The endogenous pain system may be used as a biomarker in the pharmacological treatment of patients with CLBP, enabling an individualized, mechanism-based treatment approach.
Keywords: Chronic low back pian, Endogenous pain modulation, Conditioned pain modulation, Offset analgesia, Temporal summation
Abstract
Introduction:
Chronic low back pain (CLBP) is one of the most common chronic pain conditions in pain practice.
Objectives:
In the current study, we describe phenotypes of patients with CLBP based on the status of their endogenous pain modulatory system.
Methods:
Conditioned pain modulation (a measure of central pain inhibition), temporal summation (TS, a measure of pain facilitation), and offset analgesia (a measure of temporal filtering of nociception) were evaluated in 53 patients with CLBP at painful and nonpainful sites. Next, in a double-blind, randomized, placebo-controlled trial, 40 patients with defective conditioned pain modulation responses received treatment with tapentadol prolonged-release or placebo for 3 months.
Results:
The majority of patients (87%) demonstrated loss of central pain inhibition combined with segmentally increased TS and reduced offset analgesia at the lower back region. During treatment, tapentadol reduced pain intensity more than placebo (tapentadol −19.5 ± 2.1 mm versus placebo −7.1 ± 1.8 mm, P = 0.025). Furthermore, tapentadol significantly decreased pain facilitation by reduction of TS responses at the lower back (tapentadol −0.94 ± 1.9 versus placebo 0.01 ± 1.5, P = 0.020), which correlated with pain reduction (P < 0.001).
Conclusion:
Patients with CLBP demonstrated different phenotypes of endogenous pain modulation. In patients with reduced conditioned pain modulation, tapentadol produced long-term pain relief that coincided with reduction of signs of pain facilitation. These data indicate that the endogenous pain system may be used as a biomarker in the pharmacological treatment of CLBP, enabling an individualized, mechanism-based treatment approach.
1. Introduction
Chronic low back pain (CLBP) remains one of the most common pain conditions in current pain practice. Treatment is challenging and performed by a multimodal approach, combining pharmacological therapies and nonpharmacological interventions for symptomatic pain relief.4,27 Given the often low success rate of treatment in CLBP, an individualized and mechanism-based approach may increase the probability of treatment success.3 One such approach is to use the endogenous pain modulatory system as biomarker of treatment effect.40 The endogenous pain system consists of inhibitory and facilitatory descending pain pathways that modulate the activity of nociceptive neurons at the spinal level.1,31,39 Both a loss of pain inhibition and an increase in pain facilitation have been observed in patients with CLBP.1,25,26 However, up to now, we remain uninformed on the association between the endogenous pain system and treatment efficacy in CLBP.
Sensitivity of treatment responses may depend on patient phenotypes based on the status of the endogenous pain system.23,40 Frequently used test modalities to evaluate this system are conditioned pain modulation (CPM), temporal summation (TS), and offset analgesia (OA). Conditioned pain modulation is a paradigm where inhibition of a focal noxious stimulus is observed by administration of a second noxious stimulus at a remote area and is an expression of central pain inhibition.36,41 Temporal summation is defined by the increase in pain intensity to a repetitive noxious stimulus and is an expression of pain facilitation.1,39 Offset analgesia is characterized by profound analgesia after a slight decrease in noxious stimulation and is considered an expression of temporal filtering of nociception.18 By profiling patients at noxious and non-noxious regions, the existence of regional differences in the endogenous pain modulatory system may be observed.
Although strong analgesics such as opioids are often used in the management of CLBP, the long-term efficacy has only been sparsely evaluated.7,9,12,24 Different types of opioids have unique effect and side-effect profiles.10 Tapentadol is a bifunctional opioid with activity on the μ-opioid receptor additionally with inhibition of neuronal noradrenaline reuptake.35 The drug is an attractive alternative to conventional opioids as the synergistic interaction at the 2 sites of action produces potent analgesia with less adverse effects.8,9,37 We previously showed that tapentadol induced pain relief because of improvement or restoration of CPM responses in patients with diabetes-induced polyneuropathy and fibromyalgia syndrome.13,29
In the current study, we first determined the endogenous pain modulatory system phenotype of patients with CLBP, and next, given the mechanistic nature of tapentadol-treated patients with reduced or absent CPM responses with tapentadol for 3 months. We hypothesize that pain relief coincides with improvement of the pain modulatory system at a segmental and general level, ie, improvement at noxious and non-noxious sites.
2. Methods
2.1. Patients
Patients diagnosed with nonspecific CLBP were recruited to participate in the trial. Chronic low back pain was defined by the presence of pain in the lumbar region of the spine for at least 3 consecutive months. Patients were considered suitable for inclusion if they had a pain score of at least 5 points on an 11-point numeric rating scale (NRS) for most of the day. Exclusion criteria included specific pathologies causing chronic back pain (such as spinal canal stenosis, disk herniation, spondylolisthesis, etc.), an age <18 or >75 years, a body mass index >40 kg/m2, the presence of any medical disease, pregnancy, and a history of psychosis, illicit drug, or alcohol abuse. Patients were asked to stop pain medication for at least 4 weeks before the first study visit and refrain from further analgesic medication during the entire study. Written and oral informed consent was obtained from all patients before enrollment into the study. The study protocol was approved by the local institutional review board (Leiden, the Netherlands) and the Central Committee on Research Involving Human Subjects (CCMO, The Hague). The study was registered at the trial register of the Dutch Cochrane Center (Amsterdam, the Netherlands) under identifier 6329 and at the EU clinical trials register with identification number 2015-005259-28. All procedures were performed in compliance with the Declaration of Helsinki and Good Clinical Practice guidelines.
2.2. Study design
Patients were recruited at the pain clinic and invited for a screening visit before enrollment into the study (visit 1). During this screening visit, the inclusion and exclusion criteria were documented and a physical examination was performed. When all inclusion and exclusion criteria were met and the physical examination revealed no abnormalities that precluded enrollment in the study, patients entered into part 1 of the study. Part 1 of the study involved phenotyping, ie, the determination of a footprint of the endogenous pain modulatory system by CPM, OA, and TS testing, and questionnaires to quantify symptom severity.
