Abstract
Cognitive impairment and dementia are predicted to undergo a dramatic increase in the following years with more than 131.5 million people being affected by 2030. Although vascular diseases play the most important role in the pathogenesis of memory impairment in aging men, some pre-clinical and clinical evidence has suggested a possible contribution of the age-dependent reduction of testosterone (T). In this paper we have summarized and discussed all the information derived from available animal and experimental studies. In addition, we meta-analyzed data rising from all randomized placebo controlled trials (RCTs) published so far. Only limited preclinical and clinical evidence can support a possible contribution of T in the pathogenesis of the age-dependent impairment of cognitive functions. In addition, our meta-analysis did not support the use of T replacement therapy for the improvement of several cognitive domains analyzed including attention/working memory, executive function, language, verbal memory, visual memory, visuomotor ability, and visuospatial ability. However, it is important to recognize that the vast majority of available RCTs included mixed populations of subjects with eugonadism and hypogonadism preventing any final conclusion being drawn on these issues.
Keywords: Aging, Cognitive impairment, Dementia, Hypogonadism, Testosterone
INTRODUCTION
Life expectancy has steadily increased over recent decades in all high-income countries. Data from members of the Organization for Economic Cooperation and Development, including a population of about one million people among all high-income countries worldwide, suggest that by 2030 life expectancy will increase with a probability of at least 65% for women and 85% for men [1]. In particular, projection data indicate that, for men, South Korea, Australia, and Switzerland will show the best results with a 95% probability that men's life expectancy at birth in these three countries will surpass 80 years in 2030, and 27% that it would surpass 85 years [1]. Gender differences are confirmed also by these data with women's life expectancy projections worldwide higher when compared to men [1].
Although global aging is the result of medical, social, and economic advances over disease, it also presents important challenges. In particular, aging people are characterized by chronic and progressive diseases requiring assistance during the activities of daily living. Accordingly, cognitive impairment and dementia prevalence are predicted to show a dramatic increase with a projection by 2050 of more than 131.5 million people being affected [2]. In line with these data, the economic burden of US$ 818 billion is expected to increase substantially over the next few decades [3]. In order to face this situation, the World Health Organization (WHO) in 2012 lunched a worldwide call among all the dementia stakeholders in order to finalize common solutions and plans to solve the problem [4]. This has eventually led to the organization of the first WHO Ministerial Conference on Global Action Against Dementia in March, 2015 [2]. Among the identified top ten research priorities, the most important were those related to prevention, identification, and reduction of dementia risk factors as well as those on delivery and quality of care for people with dementia and their caretakers [2].
It is quite clear that vascular diseases play an essential role in the majority of patients with dementia [5]. However, it is also important to recognize that dementia phenotype includes a wide spectrum of features with several underlying risk factors. The role of the endocrine system and, in particular, of sex steroids, is still conflicting. In men, an age-dependent reduction of testosterone (T) has been reported [6]. Late onset hypogonadism (LOH) is the most frequently used term to describe this phenomenon. A recent meta-analysis, including all available observation studies, has documented that reduced T is associated with an increased risk of cardiovascular (CV) morbidity and mortality [7]. Similarly, several data have documented a close relationship between age-dependent reduction of T levels and worse CV and metabolic profile [8,9,10]. In addition, both pre-clinical and clinical studies have also suggested a possible direct role of T in neuro-protective mechanisms [11,12].
The aim of the present study is to systematically summarize and discuss all the available evidence regarding the possible role of T in age-depended male cognition impairment and dementia. Both animal and experimental data as well as results derived from randomized placebo controlled trials (RCTs) will be considered. When possible a meta-analytic approach will be used.
METHODS
A comprehensive Medline, Embase, and Cochrane search was performed including the following words: (“testosterone” [MeSH Terms] OR “testosterone” [All Fields]) AND (“cognition” [MeSH Terms] OR “cognition” [All Fields]). Publications from January 1, 1969 up to December 31st, 2019 were included. When available, meta-analytic data were preferred. In addition, a new meta-analysis on the effect of T replacement therapy (TRT) on cognition parameters with only placebo RCTs was also performed.
PRE-CLINICAL EVIDENCE
Androgen receptors (AR) are widely expressed within the central nervous system. T, in its free form, is able to cross the blood barrier and to influence neuronal cells, acting through genomic and non-genomic pathways [11,12]. Data from animal in vitro and in vivo models have reported conflicting results regarding the possible role of androgens on neuroprotection. In fact both beneficial and deleterious effects have been reported [11,12].
1. In vitro studies
Data derived from primary neurons suggested that T can act in protecting or exacerbating experimental neuronal damage depending on the concentration [13,14,15]. In particular, supra-physiological concentrations (10 µM) can amplify glutamate-induced excitotoxic neuronal death, whereas protection has been observed at 10 nM [16]. Although indirect effects due to T aromatization to estradiol have been reported, some evidence has also documented a direct role of androgens thought AR [13,14,15]. Neurotophic effects of T have also been described [17,18]. Data obtained in cultures of human neuroblastoma cells showed that T was more effective in alleviating β-amyloid induced mitochondrial bioenergetic deficits, by regulating mitochondrial oxidative phosphorylation genes [19,20,21]. Finally, more recently, it has been reported that, under pathological conditions, astrocytes can mediate, at least partially, the neuroprotective effects of gonadal steroid hormones by reducing the release of pro-inflammatory molecules [11]. Given the side effects that sex steroids may have when administered systemically, a number of synthetic agonists of the receptors for gonadal steroid hormones in the nervous system have been developed, and may be considered for clinical use after brain injury, as potential enhancers of the neuroprotective astrocytic functions [11].
