Supplemental Digital Content is available in the text.
Keywords: eicosapentaenoic acid, icosapent ethyl, myocardial revascularization, prevention & control
Abstract
Background:
Patients with elevated triglycerides despite statin therapy have increased risk for ischemic events, including coronary revascularizations.
Methods:
REDUCE-IT (The Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial), a multicenter, double-blind, placebo-controlled trial, randomly assigned statin-treated patients with elevated triglycerides (135–499 mg/dL), controlled low-density lipoprotein (41–100 mg/dL), and either established cardiovascular disease or diabetes plus other risk factors to receive icosapent ethyl 4 g/d or placebo. The primary and key secondary composite end points were significantly reduced. Prespecified analyses examined all coronary revascularizations, recurrent revascularizations, and revascularization subtypes.
Results:
A total of 8179 randomly assigned patients were followed for 4.9 years (median). First revascularizations were reduced to 9.2% (22.5/1000 patient-years) with icosapent ethyl versus 13.3% (33.7/1000 patient-years) with placebo (hazard ratio, 0.66 [95% CI, 0.58–0.76]; P<0.0001; number needed to treat for 4.9 years=24); similar reductions were observed in total (first and subsequent) revascularizations (negative binomial rate ratio, 0.64 [95% CI, 0.56–0.74]; P<0.0001), and across elective, urgent, and emergent revascularizations. Icosapent ethyl significantly reduced percutaneous coronary intervention (hazard ratio, 0.68 [95% CI, 0.59–0.79]; P<0.0001) and coronary artery bypass grafting (hazard ratio, 0.61 [95% CI, 0.45–0.81]; P=0.0005).
Conclusions:
Icosapent ethyl reduced the need for first and subsequent coronary revascularizations in statin-treated patients with elevated triglycerides and increased cardiovascular risk. To our knowledge, icosapent ethyl is the first non–low-density lipoprotein–lowering treatment that has been shown to reduce coronary artery bypass grafting in a blinded, randomized trial.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT01492361.
Clinical Perspective.
What Is New?
Icosapent ethyl reduced first and total coronary revascularization in patients with elevated triglycerides and high cardiovascular risk, despite well-controlled low-density lipoprotein cholesterol on a statin.
This appears to be the first non–low-density lipoprotein cholesterol intervention shown to reduce coronary artery bypass grafting in a randomized, blinded clinical trial.
What Are the Clinical Implications?
Patients with elevated triglycerides have significant residual risk for coronary revascularization, despite having a well-controlled low-density lipoprotein cholesterol on a statin.
Icosapent ethyl has been shown to significantly reduce first and total cardiovascular events in this patient population, including percutaneous coronary intervention and coronary artery bypass grafting.
These findings are applicable to a significant proportion of primary and secondary cardiac risk prevention populations.
Reduction of low-density lipoprotein cholesterol (LDL-C) has been extensively documented to prevent cardiovascular events, including coronary revascularization.1–6 In addition, hypertriglyceridemia is thought to play an important role in the development and progression of coronary plaque and inflammation contributing to residual coronary atherosclerosis.7–16 However, in many clinical trials, omega-3 fatty acids, fibrates, and niacin have not shown a consistent reduction in coronary revascularization events, despite their ability to lower triglyceride levels.17–23 A moderate dose (1.8 g/d) of a highly purified ethyl-ester form of eicosapentaenoic acid (EPA), on the other hand, showed a reduction in coronary events in an open-label, prospective randomized blinded end point trial.24,25
REDUCE-IT (The Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial) enrolled 8179 statin-treated patients randomly assigned 1:1 to either 4 g/d icosapent ethyl (a highly purified, stable prescription ethyl ester of EPA) or matching placebo, with a median follow-up time of 4.9 years. Of randomly assigned patients, 70.7% had established cardiovascular disease, with or without diabetes, and 29.3% had diabetes and additional risk factors. REDUCE-IT demonstrated a 25% relative risk reduction in the primary end point and a 26% relative risk reduction in the key secondary end point (both P<0.0001) with icosapent ethyl versus placebo.26–28 This benefit of icosapent ethyl was seen both with first and subsequent cardiovascular events, with primary end point total events reduced by a significant 32% (P<0.0001).29 These results were consistent across various subgroups, including those with normal triglycerides, suggesting broad generalizability.30,31 Patients were also less likely to undergo urgent or emergent coronary revascularization as a composite end point if they were treated with icosapent ethyl.26
The aim of this analysis was to further explore the effects of icosapent ethyl versus placebo on any type of first and total (first plus subsequent) coronary revascularization events, and subtypes of coronary revascularization procedures, as well, among REDUCE-IT patients.
Methods
Study Design and Patient Characteristics
The data that support the findings of this study may be made available from the corresponding author on reasonable request. The study design and main results of the REDUCE-IT trial have been published previously.26,32 In brief, REDUCE-IT was a phase 3b, double-blind, randomized, placebo-controlled trial in which patients were treated with icosapent ethyl 4 g/d (taken as 2 g twice daily) or matching placebo. Patients were randomly assigned on a 1:1 basis. All patients provided verbal and written informed consent, and all sites were approved by institutional review boards.
Patients were included in the secondary prevention group if they had established cardiovascular disease. Patients were included in the primary prevention group if they had type 1 or type 2 diabetes on medical treatment, were ≥50 years of age, and had at least 1 other major cardiovascular risk factor. In addition, all patients were required to be on stable statin therapy for at least 4 weeks and have an LDL-C between 41 and 100 mg/dL. Patients were included if they had triglycerides between 135 and 500 mg/dL. Patients were excluded if they had severe heart failure, planned coronary intervention or surgery, severe liver disease, a hemoglobin A1c level >10%, a history of pancreatitis, or known hypersensitivity to fish, shellfish, or ingredients of icosapent ethyl or placebo.
End Points and Follow-Up
The primary composite end point of the REDUCE-IT trial was 5-point major adverse cardiovascular event (cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or unstable angina). For this analysis, we further analyzed the end point of coronary revascularization. In this article we report the prespecified analysis of all first coronary revascularization events, which included the subtypes of elective, urgent, emergent, and salvage revascularization procedures, and both categories of procedure, percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG), as well. Revascularization procedures were defined as elective when performed on an outpatient basis or during subsequent hospitalization without significant risk of myocardial infarction or death, or when performed on stable, hospitalized patients for convenience, not because the patient’s clinical situation demanded it before discharge. Procedures were considered urgent when performed on hospitalized patients before discharge because of significant concerns of myocardial ischemia risk, myocardial infarction, or death, or when performed on outpatients or patients who were in the emergency department at the time the procedure was requested and clinical presentation warranted hospital admission. Revascularizations were considered emergent when performed as soon as possible because of substantial concerns that ongoing myocardial ischemia or myocardial infarction could lead to death. A procedure was considered salvage when the patient was in cardiogenic shock as the procedure began or within the last 10 minutes of the diagnostic portion of the case, and the patient had also received chest compressions or had been on unanticipated circulatory support. Each subtype of revascularization, and elective coronary revascularization after 30 days, as well, were prespecified to be analyzed as individual end points. Analyses for time to first PCI and CABG as individual end points were post hoc. Baim Institute for Clinical Research performed the adjudication of revascularization. Individual subtypes of revascularization were determined by blinded adjudicators (elective, urgent, emergent, or salvage), as were categories of procedure (CABG or PCI).
