Abstract
In this review, we describe the role of oxidized forms of nicotinamide adenine dinucleotide (NAD+) as a molecule central to health benefits as the result from observing selected healthy lifestyle recommendations. Namely, NAD+ level can be regulated by lifestyle and nutrition approaches such as fasting, caloric restriction, sports activity, low glucose availability, and heat shocks. NAD+ is reduced with age at a cellular, tissue, and organismal level due to inflammation, defect in NAMPT-mediated NAD+ biosynthesis, and the PARP-mediated NAD+ depletion. This leads to a decrease in cellular energy production and DNA repair and modifies genomic signalling leading to an increased incidence of chronic diseases and ageing. By implementing healthy lifestyle approaches, endogenous intracellular NAD+ levels can be increased, which explains the molecular mechanisms underlying health benefits at the organismal level. Namely, adherence to here presented healthy lifestyle approaches is correlated with an extended life expectancy free of major chronic diseases.
1. Introduction
Fasting, caloric restriction, sports activity, low glucose availability, and heat shocks are lifestyle and nutrition approaches that influence NAD+ levels [1–6]. Deficiency in NAMPT-mediated NAD+ biosynthesis, increased inflammation, and the PARP-mediated NAD+ depletion are causes of reduced NAD+ levels with age at a cellular, tissue, and organismal level [7, 8].
Coenzyme nicotinamide adenine dinucleotide (NAD+), which contains two covalently joined mononucleotides (nicotinamide mononucleotide or NMN, and AMP) [9], has an important role in an energy metabolism like mitochondrial electron transport, glycolysis, and citric acid cycle [10] in order to generate adenosine triphosphate (ATP) [11]. NAD+ is also a rate-limiting substrate for many signalling enzymes such as sirtuin (SIRT) proteins SIRT1 and SIRT3, the poly (ADP-ribose) polymerase (PARP) proteins PARP1 and PARP2, a COOH-terminal binding protein (CtBP), cyclic ADP-ribose (ADPR) synthetases CD38 and CD157, and many other NAD+-dependent enzymes. These enzymes are involved in important cellular processes, like DNA repair, stress response, genomic stability, chromatin remodelling, circadian rhythm regulation, cell cycle progression, insulin secretion and sensitivity, and expression of the inflammatory cytokines, thus translating changes in energy status into metabolic adaptations [12]. NAD+ is recycling during ATP formation in processes of glycolysis, beta-oxidation, Krebs cycle, and electron transport in cytosol and mitochondria and shifts between reduced and oxidized forms as required for the continuous flow of electrons across the metabolic pathways. Therefore, the NAD+ molecule is conserved during these processes. On the other hand, the NAD+ is consumed during cellular signalling, in adenosine diphosphate (ADP)-ribosyl transfer reactions, by poly-ADP-ribose polymerases (PARPs), sirtuin deacetylases (Sirtuins), and the cluster of differentiation 38 (CD38), i.e., the nicotinamide (NAM) unit is separated. NAD+ half-life is between 1–2 h in the cytoplasm and nucleus and approximately 8 h in the mitochondria [13] and can be salvaged and reused by three pathways: (1) de novo synthesis (from L-tryptophan), (2) Preiss-Handler pathway (from nicotinic acid or nicotinic acid ribose), and (3) salvage pathway (from niacinamide/nicotinamide, nicotinamide riboside, and nicotinamide mononucleotide) [9, 14–18]. NAD+ is mainly produced by the NAD+ salvage pathway where nicotinamide phosphoribosyltransferase (NAMPT) is the rate-limiting enzyme, converting NMN into NAD+ [19–21]. NAMPT regulates processes related to the pathological processes of obesity and a type 2 diabetes mellitus by influencing lipid and glucose metabolism, insulin resistance, the oxidative stress response, apoptosis, and inflammation [22, 23].
The NAD+/NADH ratio influences also the reactive oxygen species (ROS) and oxidative stress formation through regulation of intracellular ATP production, different metabolic enzymes, and redox state. An increase of NAD+ and/or NAD+/NADH ratio can increase cell defence, can induce DNA repair and apoptosis through activation of PARPs and sirtuins, and thus plays an important role in the prevention of cancerogenesis and many other diseases [14, 24]. For example, cellular NAD+/NADH ratio regulates SIRT1 enzymatic activity, which further regulates a number of target proteins [25], such as FOXO family of transcription factors [26–28], p53 [29, 30], PGC-1a [31, 32], and NF-kB [33–35]. While chronic diseases and ageing are related to decreased NAD+ levels [16, 36, 37], different lifestyle factors have been found that ameliorate NAD+ bioavailability, which positively affects SIRT stimulation and subsequent PGC-1α and FOXO1 expression, leading to mitochondrial changes and metabolic adaptations (Figure 1) [38]. Increased available cellular energy, improved stem cell and mitochondrial function, DNA repair [39], telomere maintenance [40], and enhanced metabolic activity are prerequisites for effective health span and life span [41, 42] as demonstrated by studies where NAD+ levels were intentionally increased [23, 43–48].
Figure 1.
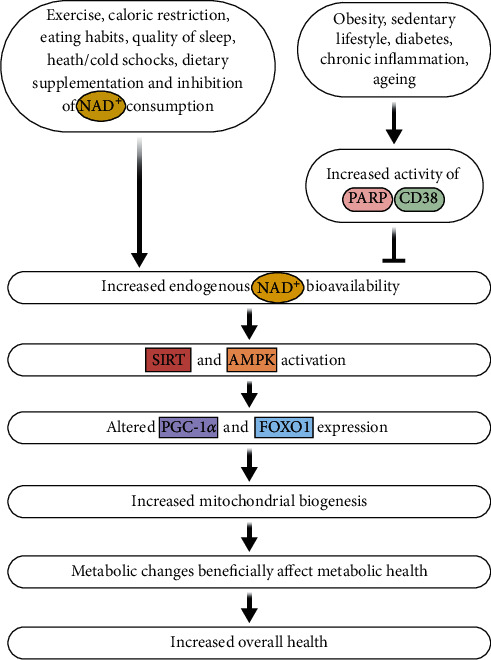
Health benefits as a result of implementing approaches to increase NAD+ bioavailability.
2. Caloric Restriction, Eating Habits, and NAD+ Levels
A well-balanced diet in macro- and micronutrients represents a basis for health and well-being. Limited calorie intake continues to be the strategy supported by the greatest evidence for ensuring increased lifespan and health [49]. In different model organisms, a significant increase in lifespan was reported if calories were restricted between 25–60% relative to normally fed control [50, 51]. How is caloric restriction connected with NAD+ levels? CR stimulates the NAD+ salvage pathway leading to increased NAD+ bioavailability by activating the expression of NAMPT, which triggers the NAD+ salvage pathway by transforming nicotinamide (NAM) to NAD+ [52]. Caloric restriction increases NAD+ levels, while lowers NADH levels and activates sirtuins [53]. For example, caloric restriction extends the yeast's life span by lowering the level of NADH, since NADH is a competitive inhibitor of Sir2 [54]. Thus, activation of sirtuins with a sufficient amount of bioavailable NAD+ is a necessary condition for the life-span extension provided by CR [55, 56]. Specifically, Sirt1 regulates CR by detecting intracellular low energy levels and provoking physiological changes relevant to health and longevity [57]. On the other hand, inactivation of SIRT1 results in the prevention of CR-mediated lifespan extension [58].
Studies on caloric restriction revealed that it is more important to improve the ratio between NAD+ and NADH than to raise the overall amount of cellular NAD+ [59]. Namely, caloric restriction reduces NADH amount more than it influences the NAD+ levels, at least in yeast [54, 60]. It seems that lowering NADH is an important factor responsible for the increased activity of the NAD+-consuming enzymes, as NADH is an inhibitor of Sirtuins and PARPs [54].
Besides by caloric restriction, NAD+ levels can be increased with food and commercially available supplements. Ingestion of the amino acid tryptophan or forms of vitamin B3 (niacin, nicotinic acid, niacinamide) as well as nicotinamide riboside (NR), nicotinamide mononucleotide (NMN), and nicotinic acid riboside (NaR) stimulates the formation of NAD+ [61–64]. Daily requirements for NAD+ synthesis can be obtained either with dietary tryptophan or with around 15 mg/d of daily niacin, a collective term for nicotinic acid (NA) and nicotinamide (NAM) [61], which can be found in meat, fish, and dairy products [65].