2. In vivo studies
Similar to what has been observed in vitro studies, data obtained in aged rodents documented that, during stroke, maintaining T, or dihydrotestosterone, plasma levels within the low physiological range confers protection [22]. The latter effect was blunted by administration of the AR antagonist flutamide, suggesting AR-mediated mechanisms [22]. In other experimental animal models, T administration is associated with an increase in neuron somal size, neuritic growth, plasticity, and synaptogenesis in both motoneurones of the spinal nucleous of the bulbocavernous [23]. Finally, in male double-transgenic mice, the increase of T levels is associated with a correspondent decrease in β-secretase, an enzyme involved in the cleavage of the amyloid β protein precursor [24]. However, other authors reported that, in castrated male rats, high androgen levels exacerbate ischemic damage [25]. Similarly, a more recent study showed that chronic high-dose T administration impairs cognitive flexibility in a rat animal model [26].
CLINICAL EVIDENCE
Aging is associated with an impairment of cognitive ability, including memory, attention, language and visuospatial ability [2,3,4,5]. Some authors have hypothesized that the age-related reduction in cognition and the T decline are temporally related, suggesting a possible role of low T in cognition impairment [27,28]. Accordingly, cognition impairment has been considered a possible component of LOH [10,28,29,30,31].
1. Chemical castration and cognition
Up to now, two systematic meta-analyses have evaluated the relationship between androgen deprivation therapy (ADT) and cognition (Table 1). The first study included 14 RCTs with only a limited number of subjects (n=417 patients and 122 controls) [32]. Total duration of ADT ranged from a mean of 23 to 31 months, whereas mean age of the ADT groups ranged from 63.2 to 71.0 years across study samples [32]. Overall, across studies, various neuropsychological tests were used, which are divided into seven cognitive domains (Table 2) [32]. The authors concluded that patients treated with ADT performed worse than controls or than their own baseline on visuomotor domain, with larger magnitude effect in studies with a shorter follow-up. No significant effect was observed on the other six cognitive domains, including attention/working memory, executive function, language, verbal memory, visual memory, and visuospatial ability [32].
Table 1. Comparisons of the available meta-analyses evaluating the relationship between androgen deprivation therapy and cognitive impairment.
| McGinty et al (2014) [32] | Sun et al (2018) [33] | |
|---|---|---|
| Inclusion criteria | ||
| No. of trials included | 14 | 6 |
| No. of patients analyzed | 414 | |
| No. of controls analyzed | 122 | |
| Outcomes evaluated | ||
| Visomotor domain | Yes | No |
| Attention/working memory | Yes | No |
| Executive function | Yes | No |
| Language | Yes | No |
| Verbal memory | Yes | No |
| Visual memory | Yes | No |
| Visuospatial ability | Yes | No |
| Any cognitive impairment retrospective studiesa | No | Yes |
| Any cognitive impairment prospective studiesb | No | Yes |
aScoring 1.5 or more standard deviations below published norms on 2 or more tests, or scoring 2.0 or more standard deviations below published norms on at least 1 test; bDefined using International Classification of Diseases-9 diagnostic or procedure codes or other system based identification scheme.
Table 2. Effect size of several cognitive test and risk of cognitive impairment in men treated with androgen deprivation therapy or controls.
| Risk factor | McGinty et al (2014) [32]a | Sun et al (2018) [33]b |
|---|---|---|
| Attention/working memory | −0.07 (−0.67–0.53) | |
| Executive function | −0.06 (−0.80–0.67) | |
| Language | −0.19 (−0.82–0.43) | |
| Verbal memory | −0.05 (−0.47–0.37) | |
| Visual memory | 0.22 (−0.19–0.63) | |
| Visuomotor ability | −0.67 (−1.17–−0.17) | |
| Visuospatial ability | 0.06 (−0.55–0.56) | |
| Any cognitive impairment prospective studiesc | 1.57 (0.50–4.92) | |
| Any cognitive impairment prospective studiesd | NA | 1.75 (0.49–6.25) |
| Any cognitive impairment retrospective studiese | NA | 1.28 (0.93–1.76) |
NA: not available.
aEffect size (95% confidence interval [CI]). bOdds ratio (95% CI). cScoring 1.5 or more standard deviations below published norms on 2 or more tests; dScoring 2.0 or more standard deviations below published norms on at least 1 test; eDefined using International Classification of Diseases-9 diagnostic or procedure codes or other system based identification scheme.
A more recent meta-analysis included two prospective and four retrospective studies, accounting for 442 and 67,644 men, respectively. Men included in the prospective studies were younger (mean 67 to 69 years old) than those evaluated in the retrospective survey studies (70 to 75 years old) [33]. When only prospective studies were considered, no difference between case and controls was observed in the risk of developing cognitive impairment, according to the International Cognition and Cancer Task Force (scoring 1.5 or more standard deviations below published norms on two or more tests, or scoring 2.0 or more standard deviations below published norms on at least one test). Similarly no increased risk of cognitive impairment was observed as defined using International Classification of Diseases-9 diagnostic or procedure codes or on other systembased identification schemes (Table 2) [33].
2. Serum levels of testosterone and cognitive function in aging men
Different population-based studies have investigated a possible relationship between T levels and cognitive function in aging men (Table 3) [34,35,36,37,38,39,40,41,42,43]. The number of subjects included ranges from 310 to up to almost 6,000. Several studies have documented an association between androgen status and cognitive impairment. In particular, two studies showed that subjects with reduced total T levels have a cognitive impairment. In addition, two studies demonstrated an inverse correlation between calculated free T, and two with free T index (FTI), and impaired cognition. However, it is important to recognize that all the aforementioned studies used radioimmunoassays for T evaluation, which have demonstrated some problems of accuracy, especially in the presence of very low levels of T [44]. In addition, the use of FTI for the assessment of androgen status has been strongly criticized [45].