Statistical Analysis
This analysis of revascularization events was prespecified in the study protocol. Baseline characteristics were compared among groups using the χ2 test for categorical variables and using the Wilcoxon rank sum test for continuous variables. Hazard ratios and 95% CIs were generated using a Cox proportional hazards model using treatment group as the covariate, and stratified by geographic region, cardiovascular risk category, and use of ezetimibe. Log-rank test statistics and P values were generated from Kaplan-Meier analyses of the individual end points, also stratified by geographic region, cardiovascular risk category, and the use of ezetimibe. P values and 95% CIs were not adjusted for multiple comparisons, and thus should be considered hypothesis generating.
As with the previously published analysis of total events with respect to the 5-point major adverse cardiovascular event primary end point and key secondary end point, we used various statistical methods in ascertaining the rate of recurrent revascularization events using both full (all events) and reduced (collapsing related events occurring close in time) data sets.29 We used the negative binomial regression model to calculate rates and rate ratios for total cardiovascular events. As supportive analyses, we used the modified Wei-Lin-Weissfeld method (Li and Lagakos modification) to calculate hazard ratios for the time to the first, second, or third event.33 Also, the Andersen-Gill model using a Cox proportional hazard with the counting-process formulation was performed to model the total events. All statistical analyses were conducted using SAS 9.4.
Results
Patient Characteristics
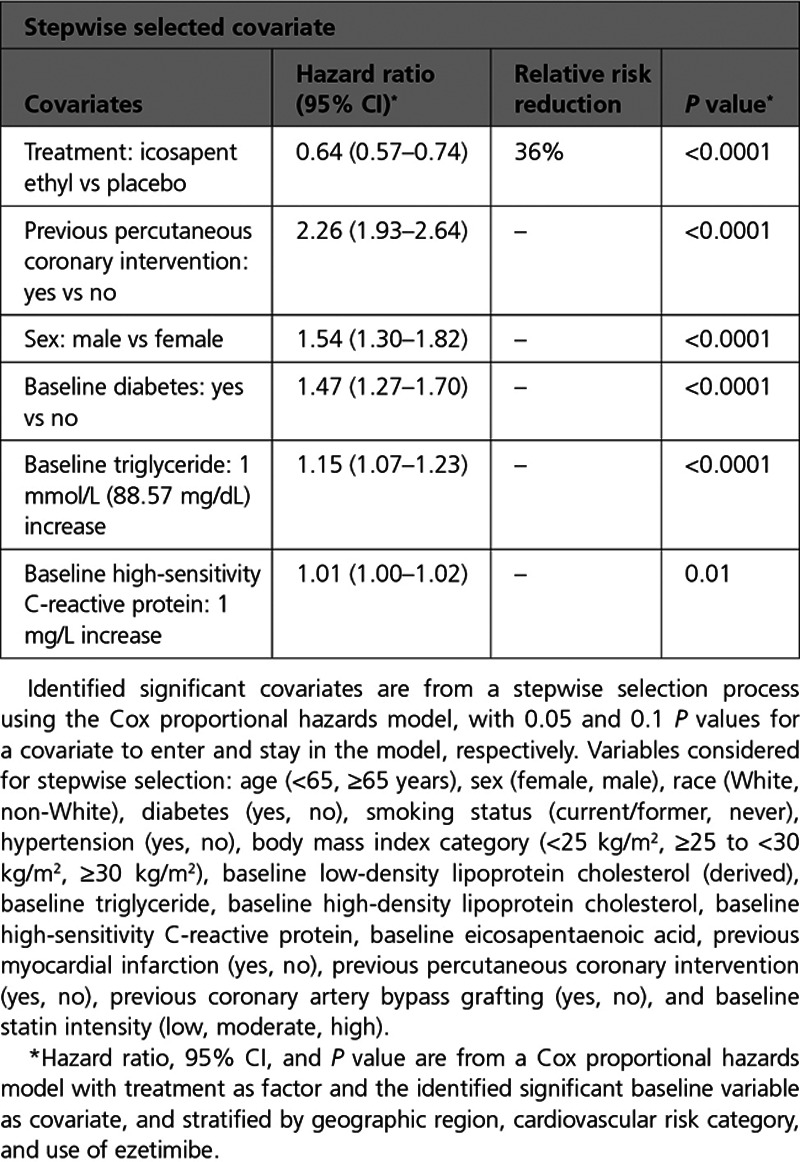
Baseline characteristics of patients who did or did not experience a postrandomization coronary revascularization event are listed in Table I in the Data Supplement. Randomization to icosapent ethyl was independently associated with a significant 36% reduction in coronary revascularization risk (P<0.0001). Independent predictors of higher coronary revascularization risk were previous PCI, male sex, baseline diabetes, elevated baseline triglyceride, and elevated baseline high sensitivity C-reactive protein levels (Table 1).
Table 1.
Independent Predictors of Revascularization
First Coronary Revascularization Events
Over a 4.9-year median follow-up, icosapent ethyl significantly reduced first coronary revascularizations by 34%, with an absolute risk reduction of 4.1% and a number needed to treat of 24 (Table 2, Figure 1). A signal of efficacy was observed early; the statistically significant difference between the treatment arms for time to first coronary revascularization became sustained at 11 months of treatment (Table II in the Data Supplement; Figure 2). There were similar statistically significant and clinically meaningful relative risk reductions of ≥32% in time to first occurrences of elective, urgent, or emergent revascularizations as individual or composite end points (Table 2, Figure 3).
Table 2.
First Coronary Revascularization End Points: Icosapent Ethyl Versus Placebo
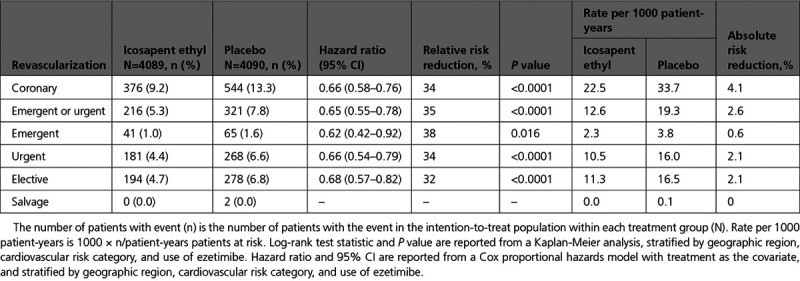
Figure 1.
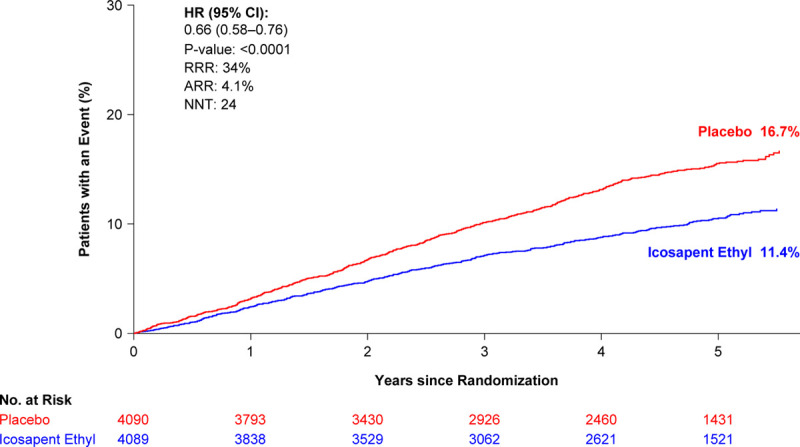
Kaplan-Meier curve of time to coronary revascularization from randomization: intention-to-treat population. The curves were visually truncated at 5.7 years because a limited number of events occurred beyond that time point; all patient data were included in the analyses. ARR is based on the observed rates of events of 9.2% for icosapent ethyl and 13.3% for placebo. ARR indicates absolute risk reduction; HR, hazard ratio; NNT, number needed to treat; No. at risk, number of patients at risk; and RRR, relative risk reduction.
