Abstract
Purpose
Seminal plasma provides a nutritive and protective milieu for spermatozoa. It contains factors/proteins required for sperm maturation, hyperactivation, capacitation and acrosome reaction. Alteration in the expression levels of seminal plasma proteins affect the fertilization process. The main objective of this study is to compare the seminal plasma proteome of healthy fertile men (control group) with varicocele patients in order to identify the differentially expressed seminal plasma proteins.
Materials and Methods
Pooled seminal plasma samples from control (n=5) and varicocele (unilateral: n=5 and bilateral: n=5) subjects were used for proteomic profiling and functional bioinformatic analysis. Key differentially expressed proteins (DEPs) associated with binding of zona pellucida (acrosin; ACR), protein folding (heat shock related 70 kDa protein 2; HSPA2), oxidative stress (peroxiredoxin 2; PRDX2), lipid peroxidation and DNA fragmentation (apolipoprotein A2; APOA2) were validated by Western blot. Statistical analysis was conducted using Mann-Whitney test.
Results
A total of 412 and 486 proteins were detected in seminal plasma of control group and varicocele patients respectively. Twenty-eight proteins were identified as DEPs between varicocele and control group. Validation of DEPs revealed downregulation of HSPA2 (p=0.0037) as well as APOA2 (p=0.0373), and upregulation of PRDX2 (p=0.0474).
Conclusions
The seminal plasma protein profile of varicocele patients differ from healthy fertile men. Aberrant expression of seminal plasma proteins serve as an indicator of sperm pathology in varicocele patients.
Keywords: Infertility, male; Proteomics; Seminal plasma; Varicocele
INTRODUCTION
Varicocele is an abnormal physical condition associated with the dilatation of pampiniform plexus that affects 15% of healthy men. It accounts for 35% and 80% of men with primary and secondary infertility, respectively [1]. Infertile men with varicocele have poor semen quality, seminal oxidative stress and sperm DNA damage [2]. Clinically, the main focus is to treat patients with varicocele having poor semen quality. Although varicocele repair could help to improve semen quality and reproductive outcomes, the information about the subcellular changes in the spermatozoa of these patients are limited.
The molecular changes associated with defective spermatozoa are predominantly due to alterations in the expression levels of their proteins. The transcriptionally silent spermatozoa depends on translated proteins to perform their biological functions [3]. Our previous reports demonstrated an altered expression of key sperm proteins associated with spermatogenesis, sperm motility, capacitation, hyperactivation and zona pellucida binding, and mitochondrial function in varicocele patients [4,5,6]. Although the cause of sperm dysfunction in varicocele patients has been explained via a sperm proteomic approach [6,7], it is also crucial to investigate the possible differential expression of seminal plasma proteins as they are important for normal sperm function [7]. The seminal plasma provides a protective environment to the spermatozoa and their proteins play a major role in the fertilization process by initiating capacitation and acrosome reaction essential for sperm-oocyte interaction [7,8]. It also harbors approximately 30% of the sperm proteins, which reflects the functional state of the spermatozoa [9]. Majority of the secretions are from testis, cauda epididymis and the accessory sexual glands. In varicocele patients, pathophysiological state, compromised testicular function and epididymal dysfunction may change the composition of the seminal plasma [10,11].
So far, very few studies were focused on the seminal plasma proteomics pertaining to varicocele condition [12,13,14,15]. Camargo et al [14], reported that the nitric oxide metabolism was enhanced in the seminal plasma of varicocele patients. Expression of seminal plasma proteins related to spermatogenesis, sperm-binding activity, inflammatory response, proliferative activity and apoptosis regulation were altered in adolescents with varicocele [16,17]. However, these studies did not report the changes in the sperm proteome. The present study is an extension of our sperm proteomics [6]. We have compared the seminal plasma proteome profile of varicocele patient with healthy fertile donors to identify the differentially expressed proteins (DEPs). The main objective was to evaluate the seminal plasma proteome profile and correlate the DEPs with sperm pathology in varicocele patients.
MATERIALS AND METHODS
1. Ethics statement
The present study involves human subject participation and was approved by the Institutional Review Board (IRB) of Cleveland Clinic (IRB # 17-422), Cleveland, Ohio, United States. Informed consent was obtained by all subjects when they were enrolled.
2. Study subjects
Semen samples were obtained from 10 healthy fertile men without varicocele (control group), and 50 varicocele patients (33 unilateral and 17 bilateral) of age 20 to 40 years. Patients who attended the clinic for infertility treatment from March 2012 to March 2014 were consented and enrolled in this study [6]. Clinical examination, patient history and semen analysis results were considered for sample selection. The clinical diagnosis of varicocele was done by genital examination and scrotal palpation performed by the physician. The control group included men who had initiated pregnancy or fathered a child in the last two years. The sperm proteome of these subjects were analyzed and reported earlier [6]. In the present study, seminal plasma from grade 1 to 3 varicocele patients were used for proteomic analysis. The study was conducted in compliance with the Minimum Information about a Proteomics Experiment (MIAPE) guidelines of the Human Proteome Organization's Proteomics Standards Initiative (HUPO-PSI) for reporting proteomics studies [18].