Table 3. Relationship between endogenous testosterone (T) levels and cognitive function in available population-based studies.
| Reference (year) | Study | Country | Population | Type of T assay used | Cognitive outcome |
|---|---|---|---|---|---|
| Barrett-Connor et al (1999) [34] | Rancho Bernardo | USA | 547 men aged 59–89 years at baseline | Radioimmunoassay | ↓ In men with reduced TT |
| Yaffe et al (2002) [35] | Study of osteoporotic risk in men | USA | 310 men aged 50 years or older | Radioimmunoassay | ↓ In men with reduced BT |
| Moffat et al (2004) [36] | Baltimore longitudinal study of aging | USA | 1,148 men aged 32 to 87 years at baseline | Radioimmunoassay | ↓ In men with reduced FTI |
| Fonda et al (2005) [37] | Massachusetts male aging study | USA | 981 men aged 40–70 years at baseline | Radioimmunoassay | ↔ |
| Muller et al (2005) [38] | Netherlands | 400 men aged 40–80 years at baseline | Radioimmunoassay | ↓ In men with reduced TT | |
| Geerlings et al (2006) [39] | Honolulu-Asia aging study | Honolulu-Asia | 2,974 men aged 71 to 93 years at baseline | Radioimmunoassay | ↔ |
| Thilers et al (2006) [40] | Betula study | Sweden | 1,107 men aged 35 to 90 years at baseline | Radioimmunoassay | ↓ In men with reduced cFT |
| Yeap et al (2008) [41] | Health in men study | Australia | 2,932 men aged 70 to 89 years at baseline | Radioimmunoassay | ↓ In men with reduced cFT |
| LeBlanc et al (2010) [42] | Osteoporotic fractures in men study | USA | 5,995 men aged 65 years or older at baseline | Mass spectometry in a random sample of 1,602 men | ↔ |
| Wu et al (2010) [43] | European male aging study | Europe | 3,369 aged 40 to 79 years at baseline | Mass spectometry | ↔ |
↓: impairment, TT: total T, BT: bioavailable T, FTI: free T index, ↔: no difference, cFT: calculated free T.
In apparent contrast with the aforementioned results, when mass-spectrometry was applied, the gold standard method for all steroid evaluation, no association between low T and cognitive problems was reported (Table 3).
1) Interventional studies – testosterone trials
In 2003, the US National Institute on Aging funded a set of clinical trials in order to better clarify the benefit and possible risks of TRT in the aging male. Hence, a set of seven, 52-week, randomized, placebo-controlled, double-blind trials, including overall 788 hypogonadal (total testosterone [TT]<9.4 nM) men older than 65 years were designed and planned. All men included in the active arm were treated with T 1% gel. One specific RCT, the Cognitive Function Trial, specifically investigated the efficacy of TRT on cognitive outcomes among 493 men with age-associated memory impairment [46]. The primary designated outcome of the study was verbal memory, as assessed by delayed paragraph recall performance. The latter test was selected based on prior findings in small RCTs and on its clinical importance. In fact, epidemiological data indicate that, in the years preceding clinical dementia, verbal memory impairment is accelerated [47,48]. In addition, it is important to recognize that delayed paragraph recall performance involves neurological areas of the hippocampus, which contains both androgen and estrogen receptors, supporting a physiological role of sex steroids [49,50]. However, despite this evidence, TRT for one year, as compared with placebo, was not associated with improved memory, as well as with the other cognitive functions evaluated, including visual memory, spatial ability, and executive function [46]. In the T trials the cognitive function test was also used in subjects without memory problems. When the analysis was extended to the whole population, T-treated men showed a small, but statistically significant, increase in executive function. However, the same authors recognized that treatment effect was small and the observed result does not justify the use of TRT in older men to improve cognition [51].
2) Interventional studies – meta-analysis of available randomized placebo controlled trials
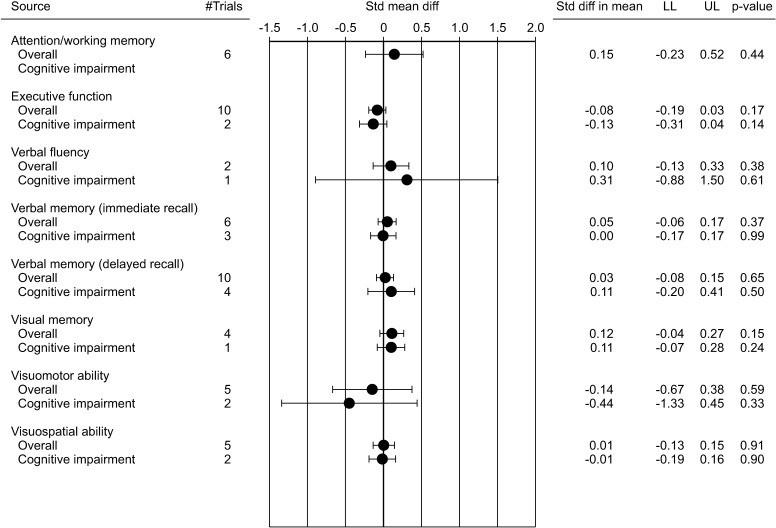
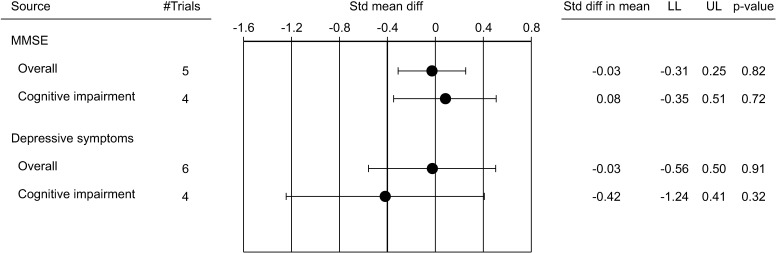
Besides the T trials, several other RCTs have evaluated the effect of TRT on cognitive function in aging men. In particular, 17 studies are available overall [46,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67]. These trials enrolled 1,438 patients with a mean age of 70.4 years and a mean follow-up of 45.6 weeks. These trials differ in basal TT levels and type of T preparation used (Table 4). In addition, nine were performed in aging men without memory problems, five in subjects with mild to moderate cognitive impairment and two in patients with Alzheimer's disease (Table 4). Since the classification of tests into cognitive domains differed among the included studies, the available neuropsychological tests were divided into seven cognitive domains, based on an established neuropsychological reference text [68], as previously reported (Supplementary Table 1) [32]. In order to obtain more comparable results, only trials with homogenous cognitive tests were analyzed. Combining the results of those trials, when TRT was compared to placebo, no difference in all the cognitive domains analyzed was observed (Fig. 1, Supplementary Fig. 1). In addition, no differences were also observed when depressive symptoms or score derived from Mini-Mental State Examination test were considered (Fig. 2). Similarly, no differences were observed when only patients with cognitive impairment were considered (Fig. 1, 2, Supplementary Fig. 2).
