy, noninvasive monitoring, transcutaneous measurements
INTRODUCTION
Patient safety is the number one issue in anesthesiology. At present, anesthesia is absolutely safe in uncomplicated patients undergoing low-risk procedures, as improvement of monitoring modalities and anesthetics, and the preparation of the perioperative process have led to optimization of care. In general, intraoperative mortality has dramatically decreased in the last decades [1]. This overall safety has led to a change of the paradigm of anesthesia, from survival of the surgery and avoiding direct side effects into concepts based on quality of life and value-based health care. This requires a new view on monitoring to optimize organ preservation by controlling local oxygenation and metabolism.
In perioperative monitoring of pediatric patients, we face specific challenges, which postponed the development of appropriate age and size-related pediatric monitors. First, it is not always possible to get baseline measurements and some equipment is not validated for children or has size limitations. Moreover, there is no consensus on safety margins of some parameters, while goal directed monitoring in adults has already been established.
Due to rapid hemodynamic and respiratory changes under anesthesia, continuous and noninvasive monitoring would be favorable. Most parameters daily used in anesthesia are only proxies for end organ function. The brain is perhaps the most vulnerable, but also the least monitored organ. Due to the development of encephalopathy in (ex)preterm neonates requiring multiple surgeries, pediatric anesthesiologists are especially interested in brain perfusion [2]. We know that a short anesthetic in healthy children is harmless, but if this is still the case in high-risk neonates and infants undergoing multiple procedures remains unknown [3▪▪]. It is unclear what exactly happens within the brain during anesthesia, due to changes in fluid status, cerebral perfusion pressure, CO2 pressure and unknown local factors.
The current review focuses on recent developments and current evidence on noninvasive monitoring in noncardiac pediatric anesthesia. We will concentrate on cardiac output (CO), near-infrared spectroscopy (NIRS) and transcutaneous blood gas analysis as monitors that may guide our interventions to optimize end organ function of our patients.
Box 1.
no caption available
HEMODYNAMIC MONITORING
Blood pressure (BP) measured noninvasively with the oscillometry technique (NIBP) has a good correlation with intra-arterial BP (IABP), also in infants and neonates [4]. However, changing the site of measurement from the arm to another location may provide less reliable information. Large deviations are common when NIBP is measured from the leg or forearm in children under anesthesia, compared with arm NIBP. Leg NIBPs are usually lower than arm measurements in children, in contrast to higher leg NIBPs in adults. In children the soft, compliant pediatric arteries produce less augmentation of the signal than stiffer adult arteries. Also a reduced sympathetic tone and a relatively reduced blood volume in the lower limbs of small children may play a role [5▪,6–8].
Continuous noninvasive BP can be measured with a finger cuff, measuring noninvasive finger arterial pressure (FINAP) by clamping the finger artery to a constant volume and varying the counter pressure [9,10]. With the Nexfin monitor (Table 1), FINAP is reconstructed into a brachial arterial pulse pressure waveform. In children, the FINAP was reliable, with a good level of agreement for DBP and mean arterial pressure between the Nexfin and IABP. However, underestimation of Nexfin SBP was observed [11,12].
Table 1.
Devices for noninvasive hemodynamic measurements
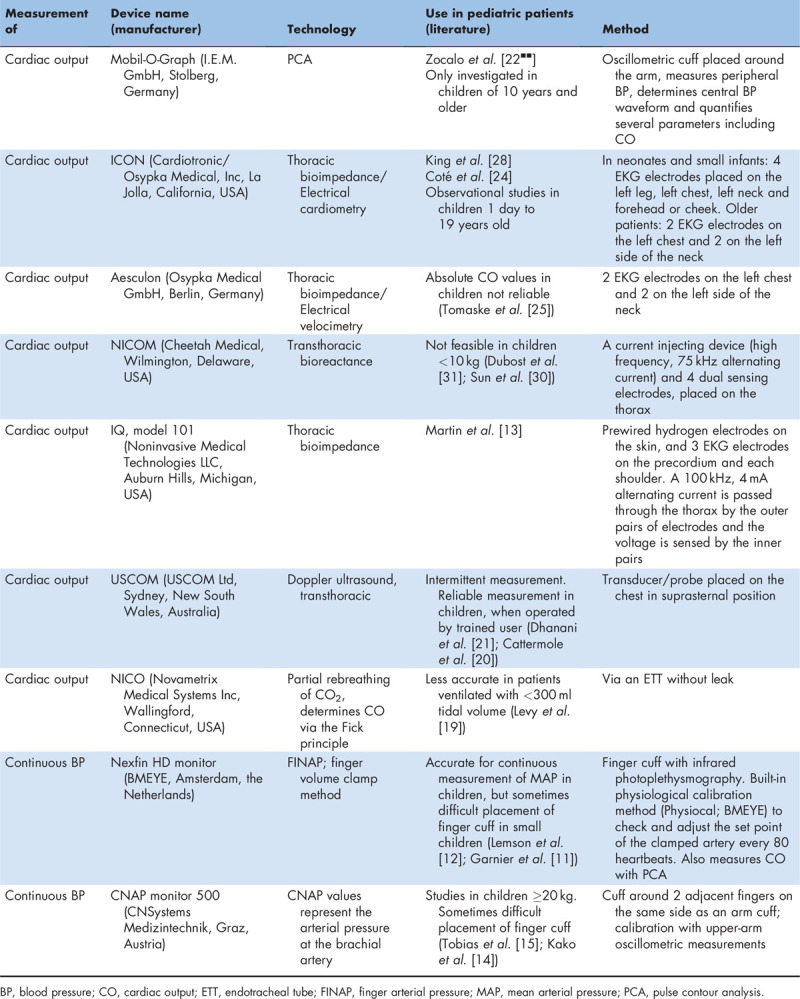