3. Inclusion and exclusion criteria
Inclusion criteria for control group included healthy men with no varicocele, no chronic diseases, normal genital examination and who had initiated pregnancy or fathered a child in the last two years.
Exclusion criteria for varicocele group included infertility of the female partner, genetic defects (Klinefelter syndrome, Y chromosome micro deletion and cystic fibrosis with congenital absence of the vas deferens), chronic prostatitis, and reproductive tract infection.
Additional exclusion criteria for both the study groups included leukocytospermia (Endtz positive), azoospermia and oligozoospermia (<106 sperm/mL), history of systemic illness, inflammation of reproductive tract (orchitis, epididymitis, urethritis, and testicular atrophy), sexually transmitted disease, smoking, and medications.
4. Semen analysis
Following 48 hours of sexual abstinence, semen samples were collected by masturbation at the Andrology Laboratory, Cleveland Clinic. Samples were allowed to liquefy completely for 20 minutes at 37℃ and routine semen analysis was performed according to World Health Organization guidelines [19]. Reactive oxygen species (ROS) levels and sperm DNA fragmentation (SDF) were measured using chemiluminescence assay and terminal deoxynucleotidyl transferase dUTP nickend labeling (TUNEL) assay, respectively [20]. Semen samples were centrifuged for 7 minutes at 1,000g, and clear seminal plasma was aspirated and stored at −80℃ for proteomic analysis.
5. Preparation of samples for proteomic studies
Seminal plasma samples were thawed at room temperature and centrifuged at 3,000g for 30 minutes to completely remove contaminating spermatozoa and somatic cells. Protein concentration was determined using a bicinchoninic acid kit (Thermo, Rockford, IL, USA). Pooled samples from varicocele group (unilateral, n=5 & bilateral, n=5) and control group (n=5) were used for proteomic analysis. Equal concentration of protein from each individual sample was used to normalize the protein concentration in each group. In general, pooling of samples is accepted in proteomic analysis and it has been well documented in previous reports [20,21,22]. All the samples were run in triplicate in one dimensional-Polyacrylamide gel electrophoresis. After electrophoresis, each gel lane was cut into 6 pieces, digested using 5 µL trypsin (10 ng/µL) and 50 mM ammonium bicarbonate, and incubated overnight at room temperature. Prior to in-gel digestion, the samples (cut lanes) were alkylated with iodoacetamine and reduced with dithiothreitol. The peptides from the digested gel were extracted in two aliquots of 30 µL acetonitrile (10%) with formic acid (5%). The two aliquots were pooled together and evaporated to <10 µL and then diluted with 1% acetic acid to make up a final volume of 30 µL.
6. Liquid chromatography-tandem mass spectrometry analysis
Proteomic profiling of seminal plasma samples were carried out using a Finnigan LTQ linear ion trap mass spectrometer liquid chromatography-tandem mass spectrometry (LC-MS/MS) system. The peptides were fractionated by injecting 5 µL into high performance liquid chromatography column (Phenomenex Jupiter C18 reversed-phase capillary chromatography column). Fractions containing the peptides were eluted and introduced into the source of the mass spectrometer online. A full spectral scan was performed by utilizing the data dependent multitask ability of the instrument to determine peptide molecular weights and amino acid sequence of the peptides [20].
7. Protein identification and quantitative proteomics
Protein identification criteria were established at >99% probability to achieve false detection rate <1% as explained in our previous study [6]. Tandem mass spectra were extracted by Proteome Discoverer version 1.4.1.288. All MS/MS results were analyzed using Mascot (Matrix Science, London, UK; ver. 2.3.02), Sequest (Thermo Fisher Scientific, San Jose, CA, USA; ver. 1.4.0.288), and X!Tandem (The GPM, thegpm.org; ver. CYCLONE [2010.12.01.1]). The search was limited to the human protein reference database (http://www.hprd.org/) and results were uploaded into the Scaffold (ver. 4.0.6.1; Proteome Software Inc., Portland, OR, USA) as previously described [6]. Annotation of proteins was performed using Gene Ontology (GO) terms from National Center for Biotechnology Information (NCBI).
Relative quantification of the proteins was performed by comparing the number of spectra, termed spectral counts in both varicocele and control groups. The abundance of the proteins was determined by matching the spectra (spectral counts or SpCs), and classified as High (H), Medium (M), Low (L), or Very Low (VL). To overcome the sample-to-sample variation, normalization of spectral counts was done using the normalized spectral abundance factor [6,23].