Table 4. Characteristics and outcomes of the randomized, controlled clinical studies included in the meta-analysis.
| Reference (year) | No. of patient | Trial duration (wk) | Age (y) | Type of population | T levels | Dose (daily) | Design | Randomization | Blinding | Drop-out | Intention to treat |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Janowsky et al (1994) [52] | 56 | 12 | 67.4 | Aging men | Mixed | T patch 15 mg/die | Parallel | A | NA | NA | NR |
| Janowsky et al (2000) [53] | 19 | 4 | 67.5 | Aging men | Eugonadal | TE 150 mg/wk | Parallel | A | NA | NA | NR |
| Cherrier et al (2001) [54] | 25 | 6 | 70.2 | Aging men | Eugonadal | TE 100 mg/wk | Parallel | NA | NA | A | A |
| Kenny et al (2002) [55] | 44 | 52 | 75.5 | Aging men | Mixed | T patch 50 mg/d | Parallel | NA | NA | A | A |
| Kenny et al (2004) [56] | 11 | 10 | 79.6 | Mild to moderate CI | Mixed | TE 200 mg/3 wk | Parallel | NA | NA | A | A |
| Cherrier et al (2005) [57] | 25 | 6 | 70.2 | AD | Eugonadal | TE 100 mg/wk | Parallel | A | NA | A | A |
| Haren et al (2005) [58] | 76 | 52 | 68.5 | Aging men | Mixed | TU 160 mg/d | Parallel | A | A | A | A |
| Lu et al (2006) [59] | 18 | 24 | 69.8 | AD | Mixed | TG 75 mg/d | Parallel | A | NA | A | A |
| Maki et al (2007) [60] | 15 | 36 | 73.9 | Aging men | <8 nM | TE 200 mg/wk | Parallel | A | A | A | A |
| Vaughan et al (2007) [61] | 47 | 156 | 70.8 | Aging men | <12 nM | TE 200 mg/2 wk | Parallel | A | A | A | A |
| Emmelot-Vonk et al (2008) [62] | 237 | 24 | 67.3 | Aging men | Mixed | TU 160 mg/d | Parallel | A | A | A | A |
| Fukai et al (2010) [63] | 24 | 24 | 81.0 | Mild CI | Mixed | TU 160 mg/d | Parallel | A | NA | NA | NA |
| Borst et al (2014) [64] | 30 | 52 | 70.5 | Aging men | <10.4 nM | T patch 150 mg/d | Parallel | A | NA | NA | NA |
| Cherrier et al (2015) [65] | 22 | 24 | 70.5 | Mild CI | <10.4 nM | TG 50 to 100 mg/d | Parallel | A | A | A | A |
| Huang et al (2016) [66] | 308 | 156 | 67.6 | Aging men | Eugonadal | TG 75 mg/d | Parallel | A | A | A | A |
| Wahjoepramono et al (2016) [67] | 44 | 52 | 61.1 | Mild CI | <10.4 nM | TG 50 mg/d | Cross-over | A | A | A | A |
| Resnick et al (2017) [46] | 493 | 52 | 72.5 | Mild CI | <8 nM | TG 50 mg/d | Parallel | A | A | A | A |
T: testosterone, CI: cognitive impairment, AD: Alzheimer's disease, TE: testosterone enanthate, TU: testosterone undecanoate, TG: testosterone gel, A: adequate, NA: not adequate, NR: not reported.
Fig. 1. Weighted standardized mean diff (with 95% confidence interval) of several cognitive domains at end point in randomized controlled trials. Std: standard deviation, diff: difference, LL: lower limit, UL: upper limit.
Fig. 2. Weighted standardized mean diff (with 95% confidence interval) of Mini-Mental State Examination test (MMSE) and depressive symptoms at end point in randomized controlled trials. Std: standard deviation, diff: difference, LL: lower limit, UL: upper limit.
Another recent meta-analysis, including a lower number of RCTs (n=14) and of patients (n=1,406), reported that TRT improved psychomotor speed and executive function. However, the same authors recognized that the effect size was very low, although statistically significant [69].
CONCLUSIONS
Limited preclinical and clinical evidence suggests that T can be involved in the pathogenesis of the agedependent impairment of cognitive functions. However, when T was evaluated with the gold standard method for sex steroid evaluation (i.e., mass spectrometry) no association between age-dependent reduction of T and memory problems was observed. In addition, the present meta-analysis does not support the use of TRT for the improvement of several cognitive domains analyzed. It is important to recognize that the vast majority of available RCTs included mixed populations of subjects with eugonadism and hypogonadism preventing any final conclusion to be drawn on these issues. In fact, positive effects of TRT were observed either on sexual function [70,71] or on body composition [72,73] only when baseline T levels were below 12 nM. Hence, further larger RCTs are advisable in order to better clarify the role of TRT in aging men, and in particular, in those with a cognitive impairment.