The CNAP monitor (Table 1) provides beat-to-beat noninvasive pressure readings. In pediatric patients, the continuous BP readings were clinically useful. However, there is some variation in accuracy, especially with SBPs. Cuff placement was sometimes problematic, so further development in finger cuffs for children is necessary [14,15].
CARDIAC OUTPUT MEASUREMENTS
CO is the product of cardiac stroke volume (SV) and heart rate (HR). CO is measured by transpulmonary dilution techniques, requiring central venous catheterization [16,17]. Bolus thermodilution is still the most accepted reference method [18]. Less invasive techniques have become available, such as pulse contour cardiac output analysis, arterial pressure curve-based CO measurements, transesophageal Doppler (TED) and partial rebreathing of CO2. Transthoracic echocardiography or ultrasonic monitors are noninvasive, but noncontinuous measures [16,17,19–21].
Pulse contour analysis (PCA) of IABP waveforms can estimate CO continuously [17]. PCA can be measured noninvasively with devices such as the Nexfin monitor or Mobil-O-Graph (Table 1). Pediatric studies using this method are limited. The PCA-derived CO values of the Mobil-O-Graph were measured in awake adults and children at least 10 years of age, and showed to be comparable with two-dimensional echocardiography CO values; however, the values were not interchangeable [22▪▪]. At low CO values, PCA-derived data were higher than data from echocardiography. This type of CO measurement needs further refining in accuracy and precision, before it can be used in pediatric anesthesia.
Another technique of measuring CO continuously is based on the bioimpedance method. Bioimpedance cardiography measures changes in thoracic electrical bioimpedance during the cardiac cycle via electrodes on the skin, from which SV, and subsequently CO can be calculated [23]. Several devices are on the market measuring bioimpedance, electrical velocimetry or bioreactance (Table 1).
Electrical velocimetry relates the maximum rate of change of impedance to peak aortic blood acceleration during the cardiac cycle. The change in orientation of the red blood cells in the aorta, from random during diastole (high-impedance state) to an aligned or parallel orientation during systole (low-impedance state), causes changes in electrical conductivity and electrical impedance [24]. In pediatric patients studies showed agreement, but not consistently [25–27]. Observational studies with the ICON monitor in 402 children, ranging from preterm neonates to teenagers, showed that continuous cardiovascular parameter assessment was feasible during anesthesia for patients of all sizes and that it provided useful, real-time information regarding adverse hemodynamic changes and the response to interventions [24,28].
Bioreactance is the analysis of the variation in the frequency spectra of a delivered oscillating current that occurs when the current traverses the thoracic cavity. It is less susceptible to interference than bioimpedance [17,29]. NICOM CO values showed a good correlation and agreement with echocardiography during anesthesia in pediatric patients with normal heart anatomy, but no agreement in pediatric patients with a cardiac defect [30]. In children undergoing major abdominal surgery, the NICOM showed poor correlation between confidence interval values obtained by bioreactance and TED [31].
A meta-analysis of CO monitoring devices in adults found that no noninvasive device or technology was interchangeable with bolus thermodilution; the percentage of error was 42% for bioimpedance and 45% for noninvasive PCA, where a maximum of 30% percentage of error is considered acceptable [32]. Still, the noninvasive CO monitors could be interesting bedside monitors, as the percentage of error was similar to that of minimally invasive CO monitors, such as FloTrac (Edward Lifesciences Corp., Irvine, California, USA).