8. Bioinformatic analysis
DEPs identified in both study groups were subjected to functional annotation and enrichment analysis using both publicly available bioinformatic annotation tools and databases such as UniProt and Reactome. Proprietary curated database such as Ingenuity pathway analysis (IPA) and Metacore™ (GeneGo Inc., St. Joseph, MI, USA) were used to analyze the involvement of DEPs in biological and cellular processes, pathways, cellular distribution, regulatory networks, and protein-protein interactions.
9. Protein selection criteria and validation by Western blot
From the list of 28 proteins identified as DEPs, 4 proteins (heat shock related 70 kDa protein 2: HSPA2, peroxiredoxin 2: PRDX2, apolipoprotein A2: APOA2 and acrosin: ACR) were selected for validation by Western Blot (WB) (n=6, fertile healthy men group and n=12, varicocele group). Criteria for selection of proteins include: 1) involvement in the top canonical pathways, 2) role of the proteins in reproductive system development and functions, 3) proteins associated with oxidative stress and sperm DNA damage, 4) proteins with a well-described function in the literature. A total of 50 µg of protein per sample was loaded into a 4% to 15% sodium dodecyl sulfate-polyacrylamide gel and electrophoresed for 2 hours at 90 V. WB using specific primary and secondary antibodies (Supplementary Table 1) was performed under standardized conditions [24]. Total protein staining was done with colloidal gold protein stain (Bio-Rad, Hercules, CA, USA) for 2 hours at room temperature by gentle shaking. Stained membranes were washed twice with distilled water for 10 minutes and the densitometry image was captured using colorimetric mode on Chemi-Doc (ChemiDoc™ MP Imaging System; Bio-Rad).
10. Statistical analysis
Data analysis was performed using MedCalc Statistical Software (V. 17.8; MedCalc Software, Ostend, Belgium). Mann-Whitney test was carried out to compare the semen parameters of the fertile donor group and the varicocele group, and the results were considered significant for p<0.05. The same test was used to compare the expression levels of the proteins validated using WB technique in both the groups.
RESULTS
1. Semen Parameters
Sperm concentration, motility and normal morphology were significantly lower in varicocele patients compared to healthy fertile men (Supplementary Table 2). Moreover, ROS and SDF levels were significantly higher in varicocele group when compared to control group (Supplementary Table 2).
2. Proteomic profile of seminal plasma
Proteomic analysis of the pooled samples from varicocele (unilateral, n=5 & bilateral, n=5) and control group (n=5) resulted in a total of 486 and 412 proteins, respectively. Based on the comparative proteomic analysis 28 proteins were identified to be differentially expressed between varicocele and control group (Fig. 1), with 4 DEPs (T-complex protein 1 subunit delta isoform a: CCT4, complement factor I preproprotein: CF1, T-complex protein 1 subunit theta isoform 1: CCT8, T-complex protein 1 subunit alpha isoform a: TCP1) unique to control group (Table 1). The DEPs differed in their abundance in both the varicocele and control groups (Table 1).
Fig. 1. The number of proteins identified in the control (n=412) and varicocele groups (n=486) along with the differentially expressed proteins (DEPs) in the control and varicocele groups.
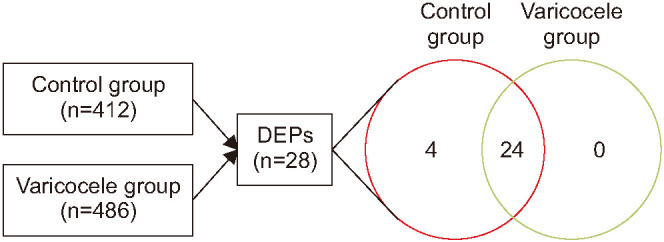
Table 1. The list of 28 differentially expressed proteins and their abundance and expression in varicocele group compared with the healthy fertile men group.
