Purpose of review
In response to the HIV–AIDS pandemic, great strides have been made in developing molecular methods that accurately quantify nucleic acid products of HIV-1 at different stages of viral replication and to assess HIV-1 sequence diversity and its effect on susceptibility to small molecule inhibitors and neutralizing antibodies. Here, we review how knowledge gained from these approaches, including viral RNA quantification and sequence analyses, have been rapidly applied to study SARS-CoV-2 and the COVID-19 pandemic.
Recent findings
Recent studies have shown detection of SARS-CoV-2 RNA in blood of infected individuals by reverse transcriptase PCR (RT-PCR); and, as in HIV-1 infection, there is growing evidence that the level of viral RNA in plasma may be related to COVID disease severity. Unlike HIV-1, SARS-CoV-2 sequences are highly conserved limiting SARS-CoV-2 sequencing applications to investigating interpatient genetic diversity for phylogenetic analysis. Sensitive sequencing technologies, originally developed for HIV-1, will be needed to investigate intrapatient SARS-CoV-2 genetic variation in response to antiviral therapeutics and vaccines.
Summary
Methods used for HIV-1 have been rapidly applied to SARS-CoV-2/COVID-19 to understand pathogenesis and prognosis. Further application of such methods should improve precision of therapy and outcome.
Keywords: COVID-19, COVID-19 prognosis, SARS-CoV-2 diagnostics, SARS-CoV-2 genetics, SARS-CoV-2 viremia
INTRODUCTION
In response to the COVID-19 pandemic, researchers across the globe have collectively shifted focus to the common goal of understanding the newly emerged SARS-CoV-2. HIV-1 researchers have accumulated nearly 40 years of experience studying an epidemic caused by a newly emerged viral pathogen, uniquely positioning them to quickly respond to a new viral pandemic. Many methods and technologies used during the HIV-1 epidemic can and have been applied to understanding the SARS-CoV-2 pandemic. In the following review, we discuss how molecular technologies developed for HIV-1 have been applied successfully and swiftly to the study of SARS-CoV-2.
Box 1.
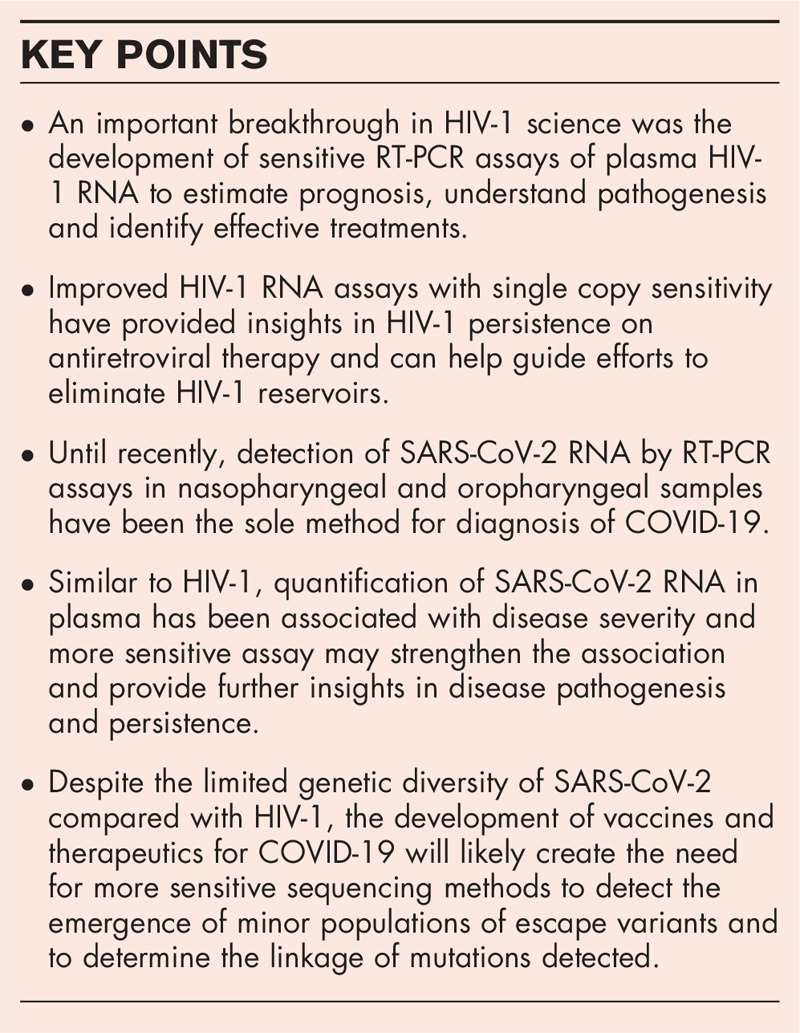
no caption available
SENSITIVE METHODS FOR MEASURING VIRAL RNA
Early in the AIDS epidemic disease was diagnosed and monitored clinically and by lymphocyte counts. Once HIV-1 was identified as the causative agent of AIDS [1,2], tests were developed to diagnose infection serologically and quantify HIV-1 RNA in blood [3–5]. The importance of quantifying HIV-1 RNA in plasma (also referred to as viral load) for prognosis was then demonstrated [6,7] and it soon became routine practice to measure viral load for disease staging and assessing response to treatment (Table 1). The importance of viral load as a marker of treatment failure and emergence of drug resistance from a highly diverse viral population became clear with the discovery of more potent inhibitors of viral replication. Indeed, the importance of viral load in disease monitoring became so widely accepted that the WHO recommended implementation of viral load testing for monitoring individuals on antiretroviral therapy (ART) in resource-limited settings in 2010 [8]. As viral load monitoring has become more important automated platforms have increased the ease and throughput of testing. Roche, Abbott, Hologic, and Cepheid offer FDA-cleared nucleic acid amplification-based HIV-1 RNA quantification tests on automated platforms that are widely used across the world (Table 1).