Footnotes
Conflicts of Interest: The authors have nothing to disclose.
- Conceptualization: GC, MM.
- Data curation: GC, GR, FG.
- Formal analysis: GC, FG.
- Funding acquisition: none.
- Investigation: GC.
- Methodology: GC.
- Project administration: GC.
- Resources: GC, FG.
- Software: GC.
- Supervision: GC, AS, MM.
- Validation: GC, MM.
- Visualization: GC, MM.
- Writing — original draft: GC, FG.
- Writing — review & editing: GC, FG, GR, AS, MM.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.200017.
Cognitive test used in the randomized, controlled clinical studies included in the meta-analysis
Weighted standardized mean diff (with 95% confidence interval [CI]) of several cognitive domains at end point in randomized controlled trials when the whole population was considered. (A) Attention/working memory; (B) Executive function; (C) Language; (D) Verbal memory (immediate recall); (E) Verbal memory (delayed recall); (F) Visual memory; (G) Visuomotor ability; (H) Visuospatial ability. Std: standard deviation, diff: difference.
Weighted standardized mean diff (with 95% confidence interval [CI]) of several cognitive domains at end point in randomized controlled trials when only patients with cognitive impairment were considered. (A) Executive function; (B) Language; (C) Verbal memory (immediate recall); (D) Verbal memory (delayed recall); (E) Visual memory; (F) Visuomotor ability; (G) Visuospatial ability. Std: standard deviation, diff: difference.
References
- 1.Kontis V, Bennett JE, Mathers CD, Li G, Foreman K, Ezzati M. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet. 2017;389:1323–1335. doi: 10.1016/S0140-6736(16)32381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah H, Albanese E, Duggan C, Rudan I, Langa KM, Carrillo MC, et al. Research priorities to reduce the global burden of dementia by 2025. Lancet Neurol. 2016;15:1285–1294. doi: 10.1016/S1474-4422(16)30235-6. [DOI] [PubMed] [Google Scholar]
- 3.Alzheimer's Disease International. World Alzheimer Report 2015: the global impact of dementia [Internet] London: Alzheimer's Disease International; c2015. [cited 2020 Jan 14]. Available from: https://www.alz.co.uk/research/world-report-2015. [Google Scholar]
- 4.World Health Organization, Alzheimer's Disease International. Dementia: a public health priority. Geneva: World Health Organization; 2012. [Google Scholar]
- 5.Gladman JT, Corriveau RA, Debette S, Dichgans M, Greenberg SM, Sachdev PS, et al. Vascular contributions to cognitive impairment and dementia: research consortia that focus on etiology and treatable targets to lessen the burden of dementia worldwide. Alzheimers Dement (N Y) 2019;5:789–796. doi: 10.1016/j.trci.2019.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corona G, Maseroli E, Rastrelli G, Francomano D, Aversa A, Hackett GI, et al. Is late-onset hypogonadotropic hypogonadism a specific age-dependent disease, or merely an epiphenomenon caused by accumulating disease-burden? Minerva Endocrinol. 2016;41:196–210. [PubMed] [Google Scholar]
- 7.Corona G, Rastrelli G, Di Pasquale G, Sforza A, Mannucci E, Maggi M. Endogenous testosterone levels and cardiovascular risk: meta-analysis of observational studies. J Sex Med. 2018;15:1260–1271. doi: 10.1016/j.jsxm.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Rastrelli G, Lotti F, Reisman Y, Sforza A, Maggi M, Corona G. Metabolically healthy and unhealthy obesity in erectile dysfunction and male infertility. Expert Rev Endocrinol Metab. 2019;14:321–334. doi: 10.1080/17446651.2019.1657827. [DOI] [PubMed] [Google Scholar]
- 9.Lotti F, Rastrelli G, Maseroli E, Cipriani S, Guaraldi F, Krausz C, et al. Impact of metabolically healthy obesity in patients with andrological problems. J Sex Med. 2019;16:821–832. doi: 10.1016/j.jsxm.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Corona G, Vignozzi L, Sforza A, Maggi M. Risks and benefits of late onset hypogonadism treatment: an expert opinion. World J Mens Health. 2013;31:103–125. doi: 10.5534/wjmh.2013.31.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Acaz-Fonseca E, Avila-Rodriguez M, Garcia-Segura LM, Barreto GE. Regulation of astroglia by gonadal steroid hormones under physiological and pathological conditions. Prog Neurobiol. 2016;144:5–26. doi: 10.1016/j.pneurobio.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Liu M, Kelley MH, Herson PS, Hurn PD. Neuroprotection of sex steroids. Minerva Endocrinol. 2010;35:127–143. [PMC free article] [PubMed] [Google Scholar]
- 13.Caruso A, Di Giorgi Gerevini V, Castiglione M, Marinelli F, Tomassini V, Pozzilli C, et al. Testosterone amplifies excitotoxic damage of cultured oligodendrocytes. J Neurochem. 2004;88:1179–1185. doi: 10.1046/j.1471-4159.2004.02284.x. [DOI] [PubMed] [Google Scholar]