| Gene | Accession No. | Protein name | Healthy fertile group | Varicocele group | NSAF ratio | Expression | ||
|---|---|---|---|---|---|---|---|---|
| SC | Abund | SC | Abund | |||||
| CCT4 | 38455427 | T-complex protein 1 subunit delta isoform a | 2.7 | VL | 0 | - | 0.00 | Unique to fertile group |
| CF1 | 119392081 | Complement factor I preproprotein | 1.3 | VL | 0 | - | 0.00 | Unique to fertile group |
| CCT8 | 48762932 | T-complex protein 1 subunit theta isoform 1 | 5.7 | VL | 0 | - | 0.00 | Unique to fertile group |
| TCP1 | 57863257 | T-complex protein 1 subunit alpha isoform a | 2.0 | VL | 0 | - | 0.00 | Unique to fertile group |
| RUVBL1 | 4506753 | RuvB-like 1 | 8.3 | L | 0.3 | VL | 0.02 | UE |
| APOA2a | 4502149 | Apolipoprotein A-II preproprotein | 1.3 | VL | 0.2 | VL | 0.04 | UE |
| RUVBL2 | 5730023 | RuvB-like 2 | 7.7 | VL | 0.5 | VL | 0.09 | UE |
| EEF1G | 4503481 | Elongation factor 1-gamma | 10.3 | L | 2.8 | VL | 0.24 | UE |
| PGK2 | 31543397 | Phosphoglycerate kinase 2 | 25.3 | M | 8.3 | L | 0.29 | UE |
| CASP12 | 300360580 | Inactive caspase-12 | 21.7 | M | 7.7 | VL | 0.31 | UE |
| ACRa | 148613878 | Acrosin precursor | 10.7 | L | 3.2 | VL | 0.32 | UE |
| SEMG1 | 4506883 | Semenogelin-1 preproprotein | 698.3 | H | 395.7 | H | 0.46 | UE |
| HSPA2a | 13676857 | Heat shock related 70 protein 2 | 46.3 | M | 37.5 | M | 0.48 | UE |
| SEMG2 | 4506885 | Semenogelin-2 precursor | 1321.7 | H | 765.0 | H | 0.48 | UE |
| ACTG1 | 4501887 | Actin, cytoplasmic 2 | 52.3 | M | 91.5 | H | 1.73 | OE |
| PRDX1 | 4505591 | Peroxiredoxin-1 | 13.3 | L | 35.7 | M | 2.09 | OE |
| PRDX2a | 32189392 | Peroxiredoxin-2 | 11.3 | L | 32.2 | M | 2.46 | OE |
| ANXA1 | 4502101 | Annexin A1 | 7.7 | VL | 24.7 | M | 3.05 | OE |
| CD63 | 383872538 | CD63 antigen isoform D precursor | 3.0 | VL | 8.3 | L | 3.15 | OE |
| TGM4 | 156627577 | Protein-glutamine gamma-glutamyltransferase 4 | 31.3 | M | 181.8 | H | 3.46 | OE |
| RAB27B | 530414276 | Ras-related protein Rab-27B isoform X1 | 2.0 | VL | 9.5 | L | 4.03 | OE |
| HIST1H4A | 4504301 | Histone H4 | 1.7 | VL | 9.8 | L | 5.67 | OE |
| CBR1 | 4502599 | Carbonyl reductase [NADPH] 1 isoform 1 | 0.7 | VL | 4.7 | VL | 6.27 | OE |
| LAMA5 | 145309326 | Laminin subunit gamma-1 precursor | 2.0 | VL | 25.7 | M | 7.69 | OE |
| LCN15 | 578817458 | Lipocalin-15 isoform X1 | 0.7 | VL | 6.2 | VL | 8.21 | OE |
| GLO1 | 118402586 | Lactoylglutathione lyase | 1.3 | VL | 11.5 | L | 9.22 | OE |
| LAMC1 | 578835999 | Laminin subunit alpha-5 isoform X1 | 1.7 | VL | 36.5 | M | 12.90 | OE |
| PIGR | 530366266 | Polymeric immunoglobulin receptor isoform X1 | 0.3 | VL | 34.0 | M | 70.67 | OE |
SC: spectral count, Abund: abundance, NSAF: normalized spectral abundance factor, VL: very low, L: low, M: medium, H: high, UE: underexpressed, OE: overexpressed.
aThe proteins HSPA2, APOA2, PRDX2 and ACR were selected for validation by Western blotting.
3. Pathways regulated by differentially expressed proteins
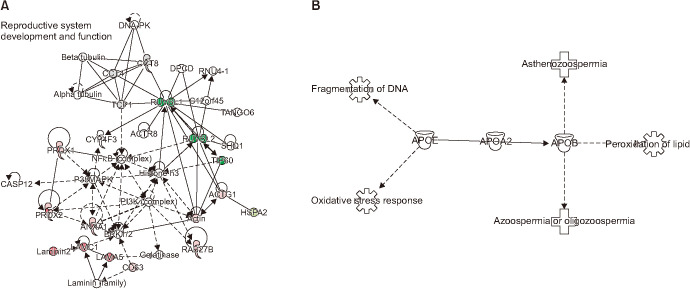
Networks generated using IPA software revealed that reproductive system development and function was the most enriched network with 15 DEPs (Fig. 2A). Furthermore, functional analysis of DEPs revealed the association of APOA2 protein with DNA fragmentation, oxidative stress response, lipid peroxidation, asthenozoospermia, azoospermia or oligozoospermia (Fig. 2B).
Fig. 2. (A) Top most functional enriched network, reproductive system development and function pathway with differentially expressed proteins in seminal plasma of infertile varicocele patient. (B) Functional analysis of the apolipoprotein (APO) A2 protein that was downregulated in the seminal plasma of infertile patients with varicocele.
