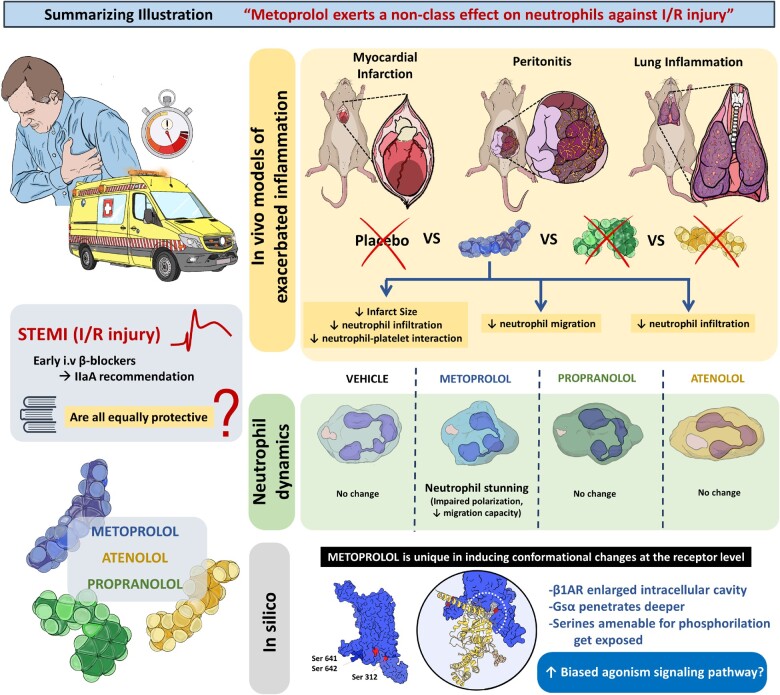
Abstract
Aims
Clinical guidelines recommend early intravenous β-blockers during ongoing myocardial infarction; however, it is unknown whether all β-blockers exert a similar cardioprotective effect. We experimentally compared three clinically approved intravenous β-blockers.
Methods and results
Mice undergoing 45 min/24 h ischaemia–reperfusion (I/R) received vehicle, metoprolol, atenolol, or propranolol at min 35. The effect on neutrophil infiltration was tested in three models of exacerbated inflammation. Neutrophil migration was evaluated in vitro and in vivo by intravital microscopy. The effect of β-blockers on the conformation of the β1 adrenergic receptor was studied in silico. Of the tested β-blockers, only metoprolol ameliorated I/R injury [infarct size (IS) = 18.0% ± 0.03% for metoprolol vs. 35.9% ± 0.03% for vehicle; P < 0.01]. Atenolol and propranolol had no effect on IS. In the three exacerbated inflammation models, neutrophil infiltration was significantly attenuated only in the presence of metoprolol (60%, 50%, and 70% reductions vs. vehicle in myocardial I/R injury, thioglycolate-induced peritonitis, and lipopolysaccharide-induced acute lung injury, respectively). Migration studies confirmed the particular ability of metoprolol to disrupt neutrophil dynamics. In silico analysis indicated different intracellular β1 adrenergic receptor conformational changes when bound to metoprolol than to the other two β-blockers.
Conclusions
Metoprolol exerts a disruptive action on neutrophil dynamics during exacerbated inflammation, resulting in an infarct-limiting effect not observed with atenolol or propranolol. The differential effect of β-blockers may be related to distinct conformational changes in the β1 adrenergic receptor upon metoprolol binding. If these data are confirmed in a clinical trial, metoprolol should become the intravenous β-blocker of choice for patients with ongoing infarction.
Keywords: β-blockers, β1AR, I/R injury, Neutrophils, Inflammation
Graphical Abstract
See page 4441 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa764)
Introduction
Acute myocardial infarction (AMI) is a leading cause of morbidity and mortality worldwide. The advent of reperfusion technologies has dramatically reduced acute mortality associated with AMI. However, the size of the infarct often leaves survivors with severe heart damage, and these patients are at high risk of future heart failure and readmission.1 , 2 Reperfusion, despite being essential for myocardial salvage, triggers an exacerbated sterile inflammatory process that contributes to final infarct size (IS). This inflammation is driven by neutrophils, which infiltrate the damaged myocardium through interactions with platelets contribute to ischaemia–reperfusion (I/R) injury (IRI).3–5 Paradoxically, blood flow restoration in the large epicardial coronary artery many times is not accompanied by efficient tissue perfusion due to the obstruction of the microvasculature. Endothelial swelling, external compression of small vessels secondary to oedema formation, and cellular aggregates (neutrophils, platelets, and erythrocytes) generating plugs that restrict tissue perfusion at the capillary level contribute to the phenomenon known as microvascular obstruction (MVO).6–8 The latter is a main contributor to IRI and final IS.1 , 2
Translational perspective
Early administration of intravenous (i.v.) β-blockers is recommended for patients with an ongoing myocardial infarction; however, it is unknown whether all approved drugs exert the same cardioprotective effect. Here, we show that metoprolol, but not atenolol or propranolol, has an ameliorative effect on neutrophil-induced tissue damage during exacerbated inflammation, including myocardial ischaemia–reperfusion injury. Metoprolol disrupts deleterious neutrophil dynamics during reperfusion, and this translates into a significant infarct-limiting effect not shared by the other tested β-blockers. Modelling shows that metoprolol binding triggers a unique conformational change in the β1 adrenergic receptor intracellular domain. If confirmed at the clinical level, early intravenous metoprolol, but not other β-blockers, should be used to treat patients with ongoing infarction before reperfusion.
The β1-selective blocker metoprolol has been demonstrated to reduce myocardial IS in several species, including humans.9–11 Metoprolol appears to limit IS largely through its inhibitory effect on neutrophils.9 Based partly on the cardioprotective effect of metoprolol injection,10 , 12 current clinical practice guidelines recommend early intravenous administration of β-blockers (as a drug class) to patients with an ongoing AMI.13 However, it is unknown whether different β-blockers exert the same cardioprotective effect, and a trial in patients with ongoing AMI undergoing reperfusion showed no infarct-limiting effect of the β1-selective blocker atenolol.14 In this study, we explored the cardioprotective effect of three β-blockers approved for clinical i.v. administration (metoprolol, atenolol, and propranolol) in a mouse model of IRI. We further explored the effect of these β-blockers on neutrophil migration and infiltration in three models of exacerbated inflammation: myocardial I/R, thioglycolate-induced peritonitis, and lipopolysaccharide (LPS)-induced acute lung injury (ALI). In silico studies were conducted to evaluate conformational changes in the β1 adrenergic receptor upon binding the different β-blockers. Our results show that metoprolol has a particular action on neutrophils during exacerbated inflammation that affords a cardioprotection not provided by other β-blockers.
Methods
Full section of material and methods can be found in the Supplementary material online.
Results
Metoprolol, but not atenolol or propranolol, limits myocardial infarct size
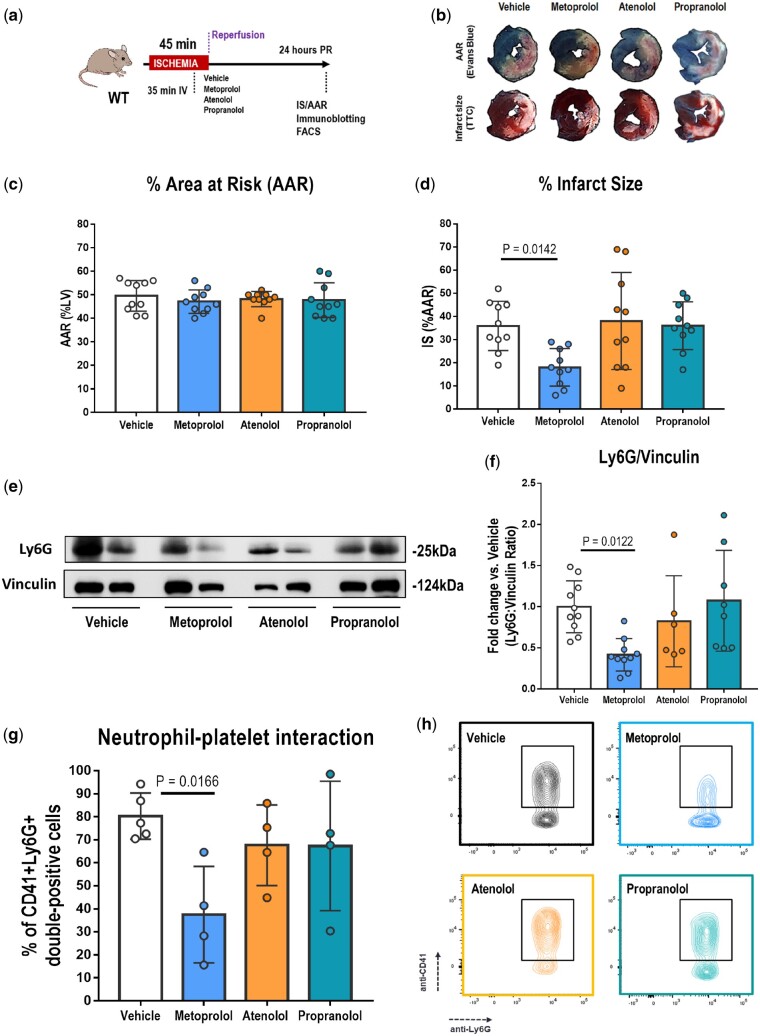
The cardioprotective activity of three clinically approved intravenous β-blocker agents was assessed in an established in vivo model of IRI (Figure 1A).9 In brief, mice were anaesthetized by i.p. administration of ketamine, xylazine, and atropine, and were placed on mechanical ventilation. The left anterior descending coronary artery was accessed by a small thoracotomy and then fully occluded by tying a silk knot around the proximal segment of the artery. After 45 min of coronary artery occlusion, the knot was released to allow reperfusion. Before reperfusion, mice were randomly allocated to i.v. metoprolol, atenolol, propranolol (all 12.5 mg/kg) or vehicle (0.9% NaCl). Operators were blinded to treatment allocation. Drug or vehicle was injected as a single bolus through the retro-orbital sinus 10 min before reperfusion (35 min after ischaemia onset). The metoprolol, atenolol, and propranolol dose was based on a dose–response study, in which 12.5 mg/kg was identified as the highest dose with moderate haemodynamic effect (<20%) for the three β-blockers (Supplementary material online, Figure S1). At 24 h post-reperfusion, mice were euthanized, and area at risk (AAR)-normalized IS was calculated.9
Figure 1.