NEAR-INFRARED SPECTROSCOPY
Almost 30 years after the introduction of the first commercially available NIRS monitor the value of NIRS and its applicability in pediatric anesthesia are still a matter of debate.
NIRS is still misunderstood while a short introduction to its technical background would help to use it in the best interest of patients at risk of inadequate tissue oxygenation [33,34▪,35]. NIRS provides blood flow independent real time information regarding regional tissue oxygenation (r-SO2), and the oxygen uptake/consumption balance. It should not be confused with pulse oximetry.
Cerebral NIRS monitoring has become a standard monitoring tool in many pediatric cardiac centers and neonatal ICUs. In noncardiac pediatric anesthesiology, however, NIRS has not yet become part of the standard monitoring equipment, and the price of the disposables certainly requires careful patient selection.
Despite significant scientific efforts during the last two decades aiming at the definition of normal ranges [36,37] and lower safety margins [38–41] of cerebral r-SO2 in children, consensus regarding these important targets has not yet been reached. Many pediatric anesthesiologists have adopted common adult patient intervention limits like baseline r-SO2 −20% or an absolute value less than 55% [35]. Gómez-Pesquera et al.[42▪▪] recently demonstrated the association of a decrease in cerebral r-SO2 of less than 20% and negative behavioral changes on postoperative day 7 in noncardiac pediatric patients.
Kamata et al.[43▪] reported a decrease in cerebral r-SO2 values during laparoscopic surgery in children, not reaching awake baseline levels, while hemodynamic and respiratory parameters remained unchanged. Costerus et al.[44▪] reported decreases in cerebral r-SO2 (≤10% from baseline) during neonatal thoracoscopic surgery and favorable neurodevelopmental outcome within 24 months despite severe intraoperative acidosis.
Two recent studies conducted in infants found no evidence of an effect of awake caudal [45▪] and spinal [46] anesthesia on cerebral r-SO2.
RECENT DEVELOPMENTS IN NEAR-INFRARED SPECTROSCOPY MONITORING
The list of new applications of NIRS monitoring in pediatric anesthesiology is continuously growing.
Combined cerebral and peripheral (muscle) NIRS monitoring is a new trend, with some initial evidence of its capability to detect early stage centralization [47].
The calculation of fractional regional tissue oxygen extraction [FTOE = (SaO2 − rSO2)/SaO2] [48], a composite parameter reflecting the regional oxygen delivery/consumption balance is also becoming increasingly used.
Jildenstål et al.[49▪] found an acceptable level of agreement between frontal and occipital recordings of cerebral rSO2, introducing the possibility to apply NIRS during surgical procedures where the forehead is not available for sensor placement.
Neunhoeffer et al.[50] found a positive effect of red blood cell transfusion on FTOE and cerebral r-SO2 in postsurgical infants, suggesting the feasibility of both parameters as transfusion triggers.
Smarius et al.[51▪] observed a significant reduction in cerebral r-SO2 induced by hyperextension of the neck during positioning for cleft palate repair surgery in children.
Lang et al.[52▪] found initial evidence of additional value of perioperative cerebral NIRS monitoring as a measure of intracranial pressure in symptomatic pediatric hydrocephalus patients.
NEAR-INFRARED SPECTROSCOPY DIRECTED HEMODYNAMIC MANAGEMENT
We recently developed a hemodynamic management algorithm using cerebral r-SO2 as the single target parameter, using BP, PaCO2, HR and SaO2 as major contributing parameters [34▪]. A preinduction awake baseline r-SO2 is defined as the lowest acceptable value during the anesthetic. Our experience from several hundred patients has confirmed the feasibility of this approach.
TRANSCUTANEOUS BLOOD GAS ANALYSIS
The principles of transcutaneous blood gas analysis have already been described in the late fifties by Clark and Stow-Severinghaus [53,54]. Although continuous and noninvasive, it was prone to errors compared with simpler techniques such as pulse oximetry. As the introduction of user-friendly transcutaneous sensors, their use is increasing. Especially, measurement of CO2 is reliable. This is particularly important due to the increase of video-assisted procedures. Insufflation of CO2 could lead to an increase in arterial CO2, which is a highly vasoactive substance. This is especially the case in neonates, whose brains are very sensitive for changes in CO2[55]. However, arterial blood gas analysis, despite the risks of invasive arterial lines, and capnography remain the gold standard. Transcutaneous CO2 measurement could also be useful during endoscopic airway procedures or in spontaneously breathing children without a definitive airway during procedural sedation. Therefore, further developments on the use of continuous and noninvasive measurements would be favorable.
