Abstract
Guselkumab appears to be safe and effective in the treatment of patients with HS, who do not respond to adalimumab and other systemic therapies. Guselkumab can be used in patients with comorbid Crohn's disease.
Keywords: Crohn's disease, guselkumab, Hidradenitis suppurativa, inflammation
Guselkumab appears to be safe and effective in the treatment of patients with HS, who do not respond to adalimumab and other systemic therapies. Guselkumab can be used in patients with comorbid Crohn's disease.

1. INTRODUCTION
We present a case of a 25‐year‐old man with hidradenitis suppurativa (HS) and comorbid Crohn's disease successfully treated with guselkumab. After 7 months of treatment, the patient experienced an objective improvement and showed remarkable symptom relief. Further, a systematic literature review of effectiveness and safety of guselkumab for HS was performed.
Hidradenitis suppurativa (HS) is a chronic, inflammatory skin condition primarily affecting the intertriginous areas. It is characterized by recurrence of inflamed nodules and abscesses, resulting in fistulae, fibrosis, and scarring. The pathogenesis of HS is not fully understood, but a dysregulation of the immune system with increased levels of several proinflammatory cytokines in lesional skin has been proposed. 1
IL‐23 is involved in the differentiation of Th17 and is thought to play a significant role in HS. 2 , 3 Studies have shown increased Th17 cells and levels of IL‐23 in lesional HS skin, and IL‐17A produced by Th17 in serum. 2 , 4 IL‐23 is also involved in psoriasis and inflammatory bowel disease, conditions frequently comorbid with HS. 2 , 5 , 6 , 7 Paradoxically, HS can develop as an adverse reaction of treatment with TNF inhibitors in patients with Crohn's disease, 8 which challenges choice of treatment for HS, as TNF inhibitors are currently the only approved biologic treatment for HS. Further, the potential risk of de novo development of, or worsening of existing, Crohn's disease by treating HS with IL‐17 inhibitors, non‐TNF/non‐IL‐17 inhibitor biologic medications are becoming a preferred treatment option for refractory HS.
Guselkumab is a monoclonal antibody targeting the p19 subunit of IL‐23, leading to inhibition of its downstream inflammatory effects. 9 The drug is approved for the treatment of moderate‐severe psoriasis and psoriatic arthritis. 10 However, only few cases on the use in HS have been published, 11 , 12 , 13 , 14 , 15 and only very little is known about its use in HS concomitantly with Crohn's disease.
2. CASE PRESENTATION
A 25‐year‐old man with HS since 2016 presented with Hurley stage II lesions in the groins and anogenital skin. In addition to HS, the patient had Crohn's disease since 2014. He had a BMI of 26 kg/m 2 and a family history of HS. He had no history of smoking. At first visit at our department, in August 2017, the patient had a Dermatology Life Quality Index (DLQI, range scale 0‐30, with high score indicating worse quality of life) score of 8 and an overall disease bother score and overall disease pain score on a visual analogue scale (VAS) of 6.8 and 7.6, respectively, out of 10. All blood samples, including CRP, leukocytes, blood glucose, cholesterol, triglycerides, and liver and kidney function, were normal. During the following 19 months, his HS worsened despite treatment with systemic tetracycline, doxycycline, rifampicin/clindamycin, adalimumab, and ustekinumab and several surgical interventions (Figure 1, Panel A).
Figure 1.
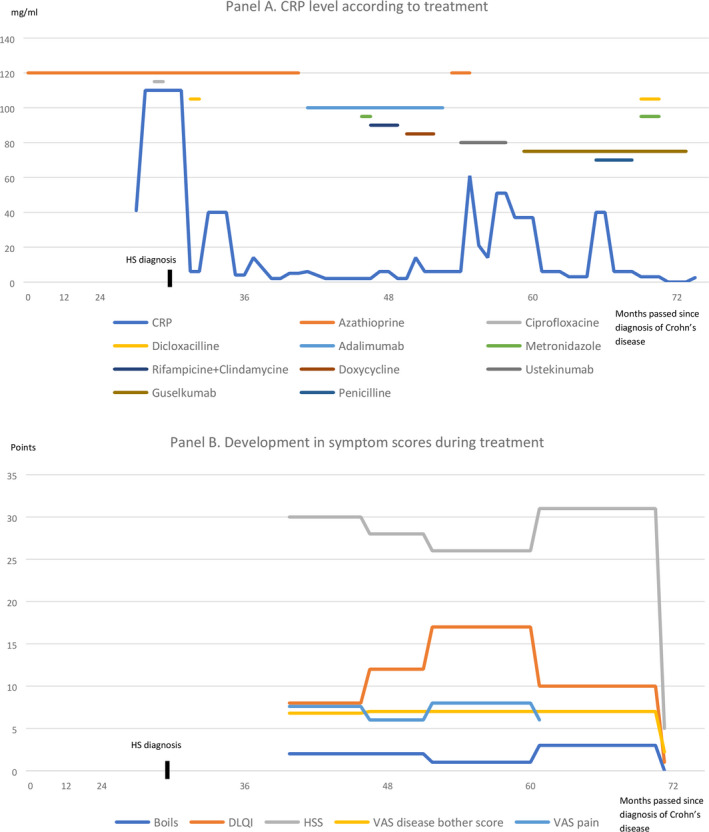
Panel A. CRP level according to treatment. Panel B. Development in symptom scores during treatment
In view of the patient's continued unmet need for treatment, we initiated treatment with guselkumab in April 2019. Guselkumab was administered in the following dosage; 100 mg by subcutaneous injection at week 0, followed by 100 mg at week 4, then maintenance dosing of 100 mg every 8 weeks, which after 6 months was increased to 100 mg every 6 weeks. After 7 months of treatment with guselkumab, the patient experienced a subjective improvement. In March 2020, the patient reported remarkable symptom relief with a reduction in number of boils in the preceding month to 0, in DLQI to 1, in overall disease bother score to 2.2 and in HSS to 5 (Figure 1, Panel B). In January 2020, excision of a fistula was performed by plastic surgeons.
