Abstract
Purpose
The aims of this study were to associate sperm kinematics and standard semen parameters with sperm DNA damage and to evaluate whether the addition of sperm kinematics improve the multivariable prediction of sperm DNA fragmentation compared to standard semen parameters alone.
Materials and Methods
We evaluated sperm kinematics, standard semen parameters, and DNA fragmentation index (DFI) in 122 men. Univariate and multivariate logistic regression models were fitted to evaluate the association of sperm kinematics and standard semen parameters with pathologically damaged sperm DNA (DFI≥26%), and receiver operating characteristics (ROC) curves were calculated for these models.
Results
On univariate analyses, average velocity, curvilinear velocity, straight-line velocity, straightness (STR), beat-cross frequency (BCF), and the percentage of progressive motile sperm cells (PPMS) were significantly associated with pathologically damaged sperm DNA. Likewise, among standard semen parameters, sperm concentration, progressive motility, normal morphology, and vitality were found to be linked with sperm DNA damage. On the multivariate analysis, vitality was the strongest predictor of pathologically damaged sperm DNA with an area under the ROC curve (AUROC) of 88.3%. Adding STR, BCF, and PPMS to vitality increased the AUROC to the significant extent of 91.5%.
Conclusions
Sperm vitality is the most accurate routine-based laboratory test for the prediction of pathologically damaged sperm DNA, but the addition of sperm kinematics increases its accuracy. Both standard semen parameters and sperm kinematics are complementary in predicting pathologically damaged sperm DNA, and might serve as a new tool to screen for fertile men.
Keywords: Computer-assisted semen analysis, Sperm DNA damage, Sperm motility, Standard semen parameters
INTRODUCTION
The assessment of the detailed movement characteristics of spermatozoa is one of the major features of the computer-aided sperm analysis (CASA) system [1]. Apart from widely used conventional motility parameters in clinical laboratories, the CASA system is capable of measuring sperm kinematics composed of sperm velocity values, sperm velocity ratios, and sperm wobble features [2].
Due to the increasing need of automation for objective and rapid analyses of a large number of spermatozoa in andrological laboratories, the use of CASA machines has increased worldwide since their introduction in mid-1980s [2]. However, the clinical application of sperm kinematic characteristics is a matter of intense and ongoing discussion. Indeed, it has been shown that sperm kinematic values, such as curvilinear velocity (VCL), straight-line velocity (VSL), average path velocity (VAP), and amplitude of lateral head displacement (ALH) are capable of predicting the outcome of in vitro fertilization (IVF) as well as intrauterine insemination (IUI) [3,4,5]. Moreover, lower levels of VCL, ALH, and straightness (STR) were reported in tobacco- and heavy metal-exposed patients [6]. VCL, ALH, and linearity (LIN) were also associated with hyperactivated motility, which enables spermatozoa to penetrate the fallopian tube and fertilize the oocyte [7]. Despite decades of research, however, a consensus concerning the use of CASA parameters and reference values has not been reached yet.
It is thought that sperm DNA damage is an important predictor of male fertility. Studies indicate that sperm DNA impairment is associated with both worse pregnancy rates in natural conception and worse outcomes in assisted reproduction, such as fertilization, blastulation, pregnancy, and live-birth rates [8]. While reports on the relationship of sperm kinematic parameters and sperm DNA impairment are scarce and not comparable owing to different study designs [9,10], the association of standard semen parameters with sperm DNA integrity was investigated in several studies with discrepant outcomes [11,12,13,14]. Although some authors support the concept that DNA impairment is ascribed to the compromised conventional sperm parameters [12,13], DNA fragmentation is regarded as an independent measure of male fertility, irrespective of conventional sperm parameters [11,14].
Due to the few numbers of studies and inconclusive results concerning the association of sperm kinematics and standard semen parameters with sperm DNA damage, we conducted this study to evaluate the relationship of sperm kinematics and standard semen parameters with sperm DNA damage and to examine whether the addition of sperm kinematics improve the multivariable prediction of sperm DNA fragmentation compared to standard semen parameters alone.