TECHNIQUE
Transcutaneous sensors locally heat the skin improving diffusion of oxygen and CO2 through the skin [56]. This results in a close approximation of arterial values, although accuracy on oxygen measurements is restricted due to limited diffusion capacity and due to increasing skin thickness with age [57▪,58]. It is mostly used on neonatal and pediatric ICUs. However, its use in the pediatric operation theatre is limited and concerns still remain on the accuracy of measured oxygen values and its usability. Membranes of the device must be switched carefully and calibration has to be taken into account afterwards. Furthermore, a short equilibration time of 10 min after skin attachment is necessary, before measurements can be interpreted safely. Nevertheless, due to improvements in sensor application [57▪], its use perioperatively has increased. During an operation, changes in hemodynamics or fluid status and anesthetic agents as well as vasoactive medication could have effect on transcutaneous measurements by influencing the microcirculation, so doubts remain about the perioperative validity of measurements.
RECENT FINDINGS
Only few studies have been published on this subject. Nosovitch et al.[59] performed the first perioperative study in children in 2002. They concluded that of noninvasive measurements of CO2, transcutaneous values were slightly more accurate than end-tidal measurements. Dullenkopf et al.[60] compared end-tidal and transcutaneous measurements of CO2 in 60 children under general anesthesia and found no significant difference in accuracy between the two methods. Karlsson et al.[61] concluded on a relatively small group of neonates under general anesthesia that measurements where technically possible but not yet accurate.
Recently, Chandrakantan et al.[62▪▪] compared end-tidal and transcutaneous CO2 to venous blood gas values in children under 10 kg and showed that transcutaneous measured CO2 has good correlation to venous values which are slightly better than standard end-tidal CO2. May et al.[63▪] reported similar results comparing single CO2 values simultaneously obtained during arterial, venous, transcutaneous and end-tidal analysis in 47 children (mean age 13.4 ± 7.8 years old) with cystic fibrosis during anesthesia. Transcutaneous monitoring was more accurate and closer to PaCO2 than capnography.
DISCUSSION
The ultimate monitor should be easy to set up and should provide the pediatric anesthesiologist of continuous, noninvasive, accurate, reproducible and real-time measurements. Ideally, this would display end organ function.
So far, this monitor has not yet been available.
Some techniques, however, seem very promising. Regarding BP measurements and CO monitoring improvements are being made with regard to availability and accuracy in children. Further development of finger cuffs for smaller children is necessary. Although the bioimpedance technique seems very promising, drawbacks are that in young children the electrodes may be difficult to place, electrocautery induces loss of data, and arrhythmia or pleural effusion may limit its use [24,29,31]. Most importantly, more research needs to be conducted on the accuracy of the absolute CO values of these devices before it can be applied routinely during anesthesia in pediatric patients.
NIRS is not the holy grail, but it is the best currently available to continuously and noninvasively measure regional tissue-oxygenation and tissue-perfusion. Using the r-SO2 as the single outcome parameter in hemodynamic monitoring requires a paradigm shift in pediatric anesthesia toward tissue oxygenation, away from BP. Additional muscle NIRS monitoring may become the ultimate addition to ensure adequate oxygenation of all tissues.
Transcutaneous measurements are complimentary to, and not a replacement of other modalities. It is, however, a great advantage that noninvasively and continuously measurements are now available. But the gold standard for assessment of gas exchange remains blood gas analysis, and for correct tube placement capnography. In the near future more studies are required confirming validity in children under anesthesia and in areas where these measurements can contribute to safety such as laryngeal surgery, video-assisted procedures and procedural sedation.
CONCLUSION
Small steps are being made to improve the monitoring modalities in pediatric anesthesiology as new techniques are available to assess a child's hemodynamic and respiratory status while anesthetized. As perioperative safety is high nowadays, we face the challenge to take these small steps and use these new monitors as complementary tools together with standard monitoring in benefit of the most vulnerable patients.
Acknowledgements
The authors wish to thank Wichor Bramer, PhD, from the Erasmus MC Medical Library for developing and updating the search strategies, and Gail Scoones, MD, from the Department of Anesthesiology, Erasmus MC Sophia Children's Hospital, for critical appraisal of the article.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Lienhart A, Auroy Y, Pequignot F, et al. Survey of anesthesia-related mortality in France. Anesthesiology 2006; 105:1087–1097. [DOI] [PubMed] [Google Scholar]
- 2.McCann ME, Schouten AN. Beyond survival; influences of blood pressure, cerebral perfusion and anesthesia on neurodevelopment. Paediatr Anaesth 2014; 24:68–73. [DOI] [PubMed] [Google Scholar]
- 3▪▪.McCann ME, de Graaff JC, Dorris L, et al. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): an international, multicentre, randomised, controlled equivalence trial. Lancet 2019; 393:664–677. [DOI] [PMC free article] [PubMed] [Google Scholar]; Extensive multicenter study on the effects of general anesthesia in children.