- 14.Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res. 2001;892:255–262. doi: 10.1016/s0006-8993(00)03155-3. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Champagne N, Beitel LK, Goodyer CG, Trifiro M, LeBlanc A. Estrogen and androgen protection of human neurons against intracellular amyloid beta1-42 toxicity through heat shock protein 70. J Neurosci. 2004;24:5315–5321. doi: 10.1523/JNEUROSCI.0913-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orlando R, Caruso A, Molinaro G, Motolese M, Matrisciano F, Togna G, et al. Nanomolar concentrations of anabolicandrogenic steroids amplify excitotoxic neuronal death in mixed mouse cortical cultures. Brain Res. 2007;1165:21–29. doi: 10.1016/j.brainres.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 17.Beyer C, Hutchison JB. Androgens stimulate the morphological maturation of embryonic hypothalamic aromataseimmunoreactive neurons in the mouse. Brain Res Dev Brain Res. 1997;98:74–81. doi: 10.1016/s0165-3806(96)00170-8. [DOI] [PubMed] [Google Scholar]
- 18.Lustig RH. Sex hormone modulation of neural development in vitro. Horm Behav. 1994;28:383–395. doi: 10.1006/hbeh.1994.1035. [DOI] [PubMed] [Google Scholar]
- 19.Grimm A, Biliouris EE, Lang UE, Götz J, Mensah-Nyagan AG, Eckert A. Sex hormone-related neurosteroids differentially rescue bioenergetic deficits induced by amyloid-β or hyperphosphorylated tau protein. Cell Mol Life Sci. 2016;73:201–215. doi: 10.1007/s00018-015-1988-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grimm A, Schmitt K, Lang UE, Mensah-Nyagan AG, Eckert A. Improvement of neuronal bioenergetics by neurosteroids: implications for age-related neurodegenerative disorders. Biochim Biophys Acta. 2014;1842(12 Pt A):2427–2438. doi: 10.1016/j.bbadis.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Vasconsuelo A, Milanesi L, Boland R. Actions of 17β-estradiol and testosterone in the mitochondria and their implications in aging. Ageing Res Rev. 2013;12:907–917. doi: 10.1016/j.arr.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Pan Y, Zhang H, Acharya AB, Patrick PH, Oliver D, Morley JE. Effect of testosterone on functional recovery in a castrate male rat stroke model. Brain Res. 2005;1043:195–204. doi: 10.1016/j.brainres.2005.02.078. [DOI] [PubMed] [Google Scholar]
- 23.Cheng J, Alkayed NJ, Hurn PD. Deleterious effects of dihydrotestosterone on cerebral ischemic injury. J Cereb Blood Flow Metab. 2007;27:1553–1562. doi: 10.1038/sj.jcbfm.9600457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McAllister C, Long J, Bowers A, Walker A, Cao P, Honda S, et al. Genetic targeting aromatase in male amyloid precursor protein transgenic mice down-regulates beta-secretase (BACE1) and prevents Alzheimer-like pathology and cognitive impairment. J Neurosci. 2010;30:7326–7334. doi: 10.1523/JNEUROSCI.1180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beauchet O. Testosterone and cognitive function: current clinical evidence of a relationship. Eur J Endocrinol. 2006;155:773–781. doi: 10.1530/eje.1.02306. [DOI] [PubMed] [Google Scholar]
- 26.Wood RI, Serpa RO. Anabolic-androgenic steroid abuse and cognitive impairment: testosterone IMPAIRS biconditional task performance in male rats. Behav Brain Res. 2020;379:112339. doi: 10.1016/j.bbr.2019.112339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yalamanchi S, Dobs A. Debate position: cognition and mood are not improved in men administered exogenous testosterone therapy. Curr Opin Urol. 2017;27:525–531. doi: 10.1097/MOU.0000000000000435. [DOI] [PubMed] [Google Scholar]
- 28.Corona G, Torres LO, Maggi M. Testosterone therapy: What we have learned from trials. J Sex Med. 2020;17:447–460. doi: 10.1016/j.jsxm.2019.11.270. [DOI] [PubMed] [Google Scholar]
- 29.Corona G, Sforza A, Maggi M. Testosterone replacement therapy: long-term safety and efficacy. World J Mens Health. 2017;35:65–76. doi: 10.5534/wjmh.2017.35.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giagulli VA, Guastamacchia E, Licchelli B, Triggiani V. Serum testosterone and cognitive function in ageing male: updating the evidence. Recent Pat Endocr Metab Immune Drug Discov. 2016;10:22–30. doi: 10.2174/1872214810999160603213743. [DOI] [PubMed] [Google Scholar]
- 31.Ciocca G, Limoncin E, Carosa E, Di Sante S, Gravina GL, Mollaioli D, et al. Is testosterone a food for the brain? Sex Med Rev. 2016;4:15–25. doi: 10.1016/j.sxmr.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 32.McGinty HL, Phillips KM, Jim HS, Cessna JM, Asvat Y, Cases MG, et al. Cognitive functioning in men receiving androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. Support Care Cancer. 2014;22:2271–2280. doi: 10.1007/s00520-014-2285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun M, Cole AP, Hanna N, Mucci LA, Berry DL, Basaria S, et al. Cognitive impairment in men with prostate cancer treated with androgen deprivation therapy: a systematic review and meta-analysis. J Urol. 2018;199:1417–1425. doi: 10.1016/j.juro.2017.11.136. [DOI] [PubMed] [Google Scholar]
- 34.Barrett-Connor E, Goodman-Gruen D, Patay B. Endogenous sex hormones and cognitive function in older men. J Clin Endocrinol Metab. 1999;84:3681–3685. doi: 10.1210/jcem.84.10.6086. [DOI] [PubMed] [Google Scholar]
- 35.Yaffe K, Lui LY, Zmuda J, Cauley J. Sex hormones and cognitive function in older men. J Am Geriatr Soc. 2002;50:707–712. doi: 10.1046/j.1532-5415.2002.50166.x. [DOI] [PubMed] [Google Scholar]
- 36.Moffat SD, Zonderman AB, Metter EJ, Kawas C, Blackman MR, Harman SM, et al. Free testosterone and risk for Alzheimer disease in older men. Neurology. 2004;62:188–193. doi: 10.1212/wnl.62.2.188. [DOI] [PubMed] [Google Scholar]