MATERIALS AND METHODS
1. Study population and semen samples
A cross-sectional study from subfertile men attending the Andrology Clinic located at the Urology Department of Krankenhaus Hietzing mit Neurologischem Zentrum Rosenhügel between May 2016 and April 2018 was performed. Patient recruitment was done at two stages. First, subfertile men without clinical signs or symptoms of genitourinary infection were screened at the Andrology Clinic located at the Urology Department of Krankenhaus Hietzing mit Neurologischem Zentrum Rosenhügel, and subsequently referred to the Andrology Laboratory, where semen analysis was carried out. At this stage, semen samples exhibiting azoospermia and cryptozoospermia were excluded (n=21). Ultimately, a cohort of 122 patients was included in the study. Semen samples were obtained in a sterile container by masturbation at the hospital after a period of sexual abstinence of 2 to 7 days, and subsequently examined in the laboratory after liquefaction at 37℃.
2. Ethics statement
The present study protocol was reviewed and approved by the institutional review board of municipal department 15–the ethics committee of the city of Vienna (EK 15-112-VK). Informed consent was submitted by all subjects when they were enrolled.
3. Sperm kinematics and computer-aided sperm analysis
Sperm kinematic values were assessed using the CASA system according to the World Health Organization (WHO) laboratory manual for semen examination [1], and included VCL, VSL, VAP, ALH, LIN (VSL/VCL), STR (VSL/VAP), beat-cross frequency (BCF), and the percentage of progressive motile sperm cells (PPMS), defined as the percentage of motile spermatozoa having VAP >25 µm/s and STR >80%.
In order to determine sperm kinematics by the IVOS analyzer (ver. 12.3; IVOS Analyzer; Hamilton Thorne Inc., Beverly, MA, USA), a 7 µL semen sample was loaded into a disposable Leja® chamber (Leja Products B.V., Nieuw Vennep, The Netherlands) with a depth of 20 µm. The chamber was then placed on the prewarmed plate of the IVOS and analysis was performed on 20 consecutive fields from each sample. The median of counted sperm cells was 350.5. The following settings were used to analyze semen samples according to the manufacturer's instructions: the number of the frames captured within one second was 60 and the number of the images captured for analysis was 30. The minimum contrast and cells size for the detection of sperm cells was 80 and 3 pixels, respectively. The default values for identifying static sperm cells, when less than five cells in the field were motile, were 6 pixels and 160 for cells size and cell intensity, respectively. The cutoff values for slow motility were 5 µm/s for VAP and 11 µm/s for VSL, meaning sperm cells with both VAP>5 µm/s and VSL>11 µm/s were classified as progressive motile.
4. Standard semen parameters
Standard semen parameters were evaluated according to WHO criteria [1] and included sperm concentration, total sperm number, progressive motility, normal morphology, and vitality. Sperm concentration and progressive motility were assessed using the IVOS analyzer. Total sperm number was obtained by multiplying the sample volume by the sperm concentration. Sperm morphology was determined by means of a Diff-Quik staining set (Medion Diagnostics AG, Düdingen, Switzerland). VitalScreen™ (FertiPro N.V., Beernem, Belgium) was used to evaluate sperm vitality based on the eosin-nigrosin staining technique, in which dead spermatozoa with damaged sperm membrane take up the eosin and stain red. Nigrosin provides a dark background, facilitating the assessment of slides.
5. Analysis of DNA fragmentation
Sperm DNA fragmentation was analyzed by the sperm chromatin dispersion (SCD) test commercially produced as the halosperm® G2 kit (Halotech DNA, S.L., Madrid, Spain) according to manufacturer's instruction and expressed as DNA fragmentation index (DFI). To avoid inaccuracies in the measurement of DNA fragmentation after chilling or cryopreservation [15], only fresh liquefied semen samples were considered for the assessment of DNA fragmentation. In brief, an aliquot of semen sample was diluted to a maximum of 20 million sperms per milliliter in phosphate buffer saline and 50 µL of the diluted semen sample was then added to the melted agarose. Of the semen-agarose mix, 8 µL was pipetted onto a slide precoated with agarose and covered with a 22-×22-mm coverslip. The slide was placed on a cold surface for 5 minutes. The coverslip was gently removed and an acid denaturant was applied onto the slide for 7 minutes. Subsequently, the slide was drained and covered with a lysing solution for 20 minutes. Following a 5 minutes wash in a tray with abundant distilled water, the slide was dehydrated in increasing concentrations of ethanol (70% and 100%) for 2 minutes each before air drying and exposing to the Diff-Quik™ staining set. To determine the DFI, 500 spermatozoa per sample were examined using conventional bright-field microscopy at ×1,000 magnification. Nucleoids with small halo, without halo and degraded nucleoids without halo were representative of spermatozoa containing fragmented DNA.