4. Transcription factors associated with differentially expressed proteins
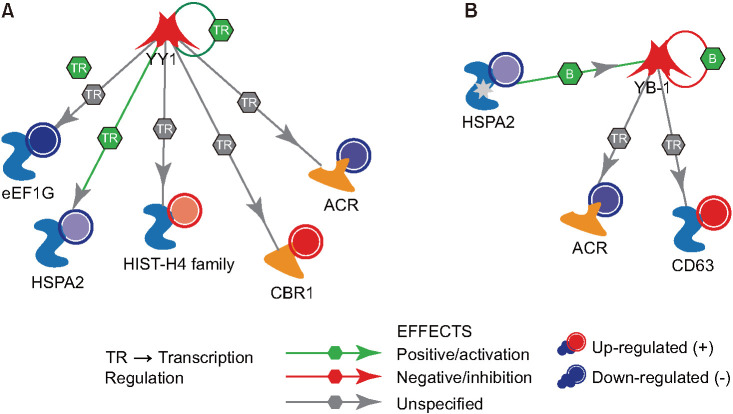
Using Metacore™, we identified 5 DEPs (elongation factor 1-gamma: eEF1G, heat shock related 70 protein 2: HSPA2, histone H4: HIST-H4, carbonyl reductase [NADPH] 1 isoform 1: CBR1, and acrosin precursor: ACR) that were predicted to be under the regulation of transcriptional repressor protein yin yang 1 (YY1) (Fig. 3A). Whereas, 3 DEPs (HSPA2, ACR, and CD63 antigen isoform D precursor: CD63) were predicted to be regulated by nuclease-sensitive element-binding protein 1 (YB-1) transcriptional factors (Fig. 3B).
Fig. 3. Upstream transcriptional factors involved in the regulation of differentially expressed proteins identified in the seminal plasma of varicocele patients relative to that of fertile men (control). (A) Transcriptional repressor protein yin yang 1 (YY1). (B) Nuclease-sensitive element-binding protein 1 (YB-1). HSPA2: heat shock related 70 kDa protein 2, ACR: acrosin.
5. Selection and validation of differentially expressed proteins by Western blot
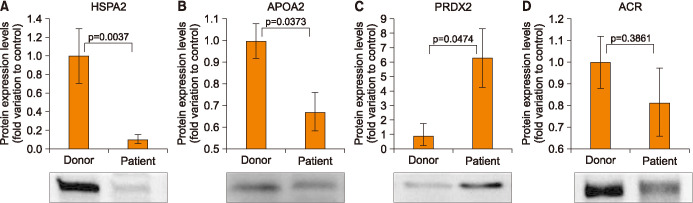
Based on the IPA results and metacore analysis we have selected four seminal plasma DEPs (HSPA2, PRDX2, APOA2, and ACR) for validation. HSPA2 and ACR proteins are under the regulation of both the transcription factors YY1 and YB-1. Whereas, the PRDX2 associated with oxidative stress response was dysregulated in the reproductive system development and function network. Additionally, APOA2 was identified as a multi-functional protein associated to oxidative stress response and SDF. WB analysis revealed that HSPA2 and APOA2 proteins were significantly downregulated (0.10-fold variation to control; p=0.0037 and 0.67-fold variation to control; p=0.0373, repectively) (Fig. 4A, 4B), whereas protein PRDX2 was significantly upregulated (1.29-fold variation to control; p=0.0474) in varicocele patients (Fig. 4C). Although, ACR was found to be downregulated (0.81-fold variation to control) in varicocele group, the relative change was not significant (p=0.3861) when compared to the control group (Fig. 4D).
Fig. 4. Protein expression levels of the differentially expressed proteins selected for validation by Western blot in varicocele group relative to fertile men. (A) Heat shock related 70 kDa protein 2 (HSPA2), (B) apolipoprotein A2 (APOA2), (C) peroxiredoxin 2 (PRDX2), (D) acrosin (ACR).
DISCUSSION
Seminal plasma, the primary nutritive medium for spermatozoa, plays a crucial role in sperm function and fertilizing potential of male gamate. Previous study from our laboratory have demostrated altered proteome profile of sperm in varicocele subjects, specifically underexpression of proteins involved in major energy metabolism pathways, transport, protein folding and proton pumps [6]. Subsequently, we were interested to explore the seminal plasma protein profile of these subjects and eluciate its possible implications on sperm pathology associated with varicocele. Therefore, the present study is an extenstion of the report on sperm proeteomics.