- 4.Friesen RH, Lichtor JL. Indirect measurement of blood pressure in neonates and infants utilizing an automatic noninvasive oscillometric monitor. Anesth Analg 1981; 60:742–745. [PubMed] [Google Scholar]
- 5▪.Hayes S, Miller R, Patel A, et al. Comparison of blood pressure measurements in the upper and lower extremities versus arterial blood pressure readings in children under general anesthesia. Med Devices Evid Res 2019; 12:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]; The study shows that in children under anesthesia, the noninvasively with the oscillometry technique measured at the leg frequently deviated from invasive blood pressure measurements, which can be of clinical importance.
- 6.Greaney D, Nakhjavani S, Desmond F, et al. Suitability of the forearm for noninvasive blood pressure measurement in children. Paediatr Anaesth 2017; 27:1125–1130. [DOI] [PubMed] [Google Scholar]
- 7.Keidan I, Sidi A, Ben-Menachem E, et al. Inconsistency between simultaneous blood pressure measurements in the arm, forearm, and leg in anesthetized children. J Clin Anesth 2014; 26:52–57. [DOI] [PubMed] [Google Scholar]
- 8.Short JA. Noninvasive blood pressure measurement in the upper and lower limbs of anaesthetized children. Paediatr Anaesth 2000; 10:591–593. [DOI] [PubMed] [Google Scholar]
- 9.Jones RDM, Brown AG, Roulson CJ, et al. The upgraded Finapres 2300e: a clinical evaluation of a continuous noninvasive blood pressure monitor. Anaesthesia 1992; 47:701–705. [DOI] [PubMed] [Google Scholar]
- 10.Penaz J, Voigt A, Teichmann W. Contribution to the continuous indirect blood pressure measurement (Beitrag zur fortlaufenden indirekten Blutdruckmessung). Z Gesamte Inn Med 1976; 31:1030–1033. [PubMed] [Google Scholar]
- 11.Garnier RP, Van Der Spoel AGE, Sibarani-Ponsen R, et al. Level of agreement between Nexfin noninvasive arterial pressure with invasive arterial pressure measurements in children. Br J Anaesth 2012; 109:609–615. [DOI] [PubMed] [Google Scholar]
- 12.Lemson J, Hofhuizen CM, Schraa O, et al. The reliability of continuous noninvasive finger blood pressure measurement in critically ill children. Anesth Analg 2009; 108:814–821. [DOI] [PubMed] [Google Scholar]
- 13.Martin M, Brown C, Bayard D, et al. Continuous noninvasive monitoring of cardiac performance and tissue perfusion in pediatric trauma patients. J Pediatr Surg 2005; 40:1957–1963. [DOI] [PubMed] [Google Scholar]
- 14.Kako H, Corridore M, Rice J, Tobias JD. Accuracy of the CNAP™ monitor, a noninvasive continuous blood pressure device, in providing beat-to-beat blood pressure readings in pediatric patients weighing 20–40 kilograms. Paediatr Anaesth 2013; 23:989–993. [DOI] [PubMed] [Google Scholar]
- 15.Tobias JD, McKee C, Herz D, et al. Accuracy of the CNAP monitor, a noninvasive continuous blood pressure device, in providing beat-to-beat blood pressure measurements during bariatric surgery in severely obese adolescents and young adults. J Anesth 2014; 28:861–865. [DOI] [PubMed] [Google Scholar]
- 16.Lemson J, Nusmeier A, van der Hoeven JG. Advanced hemodynamic monitoring in critically ill children. Pediatrics 2011; 128:560–571. [DOI] [PubMed] [Google Scholar]
- 17.Nusmeier A, van der Hoeven JG, Lemson J. Cardiac output monitoring in pediatric patients. Expert Rev Med Devices 2010; 7:503–517. [DOI] [PubMed] [Google Scholar]
- 18.Ganz W, Donoso R, Marcus HS, et al. A new technique for measurement of cardiac output by thermodilution in man. Am J Cardiol 1971; 27:392–396. [DOI] [PubMed] [Google Scholar]
- 19.Levy RJ, Chiavacci RM, Nicolson SC, et al. An evaluation of a noninvasive cardiac output measurement using partial carbon dioxide rebreathing in children. Anesth Analg 2004; 99:1642–1647. [DOI] [PubMed] [Google Scholar]
- 20.Cattermole GN, Leung PYM, Mak PSK, et al. The normal ranges of cardiovascular parameters in children measured using the Ultrasonic Cardiac Output Monitor. Crit Care Med 2010; 38:1875–1881. [DOI] [PubMed] [Google Scholar]
- 21.Dhanani S, Barrowman NJ, Ward RE, Murto KT. Intra- and inter-observer reliability using a noninvasive ultrasound cardiac output monitor in healthy anesthetized children. Paediatr Anaesth 2011; 21:858–864. [DOI] [PubMed] [Google Scholar]
- 22▪▪.Zocalo Y, Diaz A, Bia D. Cardiac output monitoring in children, adolescents and adults based on pulse contour analysis: comparison with echocardiography-derived data and identification of factors associated with their differences. Cardiovasc Eng Technol 2020; 11:67–83. [DOI] [PubMed] [Google Scholar]; Thorough study comparing noninvasive pulse contour analysis and two-dimensional or Doppler echocardiography for cardiac output monitoring in adults and children more than 10 years of age.