Table 1.
Nucleic acid amplification tests for HIV-1 and SARS-COV-2
| Automated NAAT | HIV | QuantitativeDiagnosis confirmationTreatment and drug resistance monitoring |
| SARS-CoV-2 | Qualitative/SemiquantitativeRespiratory samples onlyDiagnosis (EUA) | |
| Manual NAAT | HIV | Increased sensitivity (single copy)Nonplasma sample typesCure, treatment, and drug resistance trials |
| SARS-CoV-2 | QuantitativeNonrespiratory sample typesIncreased sensitivity |
NAAT, nucleic acid amplification tests.
Persistent HIV-1 viremia on antiretroviral therapy
Monitoring HIV-1 RNA levels below the limit of quantitation of commercial assays has become an important endpoint for many studies aimed at HIV cure. This is because, although ART results in logarithmic decreases in viral load, assays capable of detecting single copies of HIV-1 RNA have shown that the majority of those on ART have detectable HIV-1 RNA in the range of 1–3 cps/ml [9–17]. Studies of decay dynamics of virus in plasma have given important insights into disease progression and treatment, including the existence of a long-lived viral reservoir [12,13,15]. In addition, clearance of low-level persistent viremia may be a marker for HIV cure so great focus has been given to detection of viral RNA below 20 cps/ml. Some iteration of manual reverse transcriptase PCR (RT-PCR) assays have been used for most studies of persistent low-level viremia (Table 1) [9,14,16]. Since manual single copy assays are inherently low-throughput and technically difficult there have been recent efforts to adapt high-throughput commercial platforms for detection of low-level persistent viremia [18▪,19]. Incorporating high throughput single copy assays will facilitate large studies aimed at clearing persistent viremia and achieving functional cure.
Molecular testing for SARS-CoV-2 RNA
One of the largest and most critical efforts at the start of the COVID-19 pandemic was the search for a reliable, quick, and broadly available test for SARS-CoV-2 infection. Since the beginning of the HIV epidemic in the 1980s the field of molecular diagnostics has made tremendous strides, enabling the release of a molecular test for SARS-CoV-2 RNA very quickly after the sequence of the viral genome was made available [20▪▪,21,22▪,23]. In addition to significant technical advances in molecular diagnostics, the low genetic diversity of SARS-CoV-2 as compared with HIV-1 and other RNA viruses has also contributed to the quick release and implementation of molecular assays to detect SARS-CoV-2 RNA [24▪▪]. Similar to HIV-1 the first SARS-CoV-2 molecular diagnostic tests developed were largely manual, but diagnostic companies quickly followed with their own high-throughput versions with varying amounts of automation, including Roche, Abbott, Hologic, and Cepheid (Table 1). Importantly, these tests are designed to test respiratory specimens and are qualitative with some simply returning a positive or negative result. Others return a cycle threshold value, allowing approximation of levels of viral RNA in the sample. As the pandemic evolves and data accumulates the importance of quantitative tests for understanding infection dynamics is becoming clear, particularly as it relates to disseminated infection.