The infarct-limiting effect of metoprolol is not shared by atenolol or propranolol. (A) Mouse model of myocardial ischaemia–reperfusion for the estimation of area at risk (AAR) and infarct size (IS) by Evans Blue and triphenyl tetrazolium chloride (TTC) staining and the collection of left ventricle tissue and blood for immunoblotting and flow cytometry analysis. Mice were randomized to receive the indicated i.v. treatments 10 min before reperfusion. (B) Representative images of 1-mm-thick transverse left ventricle slices showing area at risk (negative for Evans Blue, white) and the extent of necrosis (triphenyl tetrazolium chloride-negative area). (C, D) Histological analysis of AAR (% left ventricle) and IS (% area at risk) in mice subjected to ischaemia–reperfusion and randomized to receive vehicle (white), metoprolol (blue), atenolol (orange), or propranolol (green). n = 10 for each condition. (E, F) Immunoblot analysis of Ly6G (25 kDa) and vinculin (124 kDa) protein expression at 24 h post-reperfusion in myocardium of mice subjected to ischaemia–reperfusion and randomized to pre-reperfusion treatments as above: vehicle, n = 10; metoprolol, n = 10; atenolol, n = 6; propranolol, n = 8. Quantified Ly6G levels in (F) are normalized to vinculin and expressed as the fold change relative to vehicle-treated mice. (G, H) Flow cytometry analysis of neutrophil–platelet interaction in peripheral citrated blood of mice subjected to ischaemia–reperfusion and randomized to receive one of the four indicated treatments. Data are presented as the percentage of neutrophils (Ly6G+) staining doubly positive for Ly6G and the platelet marker CD41. The representative flow cytometry plots in (H) illustrate the reduction in neutrophil–platelet interactions (boxed areas) in metoprolol-treated mice: n = 4 for all treatments except vehicle, n = 5. Data are presented as mean ± SD.
Confirming previous studies, i.v. metoprolol resulted in smaller IS (% AAR) than in vehicle-treated mice (metoprolol, 18.0% ± 8.11%; vehicle, 35.9% ± 10.7%; P = 0.0142). In contrast, atenolol and propranolol had no effect on IS (atenolol, 38.0% ± 20.9%; propranolol, 36.0% ± 10.3%; vehicle, 35.9% ± 8.11%) (Figure 1B–D).
Metoprolol is the only tested β-blocker that attenuates post-acute myocardial infarction neutrophil infiltration
In previous studies in pigs and mice, we showed that pre-reperfusion metoprolol injection results in reduced myocardial neutrophil infiltration,9 , 15 accounting for its cardioprotective effect. Here, assessment of myocardial Ly6G protein levels at 24 h post-reperfusion revealed significantly lower neutrophil density in metoprolol-injected mice than in vehicle-treated mice, whereas atenolol and propranolol had no effect (Figure 1A, E, and F). Ly6G protein levels in metoprolol-treated mice were almost 60% lower in left ventricles of metoprolol-treated mice than those of controls.
Metoprolol, but not atenolol or propranolol, inhibits neutrophil–platelet interactions during myocardial ischaemia–reperfusion
Neutrophil–platelet interactions are crucial for neutrophil tissue infiltration during sterile inflammation.3–5 Because the cardioprotective effect not shared by the other β-blockers was expected to be driven by altered neutrophil dynamics, we next explored neutrophil interactions with platelets in peripheral blood 24 h after reperfusion. The percentage of circulating neutrophils interacting with platelets was significantly reduced only in the case of metoprolol (metoprolol, 37.5% ± 20.9%; vehicle, 80.2% ± 10.1%; P = 0.0166), whereas atenolol (67.6% ± 17.5%) and propranolol (67.3% ± 28.1%) had no statistically significant effect (Figure 1G and H). The percentage and number of circulating neutrophil population were not affected by any of the treatment conditions (Supplementary material online, Figure S2).
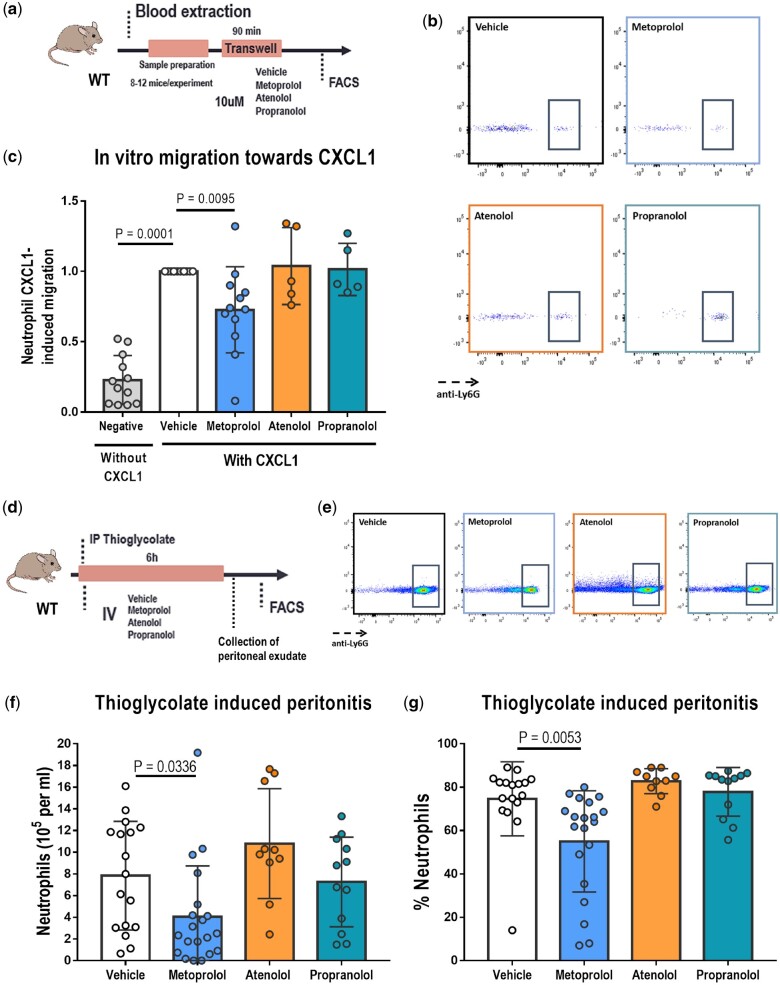
Metoprolol has a particular inhibitory effect on neutrophil migration in vitro and in vivo
We previously showed that metoprolol exerts its cardioprotective effect during I/R by targeting neutrophils.9 Here, we wanted to explore whether the action on neutrophils was a drug class effect, and thus shared by other β-blockers, or was particular to metoprolol. The effect of the tested β-blockers on neutrophil migration was assessed in a chemokine-induced transwell migration assay (Figure 2A). Mouse neutrophils were exposed across the transwell filter to the chemoattractant CXCL1 in the presence or absence of metoprolol, atenolol, or propranolol (10 µM for every condition), and the number of cells migrating across the transwell membrane was quantified by flow cytometry after 90 min. Metoprolol inhibited baseline neutrophil migration along the CXCL1 gradient (0.73 ± 0.31 vs. vehicle; P = 0.0095), whereas no effect on chemokine-induced migration was seen with either atenolol (1.04 ± 0.27) or propranolol (1.01 ± 0.19) (Figure 2B and C).