Small-scale human clinical studies have shown that NAD+ boosters such as NMN, NR, and niacin can increase the levels of NAD+ in volunteers and are relatively safe for human consumption [6, 66–72]. Most of the side effects reported during treatment with NAM, NR, and NMN are minor (e.g., diarrhea, nausea, rashes, hot flashes, cramps in the legs, erythema) and occur relatively rarely [73, 74]. Increased acetylcarnitine concentrations in skeletal muscle and minor changes in body composition and sleeping metabolic rate were reported in the recent study on NR supplementation in healthy obese humans [75]. The evidence for assessing the health risk is still limited, and long-term exposure to NAD+ booster (NR, NMN) has not yet been investigated in human clinical trials or human clinical trials are not yet completed. In addition, there is insufficient data on increasing the levels of NAD+ in various clinical disorders.
As data for some newly discovered NAD+ precursor forms are scarce, NAD+ supplements should be tested in a manner similar to drugs in development [72]. Niacin equivalents/precursors are found in animal and plant foods, mainly in the form of NA and NAM. Additionally, recently discovered NAD+ intermediates, such as NMN and NR, are also in foods, like cucumber, cabbage, and immature soybeans. Broccoli has 0.25–1.88 mg of NMN per 100 g, avocado and tomato 0.26–1.60 mg/100 g. Much less NMN can be found also in raw beef and shrimp (0.06–0.42 mg/100 g) [45] as well as human and cow milk at micromolar concentrations [76, 77]. NAD+ biosynthesis can be increased by direct activation of NAD+ biosynthetic enzymes by several AMPK and NAMPT activators, like nonflavonoid polyphenol resveratrol, metformin, 5-aminoimidazole-4-carboxamide ribonucleotide, P7C3, leucine, epigallocatechin gallate, and proanthocyanidins [78–86]. CD38, its homologue CD157, and PARP-1 inhibitors could additionally increase NAD+ availability; however, they are registered as medical drugs for cancer treatment [24], thus beyond the scope of this review.
3. Eating Habits
NAD+/sirtuin pathway could be influenced also with nutritional approaches, e.g., eating habits. At what time and what and how much food we eat influence intracellular NAD+ bioavailability by altering electron transport in mitochondria. For example, a high-fat/sugar diet causes energy overload, culminating in reduced NAD+/NADH ratio [87] and decreases NAD+ levels [23, 63]. Also, a low AMP/ATP ratio causes a decrease in NAD+ or NAD+/NADH, in situations when enormous amounts of calorically rich food (lipids and/or carbohydrates) are eaten. This additionally leads to elevated blood sugar and insulin levels, increased NADH/NAD ratio, and increased formation of ROS, which triggers the postprandial oxidative stress and oxidative damage [88–91]. Large amounts of electrons from sugars enter the mitochondria after a large portion of food that generates more superoxide at complex 1 (NADH: ubiquinone oxidoreductase) and complex III (ubiquinol: cytochrome c oxidoreductase) [92]. Efficient electron flow and avoidance of electron leaks (superoxide formation) can be achieved if ATP is regularly consumed; for example, by moderate sport activity or any kind of physical work. This increases the AMP/ATP ratio and NAD+ availability [87, 93, 94]. The link between the metabolism and NAD+ is further strengthened by observations that besides overnutrition, tissue NAD+ levels decrease also with high-fat diets and obesity [23, 63, 95–98]. Rappou et al. [99] compared SIRT1, SIRT3, SIRT7, and NAMPT expressions and total PARP activity in lean and obese subjects. Results indicated lower sirtuins and NAMPT expressions and increased total PARP activity in obese compared to lean subjects. After a moderate weight loss, SIRT1 and NAMPT expressions increased while PARP activity significantly decreased in subjects upon the weight loss. Similar results were obtained in healthy men during lipid overfeeding [100]. Other studies observed that obesity is associated with low NAD+ levels or SIRT pathway expression [101]. On the other hand, supplementation with NAD+ precursors or intermediates activates sirtuins and oxidative metabolism resulting in the protection against high-fat diet-induced obesity [63], improved glucose tolerance and hepatic insulin sensitivity [23], and lipid metabolism [45].
A high-fat caloric diet induces obesity through the protein CD38, which is a regulator of body weight and an NAD+ consumer [102]. Mice deficient in CD38 are protected against the high-fat diet-induced obesity due to boosted metabolic rate in part via a NAD-dependent stimulation of SIRT-PGC1alpha axis [102]. Adipose tissue elevates the expression of CD38 and inflammation-related genes in obese people [103, 104]. In line, low expression of CD38 protected against obesity when fed a high-fat diet in animals [102, 105].
NAD+ level is not only nutritionally controlled, but it depends also on the sports activities and other lifestyle factors.
4. Exercise and NAD+ Levels
Physical activity and exercise, as part of a healthy lifestyle, have a significant impact on health outcomes, including improved motor skills, healthy bones, enhanced aerobic fitness, efficient heart and lung function, improved cardiovascular health, lowered risk of stroke, certain types of cancer and diabetes, improved metabolic flexibility and mitochondrial function, and a positive effect on cognitive function and mental health—including on depressive symptom improvement and anxiety- or stress-related disease [38, 106–108].
How does sports activity affect NAD+ levels? Aerobic exercise training or any kind of exercise/sports activity increases the amount of NAD+ due to the induction of skeletal muscle's NAMPT expression that was shown in rodent and human studies [109–111]. Namely, in human skeletal muscle, exercise training reverses the age-dependent decline of NAD+ by stimulating the NAD+ salvage pathway, in which nicotinamide NAMPT is a rate-limiting enzyme [112]. Exercise and aerobic sports activity increases the amount of NAD+ due to the induction of skeletal muscle's NAMPT expression [109] and reverses the age-dependent decline of NAD+ by stimulating the NAD+ salvage pathway [112] through the 5′ AMP-activated protein kinase (AMPK) pathways [4].
NAD+ has an important role in the generation of intracellular ATP, which is required for exercise and sports activities. On the other hand, as already mentioned, ATP production in mitochondria represents the main source of free radical generation. The reduction state of complex I in mitochondria depends strongly on the NAD+ and NADH levels. Ameliorating the NAD+/NADH ratio by elevated ATP consumption (e.g., sports activity) or decreased ATP production (e.g., intermitted fasting, consumption of small portions of food, and CR) regulates the magnitude of superoxide-generation from the transfer of electrons to molecular oxygen at mitochondrial complexes I and III and can thus ameliorate the intensity of oxidative damage [113]. Increased demand for energy during the exercise is sensed by the cell and activates AMPK, which can modulate NAD+ bioavailability [38]. Both exercise and caloric restriction trigger the metabolic stress that follows by adaptation by inducing NAMPT expression through the AMPK [4, 109, 114] resulting in increased NAD+ levels available for sirtuins and PARPs.
A recent study by de Guia et al. revealed that different exercise training methods reverse the age-dependent decline in NAD+ salvage capacity in the human skeletal muscle [112]. Namely, both aerobic and resistance exercise training increased NAMPT levels in young and older individuals. In aged rats, exercise training also increased NAD+, NAMPT levels, and SIRT1 activity [111] and accelerates the de novo biosynthesis of NAD+ from L-tryptophan [115].
The important function of NAD+ during sports activity is its role as a hydrogen/electron transfer molecule for adenosine triphosphate (ATP) production and mitochondrial biogenesis in muscle cells [116]. Additionally, sports activity increases the NAD+ amount also at the systemic level [117] that results in health benefits at the organismal level due to the NAD+ role in multiple and diverse cellular processes, in addition to redox reactions, such as deacetylation and ADP-ribosylation [116]. During the intense sports activity, ATP is consumed; thus, the need for NADH as the electron donor increases, which in the end results in the boosted formation of oxidised NAD+ and decreased NADH, i.e., an improved NAD+/NADH ratio. The total amount of NAD+ is not significantly changed during the redox reaction; however, the NAD+/NADH (and NADP to NADPH) ratio is changed in favour of NAD+ [61], which activates sirtuins, PARPs, CD38, and other NAD+-consuming reactions. Since NAD+-consuming enzymes intervene in many crucial cellular processes, many healthy processes at the organismal level are enhanced by the implementation of exercise and sports activity.
Surprisingly, NR, the NAD+ precursor, decreases exercise performance in rats [118], most likely due to the pleiotropic metabolic and redox properties of NAD+ and NADP+. Nicotinic acid also reduced the capacity for high-intensity exercise in humans [119], which is ascribed to lower plasma free fatty acids, leading to earlier fatigue. Studies on NAD+ precursor supplementation implied prevention of vascular dysfunction, oxidative stress, and muscle age-degeneration in mice [45, 46, 120]. Accordingly, it is important to preserve a high NAD+ to NAD+/NADH ratio that can be achieved also by sports activity.