- 23.Karnegis JN, Kubicek WG. Physiological correlates of the cardiac thoracic impedance waveform. Am Heart J 1970; 79:519–523. [DOI] [PubMed] [Google Scholar]
- 24.Coté CJ, Sui J, Anderson TA, et al. Continuous noninvasive cardiac output in children: is this the next generation of operating room monitors? Initial experience in 402 pediatric patients. Paediatr Anaesth 2015; 25:150–159. [DOI] [PubMed] [Google Scholar]
- 25.Tomaske M, Knirsch W, Kretschmar O, et al. Cardiac output measurement in children: comparison of Aesculon cardiac output monitor and thermodilution. Br J Anaesth 2008; 100:517–520. [DOI] [PubMed] [Google Scholar]
- 26.Noiri E, Kobayashi N, Takamura Y, et al. Pulse total-hemoglobinometer provides accurate noninvasive monitoring. Crit Care Med 2005; 33:E2831. [DOI] [PubMed] [Google Scholar]
- 27.Taylor K, Manlhiot C, McCrindle B, et al. Poor accuracy of noninvasive cardiac output monitoring using bioimpedance cardiography [PhysioFlow(R)] compared to magnetic resonance imaging in pediatric patients. Anesth Analg 2012; 114:771–775. [DOI] [PubMed] [Google Scholar]
- 28.King MR, Anderson TA, Sui J, et al. Age-related incidence of desaturation events and the cardiac responses on stroke index, cardiac index, and heart rate measured by continuous bioimpedance noninvasive cardiac output monitoring in infants and children undergoing general anesthesia. J Clin Anesth 2016; 32:181–188. [DOI] [PubMed] [Google Scholar]
- 29.Sangkum L, Liu GL, Yu L, et al. Minimally invasive or noninvasive cardiac output measurement: an update. J Anesth 2016; 30:461–480. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y, Wu C, Wu JZ, et al. Noninvasive cardiac output monitoring using bioreactance-based technique in pediatric patients with or without ventricular septal defect during anesthesia: in comparison with echocardiography. Paediatr Anaesth 2015; 25:167–173. [DOI] [PubMed] [Google Scholar]
- 31.Dubost C, Bougle A, Hallynck C, et al. Comparison of monitoring performance of bioreactance versus esophageal Doppler in pediatric patients. Indian J Crit Care Med 2015; 19:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joosten A, Desebbe O, Suehiro K, et al. Accuracy and precision of noninvasive cardiac output monitoring devices in perioperative medicine: a systematic review and meta-analysis. Br J Anaesth 2017; 118:298–310. [DOI] [PubMed] [Google Scholar]
- 33.Marin T, Moore J. Understanding near-infrared spectroscopy. Adv Neonatal Care 2011; 11:382–388. [DOI] [PubMed] [Google Scholar]
- 34▪.Weber F, Scoones GP. A practical approach to cerebral near-infrared spectroscopy (NIRS) directed hemodynamic management in noncardiac pediatric anesthesia. Paediatr Anaesth 2019; 29:993–1001. [DOI] [PubMed] [Google Scholar]; Interesting article on the background of near-infrared spectroscopy (NIRS), including a suggestion for a new treading guideline in which baseline regional cerebral oxygenation is used as the single target parameter.