Figure 2.
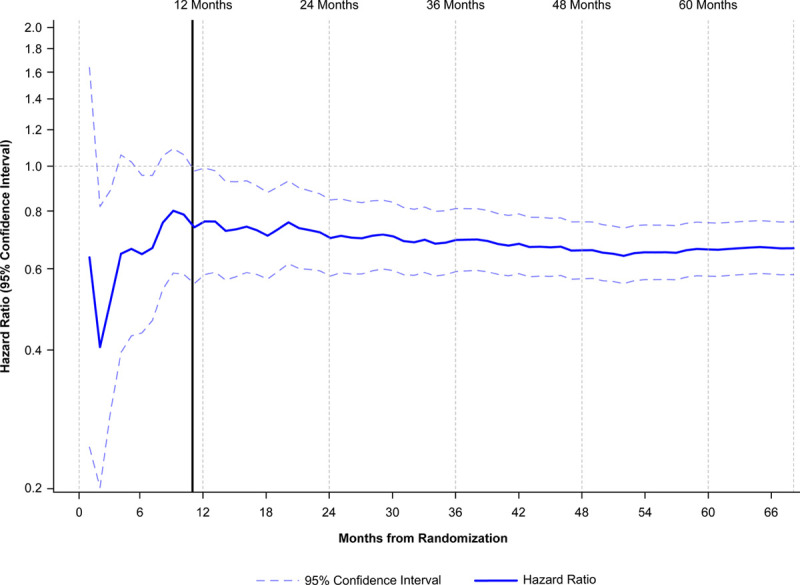
Time to coronary revascularization from date of randomization by 1-month increments: intention-to-treat population. This figure was constructed by estimating the hazard ratio and 95% CI of the coronary revascularization end point repeatedly with a stratified Cox proportional hazards model (ie, stratified by the 3 randomization factors of REDUCE-IT (The Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial): geographic region, cardiovascular risk stratum, and ezetimibe use) by censoring time-to-event data from 30 days until 6 years, with a gap of 30 days between each model. All hazard ratios and the upper and lower limits of 95% CI over the time from 30 days to 6 years were plotted accordingly.
Figure 3.
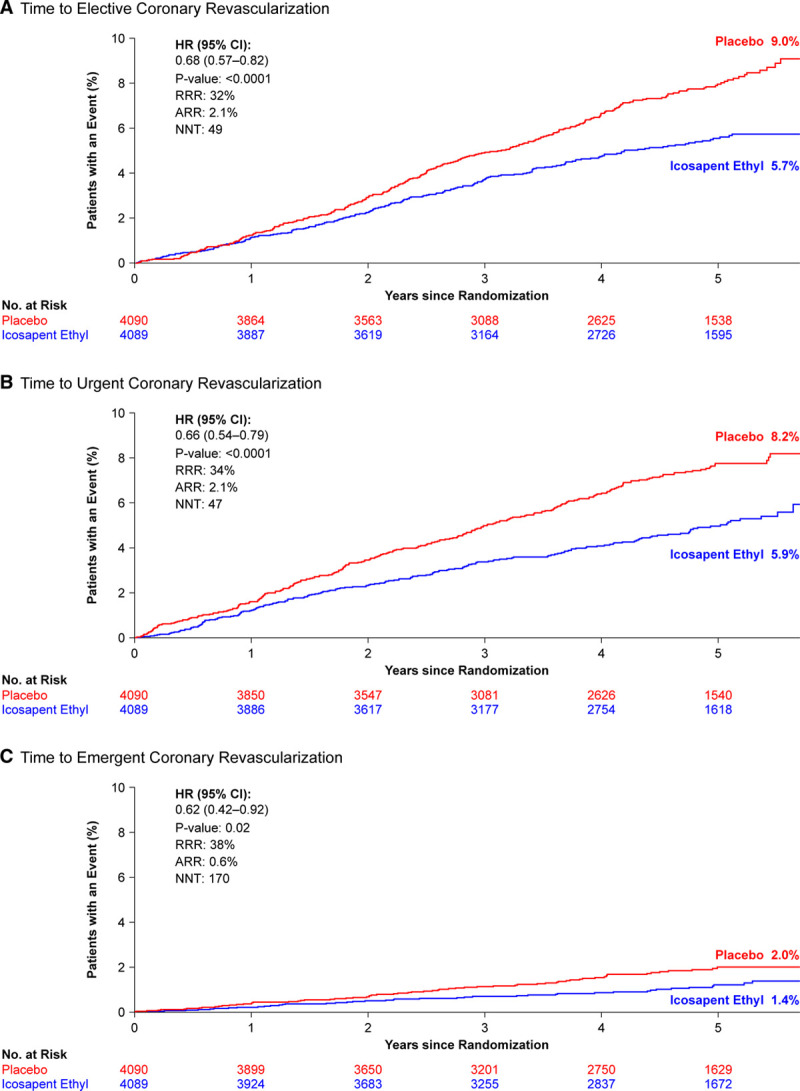
Kaplan-Meier curves: intention-to-treat population. Kaplan-Meier curves of the time from randomization to elective (A), urgent (B), and emergent (C) coronary revascularization. The curves were visually truncated at 5.7 years because a limited number of events occurred beyond that time point; all patient data were included in the analyses. Time to elective coronary revascularization ARR is based on the observed event rates of 4.7% for icosapent ethyl and 6.8% for placebo. Time to urgent coronary revascularization ARR is based on the observed rates of 4.4% for icosapent ethyl and 6.6% for placebo. Time to emergent coronary revascularization ARR is based on the observed event rates of 1.0% for icosapent ethyl and 1.6% for placebo. ARR indicates absolute risk reduction; HR, hazard ratio; NNT, number needed to treat; No. at risk, number of patients at risk; and RRR, relative risk reduction.
Consistent with the results in all revascularization and across subtypes, there was a significant reduction in the risk of undergoing PCI among patients treated with icosapent ethyl versus placebo (7.7% versus 10.9%; hazard ratio [HR], 0.68 [95% CI, 0.59–0.79]; P<0.0001), with an absolute risk reduction of 3.2% and a number needed to treat of 31. In addition, there was a significant reduction in the number of patients who underwent CABG (1.9% versus 3.0%; HR, 0.61 [95% CI, 0.45–0.81]; P=0.0005), with an absolute risk reduction of 1.1% and a number needed to treat of 87 (Figure 4).
Figure 4.