Only under the condition of a CPM response <12% on both locations, patients entered part 2 of the study. We chose 12% as cutoff value because a CPM response <12% was not observed in healthy young populations; we tested using this specific CPM paradigm (based on 100 subjects, between-day correlation coefficient 0.84 [unpublished observation]). In the current double-blind, randomized, placebo-controlled trial, the effect of treatment (tapentadol) on pain intensity, endogenous pain modulation, and symptom severity was investigated. Patients were randomly assigned to either receive a 12-week tapentadol prolonged release (Grünenthal GmbH, Aachen, Germany) or placebo treatment. The local pharmacy was responsible for randomization and dispensing of the study medication. Tapentadol and placebo tablets were repackaged by the pharmacy to ensure identical appearance without the chance of unblinding the investigator or the patient. Treatment was started at a dose of 50 mg twice daily and weekly increased depending on the amount of pain relief and side-effect profile to a maximum of 250 mg twice daily. In case of unacceptable side effects, dosages were decreased to a dose where side effects were tolerated. During the 12-week treatment period, patients visited the research unit 1, 2, and 3 months (visit 2–4) after the start of treatment and 1 month after treatment ended (visit 5). During these visits, CPM, OA, and TS tests were performed and patients were queried about symptom severity. CPM, OA, and TS were measured on the lower dominant forearm and on the most painful area on the lower back (2 times per location). In addition to these 4-weekly visits, patients were contacted on a weekly basis by telephone to query for pain scores and side effects.
2.3. Conditioned pain modulation and offset analgesia
Conditioned pain modulation was measured using heat pain as test stimulus and cold pain as conditioning stimulus.29 Heat pain was induced using the 3 × 3 thermal probe of the pathway Neurosensory Analyzer (Medoc Ltd., Ramat Yishay, Israel). During heat stimulation, patients quantified the pain intensity level of the stimulus using a computerized potentiometer that ranged from 0 mm (no pain) to 100 mm (worst pain imaginable), allowing for continuous electronic monitoring of the visual analogue scale (eVAS). Cold pain was induced by immersion of the patient's foot and lower leg in a water bath (Lauda, Lauda-Königshofen, Germany) that could be varied in temperature from 3 to 25°C. At the start of each day, baseline measurements were performed to determine the heat temperature that induced a pain score of 50 to 60 mm (target temperature) and the cold temperature that induced a pain score of 30 to 40 mm. These temperatures were used during the remainder of the study day. For each test, the temperature of the test stimulus increased with 1.5°C/s from baseline (32°C) to the target temperature and kept constant for 10 seconds after which the temperature returned to baseline. The eVAS score of the test stimulus (heat pain) with and without the conditioning stimulus (cold pain) was determined to quantify CPM. The conditioning stimulus was applied 25 seconds before the start of the test stimulus and ended simultaneously with the test stimulus. Patients were specifically instructed to only rate pain intensity of the test stimulus.
Offset analgesia was measured using a heat pain paradigm as described previously.28 In short, the temperature of the probe was increased from the baseline temperature to the target temperature (50–60 mm). After 5 seconds, the temperature of the heat probe was raised by 1°C for 5 seconds and next returned to the target temperature (decrease of 1°C) for another 20 seconds. During heat stimulation, patients continuously rated the intensity of the heat stimulus using the computerized VAS slider to quantify OA.
2.4. Temporal summation
TS was tested with a sharp pin able to apply a force of 256 mN (MRC systems, Heidelberg, Germany) without penetrating the dermis. Patients were instructed to rate pain intensity of a single pin prick stimulus on an 11-point NRS were 0 indicated no pain and 10 the most intense pain imaginable. Next, a train of 10 repetitive pin prick stimuli was administered at a rate of 1 Hz. Patients were asked to rate pain intensity of the last (10th) stimulus.1
2.5. Questionnaires
To quantify symptom severity, 3 questionnaires were used: the PainDetect,15 the Neuropathic Pain Symptom Inventory,6 and the Roland–Morris Disability Questionnaire (RMDQ).32 The PainDetect questionnaire is a tool to detect pain intensity (scale 0–100 mm) and the presence of neuropathic pain symptoms (score 0–38), where a score of 0 to 12 points indicates the presence of nociceptive pain, 13 to 18 points that neuropathic pain might be present, and 19 to 38 points that neuropathic pain is likely present. The Neuropathic Pain Symptom Inventory questionnaire is a questionnaire designed to evaluate the different symptoms of neuropathic pain. The score ranges from 0 to 1 (no symptoms to worst symptoms imaginable). The questionnaire distinguishes 5 symptom categories: burning pain, deep pressing pain, paroxysmal pain, evoked pain, and paresthesia and dysesthesia. The RMDQ is a questionnaire that queries the level of disability a patient experiences due to CLBP. The questionnaire comprises 24 statements regarding the influence of CLBP on the patients' life. The total score of the questionnaire is the amount of statements that apply to the patients' condition (0–24). For all questionnaires, the validated Dutch versions were used.
2.6. Sample size and statistical analysis
Sample size calculation was deemed necessary for the second part of the study where patients were randomized to treatment. The primary end point was the effect of tapentadol on endogenous pain modulation. No formal sample size analysis was performed because no data were available on the efficacy of tapentadol on any of the endogenous pain modulation measures in patients with CLBP. Based on the results of a previous study,29 where tapentadol enhanced CPM in patients with a chronic neuropathic pain, the inclusion of 15 patients per group resulted in a power >90% to detect a 25% increase in CPM with an SD of 20% for tapentadol treatment compared with placebo (α = 0.05, two-tailed). We included an extra 5 patients per group to consider any margin of uncertainty around the effect size and SD.
Conditioned pain modulation responses were calculated using the average peak of the eVAS data during the test stimulus with and without conditioning stimulus. To correct for variation in the magnitude of responses between sessions and between subjects, the relative CPM was calculated as: CPM% = [(mean peak value without CS − mean peak value with CS)/(mean peak value without CS)] × 100. To quantify OA, we calculated the decrease in eVAS from the peak eVAS to the eVAS nadir after the 1°C decrease in temperature (ΔeVAS). Next, the ΔeVAS was corrected for the peak eVAS to correct for the variation in peak responses between patients and calculated as: ΔeVASc = (ΔeVAS/[peak eVAS]) × 100. Temporal summation was calculated by the difference in the NRS between the single pin prick stimulus and the 10th pin prick stimulus of the train of repetitive stimuli. A paired-sample t test was used to compare CPM, OA, and TS between the different test locations.