6. Statistical analysis
Statistical analysis was performed using the freely available R statistical package (The R Project for Statistical Computing, Vienna, Austria). For all analyses, pathologically damaged sperm DNA was defined as a DFI ≥26% [16,17,18]. Univariate and multivariate logistic regression models were fitted to evaluate the association of sperm kinematics and standard semen parameters with pathologically damaged sperm DNA, and receiver operating characteristics (ROC) curves were calculated for these models. Statistically significant variables on univariate analysis (p<0.05) were assessed on multivariate analysis, which followed a backward stepwise elimination according to the likelihood ratio criterion (inclusion/exclusion criteria: p≤0.05/p>0.1). The final model contained only statistically significant variables. Areas under the ROC curve (AUROC) and 95% confidence intervals (CI) were obtained. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated at the optimal cutoff of the model according to the Youden index.
RESULTS
A descriptive data of all variables, including standard semen parameters, sperm kinematics, and age, is presented in the Table 1.
Table 1. Characteristics of the 122 patients included in the study.
| Variable | Value |
|---|---|
| Age (y) | 37 (31–42) |
| Sperm concentration (×106/mL) | 53.6 (25.8–107.3) |
| Total sperm number (×106/ejaculate) | 189 (84.4–326.7) |
| Progressive motility (%) | 34 (19–50) |
| Normal morphology (%) | 6.5 (3–14) |
| Vitality (%) | 70 (58–77) |
| VCL (μm/s) | 76.1 (67.4–85.2) |
| VSL (μm/s) | 37.6 (30.5–43.3) |
| VAP (μm/s) | 45.3 (38.1–50.7) |
| ALH (µm) | 3.7 (3.3–4.2) |
| LIN (%) | 50 (44–53) |
| STR (%) | 82 (76–85) |
| BCF (Hz) | 25.3 (22.7–27.8) |
| PPMS (%) | 19 (9–31) |
| DFI (%) | 14.2 (7.8–24.6) |
Values are presented as median (interquartile range).
VCL: curvilinear velocity, VSL: straight-line velocity, VAP: average path velocity, ALH: amplitude of lateral head displacement, LIN: linearity, STR: straightness, BCF: beat-cross frequency, PPMS: percentage of progressive motile sperm cells (VAP>25 µm/s and STR>80%), DFI: DNA fragmentation index.