Plasma RNAemia in SARS-CoV-2 infection
Little focus has been given to the potential presence of virus in the blood (viremia) in most respiratory infections since primary target cells and general symptoms point to respiratory containment of infection. However, analyses of SARS-CoV-2 RNA in the nasopharynx have not shown consistent associations with symptomatic disease, including the severity of symptomatic disease [25–30]. While the lack of consistent association is partly attributable to inconsistencies in sample collection [31], variable viral replication kinetics in the upper respiratory tract over the infection course represent another possible explanation for the lack of consistent association between diagnostic test cycle threshold value and disease severity [32]. In addition, sampling of only the nasopharynx may miss contributions to disease caused by viral replication in the lower respiratory track or dissemination of virus through blood to extrapulmonary sites. Reports of both expanded receptor expression beyond the primary target cells in the lung and presence of virus in peripheral tissues such as the gastrointestinal (GI) tract, endothelium and central nervous system (CNS) may point to disseminated viral infection as an important contributor to severe disease [33–41]. Indeed, an unusually wide range of symptoms can accompany SARS-CoV-2 infection, including thromboses, loss of smell, vomiting, and diarrhea [37,42–44]. Unlike HIV-1, current data do not suggest that SARS-CoV-2 replicates to a significant degree in peripheral blood cells; however, normal blood flow could provide a means for virus dissemination to extrapulmonary sites. Aside from a possible role in disease pathogenesis, blood–borne viral RNA could also be an important indicator of lung tissue breakdown leading to release of intact virions, viral proteins and nucleic acids, or infected cells into the bloodstream. Along these lines, a few groups have found that the detection of SARS-CoV-2 RNA in plasma (SARS-CoV-2 RNAemia) is associated with severe disease [45▪,46▪,47,48▪–50▪], although to inconsistent degrees. The inconsistency in proportion of patients with plasma RNA and the amount of plasma RNA is likely due to differences in definitions of disease severity and in the RT-PCR methods used. SARS-CoV-2 RNA has been detected in plasma from variable proportions of patients with COVID-19, ranging from 35% of hospitalized patients [46▪] to 88% of critically ill patients [49▪], with clear trends in each study toward severe disease in those with RNAemia. Our group has found a similar trend using a highly sensitive N-targeting RT-PCR assay, with ∼90% of ICU patients having RNAemia and ∼60% of hospitalized, non-ICU patients showing RNAemia (unpublished data). Longitudinal analysis from patients across the spectrum of disease severity, including those with progressive disease during the observation period, is ongoing and is expected to provide additional insight into the usefulness of SARS-CoV-2 plasma RNAemia as a prognostic marker. In addition, it is possible that application of single copy assays to blood samples similar to those discussed above for HIV-1 will increase the frequency of SARS-CoV-2 RNA detection in less severe disease (Table 1). These studies are also ongoing. Important centrifugation and antibody pull-down studies to determine whether viral nucleic acid detected in plasma corresponds to viral particles and/or infected cells are ongoing as well; and future studies should address whether any virions present are infectious, and if infected cells are present, if they are producing infectious virus by assessing virus growth in cell culture in a Biosafety Level 3 facility. These studies will help define the clinical significance of SARS-CoV-2 RNAemia by addressing whether detection of viral RNA in plasma signals uncontrolled infection and a risk for complications requiring earlier intervention. Further studies focused on whether therapies that prevent or reduce RNAemia could improve outcome will be important and could accelerate development of effective therapies for COVID-19, similar to what occurred with ART of HIV-1 infection [51].
HIV-1 SEQUENCING TECHNOLOGIES FOR SARS-COV-2
Applying sequencing analysis to HIV-1 has provided tremendous insight into its global spread, transmission patterns, pathogenesis, immune escape, diagnostics, and viral response to treatments including small molecules and neutralizing antibodies and by identifying mutations associated with viral drug resistance to ART. Although the initial epidemiology of HIV-1 and AIDS was based on serological studies, sequencing technologies provided rapid and new insights [52–54], revealing large diversity between HIV-1 isolates, and it is now understood that HIV-1 has an extraordinarily high genetic variability with a mutation rate of 4 × 10−5 nucleotide per site per replication cycle [55–57] resulting in rapid adaptation to selective pressures. Because of the high mutation rates and highly divergent lineages, no vaccine exists to prevent the transmission of HIV-1.
HIV-1 sequencing technology for HIV-1 drug resistance
In addition to applications for improving primer design for more accurate virus quantification, a major application of HIV-1 sequencing is to detect mutations in the virus that are associated with drug resistance. By identifying mutations in HIV-1 that may have been selected for in persons receiving ART or examining the effectiveness of ART regimens in individuals with viruses containing suspected drug-resistance mutations, sequencing technology was used as a tool for establishing the most effective ART regimens [58].
To this aim, first-generation Sanger-based sequencing technologies provided a foundation for the commercialization of clinical drug resistance assays, which have allowed clinicians to tailor treatment to an individual patient's mutational pattern. Although Sanger-technology was dependable for accurately identifying mutations associated with drug resistance in the majority (>20%) of viruses circulating in plasma from persons living with HIV-1, elucidating minor intraindividual variation required sequencing cDNA templates diluted to single copies, known as single-genome sequencing (SGS) [59–61] (Table 2). Although SGS increased the sensitivity for minor variants over Sanger technology, it was limited by the number of sequencing reactions required for each patient. Next-generation sequencing (NGS) increased the throughput capacity and accuracy of sequencing by allowing for the simultaneous sequencing of thousands of copies of DNA in a single run. Multiplex amplified samples with unique index sequences greatly reduced the complexity of SGS allowing multiple amplified cDNA products in the same sequencing run. Subsequently the incorporation of cDNA barcoding into NGS increased the sensitivity for minor variant detection [62,63] even more than SGS [i.e., ultrasensitive SGS (uSGS) [64]] and was recently used to uncover rare mutations linked on the same HIV-1 genome [65].