Figure 2.
Metoprolol has a particular ability to inhibit neutrophil migration. (A) Experimental scheme for CXCL1-induced transwell migration analysis. (B) Flow cytometry plots illustrating reduced migration of neutrophils (Ly6G+ cells) upon treatment with metoprolol. To allow comparison between experiments, neutrophil migration for all treatments was normalized to the mean positive control (vehicle) value in each independent experiment. (C) Particular limiting effect of metoprolol on chemokine-induced neutrophil migration. Each independent experiment was conducted with leucocytes pooled from 8 to 12 animals, and each condition was run with three to four technical replicates: n = 12 for all conditions except for atenolol and propranolol, n = 5 each. (D) Experimental scheme for thioglycolate-induced peritonitis. Mice received a 12.5 mg/kg i.v. β-blocker dose immediately after i.p. thioglycolate administration. (E) Flow cytometry plots illustrating reduced peritoneal infiltration of neutrophils (Ly6G+ cells) in metoprolol-treated mice. (F, G) Specific limiting effect of metoprolol on thioglycolate-induced peritoneal infiltration in wild-type mice. (F) Absolute number of neutrophils/mL of infiltrate 6 h after thioglycolate injection in wild-type mice. (G) Neutrophils in intraperitoneal exudate calculated as a percentage of total viable cells. Vehicle, n = 16; metoprolol, n = 18; atenolol, n = 10; propranolol, n = 12. Data are presented as mean ± SD.
To confirm these results, we investigated whether atenolol or propranolol could mimic the ability of metoprolol to inhibit neutrophil tissue infiltration in vivo in a validated mouse model of thioglycolate-induced peritonitis9 (Figure 2D). Thioglycolate induces massive leucocyte migration into the peritoneal cavity within the first 6 h, with most infiltrating cells being neutrophils. The i.v. metoprolol bolus (12.5 mg/kg) steeply inhibited thioglycolate-induced neutrophil infiltration into the mouse peritoneal cavity (4.03 ± 4.70 × 105 vs. 7.84 ± 5.01 × 105 neutrophils/mL for metoprolol and vehicle, respectively; P = 0.0336) and reduced neutrophils as a percentage of viable cells (55.2% ± 23.3% vs. 78.5% ± 17.1% for metoprolol and vehicle, respectively; P = 0.0053). In contrast, atenolol and propranolol (12.5 mg/kg each) had no anti-migratory effect on neutrophil infiltration (10.8 ± 5.06 × 105, 7.26 ± 4.14 × 105, and 7.84 ± 5.01 × 105 neutrophils/mL for atenolol, propranolol, and vehicle, respectively) or neutrophils as a percentage of viable cells (82.9% ± 5.76%, 77.9% ± 11.23%, and 78.5% ± 17.1% for atenolol, propranolol, and vehicle, respectively) (Figure 2E–G).
To exclude potential dose-dependent effects and differential potency of the three tested β-blockers, we halved and doubled the β-blocker dose in the thioglycolate-induced peritonitis model to a single 6.25 or 25 mg/kg i.v. bolus, respectively. At these β-blocker doses, the same pattern was maintained, with neutrophil migration inhibited only by metoprolol, and atenolol and propranolol having no effect (Supplementary material online, Figure S3).
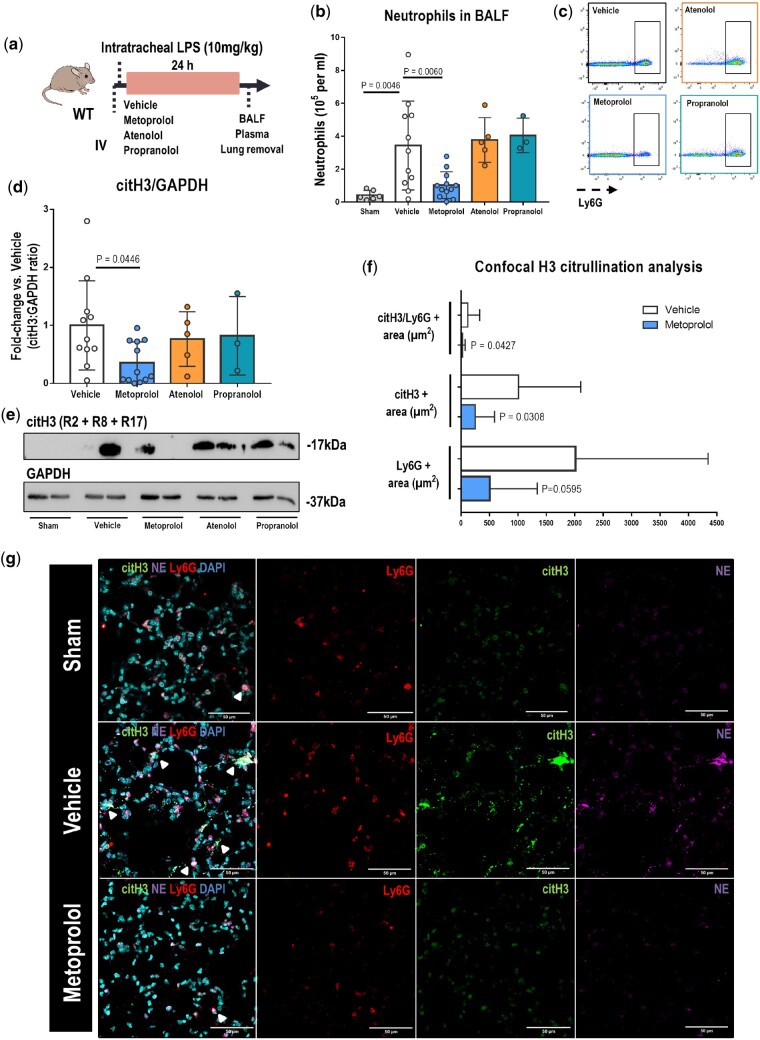
Metoprolol attenuates neutrophil infiltration during lipopolysaccharide-induced acute lung injury
We next tested the differential effects of i.v. β-blockers on neutrophil migration and infiltration in a mouse model of infection-induced inflammation: LPS-induced ALI (Figure 3A). At 24 h after LPS instillation, Broncho-alveolar lavage fluid (BALF) from metoprolol-treated mice contained significantly fewer neutrophils than BALF from vehicle-treated mice (1.03 ± 0.81 × 105 vs. 3.44 ± 2.71 × 105 neutrophils/mL for metoprolol and vehicle, respectively; P = 0.0060). Neither atenolol nor propranolol had any effect on the BALF neutrophil count (3.78 ± 1.36 × 105, 4.06 ± 1.05 × 105, and 3.44 ± 2.71 × 105 neutrophils/mL for atenolol, propranolol, and vehicle, respectively) (Figure 3B and C). Tissue damage in response to an acute inflammatory response is known to involve neutrophil release of nuclear chromatin, known as neutrophil extracellular traps (NETs).16 Given that the LPS challenge increases citH3,16 which is strongly implicated in NET formation,17 we assessed whether β-blocker treatment affects this process. Immunoblot analysis showed that mice receiving i.v. metoprolol exhibited a 65% attenuation of H3 citrullination (on R2 + R8 + R17) compared with those receiving vehicle, whereas atenolol and propranolol had no effect (Figure 3D and E). Confocal microscopy analysis of lung tissue revealed that metoprolol significantly reduced the area of lung tissue covered by citH3 and the area of co-localization between citH3 and neutrophils (Ly6G+ cells) (Figure 3F and G). Moreover, reduced H3 citrullination in the lungs of metoprolol-treated mice was accompanied by reductions in neutrophil–elastase and myeloperoxidase (Supplementary material online, Figure S4A and B), neutrophil granule proteins involved in NET generation.17 These changes were accompanied by a protection against lung tissue damage in metoprolol-treated mice (Supplementary material online, Figure S4C and D). These results confirm attenuation of NET production and the amelioration of ALI in mice receiving metoprolol.
Figure 3.
