Abstract
Purpose
We analyzed the database from the Korean National Infectious Diseases Surveillance to reveal clinical characteristics of co-infection with Neisseria gonorrhoeae (NG) and Chlamydia trachomatis (CT).
Materials and Methods
Eligible cases included a single NG infection (male/female) for 6,421 (4,975/1,446), a single CT infection for 20,436 (6,107/14,329), and co-infection for 498 (233/265) between 2011 and 2015.
Results
Cases of NG and CT have increased for 5 years; the proportion of co-infected male has increased continuously and was positively correlated with that of CT infections. But the proportion of co-infected female was positively correlated with that of NG infections, following an expanded wavelike-pattern. Generally, people with co-infection was younger than either infection alone (p=0.001). But the characteristics of co-infection revealed sex-specific differences. While the co-infected females were younger than females in NG (p=0.001) or CT group (p=0.001), the co-infected males were younger than males in CT (p=0.001) only, not males in the NG group (p=0.394). Amongst males, 4.47% with NG had CT infection, while in female 15.49% with NG had CT (p=0.001). In contrast, in male 3.68% with CT infection had NG infection and in female 1.82% of CT had NG (p=0.001). Young people in both sexes have increased risks of co-infection bi-directionally (all p=0.001), except males with NG that were also co-infected with CT (p=0.642).
Conclusions
The sex-specific findings in co-infection may improve understanding of gender-specific characteristics in NG and CT infections. Co-infected people are increasing for 5 years. Therefore, we must consider long-term complication of the co-infected people.
Keywords: Big data, Chlamydia trachomatis, Coinfection, Neisseria gonorrhoeae
INTRODUCTION
Sexually transmitted infections (STIs) have serious impacts on quality of human life. Of many STIs, Neisseria gonorrhoeae (NG) and Chlamydia trachomatis (CT) induce genito-urinary infections [1,2]. To control spreading of NG and CT infections, unbiased studies in epidemiology and clinical characteristics must be appropriately conducted [3].
Previous epidemiology studies on NG and CT infections reported a wide range of results from different clinical settings and patient groups [1,2]. In addition, as most clinical studies for co-infection, either NG co-infected with CT or CT co-infected with NG, are usually performed in selected patient groups, many biases are to be generalized. Indeed, the proportion of NG co-infection with CT in the literature varies considerably, from less than 4% up to more than 60% [4,5,6,7,8].
Characteristics of co-infection have been mostly studied in cases of NG co-infected CT [4,5,7]. In contrast, studies for CT co-infected with NG or bilateral co-infected cases are rarely reported to characterize their bi-directional clinical significance [6]. As far as we understand, no data have been published about the epidemiology of NG, CT, and bilateral co-infections in a large Korean population database.
Such combination of STIs may increase the susceptibility to long-term complications and transmissibility than each individual case. Stupiansky et al [9] revealed that NG shedding was higher in women with concurrent CT infection than in those infected with NG alone. Nsuami et al [10] reported that symptoms related to STIs are reported more frequently by co-infected students than those with a single infection case. In addition, concurrent NG infection may reactivate latent CT infections [11]. These reports are also supported by Vonck et al [12], who showed that NG colonization was significantly higher in infected female mice with preexisting infection of Chlamydia muridarum, the murine strain of chlamydia.
This study aims to characterize the co-infection of NG and CT from the database of the National Infectious Diseases Surveillance of Korea (NIDS) of the Korea Centers for Disease Control and Prevention (K-CDC). In order to do this, we categorized a single NG, a single CT, and their co-infection from the NIDS database from 2011 to 2015, and examined their trends during the periods. Finally, we calculated the proportions of either NG co-infected with CT or CT co-infected with NG, and estimated specific subpopulations at the risk of co-infection.
MATERIALS AND METHODS
1. Sentinel sites and their reports to the Korean National Infectious Diseases Surveillance of Korea
Approximately 500 medical facilities and public health centers (PHC) in Korea were appointed as sentinel sites for STIs surveillance. The number of sentinel sites depended on the size of regional population, but regions with less than 100,000 people have at least one sentinel site as an integral post for STI surveillance in the lower population density areas.
The medical providers in the sentinel sites were to report cases of NG and CT within 7 days after the diagnosis. In these reports, information such as patient's sex, age, day of diagnosis, name of STIs, whether NG were confirmed with guaranteed laboratory tests or symptomatic diagnosis, and the names of co-infection were also included (Supplement 1) [13,14,15]. Confirmed diagnostic methods for NG infections included culture, antigen detection, gram staining, and nucleic acid amplification test (NAAT), while methods for CT infections included culture, antigen detection, and NAAT.