Table 2.
Sequencing platforms used for the detection of HIV drug resistance
| Sequencing generation | Technology | Manufacturer | Applications to HIV DR testing |
| First | Sanger | ThermoFisher (Applied Biosystems) | Population-based sequencing for mutations >20% viral populationSingle-genome sequencing by limiting dilutions of cDNA to detect linkage |
| Second ‘Next’ | Pyrosequencing | Roche – discontinued | High-throughput multiplexingSensitive sequencing for mutations >5% viral populationuSGS by cDNA PrimerID tagging for minor variants (1–5%) [61,62] |
| Ion Torrent | ThermoFisher (Life Technologies) | ||
| SBS | Illumina | ||
| Third | SMRT sequencing | PacBio | Long HIV-1 readsSensitive sequencing for mutations >5% viral population (looped amplification) |
| Nanopore sequencing | Oxford Nanopore Technologies |
DR, drug resistance; SBS, sequencing-by-synthesis; SGS, single-genome sequencing; uSGS, ultrasensitive single-genome sequencing.
SARS-CoV-2 diversity and sequencing
Since the first sequence of SARS-CoV-2 was generated [22▪], more than 95 000 SARS-CoV-2 sequences have been deposited in the global initiative on sharing all influenza data (GISAID). Unlike HIV-1, which has high-level heterogeneity even within an infected individual with an estimated substitution rate of 0.8–1.7 × 10−3 per site per year [66–68], SARS-CoV-2 sequences have a high-sequence identity with a substitution rate estimated to be 8.4 × 10−4–1.1 × 10−3 per site per year at the population level [69–72]. This conservation has largely been attributed to the exoribonuclease (ExoN) activity of the nonstructural protein 14 (nsp14) as it was demonstrated that wild-type SARS-CoV has a mutation rate of 9.0 × 10−7 substitutions per nucleotide per replication cycle [73]. However, a mutant form with an inactivating substitutions in nsp14 reduced the fidelity to 1.2 × 10−5 substitutions per nucleotide per replication cycle, strikingly similar to HIV-1 and other RNA viruses [73–75]. Significantly, it was shown that a common SARS-CoV-2 mutation 14408C>T that causes a P323L substitution in the RNA dependent RNA polymerase (nsp12) may also increase the mutation rate, suggesting that mutations in regions outside of the ExoN could also alter SARS-CoV-2 fidelity [76].
In contrast to the complex HIV-1 classification systems, the conservation of SARS-CoV-2 required that slight differences in sequences were used to develop the three major nomenclature classification systems [77▪▪] (Table 3). The first two classification systems, GISAID and Nextstrain, are focused on investigating large scale diversity patterns of SARS-CoV-2 for understanding of patterns and determinants of the global spread by identifying clades that persist for at least several months and have significant geographic spread [78,79]. The PANGOLIN lineage system is the third classification system and was developed to have short-term epidemiological significance by utilizing a more dynamic system for tracking active virus lineages [77▪▪,78,80].
Table 3.
SARS-CoV-2 phylogenetic categorization systems Nextstrain, global initiative on sharing all influenza data and PANGOLIN
| Clades | Lineagesa | ||
| Nextstrain (79) | GISAID (78) | PANGOLIN (80) | GISAID Clade marker variants relative to WIV04-reference |
| 19B | S | A | C8782, T28144C |
| 19A | L | B | C241, C3037, A23403, C8782, G11083, G25563, G26144, T28144, G28882 |
| V | G11083T, G26144T NSP6-L37F + NS3-G251V | ||
| 20A | G | B.1 | C241T, C3037T, A23403G includes S-D614G |
| 20C | GH | C241T, C3037T, A23403G, G25563T includes S-D614G + NS3-Q57H | |
| 20B | GR | B.1.1 | C241T, C3037T, A23403G, G28882A includes S-D614G + N-G204R |
Pangolin lineages are also based upon phylogenetic evidence, but because they also factor in active local geographical outbreaks, they are too numerous for this table. For a more detailed information go to cov-lineages.org.