- 37.Fonda SJ, Bertrand R, O'Donnell A, Longcope C, McKinlay JB. Age, hormones, and cognitive functioning among middleaged and elderly men: cross-sectional evidence from the Massachusetts male aging study. J Gerontol A Biol Sci Med Sci. 2005;60:385–390. doi: 10.1093/gerona/60.3.385. [DOI] [PubMed] [Google Scholar]
- 38.Muller M, Aleman A, Grobbee DE, de Haan EH, van der Schouw YT. Endogenous sex hormone levels and cognitive function in aging men: Is there an optimal level? Neurology. 2005;64:866–871. doi: 10.1212/01.WNL.0000153072.54068.E3. [DOI] [PubMed] [Google Scholar]
- 39.Geerlings MI, Strozyk D, Masaki K, Remaley AT, Petrovitch H, Ross GW, et al. Endogenous sex hormones, cognitive decline, and future dementia in old men. Ann Neurol. 2006;60:346–355. doi: 10.1002/ana.20918. [DOI] [PubMed] [Google Scholar]
- 40.Thilers PP, Macdonald SW, Herlitz A. The association between endogenous free testosterone and cognitive performance: a population-based study in 35 to 90 year-old men and women. Psychoneuroendocrinology. 2006;31:565–576. doi: 10.1016/j.psyneuen.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Yeap BB, Almeida OP, Hyde Z, Chubb SA, Hankey GJ, Jamrozik K, et al. Higher serum free testosterone is associated with better cognitive function in older men, while total testosterone is not The health in men study. Clin Endocrinol (Oxf) 2008;68:404–412. doi: 10.1111/j.1365-2265.2007.03055.x. [DOI] [PubMed] [Google Scholar]
- 42.LeBlanc ES, Wang PY, Janowsky JS, Neiss MB, Fink HA, Yaffe K, et al. Osteoporotic Fractures in Men (MrOS) Research Group. Association between sex steroids and cognition in elderly men. Clin Endocrinol (Oxf) 2010;72:393–403. doi: 10.1111/j.1365-2265.2009.03692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, et al. EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363:123–135. doi: 10.1056/NEJMoa0911101. [DOI] [PubMed] [Google Scholar]
- 44.Huhtaniemi IT, Tajar A, Lee DM, O'Neill TW, Finn JD, Bartfai G, et al. EMAS Group. Comparison of serum testosterone and estradiol measurements in 3174 European men using platform immunoassay and mass spectrometry; relevance for the diagnostics in aging men. Eur J Endocrinol. 2012;166:983–991. doi: 10.1530/EJE-11-1051. [DOI] [PubMed] [Google Scholar]
- 45.Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2018;103:1715–1744. doi: 10.1210/jc.2018-00229. [DOI] [PubMed] [Google Scholar]
- 46.Resnick SM, Matsumoto AM, Stephens-Shields AJ, Ellenberg SS, Gill TM, Shumaker SA, et al. Testosterone treatment and cognitive function in older men with low testosterone and age-associated memory impairment. JAMA. 2017;317:717–727. doi: 10.1001/jama.2016.21044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tierney MC, Yao C, Kiss A, McDowell I. Neuropsychological tests accurately predict incident Alzheimer disease after 5 and 10 years. Neurology. 2005;64:1853–1859. doi: 10.1212/01.WNL.0000163773.21794.0B. [DOI] [PubMed] [Google Scholar]
- 48.Bilgel M, An Y, Lang A, Prince J, Ferrucci L, Jedynak B, et al. Trajectories of Alzheimer disease-related cognitive measures in a longitudinal sample. Alzheimers Dement. 2014;10:735–742.e4. doi: 10.1016/j.jalz.2014.04.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cherrier MM, Matsumoto AM, Amory JK, Ahmed S, Bremner W, Peskind ER, et al. The role of aromatization in testosterone supplementation: effects on cognition in older men. Neurology. 2005;64:290–296. doi: 10.1212/01.WNL.0000149639.25136.CA. [DOI] [PubMed] [Google Scholar]
- 50.Janowsky JS. The role of androgens in cognition and brain aging in men. Neuroscience. 2006;138:1015–1020. doi: 10.1016/j.neuroscience.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 51.Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, et al. Lessons from the testosterone trials. Endocr Rev. 2018;39:369–386. doi: 10.1210/er.2017-00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Janowsky JS, Oviatt SK, Orwoll ES. Testosterone influences spatial cognition in older men. Behav Neurosci. 1994;108:325–332. doi: 10.1037//0735-7044.108.2.325. [DOI] [PubMed] [Google Scholar]
- 53.Janowsky JS, Chavez B, Orwoll E. Sex steroids modify working memory. J Cogn Neurosci. 2000;12:407–414. doi: 10.1162/089892900562228. [DOI] [PubMed] [Google Scholar]
- 54.Cherrier MM, Asthana S, Plymate S, Baker L, Matsumoto AM, Peskind E, et al. Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology. 2001;57:80–88. doi: 10.1212/wnl.57.1.80. [DOI] [PubMed] [Google Scholar]
- 55.Kenny AM, Bellantonio S, Gruman CA, Acosta RD, Prestwood KM. Effects of transdermal testosterone on cognitive function and health perception in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2002;57:M321–M325. doi: 10.1093/gerona/57.5.m321. [DOI] [PubMed] [Google Scholar]
- 56.Kenny AM, Fabregas G, Song C, Biskup B, Bellantonio S. Effects of testosterone on behavior, depression, and cognitive function in older men with mild cognitive loss. J Gerontol A Biol Sci Med Sci. 2004;59:75–78. doi: 10.1093/gerona/59.1.m75. [DOI] [PubMed] [Google Scholar]
- 57.Cherrier MM, Matsumoto AM, Amory JK, Asthana S, Bremner W, Peskind ER, et al. Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology. 2005;64:2063–2068. doi: 10.1212/01.WNL.0000165995.98986.F1. [DOI] [PubMed] [Google Scholar]