In the present study, the IPA analysis identified reproductive system development and function as one of the top protein-protein interaction network enriched with 15 DEPs (Fig. 2A). PRDX1 and PRDX2 were upregulated in the varicocele patients. In response to oxidative stress PRDX1 exhibits an increased expression in seminal plasma [25]. In our previous reports, we have demonstrated an increased state of oxidative stress at the subcellular level in the spermatozoa of varicocele patients [6]. Validation results were in agreement with proteomic data, suggesting that PRDX2 may serve as potential seminal plasma biomarker in diagnosis of oxidative stress-mediated reproductive dysfunction in varicocele patients. The proteins ruvB-like 1 (RUVBL1) and ruvB-like 2 (RUVBL2), which are involved in DNA damage, were downregulated in the varicocele patients. In our previous study, we observed a significant change in the expression of these proteins in infertile men with high ROS [26]. Hence the altered expression of the DEPs in the network suggests a state of oxidative stress. This results in DNA damage in varicocele patients, which was evident with the significantly increased ROS levels and SDF compared to control group (Supplementary Table 2). The aberrant expression of seminal plasma proteins could be a major contributing factor for dysfunction of spermatozoa in the varicocele patients.
Spermatozoa of varicocele patients are under the continuous influence of oxidative stress leading to increased lipid peroxidation and DNA fragmentation [2]. In the current study, we observed downregulation of APOA2 in the seminal plasma of varicocele patients. Functional enrichment analysis using IPA revealed that APOA2 was involved in pathways such as oxidative stress response, lipid peroxidation and DNA fragmentation (Fig. 2B). Furthermore, our WB results corroborate with the proteomic results (Fig. 4B). The expression of APOA2 in body fluids is noticed during a state of oxidative stress [27]. Hence, the abnormal expression of APOA2 in varicocele subjects indicates seminal oxidative stress and its possible implication on poor semen quality.
Seminal plasma proteome revealed the complete absence of TCP1 and molecular chaperones containing TCP1 subunits such as CCT4 and CCT8 in varicocele group (Table 1). All these three proteins facilitate sperm-oocyte interaction and are essentially required for the sperm-zona pellucida binding [28]. In our previous report we have demonstrated the underexpression of these proteins in spermatozoa of varicocele patients [6]. Absence of these proteins in seminal plasma of varicocele patients reflects the defective function of the spermatozoa. Moreover, the proteomic data as well as WB analysis revealed downregulation of HSPA2, in varicocele patients. HSPA2 is under the regulation of transcription factors YB-1 and YY1 (Fig. 3) associated with sperm-zona binding and fertilization. These findings suggest that fertilizing capacity of varicocele patients is affected due to aberrant expression of seminal plasma proteins.
Post-ejaculatory changes associated with spermatozoa such as hyperactivation, capacitation and acrosome reaction play a vital role in preparing the sperm for fertilization. These events are initiated by the cascade of molecular changes at subcellular level. In the seminal plasma of varicocele group, proteins (EEF1G, phosphoglycerate kinase 2: PGK2 and ACR) associated with normal physiological sperm function were downregulated. In general, EEF1G linked to the tyrosine kinase protein is upregulated in the spermatozoa during the sperm capacitation process [26]. PGK2 involved in spermatogenesis and sperm motility [26] was also down-regulated in varicocele patients and this may be one of the reasons for the poor sperm motility and sperm concentration in varicocele group (Supplementary Table 1). Earlier reports have suggested PGK2 as a seminal plasma biomarker to differentiate between fertile and infertile men [29,30]. Besides, we observed downregulation of the protein associated with acrosome reaction of spermatozoa, ACR, in the seminal plasma of varicocele patients. ACR is the major protease of mammalian spermatozoa and stored as proacrosin in acrosome. In varicocele patients ACR was reported to be underexpressed in sperm [26]. Presence of ACR in seminal plasma confirms that acrosome integrity is compromised in varicocele patients. Our WB data showed a decreased, but not a significant, expression of ACR in varicocele patients (Fig. 4D). ACR was identified to be regulated by the transcription factors YB-1 and YY1, which play a key role in the regulation of the acrosomal matrix dispersion mechanism (Fig. 3).
The proteomic data and WB analysis revealed an altered seminal plasma proteome in varicocele subjects, which in turn elucidates the pathology associated with spermatozoa. In the present study, we validated only key DEPs associated primarily with oxidative stress and its mediators. However, validation of additional seminal plasma DEPs are warranted to explore the involvement of other relevant pathways associated with sperm pathology in varicocele patients.
CONCLUSIONS
The seminal plasma proteome is altered in varicocele subjects when compared to control group. Altered expression of PRDX2, HSPA2 and APOA2, and its association with oxidative stress and sperm function confirms the dysregulation of physiological processes in the seminal plasma of varicocele subjects.
ACKNOWLEDGEMENTS
The authors thank Tania Dias, M.Sc., Damayanthi Durairajanayagam, PhD, Banu Gopalan, PhD, Rakesh Sharma, PhD, Ana Martins, M.Sc. for their critical reading of the manuscript and helpful suggestions. Belinda Willard, PhD, Director of Proteomic Core Laboratory, Lerner Research Institute assisted with proteomic analysis while Bioinformatics data analysis was conducted by Banu Gopalan, PhD. Research support was provided by the American Center for Reproductive Medicine at Cleveland Clinic. The authors are grateful to Saradha Baskaran, PhD, for her critical review and constructive comments.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
- Conceptualization: Agarwal A.