5. Circadian Rhythms, Sleeping Habits, and NAD+ Levels
Sleep disorders predispose persons to chronic diseases like obesity, depression, diabetes, and many cardiometabolic diseases, which are significantly associated with mortality and morbidity [121, 122] [123–125]. Contrary, a steady pattern of waking and sleeping is associated with health promotion and longevity [126]. Prolonged disruptions of circadian rhythms are associated with negative health consequences [127]. NAD+ levels and sirtuin activity regulate a healthy circadian rhythm of sleep and wakefulness; concurrently, the NAD+ level is supervised by circadian rhythm and involved in the circadian clock regulation. NAD+ levels oscillate with a 24 h rhythm; these can be modified by feeding and sleeping time [128–130]. The central internal clock is in the hypothalamic suprachiasmatic nucleus, and the circadian rhythms are coordinated by intracellular proteins called “circadian clocks.” These proteins are regulated by a transcriptional negative feedback loop between transcriptional activators CLOCK and BMAL1 and repressors CRY and PER. CLOCK, the core circadian regulator, is a histone acetyltransferase whose activity is outweighed by the nicotinamide adenine dinucleotide- (NAD+-) dependent histone deacetylase SIRT1 [131, 132]. CLOCK : BMAL1 heterodimer balances the circadian expression of NAMPT, which regulates the NAD+ biosynthesis. The activity of NAMPT is constrained by light—or sleep—deprivation and upregulated by darkness and night [130].
With ageing, NAMPT activity declines, and consequently, NAD+ bioavailability drops [7, 133], leading to the deterioration of the circadian rhythm (change in amplitude, period, and phase). Disrupted circadian rhythms were reported in many pathological conditions including cardiovascular diseases, diabetes, cancer, and accelerated ageing [134, 135]. On the contrary, matching the innate circadian period results in health improvements [135–140].
6. Environmental Stress: Heat/Cold Shock and NAD+ Levels
Exposure to the elevated heat for short time periods can result in beneficial health effects. Cardiovascular responses to long-term adaptations in response to heat stress result in reduced blood pressure and arterial stiffness and improved endothelial and microvascular function [141]. For example, regular sauna bathing may be linked to several health benefits, which include decreased risk of sudden cardiac death and cardiovascular and all-cause mortality [142], reduction in the risk of neurocognitive diseases and nonvascular conditions such as pulmonary diseases, and amelioration of conditions such as arthritis, headache, and flu [143]. What is more, heat stress cardioprotection and improved postischemic functional recovery in the heat-stressed hearts after cardioplegic arrest due to increased NAD+ and NADP+ concentrations were observed [144]. Heat shock triggers an increase in the NAD+/NADH ratio as a result of decreased NADH levels and an increase in recruitment of SIRT1 to the hsp70 promoter [25]. Enzyme nicotinamide mononucleotide adenylyltransferase (NMNAT), which catalyzes nicotinamide adenine dinucleotide (NAD+) synthesis, is elevated during conditions of heat shock and transcriptionally regulated by the heat shock factor (HSF) and hypoxia-inducible factor 1α (HIF1α) in vivo [145, 146].
In addition to heat stress, also cold stress-induced physiological responses and activation of brown adipose tissue (BAT) have health benefits [147]. BAT mainly burns energy in contrast to white adipose tissue (WAT), which stores fat [141]. In mouse and human BAT, cold exposure activates NAD+ biosynthesis mediated by a rate-limiting enzyme, NAMPT [148]. BAT is abundant in mitochondria and plays a role in energy expenditure related to producing heat by an energy-dissipating process of nonshivering thermogenesis, leading to changes in lipid metabolism [149] and other health benefits like the absence of low-grade inflammation, increased insulin sensitivity, and decreased liver fat [150, 151]. Degradation, whitening, and impaired function of BAT promotes obesity [152–155].
The facts supporting the “NAD+ > SIRTs > positive effect” pathway as the mechanism of action for the beneficial effects of NAD+ repletion strategies have been presented so far. Are there indications of concerns about increasing the levels of NAD+?
7. Potential Deleterious Effects of Increased NAD+
As already discussed, NAD+ precursors, nicotinic acid (NA), and NR decreased exercise performance in young rats [118] and reduce the capacity for high-intensity exercise in humans [119], although old individuals seem to benefit from NR supplementation. Namely, increased NAD(P)H levels, decreased oxidative stress, and improved physical performance were observed only in the old subjects [156]. Kourtzidis et al. [157] expressed concern that redox agents administered exogenously in healthy young populations (not suffering from antioxidant deficiency) might lead to adverse effects. Nicotinamide (NAM) overdose was reported to cause hepatotoxicity in rare cases [158]. In addition, it was observed that a high dose of dietary NR caused glucose intolerance and dysfunction of the white adipose tissue in mice fed a slightly obesogenic diet [159].
Regarding longevity, overexpression of SIRT1 was found not to extend lifespan in mice fed standard diets, although they had better general health and fewer carcinomas [160]. Mitchell et al. [43] observed that supplementation with NAM in the mouse model did not change the lifespan, in spite of the improved healthspan. Additionally, Chen et al. [161] challenge the paradigm that CR induces SIRT1 activity in all tissues. Similarly, Frederick et al. [162] suggest that NMN and NR increase in NAD biosynthesis is cell- or tissue-specific.
It appears that the NAD+ levels could have both procancer and anticancer effects, as NAD+ is a critical protective factor in early cancer development and could become a damaging factor later in the phase of cancer progression and promotion. Namely, during cancer promotion, progression, and treatment, increased NAD+ levels could have adverse effects on the malignancy process due to increased cell survival, growth advantage, increased resistance to radio- and chemotherapy, and promotion of inflammation. In contrast, NAD+ restoration could prevent or reverse the phenotype of malignant cells in the early stages by inducing cellular repair and adaptive stress responses and regulating cell cycle arrest and apoptotic removal of damaged cells (reviewed in [24]). In addition, the compound FK866, which inhibits the nicotinamide recycling enzyme NAMPT, is a tumor apoptosis inducer due to the NAD+ depletion [163, 164] and is used as an anticancer drug.
In the area of inflammation/sepsis, there is also controversy regarding the NAD(+)-dependent sirtuin family, as elevated NAD(+) levels play a different role in the different stages of sepsis. In the initial (proinflammatory) phase, which is characterized by a cytokine storm, overproduction of reactive oxygen species (ROS), and metabolic shift [165], SIRT1 activation shows positive effects, whereas the SIRT1 expression should be inhibited in the later stages of sepsis [166]. Therefore, due to the dynamic phases of sepsis, the role of SIRT1 cannot simply be defined as beneficial or detrimental. Increased NAD+ might have also negative effects on inflammatory disorders, such as rheumatoid arthritis due to stimulated inflammatory cytokine secretion by leukocytes [167].
Another potential risk could be posed by the toxic degradation products and metabolites of NAD+ precursors, e.g., nicotinic acid adenine dinucleotide (NAAD), N-methyl nicotinamide (MeNAM), and 2-PY[71, 168]. Lastly, increased NAM levels due to the supplementation with NAD+ precursors (NAM, NR, or NMN) could inhibit PARPs and CD38 activities [169], while SIRT1 feedback inhibition in vivo by NAM may not be so important [170, 171]. Increased levels of NAM might alter also the methyl pool used to methylate DNA and proteins [171].
8. Conclusions
It is not only the NAD/NADH redox role as hydride and electron transfer in redox metabolic reactions but mainly the NAD+ as the signalling molecule and substrate for sirtuins and PARPs that is responsible for the health benefits and longevity. Cellular NAD+ content and an adequate NAD+/NADH ratio can postpone pathologic processes associated with impaired cell signalling and mitochondrial function [87, 172, 173]. Thus, for maintaining optimal cellular functioning and organismal health, it is necessary to implement the lifestyle approaches that stimulate increased NAD+ levels. The synergistic effects of different measures to ensure a healthy lifestyle are important, as there is an intimate and reciprocal relationship between them. For example, sedentary lifestyle, overeating, and excessive intake of fat and sugar are associated with disturbances in circadian rhythms [174, 175] and downregulation of NAMPT gene expression [4]. Implementation of the time-restricted feeding without reducing the caloric intake (8 h per day feeding/16 h per day fasting) improved the robustness of circadian and metabolic rhythms and prevented metabolic diseases in mice on a high-fat diet [176]. Lifestyle approaches, such as exercise and CR, can reverse insulin resistance and type 2 diabetes mellitus (T2DM) [12]. Both manipulations increase the NAMPT-mediated NAD+ generation, activate mechanistic pathways of AMPK, and enhance the SIRT1 activity and mitochondrial function [4, 114, 177, 178]. Sirtuins affect various cellular processes, including lipid metabolism, insulin secretion, and sensitivity [179]. NAD+ levels within cells are regulated by its precursors' intake, biosynthetic pathways, and degradative enzymes [180], which can be additionally balanced by selected lifestyle factors discussed here. In order to provide sufficient NAD+ bioavailability and appropriate expression of NAMPT, it is necessary to ingest sufficient amounts of NAD+ precursors/intermediates in the vitamin B3 forms, preferably as a part of a normal diet, to practice regular and moderate sports activity, and to observe time intervals between darkness and light exposure as well as the appropriate time intervals between feeding and fasting.