The overall treatment effects (corrected for baseline) on CPM, OA, TS, spontaneous pain scores, and questionnaires (visit 1–4) were analyzed using a linear mixed model with treatment as fixed effect and patient as random effect to account for repeated measurements over time. An analgesia responder rate analysis was performed to evaluate the proportion of patients who achieved predefined response rates in the range between 0% and 100%. The response rate was calculated by the proportion of pain relief during the treatment period (visit 2–4) compared with baseline. A Kolmogorov–Smirnov test was used to compare treatment distributions. Correlations between CPM, OA, TS, spontaneous pain, and questionnaires were analyzed using the Pearson correlation. All statistical analyses were performed in SPSS Statistics for Windows v25 (IBM Corp., Armonk, NY), P-values < 0.05 were considered significant. Data are presented as mean ± SD unless otherwise stated.
3. Results
3.1. Study part 1—phenotyping
A total of 68 patients were assessed for eligibility to participate in the study of whom 15 patients were excluded based on the inclusion and exclusion criteria (Fig. 1). The results of the 3 test modalities of the endogenous pain modulatory system (CPM, OA, and TS), obtained in the total population of 53 patients, are given in Figures 2A–C. On average, a pain facilitatory CPM response was observed on the arm (−7.8 ± 2.7%) and an absent response on the lower back (0.5 ± 2.4%; P = 0.08) (Fig. 2A). Offset analgesia was significantly reduced on the lower back compared with the arm (Fig. 2B): 89.1 ± 3.4% (arm) and 70.6 ± 5.2% (lower back); P = 0.004. Temporal summation was significantly increased on the lower back compared with the arm: 0.4 ± 0.2 (arm) and 1.1 ± 0.3 (lower back; P = 0.02).
Figure 1.
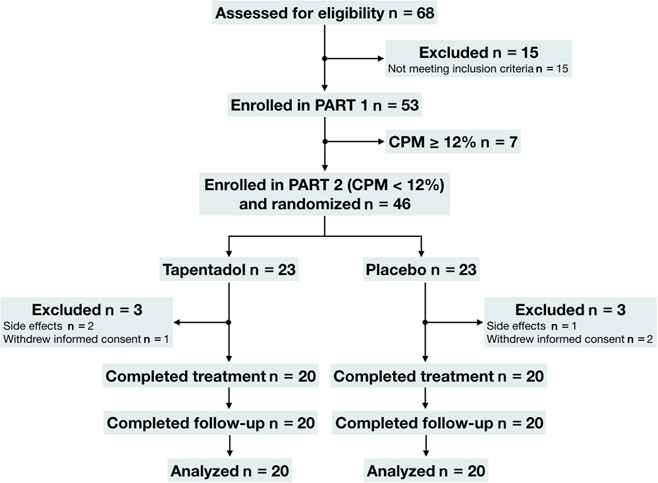
Flowchart of the study. CPM: conditioned pain modulation.
Figure 2.
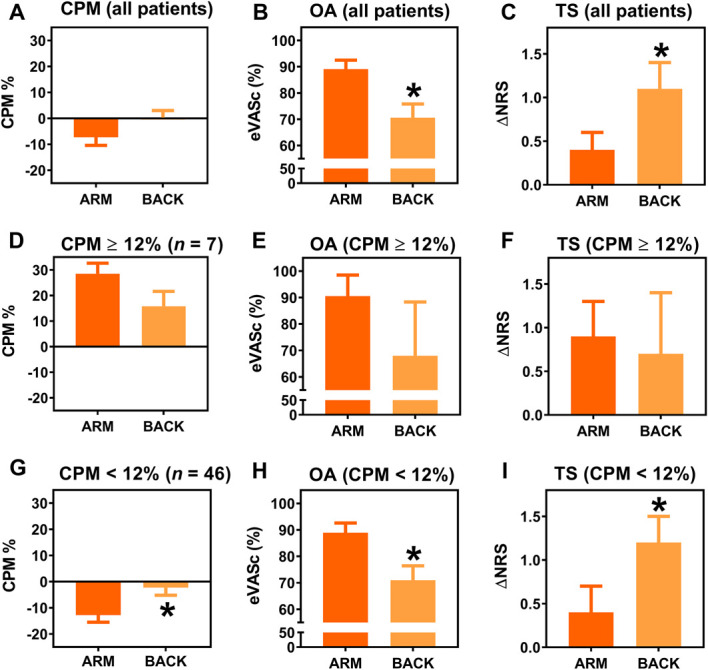
Mean values for CPM, OA, and TS on the arm (dark orange) and lower back (light orange) for all patients (A–C), for the patients with normal CPM (CPM ≥12%) (D–F), and for the patients with reduced CPM (CPM <12%) (G–I). ΔNRS, delta numerical rating score; CPM, conditioned pain modulation; eVASc, electronic visual analogue scale corrected for the peak response; OA, offset analgesia; TS, temporal summation. *Significant change between arm and back.
Patients were stratified according to their CPM response into a group with a CPM response <12% and a group with a response ≥12%.
3.1.1. Conditioned pain modulation% ≥12%
Conditioned pain modulation responses were ≥12% (both measurement sites) in 7 (13%) of 53 patients with an average CPM% of 28.5 ± 10.8% on the arm and 15.8 ± 15.3% on the lower back (P = 0.10; Fig. 2D). Offset analgesia responses (eVASc) in this population were 90.5 ± 9.5% on the arm and 68 ± 20.3% on the lower back (P = 0.23; Fig. 2E). Temporal summation was 0.9 ± 0.4 and 0.7 ± 0.7 on the arm and lower back, respectively (P = 0.85; Fig. 2F).
3.1.2. Conditioned pain modulation <12%
Conditioned pain modulation responses <12% (both measurement sites) were observed in 46 (87%) patients with an average CPM% of −12.8 ± 18.2% on the arm and −2.3 ± 19.6% on the lower back (P = 0.01; Fig. 2G). Offset analgesia responses in this population were 88.9 ± 3.7% (arm) and 71.0 ± 5.4% (lower back; P = 0.009; Fig. 2F), and TS was 0.4 ± 0.3 (arm) and 1.2 ± 0.3 (lower back; P = 0.01; Fig. 2G). No correlation was observed between the magnitudes of CPM, OA, and TS.