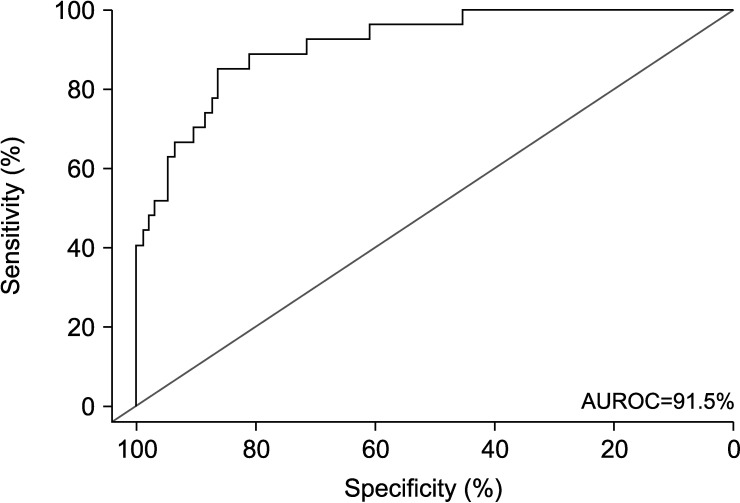
Twenty-seven of the 122 patients (22.1%) had pathologically damaged sperm DNA (i.e., DFI≥26%). On univariate analyses (Table 2), sperm kinematic parameters VCL (odds ratio [OR]: 0.93; p<0.001), VSL (OR: 0.92; p=0.001), VAP (OR: 0.90; p=0.001), STR (OR: 0.96; p=0.048), BCF (OR: 0.90; p=0.018), and PPMS (OR: 0.87; p<0.001) were all significantly associated with pathologically damaged sperm DNA. Likewise, among standard semen parameters, sperm concentration (OR: 0.99; p=0.01), progressive motility (OR: 0.91; p<0.001), normal morphology (OR: 0.88; p=0.005), and vitality (OR: 0.89; p<0.001) were found to be linked with sperm DNA damage. On multivariate analyses (Table 2), STR (OR: 1.08; p=0.042), BCF (OR: 0.86; p=0.038), PPMS (OR: 0.89; p=0.014), and vitality (OR: 0.91; p<0.001) remained to be independently associated with pathologically damaged sperm DNA. Subsequently, ROC curve analyses based on the logistic regression models were performed. Among the significant parameters vitality was the strongest predictor of pathologically damaged sperm DNA, with an AUROC of 88.3% (95% CI: 81.5%–95%). Using an optimal cutoff of 60% sperm vitality had a sensitivity of 77.8%, a specificity of 84.2%, a PPV of 60.0%, and a NPV of 93.0% to predict pathologically damaged sperm DNA (Fig. 1). Adding STR, BCF, and PPMS to vitality increased the AUROC to 91.5% (95% CI: 85.7%–97.2%). At the optimal cutoff, the sensitivity, specificity, PPV, and NPV of the multivariate model containing both sperm kinematics and vitality was 85.2%, 86.3%, 63.8%, and 95.3%, respectively (Fig. 2).
Table 2. Univariate and multivariate logistic regression analyses for prediction of pathologically damaged sperm DNA in 122 patients.
| Parameter | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | Rank | OR (95% CI) | p-value | |
| Age (y) | 1.09 (1.02–1.17) | 0.017 | 7 | 1.07 (0.98–1.17) | 0.133 |
| Sperm concentration (×106/mL) | 0.99 (0.98–0.997) | 0.01 | 6 | 0.99 (0.98–1.00) | 0.113 |
| Total sperm number (×106/ejaculate) | 1.00 (0.997–1.001) | 0.367 | |||
| Progressive motility (%) | 0.91 (0.87–0.94) | <0.001 | 2 | 1.03 (0.90–1.18) | 0.68 |
| Normal morphology (%) | 0.88 (0.80–0.96) | 0.005 | 4 | 1.12 (0.95–1.31) | 0.178 |
| Vitality (%) | 0.89 (0.85–0.93) | <0.001 | - | 0.91 (0.86–0.96) | <0.001 |
| VCL (μm/s) | 0.93 (0.89–0.97) | <0.001 | 5 | 0.96 (0.89–1.03) | 0.231 |
| VSL (μm/s) | 0.92 (0.87–0.96) | 0.001 | 1 | 0.99 (0.61–1.62) | 0.974 |
| VAP (μm/s) | 0.90 (0.85–0.96) | 0.001 | 3 | 1.18 (0.88–1.59) | 0.269 |
| ALH (µm) | 0.66 (0.42–1.05) | 0.078 | |||
| LIN (%) | 0.97 (0.93–1.01) | 0.125 | |||
| STR (%) | 0.96 (0.92–0.99) | 0.048 | - | 1.08 (1.01–1.17) | 0.042 |
| BCF (Hz) | 0.90 (0.83–0.98) | 0.018 | - | 0.86 (0.75–0.99) | 0.038 |
| PPMS (%) | 0.87 (0.82–0.93) | <0.001 | - | 0.89 (0.81–0.98) | 0.014 |
Statistically significant parameters from the univariate analysis were included in the multivariate analysis. A backward selection approach was used for the multivariate analysis, and the column “rank” indicates the rank at the time of removal (i.e., rank 1=first variable removed from the model).