The presented studies support the hypothesis that maintaining NAD+ levels leads to healthy cell metabolism, which is beneficial in terms of amelioration of metabolic diseases and ageing. It should be stressed that NAD+ is not the only factor, but rather one of the several components that influence cell health. There are many other positive effects of calorie restriction, eating habits, exercise, circadian rhythms, and environmental stress on human health that are beyond the scope of this paper. Although many animal studies have shown the link between NAD+ and healthspan, the complex role of NAD+ in the etiology of ageing and age-related chronic diseases in humans should be further elucidated. The current state of knowledge about NAD+ positive effects on ageing and healthspan is mainly based on experiments on cell cultures and model organisms, so that the positive health effects of NAD+ in humans will need to be confirmed in future in-depth studies and clinical trials.
Acknowledgments
The authors acknowledge the financial support from the Slovenian Research Agency (Research Core Funding No. P3-0388 and P3-0019).
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
References
- 1.Hipkiss A. R. Energy metabolism, altered proteins, sirtuins and ageing: converging mechanisms? Biogerontology. 2008;9(1):49–55. doi: 10.1007/s10522-007-9110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris K. C., Lin H. W., Thompson J. W., Perez-Pinzon M. A. Pathways for ischemic cytoprotection: role of sirtuins in caloric restriction, resveratrol, and ischemic preconditioning. Journal of Cerebral Blood Flow & Metabolism. 2011;31(4):1003–1019. doi: 10.1038/jcbfm.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morselli E., Maiuri M. C., Markaki M., et al. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death & Disease. 2010;1(1) doi: 10.1038/cddis.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fulco M., Cen Y., Zhao P., et al. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Developmental Cell. 2008;14(5):661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y., Cimen H., Han M. J., et al. NAD+-dependent deacetylase SIRT3 regulates mitochondrial protein synthesis by deacetylation of the ribosomal protein MRPL10. Journal of Biological Chemistry. 2010;285(10):7417–7429. doi: 10.1074/jbc.M109.053421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trammell S. A. J., Schmidt M. S., Weidemann B. J., et al. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nature Communications. 2016;7(1, article 12948):1–14. doi: 10.1038/ncomms12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braidy N., Guillemin G. J., Mansour H., Chan-Ling T., Poljak A., Grant R. Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in Wistar rats. PLoS One. 2011;6(4, article e19194) doi: 10.1371/journal.pone.0019194. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Li Y., Schoufour J., Wang D. D., et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: prospective cohort study. BMJ. 2020;368, article l6669 doi: 10.1136/bmj.l6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imai S., Guarente L. NAD+ and sirtuins in aging and disease. Trends in Cell Biology. 2014;24(8):464–471. doi: 10.1016/j.tcb.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chi Y., Sauve A. A. Nicotinamide riboside, a trace nutrient in foods, is a vitamin B3 with effects on energy metabolism and neuroprotection. Current Opinion in Clinical Nutrition and Metabolic Care. 2013;16(6):657–661. doi: 10.1097/MCO.0b013e32836510c0. [DOI] [PubMed] [Google Scholar]
- 11.Chen A. C., Damian D. L. Nicotinamide and the skin. Australasian Journal of Dermatology. 2014;55(3):169–175. doi: 10.1111/ajd.12163. [DOI] [PubMed] [Google Scholar]
- 12.Elhassan Y. S., Philp A. A., Lavery G. G. Targeting NAD+ in metabolic disease: new insights into an old molecule. Journal of the Endocrine Society. 2017;1(7):816–835. doi: 10.1210/js.2017-00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cambronne X. A., Stewart M. L., Kim D., et al. Biosensor reveals multiple sources for mitochondrial NAD+ Science. 2016;352(6292):1474–1477. doi: 10.1126/science.aad5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belenky P., Bogan K. L., Brenner C. NAD+ metabolism in health and disease. Trends in Biochemical Sciences. 2007;32(1):12–19. doi: 10.1016/j.tibs.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Stein L. R., Imai S. I. The dynamic regulation of NAD metabolism in mitochondria. Trends in Endocrinology & Metabolism. 2012;23(9):420–428. doi: 10.1016/j.tem.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houtkooper R. H., Cantó C., Wanders R. J., Auwerx J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocrine Reviews. 2010;31(2):194–223. doi: 10.1210/er.2009-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imai S. I., Guarente L. It takes two to tango: Nad+ and sirtuins in aging/longevity control. npj Aging and Mechanisms of Disease. 2016;2(1, article 16017) doi: 10.1038/npjamd.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cantó C., Menzies K. J. J., Auwerx J. NAD+ Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell metabolism. 2015;22(1):31–53. doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imai S. I. The NAD world 2.0: the importance of the inter-tissue communication mediated by NAMPT/NAD+/SIRT1 in mammalian aging and longevity control. npj Systems Biology and Applications. 2016;2(1, article 16018):1–9. doi: 10.1038/npjsba.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans J., Wang T. C., Heyes M. P., Markey S. P. LC/MS analysis of NAD biosynthesis using stable isotope pyridine precursors. Analytical Biochemistry. 2002;306(2):197–203. doi: 10.1006/abio.2002.5715. [DOI] [PubMed] [Google Scholar]
- 21.Revollo J. R., Grimm A. A., Imai S. I. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. Journal of Biological Chemistry. 2004;279(49):50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- 22.Garten A., Schuster S., Penke M., Gorski T., De Giorgis T., Kiess W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nature Reviews Endocrinology. 2015;11(9):535–546. doi: 10.1038/nrendo.2015.117. [DOI] [PubMed] [Google Scholar]
- 23.Yoshino J., Mills K. F., Yoon M. J., Imai S. I. Nicotinamide Mononucleotide, a Key NAD+ Intermediate, Treats the Pathophysiology of Diet- and Age-Induced Diabetes in Mice. Cell Metabolism. 2011;14(4):528–536. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poljsak B. NAD+ in cancer prevention and treatment: pros and cons. Journal of Clinical & Experimental Oncology. 2016;5(4) doi: 10.4172/2324-9110.1000165. [DOI] [Google Scholar]
- 25.Raynes R., Pombier K. M., Nguyen K., Brunquell J., Mendez J. E., Westerheide S. D. The SIRT1 modulators AROS and DBC1 regulate HSF1 activity and the heat shock response. PLoS One. 2013;8(1, article e54364) doi: 10.1371/journal.pone.0054364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viswanathan M., Kim S. K., Berdichevsky A., Guarente L. A Role for SIR-2.1 Regulation of ER Stress Response Genes in Determining C. elegans Life Span. Developmental Cell. 2005;9(5):605–615. doi: 10.1016/j.devcel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 27.Brunet A., Sweeney L. B., Sturgill J. F., et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303(5666):2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 28.Motta M. C., Divecha N., Lemieux M., et al. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116(4):551–563. doi: 10.1016/S0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 29.Vaziri H., Dessain S. K., Eaton E. N., et al. hSIR2SIRT1 Functions as an NAD-Dependent p53 Deacetylase. Cell. 2001;107(2):149–159. doi: 10.1016/S0092-8674(01)00527-X. [DOI] [PubMed] [Google Scholar]
- 30.Luo J., Nikolaev A. Y., Imai S., et al. Negative control of p53 by Sir2α promotes cell survival under stress. Cell. 2001;107(2):137–148. doi: 10.1016/S0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 31.Rodgers J. T., Lerin C., Haas W., Gygi S. P., Spiegelman B. M., Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434(7029):113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 32.Rodgers J. T., Lerin C., Gerhart-Hines Z., Puigserver P. Metabolic adaptations through the PGC-1α and SIRT1 pathways. FEBS Letters. 2008;582(1):46–53. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeung F., Hoberg J. E., Ramsey C. S., et al. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. The EMBO Journal. 2004;23(12):2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salminen A., Huuskonen J., Ojala J., Kauppinen A., Kaarniranta K., Suuronen T. Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Research Reviews. 2008;7(2):83–105. doi: 10.1016/j.arr.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Jung K. J., Lee E. K., Kim J. Y., et al. Effect of short term calorie restriction on pro-inflammatory NF-kB and AP-1 in aged rat kidney. Inflammation Research. 2009;58(3, article 7227):143–150. doi: 10.1007/s00011-008-7227-2. [DOI] [PubMed] [Google Scholar]