Metoprolol attenuates broncho-alveolar lavage fluid (BALF) neutrophil counts and H3 citrullination in lipopolysaccharide (LPS)-treated lungs. (A) Model of LPS-induced acute lung injury (ALI). Mice received an intratracheal instillation of LPS immediately after i.v. injection with the indicated treatments. (B, C) Flow cytometry analysis of neutrophil counts in BALF at 24 h after LPS instillation. The flow cytometry plots illustrate the reduced presence of neutrophils (Ly6G+ cells) in BALF of metoprolol-treated mice. (D, E) Immunoblot analysis of Histone 3 hypercitrullination (citH3) (17 kDa) and GAPDH (37 kDa) protein expression in the lungs of mice with acute lung injury and receiving the indicated treatments. Quantified citH3 levels in (D) are normalized to GAPDH and expressed as the fold change relative to vehicle-treated mice. Data in (B) and (D) are means ± SD. Sham (no LPS), n = 6; vehicle, n = 11; metoprolol, n = 12; atenolol, n = 5; propranolol, n = 3. (F, G) Confocal microscopy analysis of histone 3 citrullination in acute lung injury. (F) Total area of lung tissue covered by citH3 and neutrophils (Ly6G+ cells) and the area of neutrophil–citH3 co-localization 24 h after lipopolysaccharide instillation. (G) Representative confocal images of lung sections from sham-treated mice (Control, no lipopolysaccharide), and lipopolysaccharide-instilled mice receiving i.v. vehicle (saline) or metoprolol. Areas of co-localization (arrowheads) between histone 3 citrullination (citH3, green) and neutrophils (Ly6G+ cells, red) indicate generation of neutrophil extracellular traps (NETs). The neutrophil granule protein neutrophil–elastase (NE, purple) is a marker of neutrophil activation. n = 10–12 mice per condition. Data are presented as mean ± SD.
Metoprolol has a disruptive effect on neutrophil dynamics in vivo not shared by the other β-blockers tested
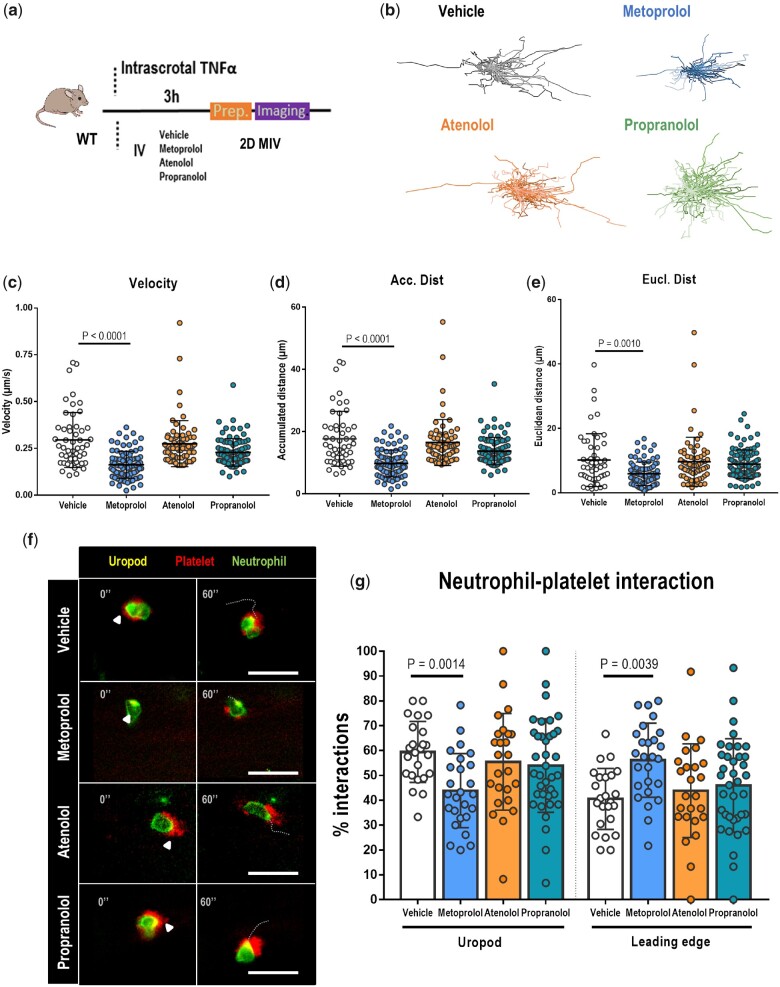
Myocardial I/R is a paradigm of acute sterile inflammation, in which chemotactic recruitment of inflammatory cells is predominantly mediated by neutrophils. To initiate an acute inflammatory response, neutrophils adhering to the activated endothelium undergo morphological rearrangements that allow them to interact with and recruit other cell types to infiltrate the tissue.4 Having observed that, unlike metoprolol, atenolol and propranolol showed no effect on neutrophil recruitment, we next explored the effect of these drugs on neutrophil dynamics. For this, we used 2D intravital microscopy (IVM) to image migration in the cremaster muscle vessels of mice injected with tumour necrosis factor α (TNFα), which triggers massive neutrophil recruitment4 (Figure 4A). Of the tested β-blockers, only metoprolol reduced neutrophil migratory velocity (0.16 ± 0.07 µm/s vs. 0.29 ± 0.15 µm/s for metoprolol and vehicle, respectively; P < 0.0001), accumulated distance (9.75 ± 4.33 µm vs. 17.7 ± 8.82 µm for metoprolol and vehicle, respectively; P < 0.0001), and euclidean crawling distance (5.90 ± 3.66 µm vs. 10.2 ± 8.18 µm for metoprolol and vehicle, respectively; P < 0.0010). Moreover, metoprolol reduced the percentage of neutrophils interacting with platelets through the uropod (42.5% ± 17.6% vs. 59.4% ± 12.3% for metoprolol and vehicle, respectively; P < 0.0014). Neither atenolol nor propranolol had any effect on any of the in vivo neutrophil dynamics parameters evaluated (Figure 4B–F and Supplementary material online, Videos S1A–D).
Figure 4.
Metoprolol has a particular disruptive effect on neutrophil dynamics in vivo. (A) Experimental scheme for 2D intravital microscopy (IVM) of neutrophil motility in inflamed cremaster muscle. (B) Representative tracks of crawling neutrophils within inflamed vessels of mice treated with vehicle, metoprolol, atenolol, or propranolol. (C–E) Two-dimensional intravascular motility parameters: velocity (µm/s), accumulated distance (µm), and euclidean distance (µm); n = 52–89 cells from 5 to 6 mice per condition. (F) Representative time-lapse images of platelets (CD41+ cells, red) with the polarized neutrophil uropod (CD62L+ domain, yellow) or leading edge (Ly6G+ domain, green) in the different conditions. Arrowheads indicate interactions with the uropod domain, and dotted lines indicate displacement of the neutrophil over 60s. (G) Percentage of platelet interactions with the neutrophil uropod or leading edge; n = 24–37 cells from three mice per condition. Data are presented as means ± SD.
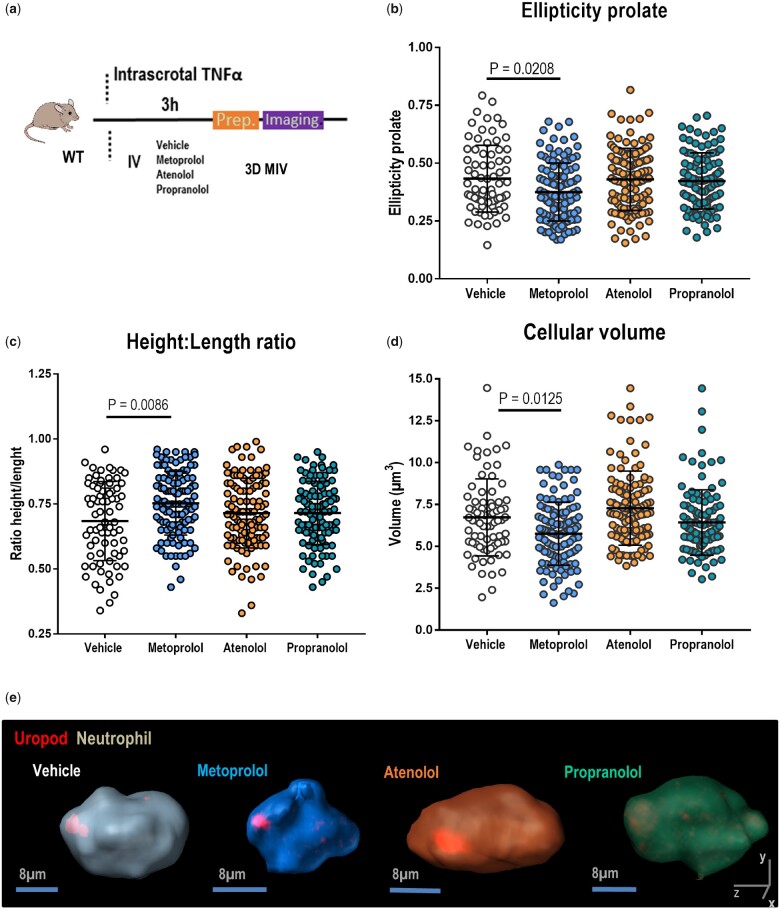
3D IVM studies were performed (Figure 5A) to test whether metoprolol specifically altered neutrophil shape or polarization during the acute inflammatory response. Consistent with the disrupted crawling dynamics observed in the 2D analysis, 3D reconstructions revealed that metoprolol impaired neutrophil polarization in TNFα-inflamed cremaster vessels, reducing neutrophil length and preventing the adoption of the typical cigar-like prolate spheroid cell shape (Figure 5B–D). These effects were not observed with atenolol and propranolol, indicating that the neutrophil morphological changes needed to initiate intercellular interactions and subsequent tissue infiltration remain intact in mice treated with these drugs. This result might explain the lack of a cardioprotective effect with these drugs during I/R. Conversely, metoprolol blocks neutrophil infiltration and migration through an effect on neutrophil dynamics, and this neutrophil-stunning effect confers a cardioprotective effect during myocardial I/R.