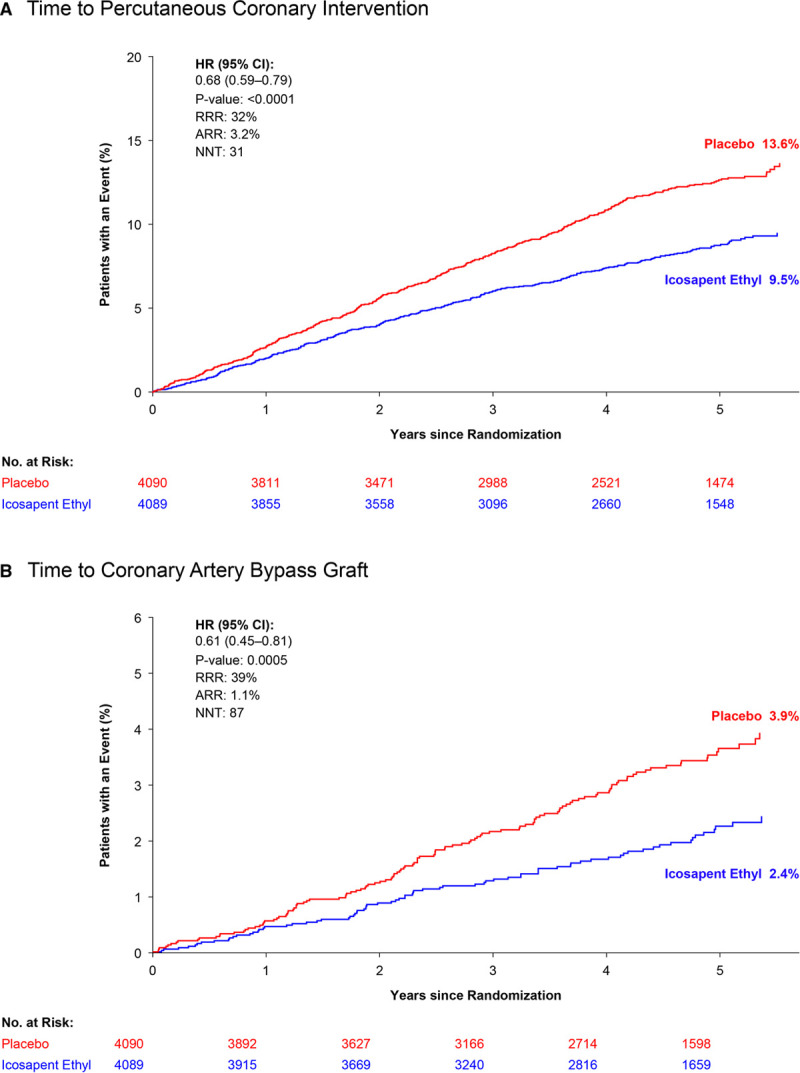
Kaplan-Meier curves: intention-to-treat population. Kaplan-Meier curves of time from randomization to percutaneous coronary intervention (A) or coronary artery bypass graft surgery (B). The curves were visually truncated at 5.7 years because a limited number of events occurred beyond that time point; all patient data were included in the analyses. Time to percutaneous coronary intervention ARR is based on the observed event rates of 7.7% for icosapent ethyl and 10.9% for placebo. Time to coronary artery bypass graft ARR is based on the observed event rates of 1.9% for icosapent ethyl and 3.0% for placebo. ARR indicates absolute risk reduction; HR, hazard ratio; NNT, number needed to treat; No. at risk, number of patients at risk; and RRR, relative risk reduction.
Efficacy Analyses Excluding Early Coronary Revascularizations
Clinical events that occur within the first 30 to 90 days of therapy are often excluded from analyses, either because these events may be occurring as a consequence of a previous recent event (eg, in studies enrolling patients with recent acute coronary events) or because there may be a delay in clinical benefit.3 We explored various efficacy analyses by excluding coronary revascularizations that occurred within the first 30 or 90 days of randomization. The observed relative risk reduction in the primary end point (Figure 1) was not substantially impacted by the exclusion of elective coronary revascularizations that occurred in the first 90 days after study enrollment (Figure I in the Data Supplement) or by excluding elective coronary revascularizations that occurred within the first 30 days (Figure II in the Data Supplement). Likewise, the relative risk reduction observed in time to the first elective coronary revascularization (Figure 3A) did not substantially change when excluding elective coronary revascularizations occurring in the first 90 days or the first 30 days after randomization (Figures III and IV in the Data Supplement). Although interaction P values suggest that some patients may experience greater benefit, results with respect to time to coronary revascularization were generally consistent across subgroups of patients by baseline characteristics (Figure V in the Data Supplement). The relative risk reductions in men and women were consistent (Pinteraction=0.63), although, because of the higher rates of revascularization in the placebo arm, the absolute risk reductions were numerically about twice as large in men as in women. The relative risk reductions for coronary revascularization with icosapent ethyl were consistent (Pinteraction=0.07) across tertiles of baseline triglyceride levels: 24%, 28%, and 46%, respectively, for lowest, middle, and highest tertiles.
Total Coronary Revascularization Events
Among the 8179 randomly assigned patients, there were 920 (76.4%) first-event coronary revascularizations and 284 (23.6%) additional revascularization events (196 second events, 52 third events, and 36 fourth or greater events) for a total of 1204 events (Table IIIA and Figure VI in the Data Supplement). The 920 first revascularization events represent 11.2% of the overall REDUCE-IT patient population. Of the subsequent events, 97 (34.2%) were from patients assigned to icosapent ethyl and 187 (65.8%) were from patients assigned to placebo. Overall, total revascularization events were reduced among patients taking icosapent ethyl versus placebo, using the negative binomial model when accounting for statistical handling of multiple end points occurring in a single calendar day by counting as a single event (rate ratio, 0.64 [95% CI, 0.56–0.74]; P<0.0001; Figure 5). Other models demonstrated similar findings: the Andersen and Gill model (HR, 0.64 [95% CI, 0.57–0.71]; P<0.0001) and the proportional means model (HR, 0.64 [95% CI, 0.55–0.73]; P<0.0001; Table III in the Data Supplement). Using the modified Wei-Lin-Weissfeld model, first occurrence events were reduced among patients taking icosapent ethyl versus placebo (HR, 0.67 [95% CI, 0.58–0.76]; P<0.0001), as were second occurrences (HR, 0.49 [95% CI, 0.36–0.66]; P<0.0001), and third or more occurrence events (rate ratio, 0.50 [95% CI, 0.26–0.97]; P=0.04; Table IIIB in the Data Supplement; Figure 5). Similar results were obtained when using the full data set (ie, including all events regardless of related events occurring close in time; Figure VI in the Data Supplement).
Figure 5.
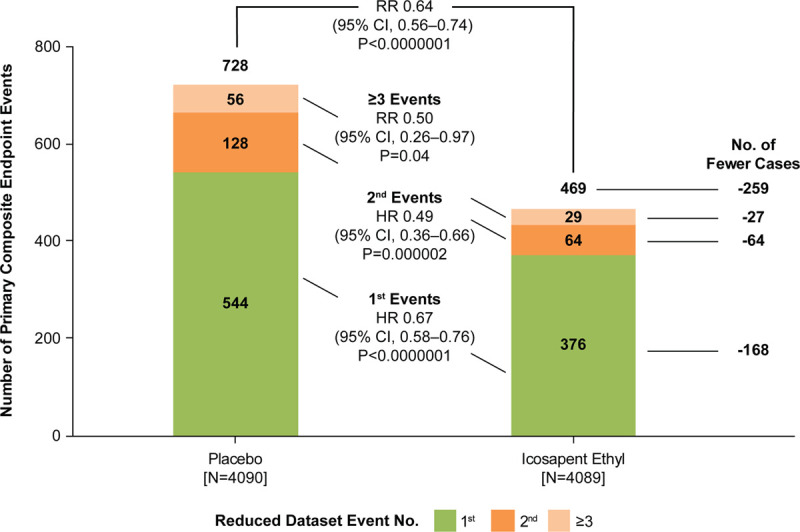
Distribution of first and subsequent coronary revascularization events in the reduced data set for patients randomly assigned 1:1 to icosapent ethyl vs placebo. Hazard ratios (HRs) and 95% CIs for between-group comparisons were generated using Li-Lagakos–modified Wei-Lin-Weissfeld method for first and second event categories. Rate ratio (RR) and 95% CI for group comparisons used a negative binomial model for additional events beyond first and second occurrences, that is, third event or more and overall treatment comparison. Analyses are based on reduced data set accounting for statistical handling of multiple end points occurring in a single calendar day by counting as a single event.