2. Definition of co-infection from the National Infectious Diseases Surveillance of Korea dataset
We obtained the reported cases of NG and CT from the NIDS dataset of Korea (http://www.cdc.go.kr/npt/biz/npp/iss/stisStatisticsMain.do) between January 1, 2011 and December 31, 2015. If the individual cases of NG or CT with identical sex and age were coincidently diagnosed on the same day at the same clinic, reported both infections to regional PHCs for review on a single day, and also deposited the reviewed data in the Korean NIDS database on a single day, such case of NG and CT was assumed to be from a single person, suggesting co-infection.
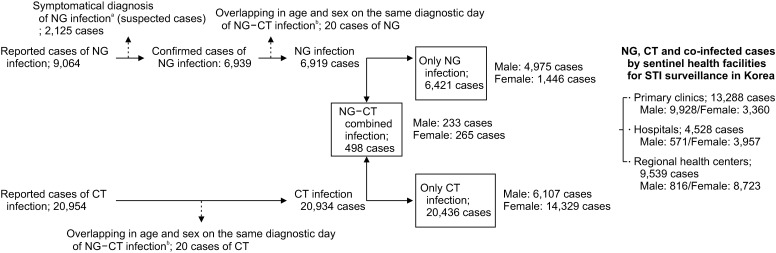
The reported cases of NG and CT in the NIDS database of the K-CDC during the five years were primarily categorized by the day of diagnosis, followed by the age parameter, and lastly by the sex parameter in a single clinic. Finally, we merged the datasets of NG and CT infections. Between these periods, 9,064 cases were reported as NG infection, and 20,954 cases as CT infection. We removed 2,125 NG cases because they were reported as suspected NG patients based on their clinical symptoms, not by the specified diagnostic tests mentioned previously. We also removed 20 cases from each NG and CT infection group that overlap in age and sex on the same day of diagnosis in the same clinic (Fig. 1, Supplement 2).
Fig. 1. Flow chart of the study. NG: Neisseria gonorrhoeae, CT: Chlamydia trachomatis, NG-CT: Neisseria gonorrhoeae and Chlamydia trachomatis co-infection. aTwo types of gonococcal infections, confirmed and suspected cases, are reported through the Korean Sentinel Surveillance System for sexually transmitted Infections (STIs). The suspected cases are defined as symptomatic persons that were not confirmed with specific laboratory tests. bWe removed 20 cases from each NG and CT infection group that overlap in age and sex parameters on the same day of diagnosis.
3. Statistical analysis
We used analysis of variance (ANOVA) to evaluate the difference in the ordinal scores among the NG, CT, and co-infection. The Tukey's test was used as a post-hoc test. Pearson chi-square test was used to evaluate the difference in categorical data. Odds ratios (OR) and 95% confidence intervals (CI) were estimated with logistic regression analysis. Two-sided null hypotheses of no difference were rejected if p-values were less than 0.05. All analyses were performed using IBM SPSS for Windows ver. 23 (IBM Corp., Armonk, NY, USA).
4. Ethics statement
This study did not require approval from the Institutional Review Board because the dataset did not contain any personal information that may discern personal identity.
RESULTS
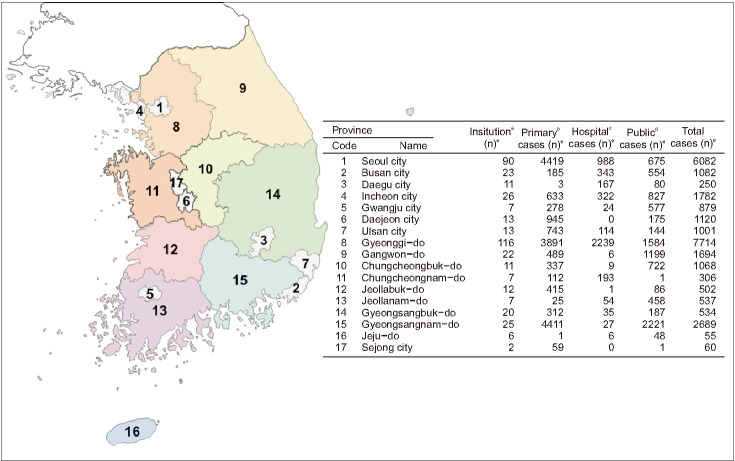
1. Locations and numbers of the health facilities that reported the Chlamydia trachomatis and Neisseria gonorrhoeae infections to the National Infectious Diseases Surveillance of Korea
A total of 411 nationwide sentinel sites from the 500 appointed facilities reported one or more cases of CT or NG infections (Fig. 2).