Figure 5.
Metoprolol alters neutrophil polarized morphology. (A) Experimental scheme for 3D intravital microscopy (IVM) of neutrophil morphology in inflamed cremaster muscle. (B–D) Three-dimensional intravascular cell morphology parameters: ellipticity prolate, height: length ratio, and volume. n = 75–118 cells from 3 to 4 mice per condition. Data are presented as mean ± SD. (E) Representative 3D reconstructions of polarized neutrophils (uropod, red) within live cremaster vessels of mice treated with vehicle (grey), metoprolol (blue), atenolol (orange), or propranolol (green).
Metoprolol-binding triggers β1 adrenergic receptor intracellular conformational change exposing phosphorylation targets involved in β-arrestin signalling cascade
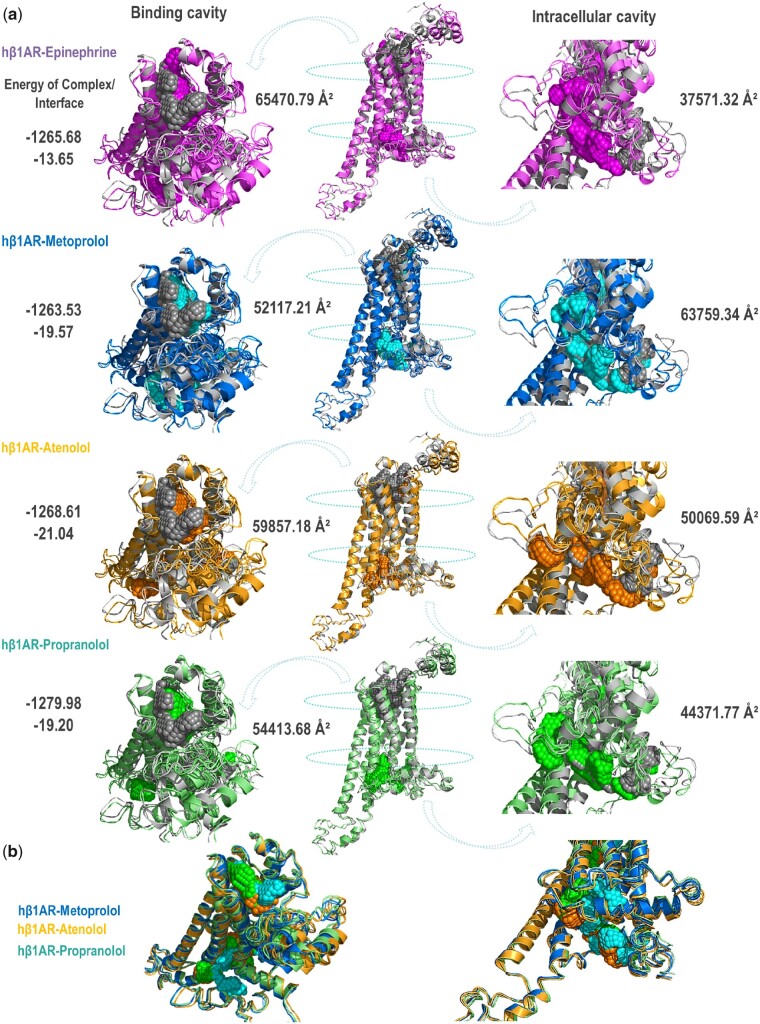
The interaction of the three β-blockers with the β1 adrenergic receptor (β1AR) was investigated by in silico approaches. All three selected β-blockers belong to the same pharmaceutical class, signal through G-protein coupled receptors (GPCRs), have high affinity for the β1AR, and are currently authorized for intravenous administration to patients. Simulated ligand binding did not substantially alter the overall topology of the human β1AR, which showed only minor differences upon binding the different β-blockers. As expected for drugs belonging to the same class, the extracellular drug-binding pocket has a small solvent accessible surface, and this pocket was moved slightly and to a similar extent with respect to the unbound protein upon binding of all tested ligands.
The model was refined by submitting it to the positioning of proteins in membranes(PPM) server, which positioned the receptor–drug complex more precisely in the membrane. The energy and stability of the ligand–β1AR complex was similar for all drugs; however, metoprolol binding induced an affinity-independent increase in the size of the internal cavity significantly greater than seen with the other tested drugs (63 759.34 Å2 for metoprolol, 37 571.32 Å2 for epinephrine, 50069.59 Å2 for atenolol, and 44 371.77Å2 for propranolol) (Figure 6A and B and Supplementary material online, Tables S1 and S2). Modelling of the mouse β1AR yielded proportionally similar differences in internal cavity size (Supplementary material online, Figure S6, Tables S3 and S4). These results strongly suggest that metoprolol binding induces a bigger conformational change in the receptor that opens the intracellular cavity, likely modifying its interactions with intracellular effectors.
Figure 6.
Metoprolol induces a conformational change in the human β1AR that increases the size of the intracellular cavity. (A) Modelling of the human β1AR modelling alone (grey) and bound to epinephrine (purple), metoprolol (blue), atenolol (orange), or propranolol (green). Each ligand-bound β1AR conformation was compared to the unbound β1AR conformation. Images were obtained with the PyMOL molecular visualization system. In silico analysis indicates that β1AR conformational changes induced by metoprolol binding differ from those induced by the other ligands, producing an enlarged intracellular receptor cavity that is more open than that of the epinephrine-, atenolol-, or propranolol-bound receptor. (B) Superposition of all β-blocker-induced β1AR conformations. The energies of the complex and interface are shown in Rosetta Energy Internal Units, whereas cavity sizes are shown in square Ångström Units (Å2).
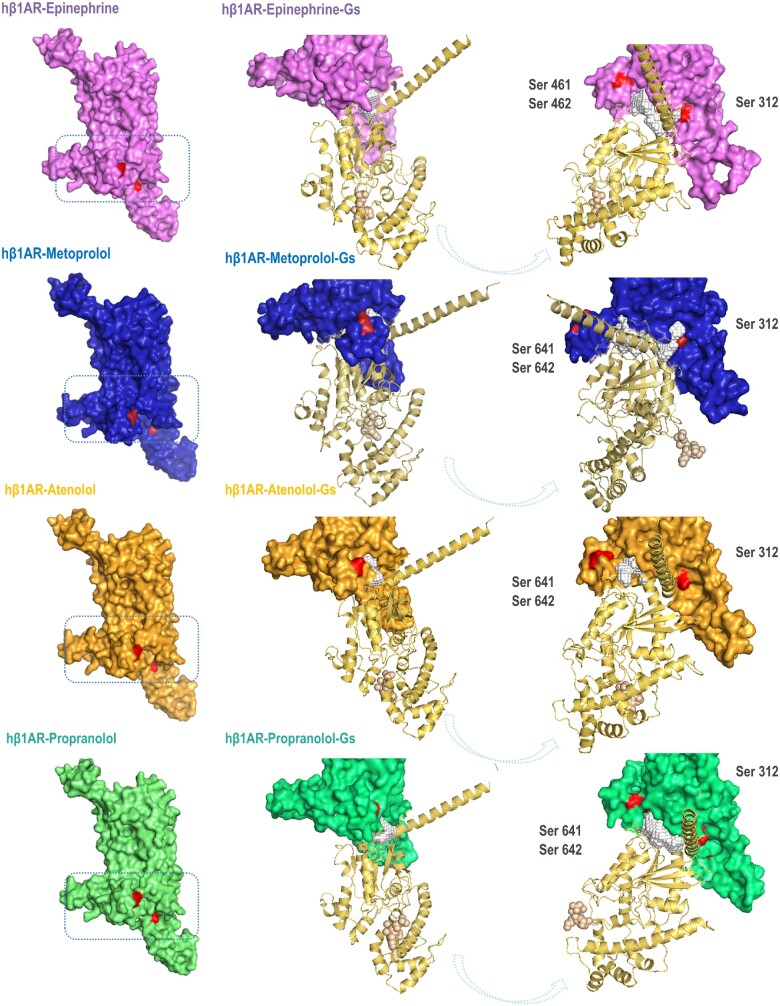
To elucidate whether the opening of the intracellular cavity of the receptor when bound to metoprolol translates into differences in Gs protein signalling, we modelled the binding of the Gsα subunit to the complexes established upon docking of the different ligands to the human β1AR. Although large differences in the energy of interface were not documented, the Gsα subunit penetrates more in the cavity of metoprolol-β1AR than in the rest of the ligand–β1AR complexes (Figure 7), possibly making it more difficult for the Gsα subunit to interact with other effectors to perpetuate the classical adenylate cyclase-AMPc signalling cascade.