Although at randomization there was no difference in EPA levels between groups, beginning at day 360, consistently and statistically significantly higher postbaseline EPA levels were observed in patients without coronary revascularization than in those who underwent coronary revascularization (Table IV in the Data Supplement).
Discussion
This analysis demonstrates a 34% relative reduction in coronary revascularization events with icosapent ethyl versus placebo among patients at high cardiovascular risk with elevated triglycerides despite appropriate treatment with a statin. This effect was consistent among different subtypes of revascularization, including elective, urgent, and emergent coronary revascularization. Also, using various methods of total events analysis, we found a significant reduction not only in first, but also subsequent revascularizations. In this blinded trial with independent adjudication of end points, there was a significant reduction in the need for CABG, objectively demonstrating the substantial decrease in atherosclerotic burden in this population of patients. It is interesting to note that, irrespective of the effects of icosapent ethyl, 11.2% of these stable but high-risk patients (primary and secondary prevention) required coronary revascularization over a 4.9-year median follow-up period.
The large degree of risk reduction in first coronary revascularization events is comparable to the reductions seen in the early statin trials, such as the 4S trial (Scandinavian Simvastatin Survival Study), in which there was a 37% reduction in coronary revascularization events among patients treated with simvastatin versus placebo (P<0.0001; Figure VII in the Data Supplement).1 In REDUCE-IT, subsequent coronary revascularization events showed an even larger relative reduction among patients treated with icosapent ethyl versus placebo: 51% (P<0.0001) for second revascularization events and 50% (P=0.04) for ≥3 revascularization events; total revascularization events were reduced by 36% (P<0.0001).
In comparison, PCSK9 (proprotein convertase subtilisin/kexin 9) inhibitors have been shown to reduce coronary revascularization by a lesser amount. There was a 22% reduction in all urgent and elective revascularizations with evolocumab and a 12% reduction in ischemia-driven revascularization with alirocumab.4,5 Drug eluting stents in comparison with bare metal stents have been shown to reduce repeat revascularization events by 24% in the contemporary era.34 Dual antiplatelet therapy after acute coronary syndrome reduced subsequent revascularization by 8%.35 Thus, the reduction in revascularization in REDUCE-IT with icosapent ethyl appears larger than with these advanced pharmacological or device therapies.
Of note, there was also a 39% reduction in CABG among patients treated with icosapent ethyl in comparison with placebo. To the best of our knowledge, this is the first non–LDL-C intervention demonstrated to reduce CABG as an end point. In the LIPID trial (Long-Term Intervention with Pravastatin in Ischemic Disease), patients treated with pravastatin had a 22% reduction in CABG in comparison with placebo (9.2% versus 11.6%).36 In the SEAS trial (Simvastatin and Ezetimibe in Aortic Stenosis), patients treated with ezetimibe plus simvastatin underwent CABG less frequently than those treated with placebo (7.3% versus 10.8%, relative risk reduction 32%).37 Other randomized trials have evaluated CABG as an end point but have not demonstrated a benefit.38–43
In REDUCE-IT, icosapent ethyl was generally very well tolerated among study participants. There was a slightly increased risk of atrial fibrillation in comparison with placebo, although there was no increase in heart failure and, in fact, a significant decrease in stroke, myocardial infarction, cardiac arrest, and sudden cardiac death. There was also a small increase in minor bleeding events, with a trend toward increased serious bleeding, but without any significant increase in fatal bleeding, intracranial bleeding, or gastrointestinal bleeding. The overall rate of serious adverse events leading to drug discontinuation was low and statistically indistinguishable between icosapent ethyl and placebo.26
The effectiveness of icosapent ethyl in reducing cardiovascular events is far greater than the modest degree of triglyceride lowering seen, suggesting additional mechanisms of risk reduction.44–48 It is important to note that the efficacy of icosapent ethyl in cardiovascular event reduction has not been shown for EPA and docosahexaenoic acid (DHA) mixtures. A randomized cardiovascular outcomes trial in statin-treated patients similar to REDUCE-IT, but using 4 g/d of a formulation of EPA plus DHA, was recently stopped early by the study’s data monitoring committee for lack of significant benefit; this unexpected result suggests that the EPA formulation studied in REDUCE-IT drove the benefit, not DHA.49 In fact, EPA and DHA have different chemical structures, leading to markedly different tissue distributions and distinct effects on cellular membrane structure, endothelial function, rates of lipid oxidation, cholesterol crystalline domain formation, and various markers of inflammation.14 EPA has been found to stabilize cell membranes, whereas DHA tends to disrupt them.46 The unique combination of fatty acid chain length and number of double bonds in EPA, in comparison with other fatty acids, including other omega-3 fatty acids such as DHA, contributes to its antioxidant activity and membrane interactions.47 The failure of mixed EPA and DHA formulations to show significant benefit suggests that the addition of DHA may diminish or even counter the cardiovascular benefits of EPA. Additionally, threshold concentrations of achieved EPA may be necessary to elicit cardiovascular benefit.
Further corroborating the unique benefits of icosapent ethyl, and consistent with the significant reduction in revascularization by 11 months seen in REDUCE-IT,50 are the interim and final data from the EVAPORATE trial (Effect of Icosapent Ethyl on Progression of Coronary Atherosclerosis in Patients with Elevated Triglycerides on Statin Therapy). The early reduction in revascularization events with icosapent ethyl 4 g/d shown in REDUCE-IT is consistent with the coronary plaque changes shown in EVAPORATE, where treatment of a REDUCE-IT–like population with icosapent ethyl 4 g/d demonstrated beneficial effects on coronary plaque by 9 months.51,52 There were significant differences between icosapent ethyl and placebo at study end (18 months) involving multiple measures of plaque volume and composition.52
Limitations of this prespecified analysis include its exploratory nature and lack of correction for multiple comparisons. All P values should be considered hypothesis generating. However, the magnitude and consistency of the effect size of icosapent ethyl on various subtypes of coronary revascularization still allow for clinical inferences. Coronary revascularization as an end point has been criticized for its subjectivity and reflection of local practice, rather than true acute coronary syndrome events. However, 537 (58.4%) of the revascularization events were urgent or emergent, suggesting that these were largely in response to acute coronary syndrome events, and each subtype of revascularization was similarly and statistically significantly reduced. It is important to note that, although the performance of revascularization may have some subjective components, this end point was adjudicated by an independent, blinded clinical end point committee evaluating data from a randomized, double-blind, placebo-controlled trial. Thus, any differences observed between icosapent ethyl and placebo can be considered causal in nature. Statistical total event models each have inherent limitations. However, the consistency of the results among various models reflects the robustness of the study’s findings. Last, because patients with planned coronary intervention or surgery were excluded from the trial, the results presented here likely represent an underestimate of the true benefits of icosapent ethyl on revascularization, especially given the early separation of the event curves with icosapent ethyl versus placebo, consistent with the early effects seen with icosapent ethyl on plaque progression in the EVAPORATE trial at the 9-month interim and 18-month final analyses.51,52
Conclusions
Among statin-treated patients having well-controlled LDL-C but persistently elevated triglyceride levels and either established cardiovascular disease or diabetes plus additional cardiovascular risk factors, we found a large reduction in first and subsequent coronary revascularization in patients treated with icosapent ethyl versus placebo. This reduction was consistent across elective, urgent, and emergent revascularization categories, and for PCI and CABG individually, as well. To the best of our knowledge, this is the first non–LDL-C intervention in a major randomized trial in which statin-treated patients underwent fewer CABG surgeries, highlighting the substantial impact of icosapent ethyl on the underlying atherothrombotic burden in this at-risk population.