- 36.Khan J. A., Forouhar F., Tao X., Tong L. Nicotinamide adenine dinucleotide metabolism as an attractive target for drug discovery. Expert Opinion on Therapeutic Targets. 2007;11(5):695–705. doi: 10.1517/14728222.11.5.695. [DOI] [PubMed] [Google Scholar]
- 37.Ying W. Therapeutic potential of NAD+ for neurological diseases. Future Neurology. 2007;2(2):129–132. doi: 10.2217/14796708.2.2.129. [DOI] [Google Scholar]
- 38.Connell N. J., Houtkooper R. H., Schrauwen P. NAD+ metabolism as a target for metabolic health: have we found the silver bullet? Diabetologia. 2019;62(6):888–899. doi: 10.1007/s00125-019-4831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J., Bonkowski M. S., Moniot S., et al. A conserved NAD+ binding pocket that regulates protein-protein interactions during aging. Science. 2017;355(6331):1312–1317. doi: 10.1126/science.aad8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang T., Kraus W. L. SIRT1-dependent regulation of chromatin and transcription: linking NAD+ metabolism and signaling to the control of cellular functions. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 2010;1804(8):1666–1675. doi: 10.1016/j.bbapap.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang H., Ryu D., Wu Y., et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352(6292):1436–1443. doi: 10.1126/science.aaf2693. [DOI] [PubMed] [Google Scholar]
- 42.Goodpaster B. H., Sparks L. M. Metabolic flexibility in health and disease. Cell Metabolism. 2017;25(5):1027–1036. doi: 10.1016/j.cmet.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchell S. J., Bernier M., Aon M. A., et al. Nicotinamide improves aspects of healthspan, but not lifespan, in mice. Cell Metabolism. 2018;27(3):667–676.e4. doi: 10.1016/j.cmet.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamoto T., Byun J., Zhai P., Ikeda Y., Oka S., Sadoshima J. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PLoS One. 2014;9(6, article e98972) doi: 10.1371/journal.pone.0098972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mills K. F., Yoshida S., Stein L. R., et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age- Associated Physiological Decline in Mice. Cell Metabolism. 2016;24(6):795–806. doi: 10.1016/j.cmet.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Picciotto N. E., Gano L. B., Johnson L. C., et al. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell. 2016;15(3):522–530. doi: 10.1111/acel.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tarantini S., Valcarcel-Ares M. N., Toth P., et al. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biology. 2019;24, article 101192 doi: 10.1016/j.redox.2019.101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uddin G. M., Youngson N. A., Chowdhury S. S., Hagan C., Sinclair D. A., Morris M. J. Administration of nicotinamide mononucleotide (NMN) reduces metabolic impairment in male mouse offspring from obese mothers. Cells. 2020;9(4) doi: 10.3390/cells9040791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carmona J. J., Michán S. Biology of healthy aging and longevity. Revista de investigacion clinica. 2016;68(1):7–16. [PubMed] [Google Scholar]
- 50.Colman R. J., Anderson R. M., Johnson S. C., et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cruzen C., Colman R. J. Effects of caloric restriction on cardiovascular aging in non-human primates and humans. Clinics in Geriatric Medicine. 2009;25(4):733–743. doi: 10.1016/j.cger.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Menssen A., Hydbring P., Kapelle K., et al. The c-MYC oncoprotein, the NAMPT enzyme, the SIRT1-inhibitor DBC1, and the SIRT1 deacetylase form a positive feedback loop. Proceedings of the National Academy of Sciences. 2012;109(4):E187–E196. doi: 10.1073/pnas.1105304109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Massudi H., Grant R., Guillemin G. J., Braidy N. NAD+ metabolism and oxidative stress: the golden nucleotide on a crown of thorns. Redox Report. 2012;17(1):28–46. doi: 10.1179/1351000212Y.0000000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin S. J., Ford E., Haigis M., Liszt G., Guarente L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes & Development. 2004;18(1):12–16. doi: 10.1101/gad.1164804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin S. J., Defossez P. A., Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in saccharomyces cerevisiae. Science. 2000;289(5487):2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 56.Leibiger I. B., Berggren P. O. Sirt1: a metabolic master switch that modulates lifespan. Nature Medicine. 2006;12(1):34–36. doi: 10.1038/nm0106-34. [DOI] [PubMed] [Google Scholar]
- 57.Guarente L., Picard F. Calorie Restriction— the SIR2 Connection. Cell. 2005;120(4):473–482. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 58.Tollefsbol T. O. Dietary epigenetics in cancer and aging. Advances in Nutrition and Cancer. 2014;159:257–267. doi: 10.1007/978-3-642-38007-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxidants & Redox Signaling. 2008;10(2):179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- 60.Evans C., Bogan K. L., Song P., Burant C. F., Kennedy R. T., Brenner C. NAD+ metabolite levels as a function of vitamins and calorie restriction: evidence for different mechanisms of longevity. BMC Chemical Biology. 2010;10(1) doi: 10.1186/1472-6769-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bogan K. L., Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annual Review of Nutrition. 2008;28(1):115–130. doi: 10.1146/annurev.nutr.28.061807.155443. [DOI] [PubMed] [Google Scholar]
- 62.Imai S. I. The NAD world: a new systemic regulatory network for metabolism and aging-Sirt1, systemic NAD biosynthesis, and their importance. Cell Biochemistry and Biophysics. 2009;53(2):65–74. doi: 10.1007/s12013-008-9041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cantó C., Houtkooper R. H., Pirinen E., et al. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metabolism. 2012;15(6):838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gomes A. P., Price N. L., Ling A. J. Y., et al. Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155(7):1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, O.B.V. and C. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington (DC): National Academies Press; 1998. [PubMed] [Google Scholar]
- 66.Irie J., Inagaki E., Fujita M., et al. Effect of oral administration of nicotinamide mononucleotide on clinical parameters and nicotinamide metabolite levels in healthy Japanese men. Endocrine Journal. 2020;67(2):153–160. doi: 10.1507/endocrj.EJ19-0313. [DOI] [PubMed] [Google Scholar]
- 67.Martens C. R., Denman B. A., Mazzo M. R., et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nature Communications. 2018;9(1) doi: 10.1038/s41467-018-03421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Airhart S. E., Shireman L. M., Risler L. J., et al. An open-label, non-randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PLoS One. 2017;12(12, article e0186459) doi: 10.1371/journal.pone.0186459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pirinen E., Auranen M., Khan N. A., et al. Niacin cures systemic NAD+ deficiency and improves muscle performance in adult-onset mitochondrial myopathy. Cell Metabolism. 2020;31(6):1078–1090.e5. doi: 10.1016/j.cmet.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 70.Conze D., Brenner C., Kruger C. L. Safety and metabolism of long-term administration of NIAGEN (nicotinamide riboside chloride) in a randomized, double-blind, placebo-controlled clinical trial of healthy overweight adults. Scientific Reports. 2019;9(1, article 9772) doi: 10.1038/s41598-019-46120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Elhassan Y. S., Kluckova K., Fletcher R. S., et al. Nicotinamide Riboside Augments the Aged Human Skeletal Muscle NAD+ Metabolome and Induces Transcriptomic and Anti- inflammatory Signatures. Cell Reports. 2019;28(7):1717–1728.e6. doi: 10.1016/j.celrep.2019.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Poljsak B., Milisav I. Vitamin B3 forms as precursors to NAD+: are they safe? Trends in Food Science & Technology. 2018;79:198–203. doi: 10.1016/j.tifs.2018.07.020. [DOI] [Google Scholar]
- 73.Dragovic J., Kim S. H., Brown S. L., Kim J. H. Nicotinamide pharmacokinetics in patients. Radiotherapy and Oncology. 1995;36(3):225–228. doi: 10.1016/0167-8140(95)01581-Z. [DOI] [PubMed] [Google Scholar]
- 74.Braidy N., Liu Y. NAD+ therapy in age-related degenerative disorders: a benefit/risk analysis. Experimental Gerontology. 2020;132, article 110831 doi: 10.1016/j.exger.2020.110831. [DOI] [PubMed] [Google Scholar]