Figure 7.
Ligand-human β1AR-Gs protein α subunit conformational changes. Modelling of the binding of the Gsα subunit to the complexes established upon docking of the different ligands (epinephrine, purple; metoprolol, blue; atenolol, orange; and propranolol, green) to the human β1AR.The binding of the Gsα subunit to the metoprolol–β1AR complex exposes phosphorylation sites in the receptor potentially triggering GRK/β-arrestin signalling cascade (see text). Red areas indicate Ser phosphorylation sites.
We further explored the impact of the β1AR–Gsα interaction (upon binding to different β-blockers) on biased agonism signalling pathways. We focused our computational analyses on the study of two conserved sites (regions) of the intracellular region of the β1AR experimentally described as containing putative phosphoryl-Ser that initiate the receptor signalling and deactivation cascade [Ser461 and Ser462, which are susceptible to being phosphorylated by G-protein-coupled receptor kinases (GRKs); and Ser312 that is susceptible to being phosphorylated by protein kinase A].
Qualitatively, it is noticeable that the Ser 461-462 positions are more exposed when the Gsα subunit binds the metoprolol–β1AR complex (Figure 7), potentially being more prone to be phosphorylated by GRKs, triggering a β-arrestin-mediated signalling cascade.
Discussion
In this study, we have evaluated the cardioprotective effect of different clinically approved i.v. β-blockers to reduce IS in a mouse model of myocardial IRI. We have explored the effect of these drugs on the hyperactive immune response during exacerbated inflammation in models of acute injury in the heart, peritoneum and lung, and neutrophil migration in vitro. Finally, we have studied in silico structural changes occurring in the β1AR when bound to the different β-blockers.
Our results show that while metoprolol significantly ameliorates myocardial IRI, atenolol and propranolol have no cardioprotective effect. Our in vitro and in vivo studies show that metoprolol is the only studied β-blocker that impairs neutrophil migration and infiltration during exacerbated inflammation, and 2D and 3D IVM studies show that metoprolol exerts a particular disruptive effect on neutrophil dynamics. The in silico analysis reveals that, upon binding to the β1AR, metoprolol provokes a significant conformational change in the intracellular domain that is not observed with atenolol or propranolol. Taken together, these results show that metoprolol has a unique ability among the β-blockers tested to target neutrophils and stun the neutrophil immune response during exacerbated inflammation (Take home figure). These findings have important clinical implications, given that since clinical practice guidelines on the use of β-blockers during AMI assume that the cardioprotective effect of metoprolol is shared by other drugs of this class.13
Take home figure.
Metoprolol exerts a particular protective effect against neutrophil-mediated ischaemia–reperfusion injury. The cardioprotective properties of metoprolol derive from its particular ability to target neutrophils and reduce ischaemia–reperfusion injury, whereas atenolol and propranolol have no effect on this cell population or on IS. Conformational changes induced in the β1AR upon binding to metoprolol differ significantly from those induced by atenolol and propranolol, and this difference may underlie the neutrophil-stunning action of metoprolol. These data have important implications because clinical practice guidelines currently recommend the use of β-blockers during acute myocardial infarction as a drug class, making no distinction among them.
The METOCARD-CNIC clinical trial demonstrated that pre-reperfusion injection of metoprolol in AMI patients significantly reduces IS and the incidence of long-term heart failure.10 , 12 In another trial in AMI patients undergoing reperfusion, atenolol administration showed no association with reduced IS.14 While these starkly different outcomes could reflect differences in trial design, they also point to possible differences in the ability of these β-blocker agents to counter injurious mechanisms. The leading mechanism of tissue injury during sterile inflammation is exaggerated neutrophil activation and tissue infiltration,18–20 and myocardial I/R serves as a paradigm of this process. A recent study showed that metoprolol ameliorates myocardial IRI through a direct action on neutrophils that prevents intercellular interactions and the cell morphological changes needed to initiate tissue infiltration.9 This prompted us to explore the potential cardioprotective effect of three clinically approved β-blockers, as well as their effect on neutrophil biology during exacerbated inflammation.
We previously showed that metoprolol-induced cardioprotection involves a ‘stunning’ effect on neutrophils. This effect is β1AR-mediated, since metoprolol did not reduce migration in neutrophils from β1KO mice.9 In the present study, we show that other β1AR-selective β-blockers do not inhibit neutrophil migration in vitro or in vivo. This result is in line with a previous in vitro study, in which metoprolol but not atenolol reduced neutrophil migration.21 The lack of an inhibitory effect with another β1AR-selective blocker prompted the authors to conclude that the metoprolol effect was independent of the β1AR. However, the lack of an anti-migratory effect of metoprolol in β1KO neutrophils suggests that the discrepancy between the effects of metoprolol and atenolol might be due to differences in the outcome of β-blocker–β1AR interaction. Our in silico studies confirm that the β1AR undergoes different conformational changes upon binding to these different β-blockers.
The lack of an IS-reducing effect with propranolol appears to contradict a classical analysis showing smaller IS upon propranolol injection in a dog model of chronic coronary occlusion.22 , 23 However, there are important differences between that study and ours, the most important being that the canine model did not include reperfusion and thus did not examine IRI.22 , 23 Our work shows that metoprolol achieves its protective effect by targeting neutrophils, which are prominent mediators of reperfusion injury. In the absence of reperfusion, the leading mechanism of death is ischaemic damage, in which neutrophils do not play such significant role.
The drugs used in this study were selected on the basis of their availability in i.v. formulations and their shared affinity for the β1AR, with no other direct vasodilatory effect and with metoprolol and atenolol being more selective than propranolol.24 This selectivity was particularly important to avoid interference from non-specific effects. Our results with the mouse IRI model unexpectedly establish that cardioprotection is not a β-blocker class effect and that metoprolol has a differential ability to limit IS by reducing neutrophil migration to cardiac tissue and impeding neutrophil–platelet interactions (Figure 1). The inhibitory effect of metoprolol on neutrophil–platelet interactions has been previously shown to be associated with less MVO9 (a major contributor to IS). The fact that atenolol and propranolol did not show any effect on these cell-to-cell interactions probably resulted in no effect on MVO. Unfortunately, in the present study we have not performed thioflavin-based MVO measurements to definitely demonstrate that only metoprolol breaks the axis neutrophil–platelet interactions-MVO-IS. This non-class effect was confirmed in the other in vitro and in vivo models of exacerbated inflammation examined. The transwell and acute peritonitis results show a characteristically strong blocking effect of metoprolol on neutrophil migration and infiltration that was not observed with atenolol or propranolol even at double the i.v. dose (Figure 2and Supplementary material online, Figure S2). The ability of metoprolol to reduce neutrophil counts in BALF from mice with LPS-induced ALI (Figure 3) confirms that the protective effect is exportable to any inflammation setting. It is also significant that metoprolol attenuated histone three hypercitrullination in the ALI model (Figure 3 and Supplementary material online, Figure S4). Histone 3 hypercitrullination is a hallmark of the generation of NETs, extracellular fibrillary networks primarily composed of neutrophil chromatin. Neutrophil extracellular trap generation is a key feature of the acute inflammatory response in a variety of settings, such as atherothrombosis.25 The ability to form NETs has recently been implicated in the organ damage and mortality associated with COVID-19.26 Impaired NET formation in the ALI model appears to be due to the scarcity of neutrophils in the inflamed lung resulting from the disruptive effect of metoprolol on neutrophil recruitment.
The single-cell in vivo 2D and 3D IVM analyses confirm that metoprolol directly targets neutrophils. Metoprolol specifically induced erratic behaviour in neutrophils and altered morphological features required for tissue infiltration. The lack of any effect on these properties in the presence of atenolol or propranolol excludes any effect of atenolol and propranolol on this immune cell type (Figures 4 and 5).
Our previous results showed that the cardioprotective effect of metoprolol is mediated by the β1AR, with no involvement of the β2AR.9 We therefore focused the in silico analysis exclusively on the β1AR. β-blockers are believed to act by occupying the βAR extracellular domain, thereby blocking ligand-dependent downstream cascade activation. Nevertheless, β-blockers with similar receptor affinities have been suggested to trigger different downstream effects. Our finding that neutrophil migration and infiltration are inhibited only with metoprolol suggests that its protective effect might involve more than simply blocking catecholamine interaction with the receptor. Indeed, the ability of metoprolol to inhibit neutrophil migration in the in vitro transwell assays shows that this metoprolol action is not dependent on the presence of catecholamines.