- Data curation: Panner Selvam MK.
- Formal analysis: Agarwal A, Panner Selvam MK.
- Funding acquisition: NA.
- Investigation: Agarwal A.
- Methodology: Panner Selvam MK.
- Project administration: Agarwal A.
- Software: Panner Selvam MK.
- Supervision: Agarwal A.
- Validation: Panner Selvam MK.
- Writing — original draft: Panner Selvam MK.
- Writing — review & editing: Agarwal A, Panner Selvam MK.
- Approval of the final manuscript: Agarwal A, Panner Selvam MK.
Data Sharing Statement
The data analyzed for this study have been deposited in HARVARD Dataverse and are available at https://doi.org/10.7910/DVN/Y1ED9R.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.180118.
Differentially expressed proteins selected for validation and the primary and secondary antibodies used in Western blotting
Semen parameters in healthy fertile men and infertile patients with varicocele
References
- 1.Alsaikhan B, Alrabeeah K, Delouya G, Zini A. Epidemiology of varicocele. Asian J Androl. 2016;18:179–181. doi: 10.4103/1008-682X.172640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho CL, Esteves SC, Agarwal A. Novel insights into the pathophysiology of varicocele and its association with reactive oxygen species and sperm DNA fragmentation. Asian J Androl. 2016;18:186–193. doi: 10.4103/1008-682X.170441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panner Selvam MK, Agarwal A. Update on the proteomics of male infertility: a systematic review. Arab J Urol. 2018;16:103–112. doi: 10.1016/j.aju.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal A, Sharma R, Durairajanayagam D, Ayaz A, Cui Z, Willard B, et al. Major protein alterations in spermatozoa from infertile men with unilateral varicocele. Reprod Biol Endocrinol. 2015;13:8. doi: 10.1186/s12958-015-0007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agarwal A, Sharma R, Durairajanayagam D, Cui Z, Ayaz A, Gupta S, et al. Differential proteomic profiling of spermatozoal proteins of infertile men with unilateral or bilateral varicocele. Urology. 2015;85:580–588. doi: 10.1016/j.urology.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal A, Sharma R, Samanta L, Durairajanayagam D, Sabanegh E. Proteomic signatures of infertile men with clinical varicocele and their validation studies reveal mitochondrial dysfunction leading to infertility. Asian J Androl. 2016;18:282–291. doi: 10.4103/1008-682X.170445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samanta L, Parida R, Dias TR, Agarwal A. The enigmatic seminal plasma: a proteomics insight from ejaculation to fertilization. Reprod Biol Endocrinol. 2018;16:41. doi: 10.1186/s12958-018-0358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milardi D, Grande G, Vincenzoni F, Messana I, Pontecorvi A, De Marinis L, et al. Proteomic approach in the identification of fertility pattern in seminal plasma of fertile men. Fertil Steril. 2012;97:67–73.e1. doi: 10.1016/j.fertnstert.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Jodar M, Soler-Ventura A, Oliva R. Semen proteomics and male infertility. J Proteomics. 2017;162:125–134. doi: 10.1016/j.jprot.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Del Giudice PT, Belardin LB, Camargo M, Zylbersztejn DS, Carvalho VM, Cardozo KH, et al. Determination of testicular function in adolescents with varicocoele: a proteomics approach. Andrology. 2016;4:447–455. doi: 10.1111/andr.12174. [DOI] [PubMed] [Google Scholar]
- 11.Vivas-Acevedo G, Lozano-Hernández R, Camejo MI. Varicocele decreases epididymal neutral α-glucosidase and is associated with alteration of nuclear DNA and plasma membrane in spermatozoa. BJU Int. 2014;113:642–649. doi: 10.1111/bju.12523. [DOI] [PubMed] [Google Scholar]
- 12.Zylbersztejn DS, Andreoni C, Del Giudice PT, Spaine DM, Borsari L, Souza GH, et al. Proteomic analysis of seminal plasma in adolescents with and without varicocele. Fertil Steril. 2013;99:92–98. doi: 10.1016/j.fertnstert.2012.08.048. [DOI] [PubMed] [Google Scholar]
- 13.Fariello RM, Pariz JR, Spaine DM, Gozzo FC, Pilau EJ, Fraietta R, et al. Effect of smoking on the functional aspects of sperm and seminal plasma protein profiles in patients with varicocele. Hum Reprod. 2012;27:3140–3149. doi: 10.1093/humrep/des287. [DOI] [PubMed] [Google Scholar]
- 14.Camargo M, Intasqui Lopes P, Del Giudice PT, Carvalho VM, Cardozo KH, Andreoni C, et al. Unbiased label-free quantitative proteomic profiling and enriched proteomic pathways in seminal plasma of adult men before and after varicocelectomy. Hum Reprod. 2013;28:33–46. doi: 10.1093/humrep/des357. [DOI] [PubMed] [Google Scholar]