As of May 2020, the patient is still treated with guselkumab 100 mg every 6 weeks and is still in remission (Figure 2). During the entire treatment period of 12 months with guselkumab, the patient experienced occurrence of two inflamed nodules that were treated with triamcinolone injections. Further, he experienced an episode of fever and abdominal pain after alcohol intake and had his guselkumab injection postponed for two weeks. No ethical approval is needed for this type of study. Formal consent was obtained for use of patient photograph.
Figure 2.
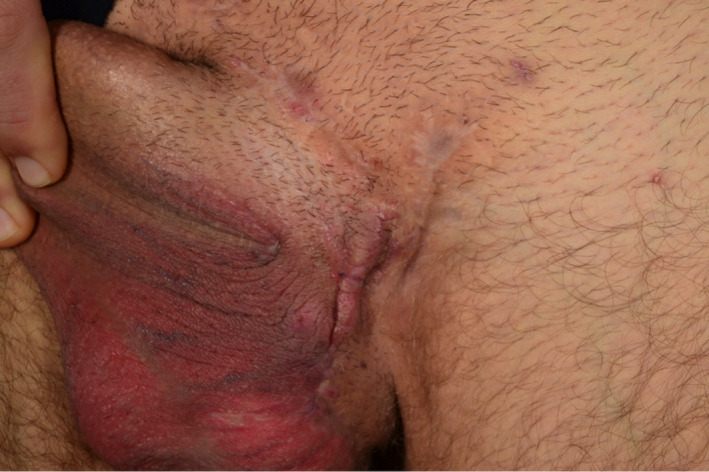
Left groin lesion after 12 months of treatment with guselkumab
3. SYSTEMATIC REVIEW OF THE LITERATURE
Five published studies were identified on the use of guselkumab for treatment of HS 11 , 12 , 13 , 14 , 15 (Table 1). A total of 17 patients with HS (8 females, 47.1%) were evaluated. Most patients had a long history of treatment with antibiotics and insufficient response to other biologic agents. The treatment regimens used followed the guidelines for psoriasis with injection of 100 mg guselkumab at baseline and week four followed by 100 mg every eight weeks in four out of five studies. Overall, a single patient experienced worsening of HS, whereas five experienced no response to treatment. The remaining 11 patients all experienced improvement from treatment, with three patients being in remission from HS. Crohn's disease was present in one patient, which was diagnosed after HS. 13 No side effects from treatment with guselkumab were reported.
Table 1.
Studies of guselkumab for treatment of hidradenitis suppurativa
| Study | Patients | Mean age, years (range) | Crohn's disease | Other comorbidities | Guselkumab dosage | Efficacy | Side effects |
|---|---|---|---|---|---|---|---|
| Kovacs et al, 2019 11 | 3 (1 female) | 27.7 (23‐35) | No | Psoriasis (1 patient) |
100 mg w0 and w4, 100 mg q8w |
3x↑↑ | None |
| Casseres et al, 2019 12 | 8 (3 females) | 35.5 (15‐68) | No | Psoriasis (4 patients) |
100 mg w0 and w4, 100 mg q8w |
4x→ 2x↑ 2x↑↑↑ |
None |
| Berman et al, 2019 13 | 1 (female) | 28 | Yes a | Psoriasis, lymphedema |
100 mg w0, w5 w12, w20, 100 mg q6w |
↑ | None |
| Kearney et al, 2020 14 | 1 (female) | 28 | No | Latent pulmonary tuberculosis |
100 mg w0 and w4, 100 mg q8w |
↑↑↑ | None |
| Montero‐Vilchez et al, 2020 15 | 4 (2 females) | 41.5 (30‐57) | No | Diabetes (1 patient), pilonidal sinus (2 patients), psoriasis (1 patient) |
100 mg w0, 100 mg q4w |
2x↑ 1x→ 1x↓ |
None |
Abbreviations: ↑, little effect; ↑↑, moderate effect; ↑↑↑, complete remission; →, no response; ↓, worsening; mg, milligram; q4w, every 4 weeks; q6w, every 6 wk; q8w, every 8 wk; w, week.