Acknowledgments
The authors thank the Amarin team members who are not authors of this article but who contributed to the success of the trial and of these analyses, including K. Diffin, A. Granger, and G. Chester, for operational support; R. Bhavanthula, R. H. Iroudayassamy, Dr Jin, D. Klevak, Dr Liu, H. Panchal, J. Shi, Dr Wang, and S.-R. Wang, for data management and statistical support; and J. Bronson for editorial assistance (limited to formatting and collation of coauthor comments); and the investigators, the study coordinators, and especially the patients who participated in REDUCE-IT (The Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial).
Sources of Funding
The main REDUCE-IT trial and this analysis have been funded by Amarin.
Disclosures
Dr Bhatt serves as the Chair and International Principal Investigator for REDUCE-IT (The Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial), with research funding from Amarin to Brigham and Women’s Hospital. Dr Bhatt discloses the following relationships: Advisory Board: Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Level Ex, Medscape Cardiology, MyoKardia, PhaseBio, PLx Pharma, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial [Portico Re-sheathable Transcatheter Aortic Valve System US IDE Trial], funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial [CENTERA THV System in Intermediate Risk Patients Who Have Symptomatic, Severe, Calcific, Aortic Stenosis], funded by Edwards), Contego Medical (Chair, PERFORMANCE 2 [Protection Against Emboli During Carotid Artery Stenting Using the Neuroguard IEP System]), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial [Edoxaban Compared to Standard Care After Heart Valve Replacement Using a Catheter in Patients With Atrial Fibrillation], funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial [Evaluation of Dual Therapy With Dabigatran vs. Triple Therapy With Warfarin in Patients With AF That Undergo a PCI With Stenting] steering committee funded by Boehringer Ingelheim; AEGIS-II [Study to Investigate SCL112 in Subjects With Acute Coronary Syndrome] executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial [A Trial Comparing Cardiovascular Safety of Degarelix Versus Leuprolide in Patients With Advanced Prostate Cancer and Cardiovascular Disease], funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national coleader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, MyoKardia, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Novo Nordisk, Takeda. Dr Miller reports receiving consulting fees from Amarin and Akcea. Dr Brinton reports receiving speaker fees from Amarin, Amgen, Kowa, Regeneron, and Sanofi-Aventis, and consulting fees from Akcea, Amarin, Amgen, Esperion, Kowa, Medicure, PTS Diagnostics, Regeneron, and Sanofi-Aventis. Dr Jacobson reports receiving consulting fees from Amgen, Esperion, Novartis, Regeneron, and Sanofi. Dr Steg reports receiving research grant funding from Amarin, Bayer, Merck, Sanofi, and Servier; and speaking or consulting fees from Amarin, Amgen, AstraZeneca, Bayer/Janssen, Boehringer-Ingelheim, Bristol-Myers Squibb, Idorsia, Lilly, Merck, Novartis, Novo-Nordisk, Pfizer, Regeneron, Sanofi, and Servier. Dr Ketchum, R.T. Doyle, Dr Juliano, Dr Jiao, and Dr Granowitz report being employed by and being stock shareholders of Amarin Pharma. Dr Tardif reports receiving grant support from AstraZeneca, Esperion, and Ionis, grant support and consulting fees from DalCor, grant support and fees for serving as cochairman of an executive committee from Pfizer, grant support and fees for serving on an executive committee from Sanofi, and grant support and consulting fees from Servier and holding a minor equity interest in DalCor and a patent (US 9,909,178 B2) on Dalcetrapib for Therapeutic Use. Dr Gibson reports research grant support and consulting fees from Amarin. Dr Pinto reports consulting fees from Abbott Vascular, Abiomed, Boston Scientific, Medtronic, Teleflex and consulting fees and stock options from NuPulseCV. Dr Giugliano reports that his institution received research grant support from Amgen, Bristol-Myers Squibb, Merck, and The Medicines Company for clinical trials in lipid therapies, and honoraria for CME programs and consulting from Akcea, Amarin, Amgen, Bristol-Myers Squibb, CVS Caremark, Daiichi Sankyo, GlaxoSmithKline, Merck, and Pfizer. Dr Budoff has received grant support and is on the speaker’s bureau for Amarin Pharmaceuticals. Dr Verma holds a Tier 1 Canada Research Chair in Cardiovascular Surgery; and reports receiving research grants and speaking honoraria from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, EOCI Pharmacomm Ltd, HLS Therapeutics, Janssen, Merck, Novartis, Novo Nordisk, Sanofi, Sun Pharmaceuticals, and the Toronto Knowledge Translation Working Group. He is the President of the Canadian Medical and Surgical Knowledge Translation Research Group, a federally incorporated not-for-profit physician organization. Dr Ballantyne reports receiving consulting fees from Arrowhead, AstraZeneca, Eli Lilly, Matinas BioPharma, Merck, Boehringer Ingelheim, Novo Nordisk, Denka Seiken, and Gilead and grant support (paid to his institution) and consulting fees from Amarin, Amgen, Esperion, Novartis, Regeneron, Sanofi-Synthelabo, and Akcea. The other authors report no conflicts.
Supplemental Materials
Data Supplement Figures I–VII
Data Supplement Tables I–IV
Supplementary Material
Footnotes
Sources of Funding, see page 42
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.120.050276.
This manuscript was sent to Prof Sripal Bangalore, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Contributor Information
Benjamin E. Peterson, Email: bepeterson@bwh.harvard.edu.
Ph. Gabriel Steg, Email: gabriel.steg@aphp.fr.
Michael Miller, Email: mmiller@som.umaryland.edu.
Eliot A. Brinton, Email: eliot.brinton@utah.edu.
Terry A. Jacobson, Email: tjaco02@emory.edu.
Steven B. Ketchum, Email: steven.ketchum@amarincorp.com.
Rebecca A. Juliano, Email: rebecca.juliano@amarincorp.com.
Lixia Jiao, Email: lisa.jiao@amarincorp.com.
Ralph T. Doyle, Jr, Email: ralph.doyle@amarincorp.com.
Craig Granowitz, Email: craig.granowitz@gmail.com.
C. Michael Gibson, Email: charlesmichaelgibson@gmail.com.
Duane Pinto, Email: dpinto@bidmc.harvard.edu.