Fig. 2. Location and number of the health facilities that participate in the Korean Sentinel Surveillance System for sexually transmitted infections (STI), and their reported cases of gonorrhea, chlamydia, and co-infection. aThere are 3 sentinel types of health institutions in Korea; medical doctor's office for primary health care, hospitals, and public health centers. bPrimary health cares are usually performed in doctor's offices where one or more doctors provide medical services to patients. The doctor for primary health care usually acts as the first point of consultation for all patients. Male STI patients usually visit the primary health care centers or walk-in clinics. cHospitals as a secondary care system usually provide the STI related services or antenatal cares. dPublic health centers provide health care interventions outside the formal health facilities. eNo, reported numbers of gonorrhea, chlamydia, and co-infections.
2. Eligible cases for a single Neisseria gonorrhoeae, a single Chlamydia trachomatis, and co-infection from the National Infectious Diseases Surveillance of Korea dataset
There were 6,421 cases of NG infection, 20,436 cases of CT infection, and 498 cases of co-infection (Fig. 1). Of these reports, 13,288 cases were reported from primary clinics, 4,528 cases were reported from hospitals, and 9,539 cases from PHCs (Fig. 1).
3. Trend of reported cases in Neisseria gonorrhoeae and Chlamydia trachomatis infections during the 5-year study period
The reported cases of NG and CT infections increased each year during the five-year period. Especially, the number of infected cases had increased dramatically in 2015 compared to any previous year (Table 1, Fig. 3). Average ages from 2011 to 2015 were significantly different in both sexes (p=0.001 for male and p=0.009) (ANOVA; Table 1). Although the average age of male patients in 2014 was significantly different from the average age of male in 2011 (p=0.002) and 2013 (p=0.004), the average age of male in 2015 was not significantly different when compared to the entire four year period (all p>0.05) (post-hoc test; Table 1). In female, we found that the average age of patients in 2015 showed a marginal difference from the average age in 2012 (p=0.047) (post-hoc test; Table 1).
Table 1. The number and average age of reported gonorrhea, chlamydia, and co-infected cases from the Korean Surveillance System for sexually transmitted infections between 2011 and 2015, by sex.
| Sex | Diagnosed year | Case | Mean age (y) |
|---|---|---|---|
| Male | 2011 | 1,579 | 32.75 (32.26–33.23) |
| 2012 | 1,813 | 33.50 (33.04–33.96) | |
| 2013 | 1,892 | 32.87 (32.45–33.29) | |
| 2014 | 2,255 | 33.99 (33.55–34.42) | |
| 2015 | 3,776 | 33.38 (33.05–33.71) | |
| Total | 11,315 | 33.35 (33.16–33.53) | |
| Female | 2011 | 2,748 | 31.08 (30.74–31.42) |
| 2012 | 2,752 | 30.96 (30.62–31.296) | |
| 2013 | 2,877 | 31.62 (31.28–31.96) | |
| 2014 | 2,971 | 31.60 (31.25–31.95) | |
| 2015 | 4,692 | 31.60 (31.299–31.89) | |
| Total | 16,040 | 31.40 (31.25–31.55) | |
| All | 2011 | 4,327 | 31.69 (31.41–31.97) |
| 2012 | 4,565 | 31.97 (31.69–32.24) | |
| 2013 | 4,769 | 32.12 (31.85–32.38) | |
| 2014 | 5,226 | 32.63 (32.35–32.91) | |
| 2015 | 8,468 | 32.39 (32.17–32.61) | |
| Total | 27,355 | 32.21 (32.09–32.32) |
Values are presented as number only or mean (95% confidence interval).
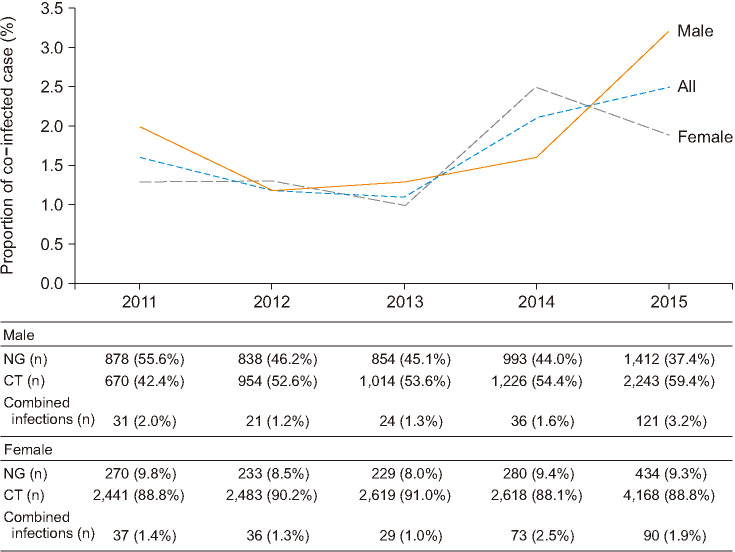
Fig. 3. Since 2013, the proportion of co-infected cases was increasing (upper) and ratios of Neisseria gonorrhoeae (NG), Chlamydia trachomatis (CT), and co-infected cases between 2011 to 2015 (lower).