3.1.3. Pain and questionnaires
Average reported pain scores in the total population were 64.0 ± 11.2 mm. Most patients reported nociceptive pain. The PainDetect questionnaire indicated that a neuropathic pain component was possibly present in 17% of patients and likely present in 9% with mild neuropathic pain symptoms according to the NPSI and moderate influence on physical disability according to the RMDQ (Table 1). No differences were observed between patients with or without CPM in pain scores or any of the items scored in the questionnaires. Furthermore, no correlation was observed between CPM, OA, and TS with the spontaneous pain scores and questionnaires.
Table 1.
Patient characteristics.
| Total population | CPM <12% | CPM ≥12% | |
|---|---|---|---|
| Men/women (n) | 33/20 | 28/18 | 5/2 |
| Age (y)—mean (SD) | 62.2 (10.9) | 62.0 (11.3) | 63.6 (8.8) |
| Weight (kg)—mean (SD) | 82.8 (17.0) | 84.4 (17.2) | 71.1 (12.2) |
| Height (cm)—mean (SD) | 174.4 (8.9) | 174.8 (9.0) | 172.1 (9.0) |
| Disease duration (y)—mean (SD) | 20.5 (17.4) | 19.9 (16.7) | 24.4 (22.6) |
| PainDetect | |||
| Pain score (mm)—mean (SD) | 64.0 (11.2) | 64.3 (11.1) | 61.4 (12.2) |
| Neuropathic symptom score—mean (SD) | 10.3 (5.7) | 11.1 (5.7) | 5.6 (3.6) |
| Score 13–18 (n, %) | 9 (17.0) | 9 (19.6) | 0 (0) |
| Score 19–38 (n, %) | 5 (9.4) | 5 (10.9) | 0 (0) |
| NPSI overall—mean (SD) | 0.2 (0.2) | 0.3 (0.2) | 0.2 (0.2) |
| Burning pain | 0.2 (0.3) | 0.2 (0.3) | 0.1 (0.1) |
| Deep pressing pain | 0.3 (0.2) | 0.3 (0.2) | 0.2 (0.1) |
| Paroxysmal pain | 0.3 (0.3) | 0.3 (0.3) | 0.2 (0.2) |
| Evoked pain | 0.2 (0.2) | 0.2 (0.2) | 0.1 (0.2) |
| Paresthesia/dysesthesia | 0.2 (0.2) | 0.2 (0.2) | 0.1 (0.2) |
| RMDQ—mean (SD) | 11.4 (4.7) | 11.0 (4.4) | 13.7 (6.6) |
CPM, conditioned pain modulation; NPSI, Neuropathic Pain Symptom Inventory; RMDQ, Roland–Morris Disability Questionnaire.
3.2. Study part 2—tapentadol treatment
A total of 46 patients with CPM <12% were eligible for treatment randomization of whom 6 patients dropped out (Fig. 1). No significant differences in baseline characteristics were observed between the 2 treatment groups (Table 2). The average daily drug dose after the titration period was 302.5 ± 93.9 mg for the tapentadol group and 357.5 ± 90.7 mg for the placebo group (P = 0.07). Side effects were reported in 18 patients randomized to tapentadol and in 8 patients randomized to placebo, with more side effects reported by patients treated with tapentadol (n = 45) than placebo (n = 17; P < 0.001; Table 3).
Table 2.
Baseline characteristics per treatment group.
| Tapentadol (n = 20) | Placebo (n = 20) | |
|---|---|---|
| Men/women (n) | 16/4 | 11/9 |
| Age (y)—mean (SD) | 64.9 (12.5) | 60.8 (9.7) |
| Weight (kg)—mean (SD) | 88.3 (19.8) | 84.3 (14.0) |
| Height (cm)—mean (SD) | 175.3 (8.0) | 175.5 (10.8) |
| Disease duration (y)—mean (SD) | 22.4 (20.0) | 15.3 (13.8) |
| PainDetect | ||
| Pain score (mm)—mean (SD) | 67.0 (12.2) | 62.5 (10.7) |
| Neuropathic symptom score—mean (SD) | 10.3 (5.9) | 12.0 (5.7) |
| Score 13–18 (n, %) | 3 (15.0) | 4 (20) |
| Score 19–38 (n, %) | 2 (10.0) | 3 (15) |
| NPSI overall—mean (SD) | 0.3 (0.2) | 0.2 (0.2 |
| Burning pain | 0.3 (0.3) | 0.2 (0.2) |
| Deep pressing pain | 0.3 (0.2) | 0.2 (0.2) |
| Paroxysmal pain | 0.4 (0.3) | 0.3 (0.2) |
| Evoked pain | 0.2 (0.2) | 0.2 (0.2) |
| Paresthesia/dysesthesia | 0.3 (0.3) | 0.2 (0.2) |
| RMDQ—mean (SD) | 11.9 (4.0) | 10.9 (5.1) |
| Heat temperature arm (°C)—mean (SD) | 46.9 (1.6) | 46.8 (1.3) |
| Heat temperature lower back (°C)—mean (SD) | 45.7 (3.0) | 46.0 (2.1) |
| Heat pain lower arm (VAS (mm)—mean (SD) | 60.2 (9.4) | 55.8 (8.3) |
| Heat pain lumbar spine (VAS (mm)—mean (SD) | 58.5 (9.4) | 55.9 (11.8) |
| Cold temperature (°C)—mean (SD) | 6.5 (4.1) | 6.7 (3.8) |
| Cold pain (VAS [mm]—mean (SD) | 28.1 (20.9) | 24.2 (17.2) |
| CPM% lower arm—mean (SD) | −14.6 (21.6) | −10.9 (11.4) |
| CPM% lumbar spine—mean (SD) | −0.06 (19.8) | −5.3 (20.1) |
| OA lower arm (eVASc)—mean (SD) | 78.9 (32.9) | 95.6 (15.9) |
| OA lumbar spine (eVASc)—mean (SD) | 64.0 (40.9) | 80.9 (26.6) |
| TS lower arm—mean (SD) | 0.6 (0.5) | 0.2 (0.4) |
| TS lumbar spine—mean (SD) | 1.6 (0.4) | 0.7 (0.4) |
CPM, conditioned pain modulation; eVASc, electronic visual analogue scale corrected; NPSI, Neuropathic Pain Symptom Inventory; OA, offset analgesia; RMDQ, Roland–Morris Disability Questionnaire; TS, temporal summation; VAS, visual analogue scale.
Table 3.