Robert P. Giugliano, Email: rgiugliano@partners.org.
Matthew J. Budoff, Email: mbudoff@labiomed.org.
Jean-Claude Tardif, Email: jean-claude.tardif@icm-mhi.org.
Subodh Verma, Email: Subodh.Verma@unityhealth.to.
Christie M. Ballantyne, Email: cmb@bcm.edu.
References
- 1.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389 [PubMed] [Google Scholar]
- 2.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, et al. JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646 [DOI] [PubMed] [Google Scholar]
- 3.Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, et al. IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489 [DOI] [PubMed] [Google Scholar]
- 4.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, et al. FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664 [DOI] [PubMed] [Google Scholar]
- 5.Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, et al. ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. doi: 10.1056/NEJMoa1801174 [DOI] [PubMed] [Google Scholar]
- 6.Steg PG, Szarek M, Bhatt DL, Bittner VA, Brégeault MF, Dalby AJ, Diaz R, Edelberg JM, Goodman SG, Hanotin C, et al. Effect of alirocumab on mortality after acute coronary syndromes. Circulation. 2019;140:103–112. doi: 10.1161/CIRCULATIONAHA.118.038840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boden WE, Bhatt DL, Toth PP, Ray KK, Chapman MJ, Lüscher TF. Profound reductions in first and total cardiovascular events with icosapent ethyl in the REDUCE-IT trial: why these results usher in a new era in dyslipidaemia therapeutics. Eur Heart J. 2020;41:2304–2312. doi: 10.1093/eurheartj/ehz778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hokanson JE, Austin MA. Plasma triglyceride levels is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a metaanalysis of population-based prospective studies. J Cardiovasc Risk. 1996;3:213–219. doi: 10.1177/174182679600300214 [PubMed] [Google Scholar]
- 9.Ganda OP, Bhatt DL, Mason RP, Miller M, Boden WE. Unmet need for adjunctive dyslipidemia therapy in hypertriglyceridemia management. J Am Coll Cardiol. 2018;72:330–343. doi: 10.1016/j.jacc.2018.04.061 [DOI] [PubMed] [Google Scholar]
- 10.Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics and biology. Circ Res. 2016;118:547–563. doi: 10.1161/circresaha.115.306249 [DOI] [PubMed] [Google Scholar]
- 11.Rosinger A, Carroll MD, Lacher D, Ogden C. Trends in total cholesterol, triglycerides, and low-density lipoprotein in US adults, 1999 –2014 JAMA Cardiol. 2017;2:339–341. doi: 10.1001/jamacardio.2016.4396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taskinen M-R, Boren J. New insights into the pathophysiology of dyslipidemia in type 2 diabetes. Atherosclerosis. 2015;239:483–495. doi: 10.1016/j.atherosclerosis.2015.01.039 [DOI] [PubMed] [Google Scholar]
- 13.Fan W, Philip S, Granowitz C, Toth PP, Wong ND. Hypertriglyceridemia in statin-treated US adults: the National Health and Nutrition Examination Survey. J Clin Lipidol. 2019;13:100–108. doi: 10.1016/j.jacl.2018.11.008 [DOI] [PubMed] [Google Scholar]
- 14.Mason RP, Libby P, Bhatt DL. Emerging mechanisms of cardiovascular protection for the omega-3 fatty acid eicosapentaenoic acid. Arterioscler Thromb Vasc Biol. 2020;40:1135–1147. doi: 10.1161/ATVBAHA.119.313286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mason RP. New insights into mechanisms of action for omega-3 fatty acids in atherothrombotic cardiovascular disease. Curr Atheroscler Rep. 2019;21:2 doi: 10.1007/s11883-019-0762-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ference BA, Kastelein JJP, Ray KK, Ginsberg HN, Chapman MJ, Packard CJ, Laufs U, Oliver-Williams C, Wood AM, et al. Association of triglyceride-lowering LPL variants and LDL-C-lowering LDLR variants with risk of coronary heart disease. JAMA. 2019;321:364–373. doi: 10.1001/jama.2018.20045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ginsberg HN, Elam MB, Lovato LC, Crouse JR, 3rd, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, et al. ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guyton JR, Slee AE, Anderson T, Fleg JL, Goldberg RB, Kashyap ML, Marcovina SM, Nash SD, O’Brien KD, Weintraub WS, et al. Relationship of lipoproteins to cardiovascular events: the AIM-HIGH Trial (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides and Impact on Global Health Outcomes). J Am Coll Cardiol. 2013;62:1580–1584. doi: 10.1016/j.jacc.2013.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, et al. HPS2-THRIVE Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–212. doi: 10.1056/NEJMoa1300955 [DOI] [PubMed] [Google Scholar]
- 20.Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, Murphy K, Aung T, Haynes R, Cox J, et al. ASCEND Study Collaborative Group. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med. 2018;379:1540–1550. doi: 10.1056/NEJMoa1804989 [DOI] [PubMed] [Google Scholar]
- 21.Bosch J, Gerstein HC, Dagenais GR, Díaz R, Dyal L, Jung H, Maggiono AP, Probstfield J, Ramachandran A, Riddle MC, et al. ORIGIN Trial Investigators. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367:309–318. doi: 10.1056/NEJMoa1203859 [DOI] [PubMed] [Google Scholar]
- 22.Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, Geleijnse JM, Rauch B, Ness A, Galan P, et al. Omega-3 Treatment Trialists’ Collaboration. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77 917 individuals. JAMA Cardiol. 2018;3:225–234. doi: 10.1001/jamacardio.2017.5205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Albert CM, Gordon D, Copeland T, et al. VITAL Research Group. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380:23–32. doi: 10.1056/NEJMoa1811403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, et al. Japan EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3 [DOI] [PubMed] [Google Scholar]
- 25.Saito Y, Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, et al. JELIS Investigators, Japan. Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: sub-analysis of primary prevention cases from the Japan EPA Lipid Intervention Study (JELIS). Atherosclerosis. 2008;200:135–140. doi: 10.1016/j.atherosclerosis.2008.06.003 [DOI] [PubMed] [Google Scholar]
- 26.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Jr, Juliano RA, Jiao L, Granowit C, et al. REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792 [DOI] [PubMed] [Google Scholar]
- 27.Bhatt DL, Juliano RA, Ketchum SB, Miller M. Response by Bhatt et al to Letter Regarding Article, “REDUCE-IT USA: Results From the 3146 Patients Randomized in the United States”. Circulation. 2020;141:e834–e835. doi: 10.1161/CIRCULATIONAHA.120.047273 [DOI] [PubMed] [Google Scholar]
- 28.Bhatt DL. REDUCE-IT: residual cardiovascular risk in statin-treated patients with elevated triglycerides: now we can REDUCE-IT! Eur Heart J. 2019;40:1174–1175. doi: 10.1093/eurheartj/ehz17930982067 [Google Scholar]