- 58.Haren MT, Wittert GA, Chapman IM, Coates P, Morley JE. Effect of oral testosterone undecanoate on visuospatial cognition, mood and quality of life in elderly men with low-normal gonadal status. Maturitas. 2005;50:124–133. doi: 10.1016/j.maturitas.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 59.Lu PH, Masterman DA, Mulnard R, Cotman C, Miller B, Yaffe K, et al. Effects of testosterone on cognition and mood in male patients with mild Alzheimer disease and healthy elderly men. Arch Neurol. 2006;63:177–185. doi: 10.1001/archneur.63.2.nct50002. [DOI] [PubMed] [Google Scholar]
- 60.Maki PM, Ernst M, London ED, Mordecai KL, Perschler P, Durso SC, et al. Intramuscular testosterone treatment in elderly men: evidence of memory decline and altered brain function. J Clin Endocrinol Metab. 2007;92:4107–4114. doi: 10.1210/jc.2006-1805. [DOI] [PubMed] [Google Scholar]
- 61.Vaughan C, Goldstein FC, Tenover JL. Exogenous testosterone alone or with finasteride does not improve measurements of cognition in healthy older men with low serum testosterone. J Androl. 2007;28:875–882. doi: 10.2164/jandrol.107.002931. [DOI] [PubMed] [Google Scholar]
- 62.Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, Aleman A, Lock TM, Bosch JL, et al. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA. 2008;299:39–52. doi: 10.1001/jama.2007.51. [DOI] [PubMed] [Google Scholar]
- 63.Fukai S, Akishita M, Yamada S, Toba K, Ouchi Y. Effects of testosterone in older men with mild-to-moderate cognitive impairment. J Am Geriatr Soc. 2010;58:1419–1421. doi: 10.1111/j.1532-5415.2010.02946.x. [DOI] [PubMed] [Google Scholar]
- 64.Borst SE, Yarrow JF, Fernandez C, Conover CF, Ye F, Meuleman JR, et al. Cognitive effects of testosterone and finasteride administration in older hypogonadal men. Clin Interv Aging. 2014;9:1327–1333. doi: 10.2147/CIA.S61760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cherrier MM, Anderson K, Shofer J, Millard S, Matsumoto AM. Testosterone treatment of men with mild cognitive impairment and low testosterone levels. Am J Alzheimers Dis Other Demen. 2015;30:421–430. doi: 10.1177/1533317514556874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang G, Wharton W, Bhasin S, Harman SM, Pencina KM, Tsitouras P, et al. Effects of long-term testosterone administration on cognition in older men with low or low-to-normal testosterone concentrations: a prespecified secondary analysis of data from the randomised, double-blind, placebo-controlled TEAAM trial. Lancet Diabetes Endocrinol. 2016;4:657–665. doi: 10.1016/S2213-8587(16)30102-4. [DOI] [PubMed] [Google Scholar]
- 67.Wahjoepramono EJ, Asih PR, Aniwiyanti V, Taddei K, Dhaliwal SS, Fuller SJ, et al. The effects of testosterone supplementation on cognitive functioning in older men. CNS Neurol Disord Drug Targets. 2016;15:337–343. doi: 10.2174/1871527315666151110125704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. 4th ed. Oxford: Oxford University Press; 2004. [Google Scholar]
- 69.Tan S, Sohrabi HR, Weinborn M, Tegg M, Bucks RS, Taddei K, et al. Effects of testosterone supplementation on separate cognitive domains in cognitively healthy older men: a metaanalysis of current randomized clinical trials. Am J Geriatr Psychiatry. 2019;27:1232–1246. doi: 10.1016/j.jagp.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 70.Rastrelli G, Guaraldi F, Reismann Y, Sforza A, Isidori AM, Maggi M, et al. Testosterone replacement therapy for sexual symptoms. Sex Med Rev. 2019;7:464–475. doi: 10.1016/j.sxmr.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 71.Rastrelli G, Corona G, Maggi M. Testosterone and sexual function in men. Maturitas. 2018;112:46–52. doi: 10.1016/j.maturitas.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 72.Corona G, Giagulli VA, Maseroli E, Vignozzi L, Aversa A, Zitzmann M, et al. Therapy of endocrine disease: testosterone supplementation and body composition: results from a metaanalysis study. Eur J Endocrinol. 2016;174:R99–R116. doi: 10.1530/EJE-15-0262. [DOI] [PubMed] [Google Scholar]
- 73.Rastrelli G, Maggi M, Corona G. Pharmacological management of late-onset hypogonadism. Expert Rev Clin Pharmacol. 2018;11:439–458. doi: 10.1080/17512433.2018.1445969. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cognitive test used in the randomized, controlled clinical studies included in the meta-analysis
Weighted standardized mean diff (with 95% confidence interval [CI]) of several cognitive domains at end point in randomized controlled trials when the whole population was considered. (A) Attention/working memory; (B) Executive function; (C) Language; (D) Verbal memory (immediate recall); (E) Verbal memory (delayed recall); (F) Visual memory; (G) Visuomotor ability; (H) Visuospatial ability. Std: standard deviation, diff: difference.
Weighted standardized mean diff (with 95% confidence interval [CI]) of several cognitive domains at end point in randomized controlled trials when only patients with cognitive impairment were considered. (A) Executive function; (B) Language; (C) Verbal memory (immediate recall); (D) Verbal memory (delayed recall); (E) Visual memory; (F) Visuomotor ability; (G) Visuospatial ability. Std: standard deviation, diff: difference.