OR: odds ratio, CI: confidence interval, VCL: curvilinear velocity, VSL: straight-line velocity, VAP: average path velocity, ALH: amplitude of lateral head displacement, LIN: linearity, STR: straightness, BCF: beat-cross frequency, PPMS: percentage of progressive motile sperm cells (VAP>25 µm/s and STR>80%).
Fig. 1. Receiver operating characteristics (ROC) curve of sperm vitality to predict pathologically damaged sperm DNA. Vitality yields an area under the ROC curve (AUROC) of 88.3% (95% confidence interval, 81.5%–95%), a sensitivity of 77.8%, and a specificity of 84.2%.
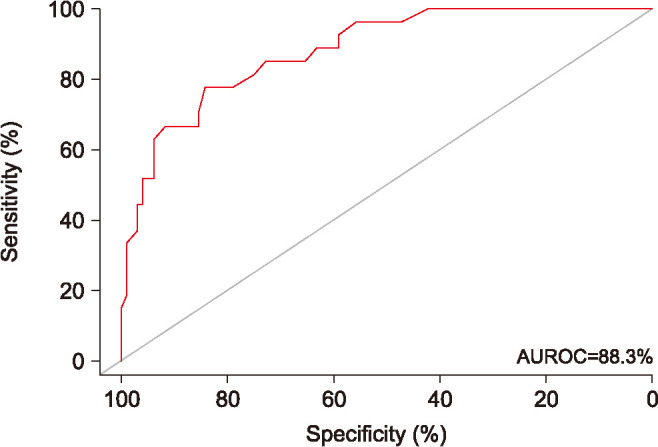
Fig. 2. Receiver operating characteristics (ROC) curve of the multivariate model to predict pathologically damaged sperm DNA, and included straightness (STR), beat-cross frequency (BCF), percentage of progressive motile sperm cells (PPMS), and vitality. The addition of STR, BCF, PPMS to vitality yields an area under the ROC curve (AUROC) of 91.5% (95% confidence interval, 85.7%–97.2%), a sensitivity of 85.2%, and a specificity of 86.3%.
DISCUSSION
Our results show that VAP, VCL, and VSL is associated with pathologically damaged sperm DNA. These results are in line with studies claiming VAP, VCL, and VSL to be the predictors of IVF and IUI outcome [3,4,5], as sperm cells having higher VAP, VCL, and VSL are associated with lower sperm DNA fragmentation and thereby higher fertility potential. The relation of VCL and VSL with sperm DNA fragmentation in our study is in line with the findings of Moskovtsev et al [10]; however, we found no significant association of LIN and ALH with DNA fragmentation rates. This might be due to different algorithms used by different CASA systems to calculate sperm kinematic parameters [1]. Instead, our results showed a significant relationship of average path linearity (STR) and average path intersections by the curvilinear path (BCF) with sperm DNA fragmentation. In agreement with our finding, Cohen-Bacrie et al [12] reported no correlation between ALH and fragmented sperm DNA. Sivanarayana et al [19] have also demonstrated significantly increased levels of DNA fragmentation in abnormal patient groups with lower levels of VAP, VCL, and VSL. Moreover, our results revealed that the PPMS characterized by both minimum VAP and STR of 25 µm/s and 80%, respectively, along with VCL had the most significant associations with sperm DNA fragmentation among sperm kinematics.
This study shows that conventional semen parameters except total sperm number were associated significantly with sperm DNA damage. Sperm vitality and progressive motility exhibited the most significant associations, which could be explained by the significant role of epididymal function in sperm pathology. Due to long exposure to overproduced reactive oxygen species (ROS) at epididymal level by both leukocytic and non-leukocytic cells, such as immature spermatozoa [20], epididymal epithelial cells [21] and spermiophages [22], ROS-induced sperm membrane peroxidation results in loss of sperm motility, membrane integrity, and consequently, loss of sperm vitality [23,24,25]. Moreover, ROS capable of penetrating sperm membrane can impair sperm DNA integrity either directly by attacking DNA backbone and by forming abasic sites and DNA adducts or indirectly by triggering the apoptotic cascade through the activation of caspases and endonucleases [26].