Number of patients reporting side effects.
| Side effects (n, %) | Tapentadol (n = 20) | Placebo (n = 20) | P |
|---|---|---|---|
| Dizziness | 9 (45) | 3 (15) | |
| Nausea | 9 (45) | 4 (20) | |
| Vomiting | 1 (5) | 0 (0) | |
| Tiredness | 6 (30) | 5 (25) | |
| Headache | 5 (25) | 1 (5) | |
| Shaking | 1 (5) | 1 (5) | |
| Itchiness | 4 (20) | 1 (5) | |
| Vivid dream | 3 (15) | 1 (5) | |
| Obstipation | 4 (20) | 0 (0) | |
| Dry mouth | 2 (10) | 0 (0) | |
| Dyspnea | 0 (0) | 1 (5) | |
| Urinary hesitancy | 1 (5) | 0 (0) | |
| Total (n) | 45 | 17 | <0.001 |
3.2.1. Pain and questionnaires
No baseline differences were observed in spontaneous pain questionnaire scores (Table 2). Tapentadol reduced spontaneous pain scores compared with placebo with an average reduction during treatment of −19.5 ± 2.1 mm (tapentadol) and −7.1 ± 1.8 mm (placebo; P = 0.03; 95% confidence interval [CI] −16.6 to −1.2 mm; Fig. 3A). Furthermore, analysis of analgesia responder rates (0%–100%) showed a treatment effect in favor of tapentadol (P < 0.001, Fig. 3B). No effect of treatment was observed on any item of the questionnaires.
Figure 3.
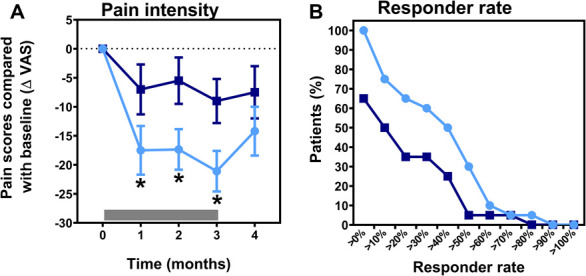
(A) Change in the pain score compared with baseline during and after treatment with tapentadol (light blue circles) and placebo (dark blue squares). The gray bar indicates the treatment period. Tapentadol significantly reduced spontaneous pain scores compared with placebo (P = 0.025). (B) Graph of the analgesia responder rate for predefined response rates in the range between 0% and 100%. ΔVAS, delta visual analogue scale; *Significant change in the pain score between tapentadol and placebo.
3.2.2. Conditioned pain modulation, offset analgesia, and temporal summation
At baseline, CPM, OA, and TS values were similar between the 2 study arms (Table 2). No effect of tapentadol was observed on CPM or OA on any location (CPM arm: 16.9 ± 4.3 versus 10.1 ± 4.3, P = 0.285, 95% CI −4.4 to 14.5; CPM lower back: 8.5 ± 5.7 versus 5.0 ± 5.7, P = 0.669, 95% CI −9.6 to 14.9; OA arm: 10.5 ± 6.8 versus −7.5 ± 6.7, P = 0.271, 95% CI −6.5 to 22.5; and OA lower back: 12.3 ± 7.3 versus −5.0 ± 7.2, P = 0.097, 95% CI −2.5 to 28.4 [all data are represented as tapentadol versus placebo]). Tapentadol did show a significant effect on TS but only on the lower back: −1.2 ± 0.4 (tapentadol) versus 0.02 ± 0.4 (placebo; P = 0.02; 95% CI −1.7 to −0.2 (Fig. 4). A significant correlation was present between these TS responses and the corresponding pain scores (r2 = 0.99; P < 0.0001; Fig. 5). No effect of tapentadol on TS was observed on the arm compared with placebo: −0.3 ± 0.4 versus 0.1 ± 0.4 (P = 0.534, 95% CI −1.2 to 0.6), and responses did not correlate to corresponding pain scores (r2 = 0.06, P = 0.695). Also, no correlations were observed between pain scores and CPM or OA responses.
Figure 4.
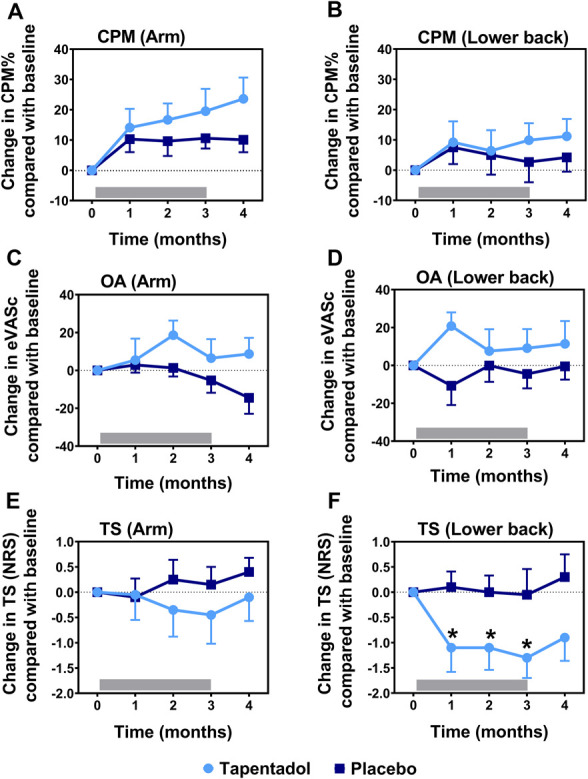
CPM, OA, and TS responses during and after treatment with tapentadol (light blue circles) and placebo (dark blue squares) for the arm (A, C, and E) and the lower back (B, D, and F). The gray bar indicates the treatment period. Tapentadol significantly decreased temporal summation responses on the lower back compared with placebo (significant change indicated by asterisk; overall effect: P = 0.020). CPM, conditioned pain modulation; eVASc, electronic visual analogue scale corrected for the peak response; NRS, numerical rating scale; OA, offset analgesia; TS, temporal summation.
Figure 5.
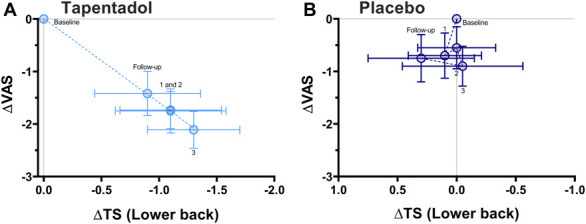
Correlation between TS responses of the lower back and spontaneous pain scores for tapentadol (A) and placebo treatment (B). A significant correlation was observed for the patients treated with tapentadol (r2 = 0.99, P < 0.001). No correlation was observed after treatment with placebo (r2 = 0.06, P = 0.695). The numbers in the graph indicate the treatment month. ΔVAS, delta visual analogue scale; ΔTS, delta temporal summation.
