Abstract
Background
Natural killer (NK) cells play a critical role in suppressing human immunodeficiency virus-1 (HIV-1) infection, but knowledge on whether and how NK cells affect immune reconstitution in HIV-1-infected individuals who receive antiretroviral therapy (ART) is limited.
Methods
We performed a case-control study with 35 healthy individuals and 66 HIV-1-infected patients including 32 immunological non-responders (INRs) with poor CD4+ T-cell recovery (<500 cells/μL after 4 years of ART) and 34 immunological responders (IRs) with improved CD4+ T-cell recovery (>500 cells/μL after 4 years of ART). NK cell phenotype, receptor repertoire, and early activation in INRs and IRs were investigated by flow cytometry.
Results
A significantly higher proportion of CD56dimCD16dim/- NK cells was observed in INRs than IRs before ART and after 4 years of ART. The number of CD56dimCD16dim/- NK cells was inversely correlated with CD4+ T-cell counts in INRs before ART (r = –0.344, P = 0.050). The more CD69-expressing NK cells there were, the lower the CD4+ T-cell counts and ΔCD4, and these correlations were observed in INRs after ART (r = –0.416, P = 0.019; r = –0.509, P = 0.003, respectively). Additionally, CD69-expressing CD56dimCD16dim/- NK cells were more abundant in INRs than those in IRs (P = 0.018) after ART, both of which had an inverse association trend towards significance with CD4+ T-cell counts. The expression of the activating receptors NKG2C, NKG2D, and NKp46 on CD56dimCD16dim/- NK cell subsets were higher in IRs than that in INRs after 4 years of ART (all P < 0.01). Strong inverse correlations were observed between CD69 expression and NKG2C, NKG2A-NKG2C+, NKG2D, and NKp46 expression on CD56dimCD16dim/- NK cells in INRs after ART (NKG2C: r = –0.491, P = 0.004; NKG2A-NKG2C+: r = –0.434, P = 0.013; NKG2D: r = –0.405, P = 0.021; NKp46: r = –0.457, P = 0.008, respectively).
Conclusions
INRs had a larger number of CD56dimCD16dim/- NK cells characterized by higher activation levels than did IRs after ART. The increase in the CD56dimCD16dim/- NK cell subset may play an adverse role in immune reconstitution. Further functional studies of CD56dimCD16dim/- NK cells in INRs are urgently needed to inform targeted interventions to optimize immune recovery.
Keywords: HIV-1 infection, Immunological non-responders, Natural killer cells, Immune reconstitution
Introduction
Antiretroviral therapy (ART) can effectively suppress human immunodeficiency virus (HIV) replication, increase HIV-infected patients’ peripheral blood CD4+ T-cell counts, and dramatically reduce acquired immunodeficiency syndrome (AIDS)-related morbidity and mortality. However, 15% to 30% of patients fail to recover their CD4+ T-cell counts despite the complete suppression of viral replication. These individuals are identified as immunological non-responders (INRs).[1–3] INRs exhibit severe immune dysfunction and have a higher rate of AIDS and non-AIDS events, such as metabolic syndrome, liver disease, nephropathy, cardiovascular disease, non-AIDS-related malignancies, and HIV-1-related neurocognitive disorder than complete immunological responders (IRs).[1,2,4,5] The reason for immunological non-response is incompletely understood. It is currently believed that decreased hematopoiesis in the bone marrow and insufficient thymic and lymph node outputs lead to a reduction in the production of CD4+ T cells, and residual viral replication, sustained immune activation, and disturbed in cytokine levels can all cause CD4+ T-cell apoptosis and depletion.[6–8] However, the current preliminary research on how dysfunction in the innate immune system affects the immune reconstitution in HIV-infected patients is limited.