Sequencing SARS-CoV-2 for clinical research
As with HIV-1, SARS-CoV-2 sequences have facilitated the investigation of transmission dynamics to guide public health action and to elucidate sequence conservation for the development of robust molecular diagnostic methods. For these reasons, SARS-CoV-2 whole genome sequencing (WGS) will continue to be essential in tracking the pandemic. In addition, as case studies have recently documented reinfections of SARS-CoV-2, the limited amount of sequence diversity in SARS-CoV-2 in any given gene region necessitates WGS for differentiating the first and second episodes by showing that each of the viruses belonged to a different lineages rather than a few mutations having been a consequence of RT-PCR or sequencing error [81,82]. Although the sequence conservation in SARS-CoV-2 genomes has limited the application of sequencing technologies relative to HIV-1, selective pressures from both host immune as well as novel vaccine and therapeutic interventions are likely to drive novel mutations in SARS-CoV-2 in the future. The SARS-CoV-2 envelope glycoprotein or Spike mediates viral entry into host cells and is thus currently the major target for vaccine and monoclonal neutralizing antibody development. As such, Spike gene sequencing will be a key focus for identifying immune escape variants. Several studies have shown that patients infected with SARS-CoV-2 strains having Gly614 variant in Spike had on average lower RT-PCR cycle threshold values relative to individuals infected with Asp614 [83▪,84] and it was also shown that the D614G substitution increases the infectivity in vitro[83▪,85]. However, there has been no conclusive evidence that any of the emerging SARS-CoV-2 substitutions have resulted in a change in transmissibility or the disease severity of COVID-19 [24▪▪]. There is also a growing body of evidence to suggest that deletions as well as mutations may be important for SARS-CoV-2 infection. For example, a deletion in Spike at the S1/S2 cleavage site that causes a premature stop codon after S1 was observed and it was speculated that this deletion may cause an increase in free S1 as a mechanism to mitigate the host response to SARS-CoV-2 infection and cause less severe disease [86]. Such deletions may also exist outside of Spike; in fact, a group recently showed that a 382-nucleotide deletion in SARS-CoV-2 ORF7b and ORF8 eliminates ORF8 transcription and, because there is a robust antibody response to ORF8, they suggest that it may be the result of immune-driven selection [69].
It is possible that more sensitive sequencing approaches are needed, such as SGS or uSGS, to completely understand the intrapatient variation and consequences of SARS-CoV-2 mutations. In addition, as the selection of mutations through immunotherapy or vaccination will often come at the expense of fitness, genetic variants will likely exist at different frequencies depending on the levels of the factor suppressing viral replication within an infected host. HIV-1 research has shown that when investigating intrapatient variation and selection it is crucial to obtain a sufficient sequence read depth to overcome the error rate of the specific sequencing platform used and to ensure that a sufficient number of templates have been sampled.
CONCLUSION
Application of the advances made through HIV-1 science to SARS-CoV-2 have provided rapid, major insights into virus quantification and sequencing in support of efforts to diagnose, treat and prevent COVID-19. Measuring HIV-1 RNA using RT-PCR-based assays greatly accelerated the development of effective therapies for HIV-1 infection. Similarly, RT-PCR-based HIV-1 for SARS-CoV-2 RNA has been routinely used for diagnosis of COVID-19 and is being extended to quantify viral dissemination and assess disease severity. Although SARS-CoV-2 isolate sequences are homogeneous and sequencing applications have been limited to WGS, it is likely that more sensitive sequencing technologies, including SGS and uSGS, will be needed to detect mutations that emerge with selective pressures from vaccines, small molecule inhibitors, and immune-based therapies.
Acknowledgements
We acknowledge Lorraine Pollini for assistance in preparing this article.
Financial support and sponsorship
Research reported in this publication was supported by a grant from the AIDS Clinical Trials Group Network (ACTG) to the University of Pittsburgh Virology Specialty Laboratory funded by National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) under Award Number UM1 AI106701. This project has also been funded in whole or in part with Federal funds from the National Cancer Institute, National Institutes of Health, under Contract Number 75N91019D00024, Task Order Number 75N91019F00129. This work was funded by the University of Pittsburgh through a Clinical and Translational Science Institute (CTSI) award supported by the National Institutes of Health through grant number UL1TR001857. The work was also funded by a University of Pittsburgh Medical Center (UPMC) Chief Medical Scientific Officer (CMSO) award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Funding for this review is provided by the National Institutes of Health, National Cancer Institute, National Institute of Allergy and Infectious Diseases.