- 35.Ghosh A, Elwell C, Smith M. Review article: cerebral near-infrared spectroscopy in adults: a work in progress. Anesth Analg 2012; 115:1373–1383. [DOI] [PubMed] [Google Scholar]
- 36.Alderliesten T, Dix L, Baerts W, et al. Reference values of regional cerebral oxygen saturation during the first 3 days of life in preterm neonates. Pediatr Res 2016; 79:55–64. [DOI] [PubMed] [Google Scholar]
- 37.Cohen E, Baerts W, Alderliesten T, et al. Growth restriction and gender influence cerebral oxygenation in preterm neonates. Arch Dis Child Fetal Neonatal Ed 2016; 101:F156–F161. [DOI] [PubMed] [Google Scholar]
- 38.Dent CL, Spaeth JP, Jones BV, et al. Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J Thorac Cardiovasc Surg 2005; 130:1523–1530. [DOI] [PubMed] [Google Scholar]
- 39.Kurth CD, Levy WJ, McCann J. Near-infrared spectroscopy cerebral oxygen saturation thresholds for hypoxia-ischemia in piglets. J Cereb Blood Flow Metab 2002; 22:335–341. [DOI] [PubMed] [Google Scholar]
- 40.Rescoe E, Tang X, Perry DA, et al. Cerebral near-infrared spectroscopy insensitively detects low cerebral venous oxygen saturations after stage 1 palliation. J Thorac Cardiovasc Surg 2017; 154:1056–1062. [DOI] [PubMed] [Google Scholar]
- 41.Kurth CD, McCann JC, Wu J, et al. Cerebral oxygen saturation-time threshold for hypoxic-ischemic injury in piglets. Anesth Analg 2009; 108:1268–1277. [DOI] [PubMed] [Google Scholar]
- 42▪▪.Gómez-Pesquera E, Poves-Alvarez R, Martinez-Rafael B, et al. Cerebral oxygen saturation and negative postoperative behavioral changes in pediatric surgery: a prospective observational study. J Pediatr 2019; 208:207–213.e1. [DOI] [PubMed] [Google Scholar]; Negative postoperative behavioral changes occurred in 38.8% of 198 children who underwent general anesthesia for noncardiac surgery. NIRS values were in almost all these patients less than 20% decreased from baseline measurements, which is a commonly used safety margin in adult perioperative care.
- 43▪.Kamata M, Hakim M, Walia H, et al. Changes in cerebral and renal oxygenation during laparoscopic pyloromyotomy. J Clin Monit Comput 2019; 34:699–703. [DOI] [PubMed] [Google Scholar]; The study showed statistical changes in cerebral regional tissue oxygenation during laparoscopic surgery in 25 neonates. The specific parameter which is responsible for these changes could not be identified.
- 44▪.Costerus S, Vlot J, Van Rosmalen J, et al. Effects of neonatal thoracoscopic surgery on tissue oxygenation: a pilot study on (neuro-) monitoring and outcomes. Eur J Pediatr Surg 2019; 29:166–172. [DOI] [PubMed] [Google Scholar]; This was a pilot study in 10 patients to show that neurodevelopmental outcomes were in normal range despite severe intraoperative acidosis. Cerebral regional tissue oxygenation was in acceptable limits from baseline values, suggesting a predictive value of NIRS monitoring.
- 45▪.Beck CE, Sumpelmann R, Nickel K, et al. Systemic and regional cerebral perfusion in small infants undergoing minor lower abdominal surgery under awake caudal anaesthesia: an observational study. Eur J Anaesthesiol 2020; 37:696–700. [DOI] [PubMed] [Google Scholar]; The authors conducted a study on 20 children for minor surgery under awake caudal anesthesia. No changes in cerebral regional tissue oxygenation were found, just as for blood pressure.
- 46.Froyshteter AB, Tumin D, Whitaker EE, et al. Changes in tissue and cerebral oxygenation following spinal anesthesia in infants: a prospective study. J Anesth 2018; 32:288–292. [DOI] [PubMed] [Google Scholar]
- 47.Pichler G, Holler N, Baik-Schneditz N, et al. Avoiding arterial hypotension in preterm neonates (AHIP) – a single center randomised controlled study investigating simultaneous near infrared spectroscopy measurements of cerebral and peripheral regional tissue oxygenation and dedicated interventions. Front Pediatr 2018; 6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vanderhaegen J, Naulaers G, Vanhole C, et al. The effect of changes in tPCO2 on the fractional tissue oxygen extraction – as measured by near-infrared spectroscopy – in neonates during the first days of life. Eur J Paediatr Neurol 2009; 13:128–134. [DOI] [PubMed] [Google Scholar]
- 49▪.Jildenstål P, Sandin J, WarrenStomberg M, et al. Agreement between frontal and occipital regional cerebral oxygen saturation in infants during surgery and general anesthesia an observational study. Paediatr Anaesth 2019; 29:1122–1127. [DOI] [PubMed] [Google Scholar]; It could be challenging in pediatric anesthesia to place frontal sensors of any kind. This study compares occipital with frontal placement of NIRS sensors in 15 children under 1 year of age. The authors found an acceptable agreement.