- 75.Remie C. M. E., Roumans K. H. M., Moonen M. P. B., et al. Nicotinamide riboside supplementation alters body composition and skeletal muscle acetylcarnitine concentrations in healthy obese humans. The American Journal of Clinical Nutrition. 2020;112(2):413–426. doi: 10.1093/ajcn/nqaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bieganowski P., Brenner C. Discoveries of Nicotinamide Riboside as a Nutrient and Conserved NRK Genes Establish a Preiss-Handler Independent Route to NAD+ in Fungi and Humans. Cell. 2004;117(4):495–502. doi: 10.1016/S0092-8674(04)00416-7. [DOI] [PubMed] [Google Scholar]
- 77.Ummarino S., Mozzon M., Zamporlini F., et al. Simultaneous quantitation of nicotinamide riboside, nicotinamide mononucleotide and nicotinamide adenine dinucleotide in milk by a novel enzyme-coupled assay. Food Chemistry. 2017;221:161–168. doi: 10.1016/j.foodchem.2016.10.032. [DOI] [PubMed] [Google Scholar]
- 78.Baur J. A. Resveratrol, sirtuins, and the promise of a DR mimetic. Mechanisms of Ageing and Development. 2010;131(4):261–269. doi: 10.1016/j.mad.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Timmers S., Auwerx J., Schrauwen P. The journey of resveratrol from yeast to human. Aging. 2012;4(3):146–158. doi: 10.18632/aging.100445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim J., Yang G., Kim Y., Kim J., Ha J. AMPK activators: mechanisms of action and physiological activities. Experimental & Molecular Medicine. 2016;48(4, article e224) doi: 10.1038/emm.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cantó C., Gerhart-Hines Z., Feige J. N., et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458(7241):1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang G., Han T., Nijhawan D., et al. P7C3 neuroprotective chemicals function by activating the rate-limiting enzyme in NAD salvage. Cell. 2014;158(6):1324–1334. doi: 10.1016/j.cell.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li H., Xu M., Lee J., He C., Xie Z. Leucine supplementation increases SIRT1 expression and prevents mitochondrial dysfunction and metabolic disorders in high-fat diet-induced obese mice. American Journal of Physiology-Endocrinology and Metabolism. 2012;303(10):E1234–E1244. doi: 10.1152/ajpendo.00198.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aragonès G., Suárez M., Ardid-Ruiz A., et al. Dietary proanthocyanidins boost hepatic NAD+ metabolism and SIRT1 expression and activity in a dose-dependent manner in healthy rats. Scientific Reports. 2016;6(1, article 24977) doi: 10.1038/srep24977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ribas-Latre A., Baselga-Escudero L., Casanova E., et al. Dietary proanthocyanidins modulate BMAL1 acetylation, Nampt expression and NAD levels in rat liver. Scientific Reports. 2015;5(1, article 10954) doi: 10.1038/srep10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berger F., Lau C., Dahlmann M., Ziegler M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. Journal of Biological Chemistry. 2005;280(43):36334–36341. doi: 10.1074/jbc.M508660200. [DOI] [PubMed] [Google Scholar]
- 87.Houtkooper R. H., Auwerx J. Exploring the therapeutic space around NAD+ Journal of Cell Biology. 2012;199(2):205–209. doi: 10.1083/jcb.201207019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kahn A. M., Allen J. C., Zhang S. Insulin increases NADH/NAD+ redox state, which stimulates guanylate cyclase in vascular smooth muscle. American Journal of Hypertension. 2002;15(3):273–279. doi: 10.1016/S0895-7061(01)02289-0. [DOI] [PubMed] [Google Scholar]
- 89.Sasaki T., Kikuchi O., Shimpuku M., et al. Hypothalamic SIRT1 prevents age-associated weight gain by improving leptin sensitivity in mice. Diabetologia. 2014;57(4, article 3140):819–831. doi: 10.1007/s00125-013-3140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cederbaum A. I. Alcohol metabolism. Clinics in Liver Disease. 2012;16(4):667–685. doi: 10.1016/j.cld.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McElfresh K. C., McDonald J. F. The effect of alcohol stress on nicotinamide adenine dinucleotide (NAD+) levels in Drosophila. Biochemical Genetics. 1983;21(3-4):365–374. doi: 10.1007/BF00499145. [DOI] [PubMed] [Google Scholar]
- 92.Bleier L., Dröse S. Superoxide generation by complex III: from mechanistic rationales to functional consequences. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 2013;1827(11-12):1320–1331. doi: 10.1016/j.bbabio.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 93.Nogueiras R., Habegger K. M., Chaudhary N., et al. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiological Reviews. 2012;92(3):1479–1514. doi: 10.1152/physrev.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yoon M. J., Yoshida M., Johnson S., et al. SIRT1-mediated eNAMPT secretion from adipose tissue regulates hypothalamic NAD+ and function in mice. Cell Metabolism. 2015;21(5):706–717. doi: 10.1016/j.cmet.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bai P., Cantó C., Oudart H., et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metabolism. 2011;13(4):461–468. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kraus D., Yang Q., Kong D., et al. Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature. 2014;508(7495):258–262. doi: 10.1038/nature13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pirinen E., Cantó C., Jo Y. S., et al. Pharmacological inhibition of poly(ADP-ribose) polymerases improves fitness and mitochondrial function in skeletal muscle. Cell Metabolism. 2014;19(6):1034–1041. doi: 10.1016/j.cmet.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang S. J., Choi J. M., Kim L., et al. Nicotinamide improves glucose metabolism and affects the hepatic NAD-sirtuin pathway in a rodent model of obesity and type 2 diabetes. The Journal of Nutritional Biochemistry. 2014;25(1):66–72. doi: 10.1016/j.jnutbio.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 99.Rappou E., Jukarainen S., Rinnankoski-Tuikka R., et al. Weight loss is associated with increased NAD+/SIRT1 expression but reduced PARP activity in white adipose tissue. The Journal of Clinical Endocrinology & Metabolism. 2016;101(3):1263–1273. doi: 10.1210/jc.2015-3054. [DOI] [PubMed] [Google Scholar]
- 100.Seyssel K., Alligier M., Meugnier E., et al. Regulation of energy metabolism and mitochondrial function in skeletal muscle during lipid overfeeding in healthy men. The Journal of Clinical Endocrinology & Metabolism. 2014;99(7):E1254–E1262. doi: 10.1210/jc.2013-4379. [DOI] [PubMed] [Google Scholar]
- 101.Jukarainen S., Heinonen S., Rämö J. T., et al. Obesity is associated with low NAD+/SIRT pathway expression in adipose tissue of BMI-discordant monozygotic twins. Journal of Clinical Endocrinology & Metabolism. 2016;101(1):275–283. doi: 10.1210/jc.2015-3095. [DOI] [PubMed] [Google Scholar]
- 102.Barbosa M. T. P., Soares S. M., Novak C. M., et al. The enzyme CD38 (a NAD glycohydrolase, EC 3.2.2.5) is necessary for the development of diet-induced obesity. The FASEB Journal. 2007;21(13):3629–3639. doi: 10.1096/fj.07-8290com. [DOI] [PubMed] [Google Scholar]
- 103.Nair S., Lee Y. H., Rousseau E., et al. Increased expression of inflammation-related genes in cultured preadipocytes/stromal vascular cells from obese compared with non-obese Pima Indians. Diabetologia. 2005;48(9):1784–1788. doi: 10.1007/s00125-005-1868-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mutch D. M., Tordjman J., Pelloux V., et al. Needle and surgical biopsy techniques differentially affect adipose tissue gene expression profiles. The American Journal of Clinical Nutrition. 2009;89(1):51–57. doi: 10.3945/ajcn.2008.26802. [DOI] [PubMed] [Google Scholar]
- 105.Wang L. F., Miao L. J., Wang X. N., et al. CD38 deficiency suppresses adipogenesis and lipogenesis in adipose tissues through activating Sirt1/PPARγ signaling pathway. Journal of Cellular and Molecular Medicine. 2018;22(1):101–110. doi: 10.1111/jcmm.13297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.WHO. Global action plan for the prevention and control of NCDs 2013-2020. Geneva: World Health Organization; 2015. [Google Scholar]