- 29.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Jr, Juliano RA, Jiao L, Granowitz C, et al. REDUCE-IT Investigators. Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J Am Coll Cardiol. 2019;73:2791–2802. doi: 10.1016/j.jacc.2019.02.032 [DOI] [PubMed] [Google Scholar]
- 30.Picard F, Bhatt DL, Ducrocq G, Elbez Y, Ferrari R, Ford I, Tardif JC, Tendera M, Fox KM, Steg PG. Generalizability of the REDUCE-IT trial in patients with stable coronary artery disease. J Am Coll Cardiol. 2019;73:1362–1364. doi: 10.1016/j.jacc.2019.01.016 [DOI] [PubMed] [Google Scholar]
- 31.Lawler PR, Kotrri G, Koh M, Goodman SG, Farkouh ME, Lee DS, Austin PC, Udell JA, Ko DT. Real-world risk of cardiovascular outcomes associated with hypertriglyceridaemia among individuals with atherosclerotic cardiovascular disease and potential eligibility for emerging therapies. Eur Heart J. 2020;41:86–94. doi: 10.1093/eurheartj/ehz767 [DOI] [PubMed] [Google Scholar]
- 32.Bhatt DL, Steg PG, Brinton EA, Jacobson TA, Miller M, Tardif JC, Ketchum SB, Doyle RT, Jr, Murphy SA, Soni PN, et al. REDUCE-IT Investigators. Rationale and design of REDUCE-IT: Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial. Clin Cardiol. 2017;40:138–148. doi: 10.1002/clc.22692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li QH, Lagakos SW. Use of the Wei-Lin-Weissfeld method for the analysis of a recurring and a terminating event. Stat Med. 1997;16:925–940. doi: 10.1002/(sici)1097-0258(19970430)16:8<925::aid-sim545>3.0.co;2-2 [DOI] [PubMed] [Google Scholar]
- 34.Bønaa KH, Mannsverk J, Wiseth R, Aaberge L, Myreng Y, Nygård O, Nilsen DW, Kløw NE, Uchto M, Trovik T, et al. NORSTENT Investigators. Drug-eluting or bare-metal stents for coronary artery disease. N Engl J Med. 2016;375:1242–1252. doi: 10.1056/NEJMoa1607991 [DOI] [PubMed] [Google Scholar]
- 35.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746 [DOI] [PubMed] [Google Scholar]
- 36.Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–1357. doi: 10.1056/NEJM199811053391902 [DOI] [PubMed] [Google Scholar]
- 37.Rossebø AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, Gerdts E, Gohlke-Bärwolf C, Holme I, Kesäniemi YA, et al. SEAS Investigators. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602 [DOI] [PubMed] [Google Scholar]
- 38.GISSI-3: effects of lisinopril and transdermal glyceryl trinitrate singly and together on 6-week mortality and ventricular function after acute myocardial infarction. Gruppo Italiano per lo Studio della Sopravvivenza nell’infarto Miocardico. Lancet. 1994;343:1115–1122 [PubMed] [Google Scholar]
- 39.Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, Espeland MA, et al. Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, De Smet BJ, Herrman JP, Adriaenssens T, Vrolix M, Heestermans AA, Vis MM, et al. WOEST study investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381:1107–1115. doi: 10.1016/S0140-6736(12)62177-1 [DOI] [PubMed] [Google Scholar]
- 41.Stub D, Smith K, Bernard S, Nehme Z, Stephenson M, Bray JE, Cameron P, Barger B, Ellims AH, Taylor AJ, et al. AVOID Investigators. Air versus oxygen in ST-segment –elevation myocardial infarction. Circulation. 2015;131:2143–2150. doi: 10.1161/CIRCULATIONAHA.114.014494 [DOI] [PubMed] [Google Scholar]
- 42.Hochman JS, Lamas GA, Buller CE, Dzavik V, Reynolds HR, Abramsky SJ, Forman S, Ruzyllo W, Maggioni AP, White H, et al. Occluded Artery Trial Investigators. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med. 2006;355:2395–2407. doi: 10.1056/NEJMoa066139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen M, Demers C, Gurfinkel EP, Turpie AG, Fromell GJ, Goodman S, Langer A, Califf RM, Fox KA, Premmereur J, et al. A comparison of low-molecular-weight heparin with unfractionated heparin for unstable coronary artery disease. Efficacy and Safety of Subcutaneous Enoxaparin in Non-Q-Wave Coronary Events Study Group. N Engl J Med. 1997;337:447–452. doi: 10.1056/NEJM199708143370702 [DOI] [PubMed] [Google Scholar]
- 44.Kastelein JJP, Stroes ESG. Fishing for the Miracle of Eicosapentaenoic Acid. N Engl J Med. 2019;380:89–90. doi: 10.1056/NEJMe1814004 [DOI] [PubMed] [Google Scholar]
- 45.Bhatt DL, Steg PG, Miller M. Cardiovascular risk reduction with icosapent ethyl. Reply. N Engl J Med. 2019;380:1678 doi: 10.1056/NEJMc1902165 [DOI] [PubMed] [Google Scholar]
- 46.Sherratt SCR, Mason RP. Eicosapentaenoic acid and docosahexaenoic acid have distinct membrane locations and lipid interactions as determined by X-ray diffraction. Chem Phys Lipids. 2018;212:73–79. doi: 10.1016/j.chemphyslip.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 47.Sherratt SCR, Juliano RA, Mason RP. Eicosapentaenoic acid (EPA) has optimal chain length and degree of unsaturation to inhibit oxidation of small dense LDL and membrane cholesterol domains as compared to related fatty acids in vitro. Biochim Biophys Acta Biomembr. 2020;1862:183254 doi: 10.1016/j.bbamem.2020.183254 [DOI] [PubMed] [Google Scholar]
- 48.Bhatt DL, Steg PG, Miller M, Juliano RA, Ballantyne CM. Reply: ischemic event reduction and triglycerides. J Am Coll Cardiol. 2019;74:1849–1850. doi: 10.1016/j.jacc.2019.08.007 [DOI] [PubMed] [Google Scholar]
- 49.Nicholls SJ, Lincoff AM, Bash D, Ballantyne CM, Barter PJ, Davidson MH, Kastelein JJP, Koenig W, McGuire DK, Mozaffarian D, et al. Assessment of omega-3 carboxylic acids in statin-treated patients with high levels of triglycerides and low levels of high-density lipoprotein cholesterol: rationale and design of the STRENGTH trial. Clin Cardiol. 2018;41:1281–1288. doi: 10.1002/clc.23055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olshansky B, Bhatt DL, Miller M, Steg G, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Jr, Juliano RA, Jiao L, et al. on behalf of the REDUCE-IT Investigators. REDUCE-IT INTERIM: accumulation of data across prespecified interim analyses to final results [published online October 3, 2020] Eur Heart J Cardiovasc Pharmacother. doi: 10.1093/ehjcvp/pvaa118. https://academic.oup.com/ehjcvp/advance-article/doi/10.1093/ehjcvp/pvaa118/5917630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Budoff MJ, Muhlestein JB, Bhatt DL, Le Pa VT, May HT, Shaikh K, Shekar C, Kinninger A, Lakshmanan S, Roy S, et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: a prospective, placebo-controlled randomized trial (EVAPORATE): interim results [published online July 1, 2020] Cardiovasc Res. doi: 10.1093/cvr/cvaa184. https://academic.oup.com/cardiovascres/advance-article-abstract/doi/10.1093/cvr/cvaa184/5865857?redirectedFrom=fulltext [DOI] [PubMed] [Google Scholar]
- 52.Budoff MJ, Bhatt DL, Kinninger A, Lakshmanan S, Muhlestein JB, Le VT, May HT, Shaikh K, Shekar C, Roy SK, et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: final results of the EVAPORATE trial. Eur Heart J. 2020:;41:3925–3932. doi: 10.1093/eurheartj/ehaa652 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