- 50.Neunhoeffer F, Hofbeck M, Schuhmann MU, et al. Cerebral oxygen metabolism before and after RBC transfusion in infants following major surgical procedures. Pediatr Crit Care Med 2018; 19:318–327. [DOI] [PubMed] [Google Scholar]
- 51▪.Smarius BJA, Breugem CC, Boasson MP, et al. Effect of hyperextension of the neck (rose position) on cerebral blood oxygenation in patients who underwent cleft palate reconstructive surgery: prospective cohort study using near-infrared spectroscopy. Clin Oral Investig 2020; 24:2909–2918. [DOI] [PubMed] [Google Scholar]; Positioning of a patient's head could reduce cerebral blood flow. In 34 patients, these authors showed a significant drop of cerebral regional tissue oxygenation. There were, however, no neurological problems postoperatively.
- 52▪.Lang SS, Khanna O, Atkin NJ, et al. Perioperative near-infrared spectroscopy cerebral oxygen saturation in symptomatic pediatric hydrocephalus patients at risk for intracranial hypertension. J Neurosurg Pediatr 2019; 1–7. Online ahead of print. [DOI] [PubMed] [Google Scholar]; The authors are searching for a noninvasive way to monitor intracranial pressure in patients who are at risk for intracranial hypertension. NIRS could be of added value as suggested in this article. In 22 patients, cerebral regional tissue oxygenation improved after drainage of liquor.
- 53.Severinghaus JW, Bradley AF. Electrodes for blood pO2 and pCO2 determination. J Appl Physiol 1958; 13:515–520. [DOI] [PubMed] [Google Scholar]
- 54.Stow RW, Baer RF, Randall BF. Rapid measurement of the tension of carbon dioxide in blood. Arch Phys Med Rehabil 1957; 38:646–650. [PubMed] [Google Scholar]
- 55.McKee LA, Fabres J, Howard G, et al. PaCO2 and neurodevelopment in extremely low birth weight infants. J Pediatr 2009; 155:217–221.e1. [DOI] [PubMed] [Google Scholar]
- 56.Lubbers DW. Theory and development of transcutaneous oxygen pressure measurement. Int Anesthesiol Clin 1987; 25:31–65. [DOI] [PubMed] [Google Scholar]
- 57▪.van Weteringen W, Goos TG, van Essen T, et al. Novel transcutaneous sensor combining optical tcPO2 and electrochemical tcPCO2 monitoring with reflectance pulse oximetry. Med Biol Eng Comput 2020; 58:239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]; Clear article about the technical specifications of transcutaneous sensors including a new sensor combining different techniques.
- 58.Hansen TN, Sonoda Y, McIlroy MB. Transfer of oxygen, nitrogen, and carbon dioxide through normal adult human skin. J Appl Physiol Respir Environ Exerc Physiol 1980; 49:438–443. [DOI] [PubMed] [Google Scholar]
- 59.Nosovitch MA, Johnson JO, Tobias JD. Noninvasive intraoperative monitoring of carbon dioxide in children: endtidal versus transcutaneous techniques. Paediatr Anaesth 2002; 12:48–52. [DOI] [PubMed] [Google Scholar]
- 60.Dullenkopf A, Di Bernardo S, Berger F, et al. Evaluation of a new combined SpO2/PtcCO2 sensor in anaesthetized paediatric patients. Paediatr Anaesth 2003; 13:777–784. [DOI] [PubMed] [Google Scholar]
- 61.Karlsson V, Sporre B, Agren J. Transcutaneous PCO2 monitoring in newborn infants during general anesthesia is technically feasible. Anesth Analg 2016; 123:1004–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62▪▪.Chandrakantan A, Jasiewicz R, Reinsel RA, et al. Transcutaneous CO2 versus end-tidal CO2 in neonates and infants undergoing surgery: a prospective study. Med Devices (Auckl) 2019; 12:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of two most recent studies in children under general anesthesia with transcutaneous measurements. The investigators conclude that transcutaneous CO2 measurements are more reliable than end-tidal measurements.
- 63▪.May A, Humston C, Rice J, et al. Noninvasive carbon dioxide monitoring in patients with cystic fibrosis during general anesthesia: end-tidal versus transcutaneous techniques. J Anesth 2020; 34:66–71. [DOI] [PubMed] [Google Scholar]; Study in 47 children with cystic fibrosis showing that transcutaneous measurements of CO2 are more accurate than capnography.