Conflicts of interest
J.W.M. is a consultant to Gilead Sciences and holds shares or share options in Co-Crystal Pharma, Inc., Abound Bio, Inc. and Infectious Disease Connect. K.D.M. and J.L.J. declare no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Barre-Sinoussi F, Chermann JC, Rey F, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 1983; 220:868–871. [DOI] [PubMed] [Google Scholar]
- 2.Gallo RC, Salahuddin SZ, Popovic M, et al. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science 1984; 224:500–503. [DOI] [PubMed] [Google Scholar]
- 3.Kievits T, van Gemen B, van Strijp D, et al. NASBA isothermal enzymatic in vitro nucleic acid amplification optimized for the diagnosis of HIV-1 infection. J Virol Methods 1991; 35:273–286. [DOI] [PubMed] [Google Scholar]
- 4.Mulder J, McKinney N, Christopherson C, et al. Rapid and simple PCR assay for quantitation of human immunodeficiency virus type 1 RNA in plasma: application to acute retroviral infection. J Clin Microbiol 1994; 32:292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pachl C, Todd JA, Kern DG, et al. Rapid and precise quantification of HIV-1 RNA in plasma using a branched DNA signal amplification assay. J Acquir Immune Defic Syndr Hum Retrovirol 1995; 8:446–454. [DOI] [PubMed] [Google Scholar]
- 6.Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med 1997; 126:946–954. [DOI] [PubMed] [Google Scholar]
- 7.Mellors JW, Rinaldo CR, Jr, Gupta P, et al. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 1996; 272:1167–1170. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents recommendations for a public health approach: 2010 revision. Geneva, Switzerland: World Health Organization; 2010. [PubMed] [Google Scholar]
- 9.Cillo AR, Vagratian D, Bedison M, et al. Improved single-copy assays for quantification of persistent HIV-1 viremia in patients on suppressive antiretroviral therapy. J Clin Microbiol 2014; 52:3944–3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dornadula G, Zhang H, VanUitert B, et al. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA 1999; 282:1627–1632. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs JL, Halvas EK, Tosiano MA, et al. Persistent HIV-1 viremia on antiretroviral therapy: measurement and mechanisms. Front Microbiol 2019; 10:2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maldarelli F, Palmer S, King MS, et al. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog 2007; 3:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmer S, Maldarelli F, Wiegand A, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A 2008; 105:3879–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmer S, Wiegand AP, Maldarelli F, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol 2003; 41:4531–4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riddler SA, Aga E, Bosch RJ, et al. Continued slow decay of residual plasma viremia in HIV-1-infected adults on long term antiretroviral therapy. J Infect Dis 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tosiano MA, Jacobs JL, Shutt KA, et al. A simpler and more sensitive single-copy HIV-1 RNA assay for quantification of persistent HIV-1 viremia in individuals on suppressive antiretroviral therapy. J Clin Microbiol 2019; 57:e01714–e01718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng L, Bosch RJ, Chan ES, et al. Predictors of residual viraemia in patients on long-term suppressive antiretroviral therapy. Antivir Ther 2013; 18:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18▪.Bakkour S, Deng X, Bacchetti P, et al. Replicate Aptima assay for quantifying residual plasma viremia in individuals on ART. J Clin Microbiol 2020; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]; Describes development of an automated highly sensitive assay for HIV-1 RNA in plasma.
- 19.Jacobs JL, Tosiano MA, Koontz DL, et al. Automated, multi-replicate quantification of persistent HIV-1 viremia in individuals on antiretroviral therapy. J Clin Microbiol 2020; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20▪▪.Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 2020; 25:2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]; Describes development of the first validated workflow for molecular detection of SARS-CoV-2 RNA.
- 21.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020; 382:929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22▪.Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature 2020; 579:265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]; First published sequence of SARS-CoV-2. They used an untargeted metagenomics sequencing approach.
- 23.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24▪▪.Day T, Gandon S, Lion S, et al. On the evolutionary epidemiology of SARS-CoV-2. Curr Biol 2020; 30:R849–R857. [DOI] [PMC free article] [PubMed] [Google Scholar]; Informative essay on modeling the evolutionary epidemiology of SARS-CoV-2 in response to recent conclusions regarding the impact of emerging mutations on the transmissibility of SARS-CoV-2.
- 25.Argyropoulos KV, Serrano A, Hu J, et al. Association of initial viral load in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) patients with outcome and symptoms. Am J Pathol 2020; 190:1881–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020; 26:672–675. [DOI] [PubMed] [Google Scholar]
- 27.Lee S, Kim T, Lee E, et al. Clinical course and molecular viral shedding among asymptomatic and symptomatic patients with SARS-CoV-2 infection in a community treatment center in the Republic of Korea. JAMA Intern Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis 2020; 20:656–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pujadas E, Chaudhry F, McBride R, et al. SARS-CoV-2 viral load predicts COVID-19 mortality. Lancet Respir Med 2020; 8:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med 2020; 382:1177–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinloch NN, Ritchie G, Brumme CJ, et al. Suboptimal biological sampling as a probable cause of false-negative COVID-19 diagnostic test results. J Infect Dis 2020; 222:899–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Zhang L, Sang L, et al. Kinetics of viral load and antibody response in relation to COVID-19 severity. J Clin Invest 2020; 130:5235–5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamers MM, Beumer J, van der Vaart J, et al. SARS-CoV-2 productively infects human gut enterocytes. Science 2020; 369:50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puelles VG, Lutgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 2020; 383:590–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solomon IH, Normandin E, Bhattacharyya S, et al. Neuropathological features of Covid-19. N Engl J Med 2020; 383:989–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song Zhang C, Israelow B, Lu-Culligan A, et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. bioRxiv 2020; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teuwen LA, Geldhof V, Pasut A, et al. COVID-19: the vasculature unleashed. Nat Rev Immunol 2020; 20:389–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020; 395:1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020; 323:1843–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 2020; 158:1831–1833.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ziegler CGK, Allon SJ, Nyquist SK, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 2020; 181:1016–1035.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cholankeril G, Podboy A, Aivaliotis VI, et al. High prevalence of concurrent gastrointestinal manifestations in patients with severe acute respiratory syndrome coronavirus 2: early experience from California. Gastroenterology 2020; 159:775–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gu J, Han B, Wang J. COVID-19: gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology 2020; 158:1518–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020; 77:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45▪.Fajnzylber J, Coxen RJ, Corry K, et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. medRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; Describes an association between SARS-CoV-2 plasma RNA presence and quantity with disease severity.