4. Changes of proportions of Neisseria gonorrhoeae, Chlamydia trachomatis, and co-infection from 2011 to 2015
Since 2013, the proportion of co-infected patients had been increasing. The proportion of co-infected males had increased steadily from 2012 to 2014 and surged up from 2014 to 2015. Among females, the proportion of co-infection showed an expanded wavelike-pattern. The pattern of the changes in proportion of co-infected male was positively associated with that of a single CT infection, while the proportion of co-infected female was positively associated with that of a single NG infection (Fig. 3).
5. Average ages among Neisseria gonorrhoeae, Chlamydia trachomatis and co-infected cases
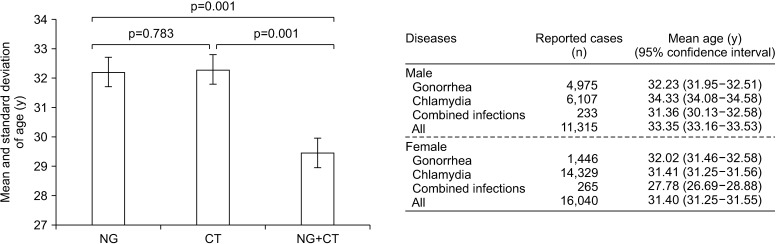
Fig. 4 shows that the mean ages and standard deviations of the reported case of NG, CT, and co-infection were 32.18±10.29, 32.28±9.34, and 29.46±9.46 year-old in both sexes (p=0.001; ANOVA); 32.23±10.12, 34.33±10.00, and 31.36±9.54 year-old in males (p=0.001), and 32.02±10.85, 31.41±9.49, and 27.78±9.08 year-old in females (p=0.001), respectively. For NG infections, the differences between the average ages of male and female patients were not statistically significant (p=0.494), while significant differences was observed between male and female patients of CT (p=0.001), and co-infection (p=0.001). Although the average age of co-infected females was younger than that of individual infections (p=0.001 for all comparisons), age distribution in the co-infected males was different from the average age in CT infected cases (p=0.001), but not in NG cases (p=0.394) (Fig. 4).
Fig. 4. Mean ages and standard deviations of the total reported case of Neisseria gonorrhoeae (NG), and Chlamydia trachomatis (CT), and co-infection (NG+CT) were 32.18±10.29, 32.28±9.34, and 29.46±9.46 year (left). NG+CT and their mean ages between male and female (right). The p-value estimated using ANOVA.
6. Changes of either Neisseria gonorrhoeae (NG) co-infected with Chlamydia trachomatis (CT) or CT co-infected with NG during the 5-year study period and their characteristics between two sexes
From 2012 to 2015, the proportion of males in either NG co-infected with CT or CT co-infected with NG groups were increasing in a stepwise pattern (p<0.0001) (Table 2). Among females, the proportion of either NG co-infected with CT and CT co-infected with NG had reached peaks in 2014 and decreased in 2015 (Table 2).
Table 2. Changes of either NG co-infected CT or CT co-infected with NG during the 5-year study period.