Human natural killer (NK) cells provide powerful innate defenses against virus-infected cells and tumor cells. Their function is crucial at the time of infection and can impact the quality of adaptive immune responses and the overall outcome of infection. NK cells can be sub-divided into several sub-populations based on the relative expression of the adhesion molecule CD56 and the activating receptor CD16. CD56brightCD16dim/− NK cells are classically viewed as immature precursors and secrete cytokines; the larger CD56dimCD16bright NK cell subset is considered the mature subset of NK cells and the more cytotoxic subset. CD56-CD16bright NK cells with low cytotoxic activity and cytokine production are considered the “dysfunctional” subset.[9,10]
NK cell function is regulated by the activating and inhibitory receptors. NK cells receive inhibitory signals through the engagement of inhibitory killer immunoglobulin (Ig)-like receptors (characterized by a long (L) cytoplasmic tail) and C-type lectin receptors, such as NKG2A. Activating signals are received by activating killer cell immunoglobulin-like receptors (KIRs, characterized by a short (S) cytoplasmic tail), as well as natural cytotoxic receptors (NKp30, NKp44, and NKp46), NKG2C, NKG2E, or NKG2D.[11,12] The downregulation of CD56 is not the only NK surface marker altered by HIV-1 viremia. The expression and functional relevance of several activating and inhibitory receptors are pathologically modified by high levels of viral replication.[13,14]
NK cell effector function during the immune response against HIV infection has been evaluated in different cohorts: HIV controls (who maintain lower levels of HIV-1 replication in the absence of ART), slow progressors, and HIV-1-exposed seronegative individuals who remain uninfected despite repeated exposure to the virus.[15,16] The distribution and activation of NK cell subsets are also relevant in the context of mother-to-child HIV-1 transmission, as they influence the susceptibility to perinatal infection.[17]
The role of NK cells in immune reconstitution has not been elucidated. HIV-1 has developed various strategies of interaction with NK cell receptors and their ligands. The HIV-1 Nef protein reduces the cell-surface expression of NKG2D ligand, MICA, ULBP1, and, especially, ULBP2, thus impairing NKG2D-dependent killing by NK cells. Such aberrancies limit NK cell immune surveillance and allow the development of opportunistic infections and cancers. Despite virus countermeasures, HIV-1-infected T cells express higher NKG2DL levels than non-infected cells and are highly susceptible to killing by NK cells through NKG2D activation.[18–20] The population expressing another activating receptor of NK cells, the natural cytotoxicity receptor NKp44, the ligand of which is expressed on the surface of uninfected CD4+ T cells, is expanded, consequently rendering these T cells sensitive to NK cell-mediated killing.[21–23] In addition to the complex effects on NK cell receptors, HIV infection also causes INR patients to have an increase in the CD56bright NK cell sub-population but causes a poor response to cytokine stimulation in terms of NKp44 upregulation and interferon (IFN)-γ production, and CD56bright NK cells from INRs show relatively high cytotoxicity against autologous activated CD4+ T cells.[24] Hence, all of these mechanisms could be involved in CD4+ T-cell depletion in HIV-1 infection and in impaired immunological recovery in ART-treated patients.
In addition, HIV causes upregulation of the expression of inhibitory NK receptors, leading to impairments in NK cell-mediated lysis of virally infected cells.[25] Notably, reduced expression/function of natural cytotoxicity receptors can also be detected in NK cells from HIV-infected patients.[14] ART reverses the effects of HIV infection on NK cells; however, there is no consensus on the degree to which the suppression of HIV replication restores the NK cell subset distribution and function.[26–28] Several changes in the NK cell receptor repertoire and functionality are observed during HIV infection; some of these changes have been related to progression, but others are still under study. These complex and possible changes may represent one of the pathogenic events associated with the drastic reduction in CD4+ T-cell counts.
To better understand the role of NK cells in INRs, in this study, we focused on the changes in the NK cell phenotype and receptor repertoire in a cohort of INRs compared to IRs and health controls (HCs). Our results showed that the CD56dimCD16dim/- subset was expanded in the INR group compared to the IR group both before and after ART. CD56dimCD16dim/- NK cells exhibited a relatively high degree of early activation even though the suppression of viral replication by ART is negatively correlated with CD4+ T-cell counts. The expansion of the CD56dimCD16dim/- subset may play an dverse role in immune reconstitution.