Our in silico analysis clearly shows that metoprolol is the tested β-blocker able to induce a more significant change in β1AR conformation, increasing the size of the intracellular cavity (Figure 6 and Supplementary material online, Tables S1 and S2). Large-scale rearrangement of GPCR residue side-chains can produce different receptor conformations that influence G-protein selectivity and generate differential effects on downstream signalling proteins.27 , 28 Our in silico analysis suggests that when metoprolol-β1AR complex binds to Gsα protein induces a specific conformational change in β1AR that affects its intracellular coupling interface, exposing Ser 461 and 462 phosphorylation sites and potentially modifying its interaction with diverse intracellular-binding partners, such as GRKs. A greater exposition of this site could boost the phosphorylation of these Serines by GRK2 (complex GRK2-Gβγ) and mediate the recruitment of β-arrestins to the receptor, which uncouples the receptor from its G protein and initiates receptor internalization and desensitization. We speculate that activated β-arrestin through these conformational changes at the receptor level might initiate a biased agonism signalling pathway.
This conformational change may deactivate constitutive β1AR function29 or activate a specific signalling profile that eventually produces cardioprotection by neutrophil stunning. These in silico outcomes suggest recent pharmacological concepts, such as inverse or biased agonism30–32 as possible mechanisms underlying metoprolol-induced neutrophil stunning through β1AR.
To date, no intervention aimed at reducing IS has demonstrated a solid clinical benefit in terms of hard endpoints reduction.33 For the case of i.v. β-blockers in the acute phase of STEMI, the acute benefits in terms of cardioprotection and primary ventricular fibrillation reduction10 have not been translated into long-term clinical benefits, as shown in a recent meta-analysis including 1150 patients.34 Several reasons might explain the lack of translation of cardioprotection into improved clinical benefits.35 , 36 The most obvious reason is the small sample size of all trials on the topic performed in the primary angioplasty era.34 In addition, key aspects, such as type, dose, and timing of β-blocker administration varied significantly between trials included in the meta-analysis.34 According to experimental data,11 the trial using the ideal dose and timing of i.v. metoprolol administration was the METOCARD-CNIC study.10 While in this trial, acute infarct-limiting effect was associated with a reduction in long-term heart failure, the small sample size (N = 270) precludes a definite conclusion. Based on these clinical data, and supported by the results provided in the present study, a definite trial with adequate dose and timing of i.v. metoprolol administration (not other β-blocker) powered to detect clinical benefits is needed to determine the clinical benefits (hard endpoints) of this strategy in haemodynamically stable STEMI patients.
In summary, the present study indicates that β-blockers should not be considered a single drug class in the treatment of myocardial IRI. The cardioprotective effect of metoprolol is mediated by a targeting of neutrophils that is not shared by other β-blockers. These findings refine cardiovascular pharmacotherapy and have major implications for clinical cardiology.
Limitations
Extrapolation of our data to the clinical scenario is limited by the fact that we have used mouse models only. Validation of these data in a more translational animal model such as the pig would have been desirable, but beyond the scope of the present mechanistic study. The in silico studies performed have intrinsic limitations, such as the lack of modelling of all molecular dynamics occurring in the in vivo setting or the lack of consideration for dose–response effects. In addition, in silico findings were not biochemically validated. Future biological studies (e.g. study of GRK2-mediated Ser 461/462 phosphorylation) should confirm the proposed mechanism responsible for the differential effect of β-blockers on IS and other exacerbated inflammation outcomes observed here. In our study, we focused on the effect of β-blockers on neutrophils, but other cell types such as macrophages play a role in final IS. Dynamics of different macrophage subtypes, and the crosstalk between these and neutrophils,37 impact post-MI healing, and it is plausible that metoprolol can affect these as well.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
We thank Verónica Labrador and Yeny Rojas-Vega from the microscopy and Advanced Imaging Units. We thank Andrés Hidalgo for sharing his knowledge of neutrophil behaviour in IRI and Carlos Galán for his expertise in graphic design and statistics. Simon Bartlett (CNIC) provided English editing.
Data availability
The individual data will be shared on reasonable request to the corresponding authors.
Funding
Ministry of Science and Innovation (‘RETOS 2019’ grant N° PID2019-107332RB-I00), Instituto de Salud Carlos III (ISCIII; PI16/02110), and European Regional Development Fund (# AC16/00021), Comunidad de Madrid (S2017/BMD-3867 RENIM-CM). B.I. is supported by an ERC-CoG grant (819775). E.O. is supported by funds from the Comunidad de Madrid Programa de Atracción de Talento (2017-T1/BMD-5185). A.C-M. and R.V-G are supported by fellowships from the Ministerio de Ciencia e Innovación (MCN) and ISCIII (FPU2017/01932 and PFIS FI17/00045). D.V.L. is supported by an Iniciativa de Empleo Juvenil grant (PEJ-2017-TL/BMD-6463) from the Comunidad de Madrid. The CNIC is supported by the ISCIII, the MCN, and the Pro CNIC Foundation and is a Severo Ochoa Center of Excellence (SEV-2015-0505).
Conflict of interest: none declared.
Contributor Information
Agustín Clemente-Moragón, Myocardial Pathophysiology Area, Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC), c/Melchor Fernandez Almagro, 3. 28029 Madrid, Spain.
Mónica Gómez, Myocardial Pathophysiology Area, Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC), c/Melchor Fernandez Almagro, 3. 28029 Madrid, Spain.
Rocío Villena-Gutiérrez, Myocardial Pathophysiology Area, Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC), c/Melchor Fernandez Almagro, 3. 28029 Madrid, Spain.
Doménica V Lalama, Myocardial Pathophysiology Area, Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC), c/Melchor Fernandez Almagro, 3. 28029 Madrid, Spain.
Jaime García-Prieto, Myocardial Pathophysiology Area, Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC), c/Melchor Fernandez Almagro, 3. 28029 Madrid, Spain; Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Instituto de Salud Carlos III, C/ Monforte de Lemos 3-5. Pabellón 11. Planta 0 28029 Madrid, Spain.
Fernando Martínez, Myocardial Pathophysiology Area, Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC), c/Melchor Fernandez Almagro, 3. 28029 Madrid, Spain; Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Instituto de Salud Carlos III, C/ Monforte de Lemos 3-5. Pabellón 11. Planta 0 28029 Madrid, Spain.
Fátima Sánchez-Cabo, Myocardial Pathophysiology Area, Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC), c/Melchor Fernandez Almagro, 3. 28029 Madrid, Spain.
Valentín Fuster, Myocardial Pathophysiology Area, Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC), c/Melchor Fernandez Almagro, 3. 28029 Madrid, Spain; Division of Cardiology, Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicina at Mount Sinai School, 1 Gustave L. Levy Place. 10029-5674 New York, NY, USA.
Eduardo Oliver, Myocardial Pathophysiology Area, Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC), c/Melchor Fernandez Almagro, 3. 28029 Madrid, Spain; Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Instituto de Salud Carlos III, C/ Monforte de Lemos 3-5. Pabellón 11. Planta 0 28029 Madrid, Spain.
Borja Ibáñez, Myocardial Pathophysiology Area, Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC), c/Melchor Fernandez Almagro, 3. 28029 Madrid, Spain; Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Instituto de Salud Carlos III, C/ Monforte de Lemos 3-5. Pabellón 11. Planta 0 28029 Madrid, Spain; Department of Cardiology, Instituto de Investigación Sanitaria (IIS)-Fundación Jiménez Díaz, Calle Isaac Peral, 42. 28015 Madrid, Spain.