| Variable | Year | Co-infection | All NG infection | NG co-infected with CT (%) | p-value | All CT infection | CT co-infected with NG (%) | p-value |
|---|---|---|---|---|---|---|---|---|
| Male | 2011 | 31 | 909 | 3.41 (2.23–4.59) | <0.0001 | 701 | 4.42 (2.90–5.94) | 0.46 |
| 2012 | 21 | 859 | 2.44 (1.41–3.47) | <0.0001 | 975 | 2.15 (1.24–3.06) | 0.0001 | |
| 2013 | 24 | 878 | 2.73 (1.65–3.81) | <0.0001 | 1,038 | 2.31 (1.40–3.22) | 0.0002 | |
| 2014 | 36 | 1,029 | 3.50 (2.38–4.62) | <0.0001 | 1,262 | 2.85 (1.93–3.77) | 0.001 | |
| 2015 | 121 | 1,533 | 7.89 (6.54–9.24) | Ref. | 2,364 | 5.12 (4.23–6.01) | Ref. | |
| All | 233 | 5,208 | 4.47 (3.91–5.03) | 6,340 | 3.68 (3.22–4.14) | |||
| Female | 2011 | 37 | 307 | 12.05 (8.41–15.69) | 0.05 | 2,478 | 1.49 (1.01–1.97) | 0.07 |
| 2012 | 36 | 269 | 13.38 (9.31–17.45) | 0.17 | 2,519 | 1.43 (0.97–1.89) | 0.04 | |
| 2013 | 29 | 258 | 11.24 (7.39–15.09) | 0.03 | 2,648 | 1.10 (0.70–1.50) | 0.002 | |
| 2014 | 73 | 353 | 20.68 (16.45–24.91) | 0.19 | 2,691 | 2.71 (2.10–3.32) | 0.11 | |
| 2015 | 90 | 524 | 17.18 (13.95–20.41) | Ref. | 4,258 | 2.11 (1.68–2.54) | Ref. | |
| All | 265 | 1,711 | 15.49 (13.78–17.20) | 14,594 | 1.82 (1.60–2.04) |
Values are presented as number only or odds ratio (95% confidence interval).
NG: Neisseria gonorrhoeae, CT: Chlamydia trachomatis, Ref.: reference.
Overall, 4.47% (95% CI, 3.91% to 5.03%) of males and 15.49% (95% CI, 13.78% to 17.20%) of females with NG also had CT (p=0.001), and 3.68% (95% CI, 3.22% to 4.14%) of males and 1.82% (95% CI, 1.60% to 2.04%) of females with CT also had NG (p=0.001). Age was a universal risk factor for co-infected females; the risk of co-infection decreased with older age in females of both NG co-infected with CT (p=0.0001) or CT co-infected with NG (p=0.0001). Although CT infected males under 25 years of age had increased risk of NG infection than other ages (p=0.001), males with NG infection was not found to have age-associated risk of co-infection (p=0.642) (Table 3).
Table 3. Age factor for co-infection of NG and CT and their 95% CI of person with NG who also had CT and person with CT who also had NG.
| Variable | Age (y) | Co-infection | NG co-infected with CT (%) | p-value | All CT infection (%) | p-value |
|---|---|---|---|---|---|---|
| Male | <25 | 61 | 4.76 (3.59–5.93) | Ref. | 1,049 (4.40–7.24) | Ref. |
| 25–55 | 168 | 4.43 (3.78–5.08) | 0.62 | 5,090 (2.81–3.79) | <0.0001 | |
| >55 | 4 | 3.05 (0.10–6.00) | 0.37 | 201 (0.06–3.92) | 0.03 | |
| All | 233 | 4.47 (3.91–5.03) | 6,340 (3.22–4.14) | |||
| Probability (p-value) | 0.642 | |||||
| Female | <25 | 118 | 22.56 (18.98–26.14) | Ref. | 3,914 (2.47–3.55) | Ref. |
| 25–55 | 145 | 12.79 (10.85–14.73) | <0.0001 | 10,436 (1.17–1.61) | <0.0001 | |
| >55 | 2 | 3.70 (−1.34–8.74) | 0.001 | 244 (−0.31–1.95) | 0.05 | |
| All | 265 | 15.49 (13.78–17.20) | 14,594 (1.60–2.04) | |||
| Probability (p-value) | 0.001 |
Values are presented as number only or odds ratio (95% CI).
NG: Neisseria gonorrhoeae, CT: Chlamydia trachomatis, CI: confidence interval, Ref.: reference.
DISCUSSION
The considerable variations of co-infection in literatures are likely because these studies have been performed in clinical settings with small number of selected patients [4,5,6,7,8]. Therefore, to reduce this variation we analyzed the NIDS dataset in Korea.
Recent updates in clinical practice may have influenced on epidemiology of STIs in Korea [13,15,16], and have thus motivated us to design this study.
First of all, in 2011 the K-CDC updated its surveillance system of STIs by removing non-gonococcal infections from the previous list, while also broadening the report form from two (confirmed and suspicious infection) to three categories (confirmed, suspicious and carriers) [13,15]. This revision reflects the recent changes in the diagnostic field of micro-organisms. To follow the new regulation, almost all of the reported NG and CT infections to the NIDS through primary clinics and hospitals were to be diagnosed through multiplex-NAAT methods. Practically, the diagnostic practices for the infections through culturing NG and CT have not been routinely performed in Korean clinics. In addition, only a negligible number of CT infected cases are reported by PHCs by some antigen detection methods in small-sized rural regions in Korea (We sent e-mails to all PHCs to survey the currently employed methods for both infections, and to estimate the numbers of infections) [15]. Accordingly, we confirmed that the reported cases of NG and CT infections from the sentinel sites were mainly by multiplex NAAT assays.