- 107.Malm C., Jakobsson J., Isaksson A. Physical activity and sports—real health benefits: a review with insight into the public health of Sweden. Sports. 2019;7(5) doi: 10.3390/sports7050127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jenkin C. R., Eime R. M., Westerbeek H., O’Sullivan G., Van Uffelen J. G. Z. Sport and ageing: a systematic review of the determinants and trends of participation in sport for older adults. BMC Public Health. 2017;17(1) doi: 10.1186/s12889-017-4970-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brandauer J., Vienberg S. G., Andersen M. A., et al. AMP-activated protein kinase regulates nicotinamide phosphoribosyl transferase expression in skeletal muscle. The Journal of Physiology. 2013;591(20):5207–5220. doi: 10.1113/jphysiol.2013.259515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Costford S. R., Bajpeyi S., Pasarica M., et al. Skeletal muscle NAMPT is induced by exercise in humans. American Journal of Physiology-Endocrinology and Metabolism. 2010;298(1):E117–E126. doi: 10.1152/ajpendo.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Koltai E., Szabo Z., Atalay M., et al. Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mechanisms of Ageing and Development. 2010;131(1):21–28. doi: 10.1016/j.mad.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.de Guia R. M., Agerholm M., Nielsen T. S., et al. Aerobic and resistance exercise training reverses age-dependent decline in NAD+ salvage capacity in human skeletal muscle. Physiological Reports. 2019;7(12, article e14139) doi: 10.14814/phy2.14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Poljsak B., Milisav I. NAD+ as the link between oxidative stress, inflammation, caloric restriction, exercise, DNA repair, longevity, and health span. Rejuvenation Research. 2016;19(5):406–413. doi: 10.1089/rej.2015.1767. [DOI] [PubMed] [Google Scholar]
- 114.Cantó C., Jiang L. Q., Deshmukh A. S., et al. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metabolism. 2010;11(3):213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ito Y., Yonekura R., Nakagami Y., et al. Developments in Tryptophan and Serotonin Metabolism. Vol. 527. Boston, MA: Springer; 2003. Tryptophan metabolism was accelerated by exercise in rat; pp. 531–535. (Advances in Experimental Medicine and Biology). [DOI] [PubMed] [Google Scholar]
- 116.Goody M. F., Henry C. A. A need for NAD+ in muscle development, homeostasis, and aging. Skeletal Muscle. 2018;8(1) doi: 10.1186/s13395-018-0154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bugaj O., Zieliński J., Kusy K., Kantanista A., Wieliński D., Guzik P. The effect of exercise on the skin content of the reduced form of NAD and its response to transient ischemia and reperfusion in highly trained athletes. Frontiers in Physiology. 2019;10 doi: 10.3389/fphys.2019.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kourtzidis I. A., Stoupas A. T., Gioris I. S., et al. The NAD+ precursor nicotinamide riboside decreases exercise performance in rats. Journal of the International Society of Sports Nutrition. 2016;13(1) doi: 10.1186/s12970-016-0143-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Murray R., Bartoli W. P., Eddy D. E., Horn M. K. Physiological and performance responses to nicotinic-acid ingestion during exercise. Medicine & Science in Sports & Exercise. 1995;27(7):1057–1062. doi: 10.1249/00005768-199507000-00015. [DOI] [PubMed] [Google Scholar]
- 120.Frederick D. W. W., Loro E., Liu L., et al. Loss of NAD homeostasis leads to progressive and reversible degeneration of skeletal muscle. Cell Metabolism. 2016;24(2):269–282. doi: 10.1016/j.cmet.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dew M. A., Hoch C. C., Buysse D. J., et al. Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosomatic Medicine. 2003;65(1):63–73. doi: 10.1097/01.PSY.0000039756.23250.7C. [DOI] [PubMed] [Google Scholar]
- 122.Mazzotti D. R., Guindalini C., Sosa A. L., Ferri C. P., Tufik S. Prevalence and correlates for sleep complaints in older adults in low and middle income countries: a 10/66 Dementia Research Group study. Sleep Medicine. 2012;13(6):697–702. doi: 10.1016/j.sleep.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 123.Beest V. Statin users risk heart attacks by dropping treatment or taking low doses doctors must emphasise importance of complying with treatment say researchers. Heart. 2006;91:250–256. doi: 10.1093/eurheartj. [DOI] [Google Scholar]
- 124.Enright P. L., Newman A. B., Wahl P. W., Manolio T. A., Haponik F. E., Boyle P. J. R. Prevalence and correlates of snoring and observed apneas in 5,201 older adults. Sleep. 1996;19(7):531–538. doi: 10.1093/sleep/19.7.531. [DOI] [PubMed] [Google Scholar]
- 125.Cappuccio F. P., D'Elia L., Strazzullo P., Miller M. A. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Klein L., Gao T., Barzilai N., Milman S. Association between sleep patterns and health in families with exceptional longevity. Frontiers in Medicine. 2017;4 doi: 10.3389/FMED.2017.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hood S., Amir S. The aging clock: circadian rhythms and later life. Journal of Clinical Investigation. 2017;127(2):437–446. doi: 10.1172/JCI90328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nakahata Y., Sahar S., Astarita G., Kaluzova M., Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324(5927):654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Asher G., Reinke H., Altmeyer M., Gutierrez-Arcelus M., Hottiger M. O., Schibler U. Poly(ADP-ribose) polymerase 1 participates in the phase entrainment of circadian clocks to feeding. Cell. 2010;142(6):943–953. doi: 10.1016/j.cell.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 130.Ramsey K. M., Yoshino J., Brace C. S., et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324(5927):651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Poljsak B. NAMPT-mediated NAD biosynthesis as the internal timing mechanism: in NAD+ world, time is running in its own way. Rejuvenation Research. 2018;21(3):210–224. doi: 10.1089/rej.2017.1975. [DOI] [PubMed] [Google Scholar]
- 132.Poljsak B., Ribarič S., Milisav I. Yin and Yang: why did evolution implement and preserve the circadian rhythmicity? Medical Hypotheses. 2019;131, article 109306 doi: 10.1016/j.mehy.2019.109306. [DOI] [PubMed] [Google Scholar]
- 133.Pelicano H., Xu R. H., du M., et al. Mitochondrial respiration defects in cancer cells cause activation of Akt survival pathway through a redox-mediated mechanism. Journal of Cell Biology. 2006;175(6):913–923. doi: 10.1083/jcb.200512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Milne J. C., Lambert P. D., Schenk S., et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450(7170):712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Roenneberg T., Merrow M. The circadian clock and human health. Current Biology. 2016;26(10):R432–R443. doi: 10.1016/j.cub.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 136.Libert S., Bonkowski M. S., Pointer K., Pletcher S. D., Guarente L. Deviation of innate circadian period from 24 h reduces longevity in mice. Aging Cell. 2012;11(5):794–800. doi: 10.1111/j.1474-9726.2012.00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pittelli M., Felici R., Pitozzi V., et al. Pharmacological effects of exogenous NAD on mitochondrial bioenergetics, DNA repair, and apoptosis. Molecular Pharmacology. 2011;80(6):1136–1146. doi: 10.1124/mol.111.073916. [DOI] [PubMed] [Google Scholar]
- 138.Kondratov R. V., Kondratova A. A., Gorbacheva V. Y., Vykhovanets O. V., Antoch M. P. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes & Development. 2006;20(14):1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Libert S., Pointer K., Bell E. L., et al. SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive. Cell. 2011;147(7):1459–1472. doi: 10.1016/j.cell.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kishi T., Yoshimura R., Kitajima T., et al. SIRT1 gene is associated with major depressive disorder in the Japanese population. Journal of Affective Disorders. 2010;126(1-2):167–173. doi: 10.1016/j.jad.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 141.Heinonen I., Laukkanen J. A. Effects of heat and cold on health, with special reference to Finnish sauna bathing. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2018;314(5):R629–R638. doi: 10.1152/ajpregu.00115.2017. [DOI] [PubMed] [Google Scholar]
- 142.Laukkanen T., Khan H., Zaccardi F., Laukkanen J. A. Association between sauna bathing and fatal cardiovascular and all-cause mortality events. JAMA Internal Medicine. 2015;175(4):542–548. doi: 10.1001/jamainternmed.2014.8187. [DOI] [PubMed] [Google Scholar]
- 143.Laukkanen J. A., Laukkanen T., Kunutsor S. K. Cardiovascular and other health benefits of sauna bathing: a review of the evidence. Mayo Clinic Proceedings. 2018;93(8):1111–1121. doi: 10.1016/j.mayocp.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 144.Gray C. C., Amrani M., Smolenski R. T., Taylor G. L., Yacoub M. H. Age dependence of heat stress mediated cardioprotection. The Annals of Thoracic Surgery. 2000;70:621–626. doi: 10.1016/S0003-4975(00)01445-4. [DOI] [PubMed] [Google Scholar]