Sperm vitality, i.e., the evaluation of the percentage of dead and live sperm cells based on resistance of intact sperm membrane to take up certain stains [1], is technically simple and cost-efficient laboratory testing suitable for routine diagnostic use. It was worth noting that sperm vitality was the strongest predictor of sperm DNA fragmentation among all measured parameters. Indeed, this parameter alone was highly accurate with an AUROC of 88.3%. In agreement with this finding, Samplaski et al [27] concluded that DFI testing may not be necessary in men with high (≥75%) and low (≤30%) levels of sperm vitality. In addition, our results support the results of Brahem et al [24], who found high levels of sperm DNA fragmentation in patients with necrozoospermia, a condition in which the proportion of non-viable spermatozoa in the ejaculate is increased significantly to more than 42%.
Apart from vitality, sperm kinematic parameters BCF, STR, and PPMS were identified as significant predictors of sperm DNA fragmentation. Indeed, the combination of sperm vitality with BCF, STR and PPMS increased the AUROC to a significant extent from 88.3% to 91.5%. Thus, sperm kinematics can be complementary to standard semen parameters, specifically to vitality, for predicting sperm DNA fragmentation more accurately. Owing to the increasing application of CASA machines in andrological laboratories, the use of sperm kinematics in combination with conventional semen parameters to precisely detect semen samples with high level of DNA integrity and thereby high fertility potential might serve as a new screening tool. Two major obstacles must be overcome to achieve that, first, the introduction of reference values for sperm kinematics using larger patient populations, and second, the standardization of CASA machines to minimize the differences in calculating sperm kinematic parameters in order to achieve comparable outcomes between andrological laboratories.
Male age was associated with sperm DNA fragmentation in our study, although it was not significant on the multivariate analysis. This result corroborates the findings of authors who associated male aging with compromised sperm DNA integrity [28].
Our study has several limitations. First and foremost, sperm DNA fragmentation was determined by SCD test. Using more precise methods based on flow cytometric measurements including the sperm chromatin structure assay (SCSA) [15] as well as the terminal deoxynucleotidyl transferase-mediated fluorescein-dUTP nick end labelling (TUNEL) assay [8] might have yielded different results. Moreover, the outcome of our study is dependent on the cutoff value used for the determination of pathologically damaged sperm DNA. We adopted the threshold value of DFI ≥26% for our study, as this was shown to be a powerful discriminator between fertile and infertile men [18]. This cutoff was also found to be the best predictor of pregnancy after assisted reproduction treatment [16,17]. However, it is noteworthy that alternative methods such as TUNEL assay [8] and SCSA [11,29] yielded the different cutoffs of 16.8% and 30%, respectively. Finally, we did not measure ROS and we were not able to account for smoking status as this was not well documented. Both excessive ROS [25] and smoking [30] can impact sperm quality, and these data could have contributed to interpret the outcome of the study.
CONCLUSIONS
Sperm vitality is the most accurate routine-based laboratory test for the prediction of pathologically damaged sperm DNA, but the addition of sperm kinematics increases its accuracy. Both standard semen parameters and sperm kinematics are complementary in predicting pathologically damaged sperm DNA, and might serve as a new tool to screen for fertile men.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
- Conceptualization: AA, HP.
- Data curation: AA.
- Formal analysis: WH, TK.
- Investigation: AA.
- Methodology: AA.
- Project administration: AA.
- Supervision: HP, TK.
- Validation: AA.
- Visualization: WH, TK.
- Writing—original draft: AA.
- Writing—review & editing: WH, HP, TK.