Another change to the STI guideline was that uncomplicated NG are now to be treated with a combination therapy [16]. The proportion of reported co-infection in previous literatures varied from less than 4% up to more than 60% [4,5,6]. If more than 60% NG infected persons are in fact co-infected, the mandatory combination with anti-chlamydial regimen may reduce chlamydial prevalence in near future. In contrast, if co-infected cases are less than 4% of the NG infected cases, the effect of combination therapy on chlamydial prevalence would be marginal in future. Our result showed that the proportions of NG co-infected with CT was only 4.47% in male (Table 2). Because of small numbers of co-infection in males, the mandatory combination therapy did not reduce CT infections in 2013, 2014, and 2015 based on our data analysis.
Thirdly, clinical practices with multiplex NAATs for the diagnosis of STIs was enlisted on the Korean National Medical Insurance since January 1, 2015. Accordingly, NG or CT infected cases have been detected more frequently than the previous years. Our results showed that the reported cases of NG or CT infection in 2015 have increased abruptly from the previous years (Table 1, Fig. 3). Interestingly, while the number of reported cases increased dramatically, we could not find any statistical difference in patients' age between 2014 and 2015 (Table 1). This suggests that the increased number of reports on NG and CT infections in 2015 may be due to the increased application of multiplex NAATs.
As the reported data in the NIDS on NG and CT infections does not contain detailed personal information of each individual case, we could not identify the co-infected cases based on personal information [13] (Supplement 1). Therefore, we hypothesized that because reported cases of NG and CT from sentinel sites are mainly based on the results of multiplex NAAT assays, co-infected cases should be considered only if individual cases of NG or CT infections with identical sex and age are coincidently diagnosed on the same day at the same clinic. Nevertheless, if more than two cases of the hypothetically defined co-infection were found within the merged dataset, it would be difficult to distinguish such cases. Therefore, we identified multiple overlapping cases of matching age-sex-diagnosed date from 11 medical institutions (Supplement 2) and removed 20 overlapping cases of each NG and CT infection (Fig. 1, Supplement 2). In addition, we also considered the scenario in which the matched case of sex-age-date of diagnosis from the same medical institution does not actually identify a single patient but rather a combination of multiple patients by chance. To estimate such accidental occurrence, we reviewed the numbers of reported NG cases age by age and institution by institution during the 5-year study period. We found that the total numbers of reported NG cases did not exceed 30 patients by each age group over 1,825 days or 5 years in all individual institutions (Supplement 3). In other words, the probability of NG diagnosis from a single medical facility was smaller than 30/1,825 per day for each age group. This number gets smaller if we include sex parameter, suggesting that the probability of accidently matched cases are rare. Therefore, we concluded that it is highly unlikely that such scenarios would influence the statistical significance.
The proportion in males of either NG co-infected with CT or CT co-infected with NG reached peak in 2015, while in female the proportion peaked in 2014 and slightly decreased in 2015 (Table 2). The increasing pattern of co-infection in male and an expanding wave-like pattern in female suggest that the proportion of co-infection among the population are increasing among the two sexes, albeit with different patterns (Fig. 3). Reasons for these differences are not well understood, but natural history and immunobiology between two infections may account for the differences [1,2,17]. In near future, we would examine the influential factors that affect the differences between the sexes.
Our nationwide study showed that the proportions of co-infection in both sexes were lower than what was initially perceived. Furthermore, our data would consolidate the general belief that the rates of co-infection decreased with age. The highest age-specific rates of reported cases of CT and NG infections in USA were among those aged 20 to 24 years. Yet, surveillance data in the NIDS database of K-CDC showed that those aged 25 to 39 years had the highest rates of both NG and CT infections among the reported cases [16]. We verified the NIDS data from sentinel sites with the data from the Health Insurance Review & Assessment Serve (HIRA) of Korea. We found that the trends and patterns in sex and age parameters of NG and CT patients were very similar between the dataset from NIDS and the HIRA from 2011 to 2015 [15]. Therefore, the lower proportion of co-infections in this study may be derived from the relatively older age of NG and CT infections found in the Korean population compared to the Americans and averaging effects in patients' characteristics in the nationwide dataset.
According to our results, female patients with NG infections are 4 times more likely to be exposed to concurrent CT infection than male patients (Table 3). This relationship is consistently found in other literatures and therefore justifies the policy of administering combined antibiotic therapy [2,4,5,16,18]. However, the opposite scenario in which CT infected patients are evaluated for NG co-infection is less well defined. Our results showed that only 3.68% of males and 1.82% of females with CT infections also had NG. Therefore, people with NG infections have higher risk for co-infection than persons with CT infections (Table 2).