- 46▪.Hagman K, Hedenstierna M, Gille-Johnson P, et al. SARS-CoV-2 RNA in serum as predictor of severe outcome in COVID-19: a retrospective cohort study. Clin Infect Dis 2020; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]; Describes an association between SARS-CoV-2 RNA detection in plasma and progression to severe disease.
- 47.Jacobs JL, Mellors JW. Detection of SARS-CoV-2 RNA in blood of patients with COVID-19: what does it mean? Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48▪.Prebensen C, Hre PLM, Jonassen C, et al. SARS-CoV-2 RNA in plasma is associated with ICU admission and mortality in patients hospitalized with COVID-19. Clin Infect Dis 2020; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]; Describes an association between plasma SARS-CoV-2 RNA levels and disease severity, but no association was found between nasopharynx swab SARS-CoV-2 RNA levels and disease severity.
- 49▪.Veyer D, Kerneis S, Poulet G, et al. Highly sensitive quantification of plasma SARS-CoV-2 RNA shelds light on its potential clinical value. Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; Describes associations between presence and levels of SARS-CoV-2 RNA in plasma and disease severity and outcome.
- 50▪.Xu D, Zhou F, Sun W, et al. Relationship between serum SARS-CoV-2 nucleic acid(RNAemia) and organ damage in COVID-19 patients: a cohort study. Clin Infect Dis 2020; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]; Describes an association between presence of plasma SARS-CoV-2 RNA and organ damage.
- 51.Katzenstein DA, Hammer SM, Hughes MD, et al. The relation of virologic and immunologic markers to clinical outcomes after nucleoside therapy in HIV-infected adults with 200 to 500 CD4 cells per cubic millimeter. AIDS Clinical Trials Group Study 175 Virology Study Team. N Engl J Med 1996; 335:1091–1098. [DOI] [PubMed] [Google Scholar]
- 52.Desai SM, Kalyanaraman VS, Casey JM, et al. Molecular cloning and primary nucleotide sequence analysis of a distinct human immunodeficiency virus isolate reveal significant divergence in its genomic sequences. Proc Natl Acad Sci U S A 1986; 83:8380–8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hahn BH, Shaw GM, Arya SK, et al. Molecular cloning and characterization of the HTLV-III virus associated with AIDS. Nature 1984; 312:166–169. [DOI] [PubMed] [Google Scholar]
- 54.Wong-Staal F, Hahn BH, Shaw GM, et al. Molecular characterization of human T-lymphotropic leukemia virus type III associated with the acquired immunodeficiency syndrome. Princess Takamatsu Symp 1984; 15:291–300. [PubMed] [Google Scholar]
- 55.Mansky LM, Bernard LC. 3′-Azido-3′-deoxythymidine (AZT) and AZT-resistant reverse transcriptase can increase the in vivo mutation rate of human immunodeficiency virus type 1. J Virol 2000; 74:9532–9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mansky LM, Preveral S, Selig L, et al. The interaction of Vpr with uracil DNA glycosylase modulates the human immunodeficiency virus type 1 in vivo mutation rate. J Virol 2000; 74:7039–7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mansky LM, Temin HM. Lower in vivo mutation rate of human immunodeficiency virus type 1 than that predicted from the fidelity of purified reverse transcriptase. J Virol 1995; 69:5087–5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DeGruttola V, Dix L, D’Aquila R, et al. The relation between baseline HIV drug resistance and response to antiretroviral therapy: re-analysis of retrospective and prospective studies using a standardized data analysis plan. Antivir Ther 2000; 5:41–48. [DOI] [PubMed] [Google Scholar]
- 59.Kearney M, Maldarelli F, Shao W, et al. Human immunodeficiency virus type 1 population genetics and adaptation in newly infected individuals. J Virol 2009; 83:2715–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McKinnon JE, Delgado R, Pulido F, et al. Single genome sequencing of HIV-1 gag and protease resistance mutations at virologic failure during the OK04 trial of simplified versus standard maintenance therapy. Antivir Ther 2011; 16:725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palmer S, Kearney M, Maldarelli F, et al. Multiple, linked human immunodeficiency virus type 1 drug resistance mutations in treatment-experienced patients are missed by standard genotype analysis. J Clin Microbiol 2005; 43:406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jabara CB, Jones CD, Roach J, et al. Accurate sampling and deep sequencing of the HIV-1 protease gene using a Primer ID. Proc Natl Acad Sci U S A 2011; 108:20166–20171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Keys JR, Zhou S, Anderson JA, et al. Primer ID informs next-generation sequencing platforms and reveals preexisting drug resistance mutations in the HIV-1 reverse transcriptase coding domain. AIDS Res Hum Retroviruses 2015; 31:658–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boltz VF, Rausch J, Shao W, et al. Ultrasensitive single-genome sequencing: accurate, targeted, next generation sequencing of HIV-1 RNA. Retrovirology 2016; 13:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boltz VF, Shao W, Bale MJ, et al. Linked dual-class HIV resistance mutations are associated with treatment failure. JCI Insight 2019; 4:e130118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li W, Tanimura M, Sharp P. Rates and dates of divergence between AIDS virus nucleotide sequences. Mol Biol Evol 1988; 5:313–330. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki Y, Yamaguchi-Kabata Y, Gojobori T. Nucleotide substitution rates of HIV-1. AIDS Rev 2000; 2:39–47. [Google Scholar]