- 15.Del Giudice PT, da Silva BF, Lo Turco EG, Fraietta R, Spaine DM, Santos LF, et al. Changes in the seminal plasma proteome of adolescents before and after varicocelectomy. Fertil Steril. 2013;100:667–672. doi: 10.1016/j.fertnstert.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 16.Belardin LB, Del Giudice PT, Camargo M, Intasqui P, Antoniassi MP, Bertolla RP, et al. Alterations in the proliferative/apoptotic equilibrium in semen of adolescents with varicocele. J Assist Reprod Genet. 2016;33:1657–1664. doi: 10.1007/s10815-016-0808-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camargo M, Intasqui P, Bertolla RP. Proteomic profile of seminal plasma in adolescents and adults with treated and untreated varicocele. Asian J Androl. 2016;18:194–201. doi: 10.4103/1008-682X.168788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martínez-Bartolomé S, Deutsch EW, Binz PA, Jones AR, Eisenacher M, Mayer G, et al. Guidelines for reporting quantitative mass spectrometry based experiments in proteomics. J Proteomics. 2013;95:84–88. doi: 10.1016/j.jprot.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization (WHO) WHO laboratory manual for the examination and processing of human semen. Geneva: WHO; 2010. [Google Scholar]
- 20.Sharma R, Agarwal A, Mohanty G, Du Plessis SS, Gopalan B, Willard B, et al. Proteomic analysis of seminal fluid from men exhibiting oxidative stress. Reprod Biol Endocrinol. 2013;11:85. doi: 10.1186/1477-7827-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diz AP, Truebano M, Skibinski DO. The consequences of sample pooling in proteomics: an empirical study. Electrophoresis. 2009;30:2967–2975. doi: 10.1002/elps.200900210. [DOI] [PubMed] [Google Scholar]
- 22.Bogle OA, Kumar K, Attardo-Parrinello C, Lewis SE, Estanyol JM, Ballescà JL, et al. Identification of protein changes in human spermatozoa throughout the cryopreservation process. Andrology. 2017;5:10–22. doi: 10.1111/andr.12279. [DOI] [PubMed] [Google Scholar]
- 23.Zybailov BL, Florens L, Washburn MP. Quantitative shotgun proteomics using a protease with broad specificity and normalized spectral abundance factors. Mol Biosyst. 2007;3:354–360. doi: 10.1039/b701483j. [DOI] [PubMed] [Google Scholar]
- 24.Panner Selvam MK, Agarwal A, Sharma R, Samanta L. Treatment of semen samples with α-chymotrypsin alters the expression pattern of sperm functional proteins-a pilot study. Andrology. 2018;6:345–350. doi: 10.1111/andr.12466. [DOI] [PubMed] [Google Scholar]
- 25.Intasqui P, Antoniassi MP, Camargo M, Nichi M, Carvalho VM, Cardozo KH, et al. Differences in the seminal plasma proteome are associated with oxidative stress levels in men with normal semen parameters. Fertil Steril. 2015;104:292–301. doi: 10.1016/j.fertnstert.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 26.Ayaz A, Agarwal A, Sharma R, Arafa M, Elbardisi H, Cui Z. Impact of precise modulation of reactive oxygen species levels on spermatozoa proteins in infertile men. Clin Proteomics. 2015;12:4. doi: 10.1186/1559-0275-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma C, Li J, Bao Z, Ruan Q, Yu Z. Serum levels of ApoA1 and ApoA2 are associated with cognitive status in older men. Biomed Res Int. 2015;2015:481621. doi: 10.1155/2015/481621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dun MD, Smith ND, Baker MA, Lin M, Aitken RJ, Nixon B. The chaperonin containing TCP1 complex (CCT/TRiC) is involved in mediating sperm-oocyte interaction. J Biol Chem. 2011;286:36875–36887. doi: 10.1074/jbc.M110.188888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rolland AD, Lavigne R, Dauly C, Calvel P, Kervarrec C, Freour T, et al. Identification of genital tract markers in the human seminal plasma using an integrative genomics approach. Hum Reprod. 2013;28:199–209. doi: 10.1093/humrep/des360. [DOI] [PubMed] [Google Scholar]
- 30.Cui Z, Agarwal A, da Silva BF, Sharma R, Sabanegh E. Evaluation of seminal plasma proteomics and relevance of FSH in identification of nonobstructive azoospermia: a preliminary study. Andrologia. 2018;50:e12999. doi: 10.1111/and.12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Differentially expressed proteins selected for validation and the primary and secondary antibodies used in Western blotting
Semen parameters in healthy fertile men and infertile patients with varicocele
Data Availability Statement
The data analyzed for this study have been deposited in HARVARD Dataverse and are available at https://doi.org/10.7910/DVN/Y1ED9R.