Adolescents are associated with higher incidences of NG and CT infections [1,2,17,19], and they have higher rates of co-infection when compared to individuals in older age groups [4,5,10]. Our results were also similar, with co-infection being commonly found in younger female population. Specifically, the risk of co-infection in female when NG was detected was attenuated by older age (one year OR, 0.957; 95% CI, 0.943–0.971; p=0.001). Similarly, the risk of co-infection in female when CT is detected was also correlated with age (one year OR, 0.952; 95% CI, 0.937–0.967; p=0.001). In addition, the risk of co-infection in male when CT is detected was significantly decreased with older age (one year OR, 0.967; 95% CI, 0.953–0.981; p=0.001). However, males with NG infection was not affected with age, as their risk of co-infection was relatively steady regardless of age (one year OR, 0.991; 95% CI, 0.978–1.005; p=0.195) (Table 3).
It is unclear if screening tests for NG and CT infections in males would be of additional benefit over screening females. However, we revealed some ominous findings in STIs in Korean males. Therefore, we must watch the trends of STIs in Korean males, as well as in females.
CONCLUSIONS
Our study report that both NG and CT cases have increased and the proportion of their co-infection has also increased over the 5-year study period. The average age of the co-infection group was younger than either infection alone. Nevertheless, characteristics of co-infection in each sex were not always consistent. When we consider that co-infection is frequently found in younger ages, we must take account of long-term complications of young people and easy transmission to sexual partners.
ACKNOWLEDGEMENTS
This research was supported by a Research Fund of Dankook University in 2018.
The authors thank the members in Department of Infectious Disease Surveillance of the Korean Center for Infectious Diseases Control for providing the invaluable surveillance dataset for this study.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
- Conceptualization: GL.
- Data curation: YS, KHC, GL.
- Formal analysis: YS, KHC, GL.
- Funding acquisition: GL.
- Investigation: YS, KHC, GL.
- Methodology: YS, KHC, GL.
- Project administration: GL.
- Resources: YS, KHC, GL.
- Software: KHC.
- Supervision: GL.
- Validation: YS, KHC, GL.
- Visualization: KHC, GL.
- Writing — original draft: YS, GL.
- Writing — review & editing: YS, KHC, GL.
Data Sharing Statement
The data analyzed for this study have been deposited in HARVARD Dataverse and are available at https://doi.org/10.7910/DVN/P98SOR.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.190116.
Official report form for sexually transmitted diseases from the Korean National Infectious Diseases Surveillance of the Korea Centers for Disease Control and Prevention.
Multiple overlapping in age and sex parameters in the same diagnostic day of Neisseria gonorrhoeae (NG) and Chlamydia trachomatis (CT) infections
Numbers of reported gonococcal infected case (X axis: Hospital code. Y axis: Age code. Z axis: numbers of reported case) between 2011 to 2015.
References
- 1.Stamm WE. Chlamydia trachomatis infections of the adult. In: Holmes KK, Sparling PF, Mardh PA, Lemon SM, Stamm WE, Piot P, et al., editors. Sexually transmitted diseases. 3rd ed. New York: McGraw-Hill; 1999. pp. 407–422. [Google Scholar]
- 2.Hook EW, III, Handsfield HH.> . Gonococcal infections in the adult. In: Holmes KK, Sparling PF, Mardh PA, Lemon SM, Stamm WE, Piot P, et al., editors. Sexually transmitted diseases. 3rd ed. New York: McGraw-Hill; 1999. pp. 451–466. [Google Scholar]
- 3.Garrett NJ, Osman F, Maharaj B, Naicker N, Gibbs A, Norman E, et al. Beyond syndromic management: opportunities for diagnosis-based treatment of sexually transmitted infections in low- and middle-income countries. PLoS One. 2018;13:e0196209. doi: 10.1371/journal.pone.0196209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwan CK, Ho HF, Ho KM. Co-infection rate of chlamydial urethritis in patients with gonorrhea in Hong Kong. Hong Kong J Dermatol Venereol. 2013;21:56–63. [Google Scholar]
- 5.Dragovic B, Greaves K, Vashisht A, Straughair G, Sabin C, Smith NA. Chlamydial co-infection among patients with gonorrhoea. Int J STD AIDS. 2002;13:261–263. doi: 10.1258/0956462021925063. [DOI] [PubMed] [Google Scholar]