References
- 1. Ibanez B, Heusch G, Ovize M, Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol 2015;65:1454–1471. [DOI] [PubMed] [Google Scholar]
- 2. Heusch G, Libby P, Gersh B, Yellon D, Bohm M, Lopaschuk G, Opie L. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 2014;383:1933–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res 2004;61:481–497. [DOI] [PubMed] [Google Scholar]
- 4. Sreeramkumar V, Adrover JM, Ballesteros I, Cuartero MI, Rossaint J, Bilbao I, Nacher M, Pitaval C, Radovanovic I, Fukui Y, McEver RP, Filippi MD, Lizasoain I, Ruiz-Cabello J, Zarbock A, Moro MA, Hidalgo A. Neutrophils scan for activated platelets to initiate inflammation. Science 2014;346:1234–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fernández-Jiménez R, García-Prieto J, Sánchez-González J, Agüero J, López-Martín GJ, Galán-Arriola C, Molina-Iracheta A, Doohan R, Fuster V, Ibáñez B. Pathophysiology underlying the bimodal edema phenomenon after myocardial ischemia/reperfusion. J Am Coll Cardiol 2015;66:816–828. [DOI] [PubMed] [Google Scholar]
- 6. Kloner RA, King KS, Harrington MG. No-reflow phenomenon in the heart and brain. Am J Physiol Heart Circ Physiol 2018;315:H550–H562. [DOI] [PubMed] [Google Scholar]
- 7. Heusch G. The coronary circulation as a target of cardioprotection. Circ Res 2016;118:1643–1658. [DOI] [PubMed] [Google Scholar]
- 8. Heusch G. Coronary microvascular obstruction: the new frontier in cardioprotection. Basic Res Cardiol 2019;114:45. [DOI] [PubMed] [Google Scholar]
- 9. García-Prieto J, Villena-Gutiérrez R, Gómez M, Bernardo E, Pun-García A, García-Lunar I, Crainiciuc G, Fernández-Jiménez R, Sreeramkumar V, Bourio-Martínez R, García-Ruiz JM, del Valle AS, Sanz-Rosa D, Pizarro G, Fernández-Ortiz A, Hidalgo A, Fuster V, Ibanez B. Neutrophil stunning by metoprolol reduces infarct size. Nat Commun 2017;8:14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ibanez B, Macaya C, Sánchez-Brunete V, Pizarro G, Fernández-Friera L, Mateos A, Fernández-Ortiz A, García-Ruiz JM, García-Álvarez A, Iñiguez A, Jiménez-Borreguero J, López-Romero P, Fernández-Jiménez R, Goicolea J, Ruiz-Mateos B, Bastante T, Arias M, Iglesias-Vázquez JA, Rodriguez MD, Escalera N, Acebal C, Cabrera JA, Valenciano J, Pérez de Prado A, Fernández-Campos MJ, Casado I, García-Rubira JC, García-Prieto J, Sanz-Rosa D, Cuellas C, Hernández-Antolín R, Albarrán A, Fernández-Vázquez F, de la Torre-Hernández JM, Pocock S, Sanz G, Fuster V. Effect of early metoprolol on infarct size in ST-segment-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention: the Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction (METOCARD-CNIC) Trial. Circulation 2013;128:1495–1503. [DOI] [PubMed] [Google Scholar]
- 11. García-Ruiz JM, Fernández-Jiménez R, García-Alvarez A, Pizarro G, Galán-Arriola C, Fernández-Friera L, Mateos A, Nuno-Ayala M, Aguero J, Sánchez-González J, García-Prieto J, López-Melgar B, Martínez-Tenorio P, López-Martín GJ, Macías A, Pérez-Asenjo B, Cabrera JA, Fernández-Ortiz A, Fuster V, Ibáñez B. Impact of the timing of metoprolol administration during STEMI on infarct size and ventricular function. J Am Coll Cardiol 2016;67:2093–2104. [DOI] [PubMed] [Google Scholar]
- 12. Pizarro G, Fernández-Friera L, Fuster V, Fernández-Jiménez R, García-Ruiz JM, García-Álvarez A, Mateos A, Barreiro MV, Escalera N, Rodriguez MD, de Miguel A, García-Lunar I, Parra-Fuertes JJ, Sánchez-González J, Pardillos L, Nieto B, Jiménez A, Abejón R, Bastante T, Martínez de Vega V, Cabrera JA, López-Melgar B, Guzman G, García-Prieto J, Mirelis JG, Zamorano JL, Albarrán A, Goicolea J, Escaned J, Pocock S, Iñiguez A, Fernández-Ortiz A, Sánchez-Brunete V, Macaya C, Ibanez B. Long-term benefit of early pre-reperfusion metoprolol administration in patients with acute myocardial infarction: results from the METOCARD-CNIC Trial (Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction). J Am Coll Cardiol 2014;63:2356–2362. [DOI] [PubMed] [Google Scholar]
- 13. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 14. Van De Werf F, Janssens L, Brzostek T, Mortelmans L, Wackers JTH, Willems GM, HeidbÜchel H, Lesaffre E, Scheys I, Collen D, De Geest H. Short-term effects of early intravenous treatment with a beta-adrenergic blocking agent or a specific bradycardiac agent in patients with acute myocardial infarction receiving thrombolytic therapy. J Am Coll Cardiol 1993;22:407–416. [DOI] [PubMed] [Google Scholar]
- 15. Ibanez B, Cimmino G, Prat-Gonzalez S, Vilahur G, Hutter R, Garcia MJ, Fuster V, Sanz J, Badimon L, Badimon JJ. The cardioprotection granted by metoprolol is restricted to its administration prior to coronary reperfusion. Int J Cardiol 2011;147:428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu S, Su X, Pan P, Zhang L, Hu Y, Tan H, Wu D, Liu B, Li H, Li H, Li Y, Dai M, Li Y, Hu C, Tsung A. Neutrophil extracellular traps are indirectly triggered by lipopolysaccharide and contribute to acute lung injury. Sci Rep 2016;6:37252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science 2004;303:1532–1535. [DOI] [PubMed] [Google Scholar]
- 18. Epelman S, Liu PP, Mann DL. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol 2015;15:117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nat Med 2011;17:1381–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 2010;330:362–366. [DOI] [PubMed] [Google Scholar]
- 21. Dunzendorfer S, Wiedermann CJ. Modulation of neutrophil migration and superoxide anion release by metoprolol. J Mol Cell Cardiol 2000;32:915–924. [DOI] [PubMed] [Google Scholar]
- 22. Rasmussen MM, Reimer KA, Kloner RA, Jennings RB. Infarct size reduction by propranolol before and after coronary ligation in dogs. Circulation 1977;56:794–798. [DOI] [PubMed] [Google Scholar]
- 23. Maroko PR, Kjekshus JK, Sobel BE, Watanabe T, Covell JW, Ross J Jr, Braunwald E. Factors influencing infarct size following experimental coronary artery occlusions. Circulation 1971;43:67–82. [DOI] [PubMed] [Google Scholar]
- 24. Oliver E, Mayor F Jr, D'Ocon P. Beta-blockers: historical perspective and mechanisms of action. Rev Esp Cardiol (Engl Ed) 2019;72:853–862. [DOI] [PubMed] [Google Scholar]
- 25. Badimon L, Vilahur G. Neutrophil extracellular traps: a new source of tissue factor in atherothrombosis. Eur Heart J 2015;36:1364–1366. [DOI] [PubMed] [Google Scholar]
- 26. Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, Dassler-Plenker J, Guerci P, Huynh C, Knight JS, Loda M, Looney MR, McAllister F, Rayes R, Renaud S, Rousseau S, Salvatore S, Schwartz RE, Spicer JD, Yost CC, Weber A, Zuo Y, Egeblad M. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med 2020;217:e20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lu M, Wu B. Structural studies of G protein-coupled receptors. IUBMB Life 2016;68:894–903. [DOI] [PubMed] [Google Scholar]
- 28. Sandhu M, Touma AM, Dysthe M, Sadler F, Sivaramakrishnan S, Vaidehi N. Conformational plasticity of the intracellular cavity of GPCR-G-protein complexes leads to G-protein promiscuity and selectivity. Proc Natl Acad Sci U S A 2019;116:11956–11965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Engelhardt S, Grimmer Y, Fan GH, Lohse MJ. Constitutive activity of the human beta(1)-adrenergic receptor in beta(1)-receptor transgenic mice. Mol Pharmacol 2001;60:712–717. [PubMed] [Google Scholar]
- 30. Wisler JW, Rockman HA, Lefkowitz RJ. Biased G protein-coupled receptor signaling: changing the paradigm of drug discovery. Circulation 2018;137:2315–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wootten D, Christopoulos A, Marti-Solano M, Babu MM, Sexton PM. Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nat Rev Mol Cell Biol 2018;19:638–653. [DOI] [PubMed] [Google Scholar]
- 32. Berg KA, Clarke WP. Making sense of pharmacology: inverse agonism and functional selectivity. Int J Neuropsychopharmacol 2018;21:962–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hausenloy DJ, Botker HE, Engstrom T, Erlinge D, Heusch G, Ibanez B, Kloner RA, Ovize M, Yellon DM, Garcia-Dorado D. Targeting reperfusion injury in patients with ST-segment elevation myocardial infarction: trials and tribulations. Eur Heart J 2017;38:935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hoedemaker NP, Roolvink V, de Winter RJ, van Royen N, Fuster V, García-Ruiz JM, Er F, Gassanov N, Hanada K, Okumura K, Ibáñez B, van ’t Hof AW, Damman P. Early intravenous beta-blockers in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: a patient-pooled meta-analysis of randomized clinical trials. Eur Heart J Acute Cardiovasc Care 2019;204887261983060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heusch G. Critical issues for the translation of cardioprotection. Circ Res 2017;120:1477–1486. [DOI] [PubMed] [Google Scholar]
- 36. Heusch G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol 2020;doi: 10.1038/s41569-020-0403-y. [DOI] [PubMed]
- 37. Horckmans M, Ring L, Duchene J, Santovito D, Schloss MJ, Drechsler M, Weber C, Soehnlein O, Steffens S. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J 2017;38:187–197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The individual data will be shared on reasonable request to the corresponding authors.