4. Discussion
In the first part of this study, we characterized the pain modulatory system in patients with CLBP at the location of their most intense pain and a reference site (arm) using CPM, OA, and TS. Segmental differences were observed for all 3 test modalities. In the second part of the study, patients with defective CPM treated with tapentadol for 3 months had significant consistent pain relief during the treatment period with a correlated reduction in TS responses at the painful lower back. No effect on CPM was observed.
4.1. Phenotype of the pain modulatory system
Three test modalities were used to evaluate the efficacy of the pain modulatory system: CPM, TS, and OA. Conditioned pain modulation, which is considered a surrogate marker for central pain inhibition, has been extensively studied in many chronic pain syndromes including CLBP.25,26,39 A recent meta-analysis evaluating CPM in patients with CLBP demonstrated reduced CPM responses in patients compared with controls.26 This agrees with the results of this study where the majority of patients with CLBP (87%) showed aberrant CPM responses. A segmental effect of CPM was observed in this population of CLBP with absent CPM responses on the lower back and a pain facilitatory response on the arm (negative CPM). These findings suggest multisegmental pain facilitation possibly in combination with segmental inhibition at the site of the lower back.38 This is in agreement with earlier studies in patients with CLBP, which show, eg, widespread hyperalgesia in subpopulations.2,5,17
In patients with reduced CPM responses, TS responses were significantly increased on the lower back relative to the arm, which we suggest to be related to segmental pain facilitation. Although several studies found indications of central sensitization in patients with CLBP,26 only a few show differences in magnitude of TS at the site of pain compared with a reference site.11,19,21 In line with our findings, Gerhardt et al.16 showed segmental pain facilitation with greater TS responses on the lower back compared with the hand. Interestingly, this was observed only in a subgroup of patients with chronic widespread pain. This possibly reflects that CPM and TS responses are differentially impaired in subsets of patients with CLBP.
Offset analgesia responses were reduced on the lower back compared with the arm in our population of patients with CLBP. OA is considered a temporal and spatial filtering mechanism of pain able to induce poststimulus pain inhibition.18 The underlying neurophysiological mechanism has not been fully understood where both central and peripheral processes have been proposed.22,35 Reduced OA responses have been observed in patients with diabetic polyneuropathy, fibromyalgia, and CRPS.22,35,42 The current study is the first to study OA responses in a large population of patients with CLBP and also the first to evaluate OA on 2 separate body locations. We previously showed that a ΔeVASc of 88% separates normal from abnormal OA responses in neuropathic pain patients (with responses <88% defined as abnormal).28 Extrapolation of this cutoff to this study suggests that OA was abnormal on the lower back (but not arm) in the majority of our patients irrespective of CPM condition. This suggests that OA is segmentally affected comparable with our observations in TS. Of note, as this is the first study which performed OA responses on the lower back, we are not informed on OA responses at this location in a healthy population.
In agreement with literature, our data indicate that multiple phenotypes exist within the CLBP population.2,5,16,17,19,25 Most patients in our sample displayed reduced pain inhibitory responses combined with segmental sensitization and segmentally reduced OA responses. This phenotype may require a specific treatment approach aimed at restoration of these specific responses to cause effective and long-term pain relief.
4.2. Tapentadol treatment
We were unable to detect a significant effect of tapentadol treatment on CPM. We relate this to an appreciable increase of CPM during placebo treatment. The magnitude of the CPM increase (about 20%) was in accordance with previously observed increases in patients with diabetic polyneuropathy and fibromyalgia syndrome.13,29 In agreement with literature,8 we did observe a significant analgesic effect with almost 50% of patients that experienced 50% of pain relief during treatment. In contrast to our previous studies,13,29 we did not observe a correlation between analgesia and CPM% responses. This might indicate that in patients with CLBP, pain relief is to a lesser extent or less consistently associated with CPM improvement and suggests a rather distinct mechanistic effect of tapentadol in different pain syndromes.
Tapentadol significantly reduced TS on the lower back. To the best of our knowledge, no other studies investigated the influence of tapentadol on pain facilitation. However, previous studies did find evidence for an effect of opioids on TS. For example, codeine, morphine, oxycodone, and remifentanil reduce TS responses.14,20,33,34 Whether the observed reduction of TS in the current study is mainly related to the effect of tapentadol on the µ-opioid receptor or to the noradrenergic component of the drug is unknown. In contrast to CPM, the effect of tapentadol on TS responses at the lower back region was significantly correlated to spontaneous pain scores. This suggests that pain relief by tapentadol in our CLBP population may be mediated by a reduction in pain facilitation rather than by an improvement of top-down pain inhibition. Tapentadol had no effect on TS responses on the reference site, but this was expected because these responses were normal before treatment. Giving the specific phenotype of patients in our study, it remains unknown what the effect of tapentadol would be in patients with normal TS responses at the painful back.
Finally, despite the observation of profound and long-term analgesia, no effect of tapentadol was observed on OA irrespective of location. This agrees with previous studies showing that centrally acting drugs, including tapentadol, do not influence OA responses.28–30 This suggests that OA is a biomarker of defective pain modulation which is not affected by either the opioidergic or the noradrenergic components of tapentadol treatment. It additionally suggests that OA is a secondary phenomenon that is not part of any of the mechanistic pathways through which pain in patients with CLBP is experienced or may be influenced.
Some limitations need to be addressed for this study. First, the study has a small sample size and should be considered as hypothesis generating. Further research needs to be performed to confirm our findings. Second, outcome measurements such as pain, CPM, and TS may spontaneously change over time (without intervention). We performed our study as standardized as possible but cannot rule out any influence of spontaneous changes over time. Third, we used a CPM cutoff value of 12% as biomarker of impaired CPM. Further studies should address the influence of CPM cutoff values on tapentadol treatment effect.
Disclosures
The authors have no conflicts of interest to declare. This investigator initiated trial study was sponsored in part by Grünenthal GmbH, Aachen, Germany. Grünenthal had no influence on the design of the study and was not involved in the study execution, data analysis, or writing of the manuscript. T. van de Donk and A. Dahan received speakers fee from Grünenthal BV (the Netherlands).