- 6.Chen XS, Yin YP, Liang GJ, Gong XD, Li HS, Shi MQ, et al. Co-infection with genital gonorrhoea and genital chlamydia in female sex workers in Yunnan, China. Int J STD AIDS. 2006;17:329–332. doi: 10.1258/095646206776790088. [DOI] [PubMed] [Google Scholar]
- 7.Creighton S, Tenant-Flowers M, Taylor CB, Miller R, Low N. Co-infection with gonorrhoea and chlamydia: How much is there and what does it mean? Int J STD AIDS. 2003;14:109–113. doi: 10.1258/095646203321156872. [DOI] [PubMed] [Google Scholar]
- 8.Dicker LW, Mosure DJ, Berman SM, Levine WC, The Resional. Gonorrhea prevalence and coinfection with chlamydia in women in the United States, 2000. Sex Transm Dis. 2003;30:472–476. doi: 10.1097/00007435-200305000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Stupiansky NW, Van Der Pol B, Williams JA, Weaver B, Taylor SE, Fortenberry JD. The natural history of incident gonococcal infection in adolescent women. Sex Transm Dis. 2011;38:750–754. doi: 10.1097/OLQ.0b013e31820ff9a4. [DOI] [PubMed] [Google Scholar]
- 10.Nsuami M, Cammarata CL, Brooks BN, Taylor SN, Martin DH. Chlamydia and gonorrhea co-occurrence in a high school population. Sex Transm Dis. 2004;31:424–427. doi: 10.1097/01.olq.0000130535.96576.d3. [DOI] [PubMed] [Google Scholar]
- 11.Batteiger BE, Fraiz J, Newhall WJ, Katz BP, Jones RB. Association of recurrent chlamydial infection with gonorrhea. J Infect Dis. 1989;159:661–669. doi: 10.1093/infdis/159.4.661. [DOI] [PubMed] [Google Scholar]
- 12.Vonck RA, Darville T, O'Connell CM, Jerse AE. Chlamydial infection increases gonococcal colonization in a novel murine coinfection model. Infect Immun. 2011;79:1566–1577. doi: 10.1128/IAI.01155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park S, Cho E. National Infectious Diseases Surveillance data of South Korea. Epidemiol Health. 2014;36:e2014030. doi: 10.4178/epih/e2014030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korea Centers for Disease Control and Prevention. 2017 Criteria for diagnosing infectious diseases [Internet] Cheongju: Korea Centers for Disease Control and Prevention; c2017. [cited 2019 Sep 3]. Available from: http://www.cdc.go.kr/board.es?mid=a20507020000&bid=0019&act=view&list_no=138040. [Google Scholar]
- 15.Lee GH. The current surveillance system for sexually transmitted diseases in Korea and plans for its improvement [Internet] Sejong: Ministry of Health and Welfare; c2017. [cited 2019 Sep 3]. Available from: http://www.prism.go.kr/homepage/lately/retrieveLatelyDetail.do;jsessionid=64F502EBF826EBD137216369710C711B.node02?research_id=1351000-201600272. [Google Scholar]
- 16.Korea Centers for Disease Control and Prevention. Update to sexually transmitted infection Korean treatment guidelines [Internet] Cheongju: Korea Centers for Disease Control and Prevention; c2013. [cited 2019 Sep 3]. Available from: http://www.cdc.go.kr/board.es?mid=a40303030000&bid=0034&act=view&list_no=21703. [Google Scholar]
- 17.Gottlieb SL, Martin DH, Xu F, Byrne GI, Brunham RC. Summary: the natural history and immunobiology of Chlamydia trachomatis genital infection and implications for chlamydia control. J Infect Dis. 2010;201 Suppl 2:S190–S204. doi: 10.1086/652401. [DOI] [PubMed] [Google Scholar]
- 18.Forward KR. Risk of coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in Nova Scotia. Can J Infect Dis Med Microbiol. 2010;21:e84–e86. doi: 10.1155/2010/760218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berman SM, Hein K. Adolescents and STD. In: Holmes KK, Sparling PF, Mardh PA, Lemon SM, Stamm WE, Piot P, et al., editors. Sexually transmitted diseases. 3rd ed. New York: McGraw-Hill; 1999. pp. 129–142. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Official report form for sexually transmitted diseases from the Korean National Infectious Diseases Surveillance of the Korea Centers for Disease Control and Prevention.
Multiple overlapping in age and sex parameters in the same diagnostic day of Neisseria gonorrhoeae (NG) and Chlamydia trachomatis (CT) infections
Numbers of reported gonococcal infected case (X axis: Hospital code. Y axis: Age code. Z axis: numbers of reported case) between 2011 to 2015.
Data Availability Statement
The data analyzed for this study have been deposited in HARVARD Dataverse and are available at https://doi.org/10.7910/DVN/P98SOR.