- 68.Zhang L, Diaz RS, Ho DD, et al. Host-specific driving force in human immunodeficiency virus type 1 evolution in vivo. J Virol 1997; 71:2555–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Su YCF, Anderson DE, Young BE, et al. Discovery and genomic characterization of a 382-nucleotide deletion in ORF7b and ORF8 during the early evolution of SARS-CoV-2. mBio 2020; 11:e01610–e01620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Day T, Gandon S, Lion S, et al. On the evolutionary epidemiology of SARS-CoV-2. Curr Biol 2020; 30:R849–R857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duchene S, Featherstone L, Haritopoulou-Sinanidou M, et al. Temporal signal and the phylodynamic threshold of SARS-CoV-2. Virus Evol 2020; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pereson MJ, Mojsiejczuk L, Martinez AP, et al. Phylogenetic analysis of SARS-CoV-2 in the first few months since its emergence. J Med Virol 2020; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eckerle LD, Becker MM, Halpin RA, et al. Infidelity of SARS-CoV nsp14-exonuclease mutant virus replication is revealed by complete genome sequencing. PLoS Pathog 2010; 6:e1000896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Denison MR, Graham RL, Donaldson EF, et al. Coronaviruses: an RNA proofreading machine regulates replication fidelity and diversity. RNA Biol 2011; 8:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sanjuán R, Nebot MR, Chirico N, et al. Viral mutation rates. J Virol 2010; 84:9733–9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eskier D, Karakulah G, Suner A, et al. RdRp mutations are associated with SARS-CoV-2 genome evolution. PeerJ 2020; 8:e9587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77▪▪.Alm E, Broberg EK, Connor T, et al. Geographical and temporal distribution of SARS-CoV-2 clades in the WHO European Region, January to June 2020. Euro Surveill 2020; 25:2001410. [DOI] [PMC free article] [PubMed] [Google Scholar]; Article showing the distribution of SARS-CoV-2 genetic clades with an outline of potential genomic surveillance objectives. Provides an overview of the three genomic nomenclature systems with informative graphics to help explain the commonalities and differences between systems.
- 78.(GISAID) GISAID. Clade and lineage nomenclature aids in genomic epidemiology studies of active hCoV-19 viruses. 2020; https://www.gisaid.org/references/statements-clarifications/clade-and-lineage-nomenclature-aidsin-genomic-epidemiology-of-activehcov-19-viruses/ [Google Scholar]
- 79.Hodcroft EB, Hadfield J, Neher RA, et al. Year-letter genetic clade naming for SARS-CoV-2 on Nextstain.org. NextStrain 2020. [Google Scholar]
- 80.Rambaut A, Holmes EC, O’Toole Á, et al. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol 2020; 5:1403–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tillett R, Sevinsky J, Hartley P, et al. Genomic evidence for a case of reinfection with SARS-CoV-2. SSRN Electron J 2020; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.To KK-W, Hung IF-N, Ip JD, et al. COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin Infect Dis 2020; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83▪.Korber B, Fischer WM, Gnanakaran S, et al. Tracking changes in SARS-CoV-2 Spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell 2020; 182:812–827.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]; First article to show lower cycle threshold values in individuals infected with viral variants at S-D614G, indicating that patients carrying the G614 mutation had higher viral loads.
- 84.Long SW, Olsen RJ, Christensen PA, et al. Molecular architecture of early dissemination and massive second wave of the SARS-CoV-2 virus in a major metropolitan area. medRxiv 2020; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang L, Jackson CB, Mou H, et al. The D614G mutation in the SARS-CoV-2 spike protein reduces S1 shedding and increases infectivity. bioRxiv 2020; Epub ahead of print. [Google Scholar]
- 86.Andres C, Garcia-Cehic D, Gregori J, et al. Naturally occurring SARS-CoV-2 gene deletions close to the spike S1/S2 cleavage site in the viral quasispecies of COVID19 patients. Emerg Microbes Infect 2020; 9:1900–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]