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Contributor Information
Tine van de Donk, Email: T.van_de_Donk@lumc.nl.
Jurjan van Cosburgh, Email: j.vancosburgh@rdgg.nl.
Tom van Dasselaar, Email: T.M.van_Dasselaar@lumc.nl.
Monique van Velzen, Email: m.van_velzen@lumc.nl.
Asbjørn Mohr Drewes, Email: amd@rn.dk.
Albert Dahan, Email: A.dahan@lumc.nl.
References
- [1].Arendt-Nielsen, Morlion B, Perrot S, Dahan A, Dickenson A, Kress HG, Wells C, Bouhassira A, Mohr Drewes A. Assessment and manifestation of central sensitisation across different chronic pain conditions. Eur J Pain 2018;22:216–41. [DOI] [PubMed] [Google Scholar]
- [2].Aoyagi K, He J, Nicol AL, Clauw DJ, Kluding PM, Jernigan S, Sharma NK. A subgroup of chronic low back pain patients with central sensitization. Clin J Pain 2019;35:869–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bannister K, Sachau J, Baron R, Dickenson AH. Neuropathic pain: mechanism-based therapeutics. Annu Rev Pharmacol Toxicol 2020;6:257–74. [DOI] [PubMed] [Google Scholar]
- [4].Baron R, Binder A, Attal N, Casale R, Dickenson AH, Treede RD. Neuropathic low back pain in clinical practice. Eur J Pain 2016;20:861–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Blumenstiel K, Gerhardt A, Rolke R, Bieber C, Tesarz J, Griederich HC, Eich W, Treede RD. Quantitative sensory testing profiles in chronic back pain are distinct from those in fibromyalgia. Clin J Pain 2011;27:682–90. [DOI] [PubMed] [Google Scholar]
- [6].Bouhassira D, Attal N, Fermanian J, Alchaar H, Gautron M, Masquelier E, Rostaing S, Lanteri-Minet M, Collin E, Grisart J, Boureau F. Development and validation of the neuropathic pain symptom inventory. PAIN 2004;108:248–57. [DOI] [PubMed] [Google Scholar]
- [7].Busse JW, Craigie S, Juurlink DN, Buckley DN, Wang LI, Couban RJ, Agoritsas T, Akl EA, Carrasco-Labra A, Cooper L, Cull C, da Costa BR, Frank JW, Grant G, Iorio A, Persaud N, Stern S, Tugwell P, Vandvik PO, Guyatt GH. Guideline for opioid therapy and chronic noncancer pain. CMAJ 2017;189:E659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Buynak R, Shapiro DY, Okamoto A, Van Hove I, Rauschkolb C, Steup A, Lange B, Lange C, Etropolski M. Efficacy and safety of tapentadol extended release for the management of chronic low back pain: results of a prospective, randomized, double-blind, placebo and active-controlled phase III study. Expert Opin Parmacother 2010;11:1787–804. [DOI] [PubMed] [Google Scholar]
- [9].Coluzzi F, Polati E, Freo U, Grili M. Tapentadol: an effective option for the treatment of back pain. J Pain Res 2019;12:1521–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].van Dam CJ, Algera MH, Olofsen E, Aarts L, Smith T, van Velzen M, Sarton E, Niesters M, Dahan A. Opioid utility function: methods and implications. Ann Palliat Med 2020;9:528–36. [DOI] [PubMed] [Google Scholar]
- [11].Diers M, Koeppe C, Diesch E, Stolle AM, Hölz R, Schiltenwolf M, Van Ackern K, Flor H. Central processing of acute muscle pain in chronic low back pain patients: an EEG mapping study. J Clin Neurophysiol 2007;24:76–83. [DOI] [PubMed] [Google Scholar]
- [12].Drewes AM, Jensen RD, Nielsen LM, Droney J, Christrup LL, Arendt-Nielsen L, Riley J, Dahan A. Differences between opioids. Pharmacological, experimental, clinical and economical perspectives. Br J Clin Pharmacol 2013;75:60–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Van de Donk T, van Velzen M, Dahan A, Niesters M. Cornea nerve fibre state determines analgesic response to tapentadol in fibromyalgia patients without effective endogenous pain modulation. Eur J Pain 2019;23:1586–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Enggaard TP, Poulsen L, Arendt-Nielsen T, Hansen SH, Bjørnsdottir I, Gram LF, Sindrup SH. The analgesic effect of codeine as compared to imipramine in different human experimental pain models. PAIN 2001;92:277–82. [DOI] [PubMed] [Google Scholar]
- [15].Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22:1911–20. [DOI] [PubMed] [Google Scholar]
- [16].Gerhardt A, Eich W, Janke S, Leisner S, Treede RD, Tesarz J. Chronic widespread back pain is distinct from chronic local back pain. Clin J Pain 2016;32:568–79. [DOI] [PubMed] [Google Scholar]
- [17].Gieseke T, Gracely RH, Grant MAB, Nachemson A, Petzke F, Wiliams DA, Clauw DJ. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum 2004;50:613–23. [DOI] [PubMed] [Google Scholar]
- [18].Grill JD, Coghill RC. Transient analgesia evoked by noxious stimulus offset. J Neurophysiol 2002;87:2205–8. [DOI] [PubMed] [Google Scholar]
- [19].Goubert D, Danneels L, Graven-Nielsen T, Descheemaeker F, Meeus M. Differences in pain processing between patients with chronic low back pain, recurrent low back pain, and fibromyalgia. Pain Physician 2017;20:307–18. [PubMed] [Google Scholar]
- [20].Hermans L, Nijs J, Calders P, De Clerck L, Moorkens G, Hans G, Grosemans S, Roman de Mettelinge T, Tuyman J, Meeus M. Influence of morphine and naloxone on pain modulation in rheumatoid arthritis, chronic fatigue syndrome/fibromyalgia, and controls: a double-blind, randomized, placebo-controlled, cross-over study. Pain Pract 2018;18:418–30. [DOI] [PubMed] [Google Scholar]
- [21].Hubscher M, Moloney N, Rebbeck T, Traeger A, Refshauge KM. Contributions of mood, pain catastrophizing, and cold hyperalgesia in acute and chronic low back pain: a comparison with pain-free controls. Clin J Pain 2014;30:886–93. [DOI] [PubMed] [Google Scholar]