- 145.Brazill J. M., Li C., Zhu Y., Zhai R. G. NMNAT: it’s an NAD+ synthase… it’s a chaperone… it’s a neuroprotector. Current Opinion in Genetics & Development. 2017;44:156–162. doi: 10.1016/j.gde.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ali Y. O., McCormack R., Darr A., Zhai R. G. Nicotinamide mononucleotide adenylyltransferase is a stress response protein regulated by the heat shock factor/hypoxia-inducible factor 1α pathway. Journal of Biological Chemistry. 2011;286:19089–19099. doi: 10.1074/jbc.M111.219295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Virtanen K. A., Lidell M. E., Orava J., et al. Functional brown adipose tissue in healthy adults. New England Journal of Medicine. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 148.Rajman L., Chwalek K., Sinclair D. A. Therapeutic potential of NAD-boosting molecules: the in vivo evidence. Cell Metabolism. 2018;27:529–547. doi: 10.1016/j.cmet.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jokinen R., Pirnes-Karhu S., Pietiläinen K. H., Pirinen E. Adipose tissue NAD+-homeostasis, sirtuins and poly(ADP-ribose) polymerases - important players in mitochondrial metabolism and metabolic health. Redox Biology. 2017;12:246–263. doi: 10.1016/j.redox.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Björntorp P., Bengtsson C., Blohmé G., et al. Adipose tissue fat cell size and number in relation to metabolism in randomly selected middle-aged men and women. Metabolism. 1971;20:927–935. doi: 10.1016/0026-0495(71)90013-8. [DOI] [PubMed] [Google Scholar]
- 151.Heinonen S., Saarinen L., Naukkarinen J., et al. Adipocyte morphology and implications for metabolic derangements in acquired obesity. International Journal of Obesity. 2014;38:1423–1431. doi: 10.1038/ijo.2014.31. [DOI] [PubMed] [Google Scholar]
- 152.Goodbody A. E., Trayhurn P. GDP binding to brown-adipose-tissue mitochondria of diabetic-obese (db/db) mice. Decreased binding in both the obese and pre-obese states. Biochemical Journal. 1981;194:1019–1022. doi: 10.1042/bj1941019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Himms-Hagen J., Desautels M. A mitochondrial defect in brown adipose tissue of the obese (obob) mouse: reduced binding of purine nucleotides and a failure to respond to cold by an increase in binding. Biochemical and Biophysical Research Communications. 1978;83:628–634. doi: 10.1016/0006-291X(78)91036-7. [DOI] [PubMed] [Google Scholar]
- 154.Shimizu I., Aprahamian T., Kikuchi R., et al. Vascular rarefaction mediates whitening of brown fat in obesity. Journal of Clinical Investigation. 2014;124:2099–2112. doi: 10.1172/JCI71643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vijgen G. H. E. J., Bouvy N. D., Teule G. J. J., Brans B., Schrauwen P., van Marken Lichtenbelt W. D. Brown adipose tissue in morbidly obese subjects. PLoS One. 2011;6 doi: 10.1371/journal.pone.0017247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dolopikou C. F., Kourtzidis I. A., Margaritelis N. V., et al. Acute nicotinamide riboside supplementation improves redox homeostasis and exercise performance in old individuals: a double-blind cross-over study. European Journal of Nutrition. 2020;59:505–515. doi: 10.1007/s00394-019-01919-4. [DOI] [PubMed] [Google Scholar]
- 157.Kourtzidis I. A., Dolopikou C. F., Tsiftsis A. N., et al. Nicotinamide riboside supplementation dysregulates redox and energy metabolism in rats: implications for exercise performance. Experimental Physiology. 2018;103:1357–1366. doi: 10.1113/EP086964. [DOI] [PubMed] [Google Scholar]
- 158.Knip M., Douek I. F., Moore W. P. T., et al. Safety of high-dose nicotinamide: a review. Diabetologia. 2000;43:1337–1345. doi: 10.1007/s001250051536. [DOI] [PubMed] [Google Scholar]
- 159.Shi W., Hegeman M. A., Doncheva A., Bekkenkamp-Grovenstein M., de Boer V. C. J., Keijer J. High dose of dietary nicotinamide riboside induces glucose intolerance and white adipose tissue dysfunction in mice fed a mildly obesogenic diet. Nutrients. 2019;11(10) doi: 10.3390/nu11102439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Herranz D., Muñoz-Martin M., Cañamero M., et al. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nature Communications. 2010;1:p. 3. doi: 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen D., Bruno J., Easlon E., et al. Tissue-specific regulation of SIRT1 by calorie restriction. Genes & Development. 2008;22:1753–1757. doi: 10.1101/gad.1650608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Frederick D. W., Davis J. G., Dávila A., et al. Increasing NAD synthesis in muscle via nicotinamide phosphoribosyltransferase is not sufficient to promote oxidative metabolism. Journal of Biological Chemistry. 2015;290:1546–1558. doi: 10.1074/jbc.M114.579565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Muruganandham M., Alfieri A. A., Matei C., et al. Metabolic signatures associated with a NAD synthesis inhibitor-induced tumor apoptosis identified by 1H-decoupled-31P magnetic resonance spectroscopy. Clinical Cancer Research. 2005;11:3503–3513. doi: 10.1158/1078-0432.CCR-04-1399. [DOI] [PubMed] [Google Scholar]
- 164.Pogrebniak A., Schemainda I., Azzam K., Pelka-Fleischer R., Nüssler V., Hasmann M. Chemopotentiating effects of a novel NAD biosynthesis inhibitor, FK866, in combination with antineoplastic agents. European Journal of Medical Research. 2006;11:313–321. [PubMed] [Google Scholar]
- 165.Liu T. F., Brown C. M., El Gazzar M., et al. Fueling the flame: bioenergy couples metabolism and inflammation. Journal of Leukocyte Biology. 2012;92:499–507. doi: 10.1189/jlb.0212078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li L., Chen Z., Fu W., Cai S., Zeng Z. Emerging evidence concerning the role of sirtuins in sepsis. Critical Care Research and Practice. 2018;2018:8. doi: 10.1155/2018/5489571.5489571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Busso N., Karababa M., Nobile M., et al. Pharmacological inhibition of nicotinamide phosphoribosyltransferase/visfatin enzymatic activity identifies a new inflammatory pathway linked to NAD. PLoS One. 2008;3(5, article e2267) doi: 10.1371/journal.pone.0002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Trammell S. A. J., Weidemann B. J., Chadda A., et al. Nicotinamide riboside opposes type 2 diabetes and neuropathy in mice. Scientific Reports. 2016;6(1) doi: 10.1038/srep26933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bockwoldt M., Houry D., Niere M., et al. Identification of evolutionary and kinetic drivers of NAD-dependent signaling. Proceedings of the National Academy of Sciences. 2019;116(32):15957–15966. doi: 10.1073/pnas.1902346116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hwang E. S., Song S. B. Nicotinamide is an inhibitor of SIRT1 in vitro, but can be a stimulator in cells. Cellular and Molecular Life Sciences. 2017;74(18):3347–3362. doi: 10.1007/s00018-017-2527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hwang E. S., Song S. B. Possible adverse effects of high-dose nicotinamide: mechanisms and safety assessment. Biomolecules. 2020;10(5):p. 687. doi: 10.3390/biom10050687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sebastián C., Satterstrom F. K., Haigis M. C., Mostoslavsky R. From sirtuin biology to human diseases: an update. Journal of Biological Chemistry. 2012;287(51):42444–42452. doi: 10.1074/jbc.r112.402768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Imai S.-i. A possibility of nutriceuticals as an anti-aging intervention: activation of sirtuins by promoting mammalian NAD biosynthesis. Pharmacological Research. 2010;62(1):42–47. doi: 10.1016/j.phrs.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Puig L. S., Valera-Alberni M., Cantó C., Pillon N. J. Circadian rhythms and mitochondria: connecting the dots. Frontiers in Genetics. 2018;9:p. 452. doi: 10.3389/fgene.2018.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kohsaka A., Laposky A. D., Ramsey K. M., et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metabolism. 2007;6(5):414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 176.Hatori M., Vollmers C., Zarrinpar A., et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metabolism. 2012;15(6):848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Meex R. C. R., Schrauwen-Hinderling V. B., Moonen-Kornips E., et al. Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes. 2010;59(3):572–579. doi: 10.2337/db09-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Phielix E., Meex R., Moonen-Kornips E., Hesselink M. K. C., Schrauwen P. Exercise training increases mitochondrial content and ex vivo mitochondrial function similarly in patients with type 2 diabetes and in control individuals. Diabetologia. 2010;53(8):1714–1721. doi: 10.1007/s00125-010-1764-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dali‐Youcef N., Lagouge M., Froelich S., Koehl C., Schoonjans K., Auwerx J. Sirtuins: the “magnificent seven”, function, metabolism and longevity. Annals of Medicine. 2009;39:335–345. doi: 10.1080/07853890701408194. [DOI] [PubMed] [Google Scholar]
- 180.Ralto K. M., Rhee E. P., Parikh S. M. NAD+ homeostasis in renal health and disease. Nature Reviews Nephrology. 2020;16(2):99–111. doi: 10.1038/s41581-019-0216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