Data Sharing Statement
The data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
References
- 1.World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: World Health Organization; 2010. [Google Scholar]
- 2.Lu JC, Huang YF, Lü NQ. Computer-aided sperm analysis: past, present and future. Andrologia. 2014;46:329–338. doi: 10.1111/and.12093. [DOI] [PubMed] [Google Scholar]
- 3.Hirano Y, Shibahara H, Obara H, Suzuki T, Takamizawa S, Yamaguchi C, et al. Relationships between sperm motility characteristics assessed by the computer-aided sperm analysis (CASA) and fertilization rates in vitro. J Assist Reprod Genet. 2001;18:213–218. doi: 10.1023/A:1009420432234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shibahara H, Obara H, Ayustawati, Hirano Y, Suzuki T, Ohno A, et al. Prediction of pregnancy by intrauterine insemination using CASA estimates and strict criteria in patients with male factor infertility. Int J Androl. 2004;27:63–68. doi: 10.1111/j.0105-6263.2004.00437.x. [DOI] [PubMed] [Google Scholar]
- 5.Fréour T, Jean M, Mirallié S, Dubourdieu S, Barrière P. Computer-Assisted Sperm Analysis (CASA) parameters and their evolution during preparation as predictors of pregnancy in intrauterine insemination with frozen-thawed donor semen cycles. Eur J Obstet Gynecol Reprod Biol. 2010;149:186–189. doi: 10.1016/j.ejogrb.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 6.Mukhopadhyay D, Varghese AC, Nandi P, Banerjee SK, Bhattacharyya AK. CASA-based sperm kinematics of environmental risk factor-exposed human semen samples designated as normozoospermic in conventional analysis. Andrologia. 2010;42:242–246. doi: 10.1111/j.1439-0272.2009.00984.x. [DOI] [PubMed] [Google Scholar]
- 7.Mortimer ST. CASA: practical aspects. J Androl. 2000;21:515–524. [PubMed] [Google Scholar]
- 8.Panner Selvam MK, Agarwal A. A systematic review on sperm DNA fragmentation in male factor infertility: laboratory assessment. Arab J Urol. 2018;16:65–76. doi: 10.1016/j.aju.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irvine DS, Twigg JP, Gordon EL, Fulton N, Milne PA, Aitken RJ. DNA integrity in human spermatozoa: relationships with semen quality. J Androl. 2000;21:33–44. [PubMed] [Google Scholar]
- 10.Moskovtsev SI, Willis J, White J, Mullen JB. Sperm DNA damage: correlation to severity of semen abnormalities. Urology. 2009;74:789–793. doi: 10.1016/j.urology.2009.05.043. [DOI] [PubMed] [Google Scholar]
- 11.Evenson DP, Jost LK, Marshall D, Zinaman MJ, Clegg E, Purvis K, et al. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod. 1999;14:1039–1049. doi: 10.1093/humrep/14.4.1039. [DOI] [PubMed] [Google Scholar]
- 12.Cohen-Bacrie P, Belloc S, Ménézo YJ, Clement P, Hamidi J, Benkhalifa M. Correlation between DNA damage and sperm parameters: a prospective study of 1,633 patients. Fertil Steril. 2009;91:1801–1805. doi: 10.1016/j.fertnstert.2008.01.086. [DOI] [PubMed] [Google Scholar]
- 13.Varghese AC, Bragais FM, Mukhopadhyay D, Kundu S, Pal M, Bhattacharyya AK, et al. Human sperm DNA integrity in normal and abnormal semen samples and its correlation with sperm characteristics. Andrologia. 2009;41:207–215. doi: 10.1111/j.1439-0272.2009.00917.x. [DOI] [PubMed] [Google Scholar]
- 14.Giwercman A, Lindstedt L, Larsson M, Bungum M, Spano M, Levine RJ, et al. Sperm chromatin structure assay as an independent predictor of fertility in vivo: a case-control study. Int J Androl. 2010;33:e221–e227. doi: 10.1111/j.1365-2605.2009.00995.x. [DOI] [PubMed] [Google Scholar]