- [22].Kobinata H, Ikeda E, Zhang S, Li T, Makita K, Kurata J. Disrupted offset analgesia distinguishes patients with chronic pain from healthy controls. PAIN 2017;158:1951–9. [DOI] [PubMed] [Google Scholar]
- [23].Kuhlmann L Olesen SS Grønlund D Olesen AE. Phillips AE Faghih M Drewes AM. Clinical pain phenotype associates with sensory testing results in chronic pancreatitis. Clin J Pain 2019;35:786–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Manchikanti L, Kaye AM, Knezevic NN, McAnally H, Slavin K, Trescot AM, Blank S, Pampati V, Abdi S, Grider JS, Kaye AD, Manchikanti KN, Cordner H, Gharibo CG, Harned ME, Albers SL, Atluri S, Aydin SM, Bakshi S, Barkin RL, Benyamin RM, Boswell MV, Buenaventura RM, Calodney AK, Cedeno DL, Datta S, Deer TR, Fellows B, Galan V, Grami V, Hansen H, Helm S, II, Justiz R, Koyyalagunta D, Malla Y, Navani A, Nouri KH, Pasupuleti R, Sehgal N, Silverman SM, Simopoulos TT, Singh V, Solanki DR, Staats PS, Vallejo R, Wargo BW, Watanabe A, Hirsch JA. Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: American Society of Interventional Pain Physicians (ASIPP) Guidelines. Pain Physician 2017;20:S3–S92. [PubMed] [Google Scholar]
- [25].McPhee ME, Graven-Nielsen T. Recurrent low back pain patients demonstrate facilitated pronociceptive mechanisms when in pain, and impaired antinociceptive mechanisms with and without pain. PAIN 2019;160:2866–76. [DOI] [PubMed] [Google Scholar]
- [26].McPhee ME, Vaegter HB, Graven-Nielsen T. Alterations in pro-nociceptive and anti-nociceptive mechanisms in patients with low back pain: a systematic review with meta-analysis. PAIN 2020:161:464–75. [DOI] [PubMed] [Google Scholar]
- [27].Morlion B. Chronic low back pain: pharmacological, interventional and surgical strategies. Nat Rev Neurol 2013;9:462–73. [DOI] [PubMed] [Google Scholar]
- [28].Niesters M, Hoitsma E, Sarton EY, Aarts LPHJ, Dahan A. Offset analgesia in neuropathic pain patients and effect of treatment with morphine and ketamine. Anesthesiology 2011;115:1063–71. [DOI] [PubMed] [Google Scholar]
- [29].Niesters M, Proto PL, Aarts L, Sarton EY, Drewes AM, Dahan A. Tapentadol potentiates descending pain inhibition in chronic pain patients with diabetic polyneuropathy. Br J Aneaesth 2014;113:148–56. [DOI] [PubMed] [Google Scholar]
- [30].Olesen AE, Nissen T, Nilsson M, Lelic D, Brock C, Christrup LL, Drewes AM. Offset analgesia and the impact of treatment with oxycodone and venlafaxine. A placebo controlled, randomized trial in healthy volunteers. Basic Clin Pharmacol Toxicol 2018;123:727–31. [DOI] [PubMed] [Google Scholar]
- [31].Ossipov MH, Dussor GO, Porecca F. Central modulation of pain. J Clin Invest 2010;120:3779–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Roland M, Morris R. A study of the natural history of back pain part I: development of a reliable and sensitive measure of disability in low-back pain. Spine 1983;8:141–4. [DOI] [PubMed] [Google Scholar]
- [33].Suzan E, Midbari A, Pud D, Hadad S, Eisenberg E. Clinical analgesia correlates with decline in temporal summation in response to remifentanil infusion in patients with chronic neuropathic (radicular) pain. J Opioid Manag 2016;12:251–8. [DOI] [PubMed] [Google Scholar]
- [34].Suzan E, Midbari A, Treister R, Haddad M, Pud D, Eisenberg E. Oxycodone alters temporal summation but not conditioned pain modulation: preclinical findings and possible relations to mechanism of opioid analgesia. PAIN 2013;154:1413–8. [DOI] [PubMed] [Google Scholar]
- [35].Szikszay TM, Adamczyk WM, Luedtke K. The magnitude of offset analgesia as a measure of endogenous pain modulation in healthy participants and patients with chronic pain. A systematic review and meta-analysis. Clin J Pain 2019;35:189–204. [DOI] [PubMed] [Google Scholar]
- [36].Talbot JD, Duncan GH, Bushnell MC, Boyer M. Diffuse noxious inhibitory controls (DNICs): psychophysical evidence in man for intersegmental suppression of noxious heat perception by cold pressor pain. PAIN 1987;30:221–32. [DOI] [PubMed] [Google Scholar]
- [37].Tzschentke TM, Christoph T, Kögel BY. The mu-opioid receptor agonist/noradrenaline reuptake inhibition (MOR-NRI) concept in analgesia: the case of tapentadol. CNS Drugs 2014;28:319–29. [DOI] [PubMed] [Google Scholar]
- [38].Valeriani M, le Pera D, Restuccia D, de Armas L, Maiese T, Tonali P, Vigevano F, Arendt-Nielsen L. Segmental inhibition of cutaneous heat sensation and of laser-evoked potentials by experimental muscle pain. Neuroscience 2005;136:301–9. [DOI] [PubMed] [Google Scholar]
- [39].Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature 1983;306:686–88. [DOI] [PubMed] [Google Scholar]
- [40].Yarnitsky D. Role of endogenous pain modulation in chronic pain mechanisms and treatment. PAIN 2015;156:S24–31. [DOI] [PubMed] [Google Scholar]
- [41].Yarnitsky D, Arendt-Nielsen L, Bouhassira D, Edwards RR, Fillingim RB, Granot M, Hansson P, Lautenbacher S, Marchand S, Wilder-Smith O. Recommendations on terminology and practice of psychophysical DNIC testing. Eur J Pain 2010;14:339. [DOI] [PubMed] [Google Scholar]
- [42].Zhang S, Li T, Kobinata H, Ikeda E, Ota T, Kurata J. Attenuation of offset analgesia is associated with suppression of descending pain modulatory and reward systems in patients with chronic pain. Mol Pain 2018;14:1744806918767512. [DOI] [PMC free article] [PubMed] [Google Scholar]