Crohn's disease diagnosed after hidradenitis suppurativa.
4. DISCUSSION
To our knowledge, only 17 cases of HS treated with guselkumab have been published 11 , 12 , 13 , 14 , 15 and only one of these patients had concomitant Crohn's disease. Most patients experienced some effect from treatment in accordance with our patient, who reported great satisfaction and rapid and significant improvement. Only the study by Kovacs et al 10 reported improvement on similar validated outcome measures as described for our patient, quality of life, clinical parameters including number of boils reported, and VAS score.
Patients with HS suffer from multiple comorbidities, and effective treatment to control their symptoms is challenging. Based on the data from our patient and the data identified through systematic literature search, there is some effect from treatment with guselkumab in most patients with HS. Further studies on the role of IL‐23 in HS pathogenesis are needed, and widespread use of guselkumab in HS awaits evidence from future larger clinical trials.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Dr Jørgensen, A‐HR, and Dr Holm, JG: contributed to conceptualization, data curation, formal analysis, investigation, methodology, and writing‐original draft; Dr Thomsen, SF: contributed to conceptualization, data curation, project administration, supervision, and writing‐review and editing.
ACKNOWLEDGMENTS
Published with written consent of the patient.
Jørgensen A‐HR, Holm JG, Thomsen SF. Guselkumab for hidradenitis suppurativa in a patient with concomitant Crohn’s disease: Report and systematic literature review of effectiveness and safety. Clin Case Rep. 2020;8:2874–2877. 10.1002/ccr3.3090
REFERENCES
- 1. Kelly G, Sweeney CM, Tobin A‐M, Kirby B. Hidradenitis suppurativa: the role of immune dysregulation. Int J Dermatol. 2014;53(10):1186‐1196. [DOI] [PubMed] [Google Scholar]
- 2. Schlapbach C, Hänni T, Yawalkar N, Hunger RE. Expression of the IL‐23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65(4):790‐798. [DOI] [PubMed] [Google Scholar]
- 3. Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140(6):845‐858. [DOI] [PubMed] [Google Scholar]
- 4. Jiménez‐Gallo D, de la Varga‐Martínez R, Ossorio‐García L, Albarrán‐Planelles C, Rodríguez C, Linares‐Barrios M. The Clinical Significance of Increased Serum Proinflammatory Cytokines, C‐Reactive Protein, and Erythrocyte Sedimentation Rate in Patients with Hidradenitis Suppurativa. Mediators Inflamm. 2017;2017:2450401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kimball AB, Sundaram M, Gauthier G, et al. The Comorbidity Burden of Hidradenitis Suppurativa in the United States: A Claims Data Analysis. Dermatol Ther (Heidelb). 2018;8(4):557‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matusiak Ł, Jemec GB, Szepietowski JC. Pharmacological development in hidradenitis suppurativa. Curr Opin Pharmacol. 2019;46:65‐72. [DOI] [PubMed] [Google Scholar]
- 7. Di Cesare A, Di Meglio P, Nestle FO. The IL‐23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129(6):1339‐1350. [DOI] [PubMed] [Google Scholar]
- 8. Toussirot É, Aubin F. Paradoxical reactions under TNF‐ α blocking agents and other biological agents given for chronic immune‐ mediated diseases : an analytical and comprehensive overview. RMD Open. 2016;1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Megna M, Balato A, Raimondo A, Balato N. Guselkumab for the treatment of psoriasis. Expert Opin Biol Ther. 2018;18(4):459‐468. [DOI] [PubMed] [Google Scholar]
- 10. Liu L, Lu J, Allan BW, et al. Generation and characterization of ixekizumab, a humanized monoclonal antibody that neutralizes interleukin‐17A. J Inflamm Res. 2016;9:39‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kovacs M, Podda M. Guselkumab in the treatment of severe hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2019;33(3):e140‐e141. [DOI] [PubMed] [Google Scholar]
- 12. Casseres RG, Kahn JS, Her MJ, Rosmarin D. Guselkumab in the treatment of hidradenitis suppurativa: A retrospective chart review. J Am Acad Dermatol. 2019;81(1):265‐267. [DOI] [PubMed] [Google Scholar]
- 13. Berman HS, Villa NM, Shi VY, Hsiao JL. Guselkumab in the treatment of concomitant hidradenitis suppurativa, psoriasis, and Crohn’s disease. J Dermatol Treat. 2019;1‐3. [DOI] [PubMed] [Google Scholar]
- 14. Kearney N, Byrne N, Kirby B, Hughes R. Successful use of Guselkumab in the treatment of severe hidradenitis suppurativa. Clin Exp Dermatol. 2020;45(5):618‐619. [DOI] [PubMed] [Google Scholar]
- 15. Montero‐Vilchez T, Martinez‐Lopez A, Salvador‐Rodriguez L, Arias‐Santiago S, Molina‐Leyva A. The use of guselkumab 100mg every 4weeks on patients with hidradenitis suppurativa and a literature review. Dermatol Ther. 2020;33(3):1–4. [DOI] [PubMed] [Google Scholar]