- 15.Evenson DP. The Sperm Chromatin Structure Assay (SCSA(®)) and other sperm DNA fragmentation tests for evaluation of sperm nuclear DNA integrity as related to fertility. Anim Reprod Sci. 2016;169:56–75. doi: 10.1016/j.anireprosci.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Gosálvez J, Caballero P, López-Fernández C, Ortega L, Guijarro JA, Fernández JL, et al. Can DNA fragmentation of neat or swim-up spermatozoa be used to predict pregnancy following ICSI of fertile oocyte donors? Asian J Androl. 2013;15:812–818. doi: 10.1038/aja.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.López G, Lafuente R, Checa MA, Carreras R, Brassesco M. Diagnostic value of sperm DNA fragmentation and sperm high-magnification for predicting outcome of assisted reproduction treatment. Asian J Androl. 2013;15:790–794. doi: 10.1038/aja.2013.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiweko B, Utami P. Predictive value of sperm deoxyribonucleic acid (DNA) fragmentation index in male infertility. Basic Clin Androl. 2017;27:1. doi: 10.1186/s12610-016-0046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sivanarayana T, Krishna ChR, Prakash GJ, Krishna KM, Madan K, Rani BS, et al. CASA derived human sperm abnormalities: correlation with chromatin packing and DNA fragmentation. J Assist Reprod Genet. 2012;29:1327–1334. doi: 10.1007/s10815-012-9885-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ollero M, Gil-Guzman E, Lopez MC, Sharma RK, Agarwal A, Larson K, et al. Characterization of subsets of human spermatozoa at different stages of maturation: implications in the diagnosis and treatment of male infertility. Hum Reprod. 2001;16:1912–1921. doi: 10.1093/humrep/16.9.1912. [DOI] [PubMed] [Google Scholar]
- 21.Sakkas D, Alvarez JG. Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril. 2010;93:1027–1036. doi: 10.1016/j.fertnstert.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 22.Pelliccione F, D'Angeli A, Cordeschi G, Mihalca R, Ciociola F, Necozione S, et al. Seminal macrophages in ejaculates from men with couple infertility. Int J Androl. 2009;32:623–628. doi: 10.1111/j.1365-2605.2008.00909.x. [DOI] [PubMed] [Google Scholar]
- 23.Tremellen K. Oxidative stress and male infertility: a clinical perspective. Hum Reprod Update. 2008;14:243–258. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 24.Brahem S, Jellad S, Ibala S, Saad A, Mehdi M. DNA fragmentation status in patients with necrozoospermia. Syst Biol Reprod Med. 2012;58:319–323. doi: 10.3109/19396368.2012.710869. [DOI] [PubMed] [Google Scholar]
- 25.Ko EY, Sabanegh ES, Jr, Agarwal A. Male infertility testing: reactive oxygen species and antioxidant capacity. Fertil Steril. 2014;102:1518–1527. doi: 10.1016/j.fertnstert.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 26.Aitken RJ, De Iuliis GN, McLachlan RI. Biological and clinical significance of DNA damage in the male germ line. Int J Androl. 2009;32:46–56. doi: 10.1111/j.1365-2605.2008.00943.x. [DOI] [PubMed] [Google Scholar]
- 27.Samplaski MK, Dimitromanolakis A, Lo KC, Grober ED, Mullen B, Garbens A, et al. The relationship between sperm viability and DNA fragmentation rates. Reprod Biol Endocrinol. 2015;13:42. doi: 10.1186/s12958-015-0035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moskovtsev SI, Willis J, Mullen JB. Age-related decline in sperm deoxyribonucleic acid integrity in patients evaluated for male infertility. Fertil Steril. 2006;85:496–499. doi: 10.1016/j.fertnstert.2005.05.075. [DOI] [PubMed] [Google Scholar]
- 29.Spanò M, Bonde JP, Hjøllund HI, Kolstad HA, Cordelli E, Leter G. Sperm chromatin damage impairs human fertility. The Danish First Pregnancy Planner Study Team. Fertil Steril. 2000;73:43–50. doi: 10.1016/s0015-0282(99)00462-8. [DOI] [PubMed] [Google Scholar]
- 30.Antoniassi MP, Intasqui P, Camargo M, Zylbersztejn DS, Carvalho VM, Cardozo KH, et al. Analysis of the functional aspects and seminal plasma proteomic profile of sperm from smokers. BJU Int. 2016;118:814–822. doi: 10.1111/bju.13539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.