Methods
Ethical statement
All the participants came from the Beijing Primo Clinical Cohort, which ran from 2011 to 2012. They provided written informed consent for participation in the study. This study and other related experiments were approved by the Beijing Youan Hospital Research Ethics Committee (No. 2017-13), and informed written consent was obtained in accordance with the Declaration of Helsinki. The study was carried out in accordance with approved guidelines and regulations.
Study participants
Sixty-six HIV-1-infected patients from the Primo cohort of men who have sex with men that were collected at the Beijing Youan Hospital between 2011 and 2012 were enrolled: 32 INRs with poor CD4+ T-cell recovery (<500 cells/μL after four years of ART) and 34 IRs with immunological restoration (>500 cells/μL after four years of ART). We also included 35 HCs with ages similar to the patients. All participants were divided into 5 groups: HCs, INRs before ART (INR pre-ART), INRs after ART (INR-ART), IRs before ART (IR pre-ART), and IRs after ART (IR-ART). All subjects without ongoing co-infections or other diseases and their characteristics are summarized in Table 1.
Table 1.
Basic characteristics of HIV-infected patients enrolled in this study.
| HIV-infected patients | p values | |||
| Parameters | Healthy controls | INRs | IRs | INRs vs IRs |
| Number of subjects | 35 | 32 | 34 | NS |
| Mean age (years) | 32 (26–53) | 34 (21–57) | 33 (23–55) | NS |
| CD4+ T-cell counts (cells/μL) | ||||
| Pre-ART | NA | 207.00 (100–313) | 227.00 (130–313) | 0.021 |
| ART | NA | 380.00 (163–497) | 680.00 (505–1240) | <0.0001 |
| CD8+ T-cell counts (cells/μL) | ||||
| Pre-ART | NA | 664.50 (255–1716) | 766.50 (331–1928) | NS |
| ART | NA | 570.00 (229–1192) | 919.00 (515–1909) | <0.0001 |
| CD4/CD8 ratio | ||||
| Pre-ART | NA | 0.25 (0.12–0.76) | 0.28 (0.11-0.67) | NS |
| ART | NA | 0.62 (0.34–1.31) | 0.70 (0.45–1.36) | NS |
| Viral load (log10, copies/mL) | ||||
| Pre-ART | NA | 4.41 (1.97–5.26) | 4.06 (1.70–5.97) | NS |
| ART | NA | ND | ND | |
Data are presented as n or median (range). p values were determined by Mann-Whitney U two-tailed t-test. NA: Not applicable; NS: Not significant; ND: Not detected.
Cell-surface staining and flow cytometry analysis
Peripheral blood mononuclear cells (PBMCs) were thawed in a water bath at 37°C for one minute, washed and resuspended in Roswell Park Memorial Institute (RPMI) medium containing 10% v/v fetal calf serum. Antibodies were incubated at 4°C for surface staining, and PBMCs were stained with the following fluorophore-conjugated human monoclonal antibodies at room temperature for 20 min: anti-CD3/PE-Cy7 (clone HIT3a; BioLegend, San Diego, CA, USA), anti-CD16/APC (clone 3G8; BioLegend, San Diego, CA, USA) or PerCP (clone 3G8; BioLegend, San Diego, CA, USA), anti-CD56/APC-Cy7 (clone HCD56; BioLegend, San Diego, CA, USA), anti-NKG2A/FITC (clone REA110; Miltenyi Biotec), anti-NKG2C/PE (clone REA205; Miltenyi Biotec), anti-NKG2D/BV421 (clone 1D11; BioLegend, San Diego, CA, USA), anti-CD69/FITC (clone FN50; BioLegend, San Diego, CA, USA), anti-NKp30/APC (clone P30-15; BioLegend, San Diego, CA, USA), anti-NKp44/PE (clone P44-8; BioLegend, San Diego, CA, USA), and anti-NKp46/BV421 (clone 9E2; BioLegend, San Diego, CA, USA). Cell viability was determined using Live/Dead fixable viability stain 510 (BD Biosciences, San Jose, CA, USA). Cells were washed and fixed with 2% paraformaldehyde. Cytometer setup and tracking calibration particles were used to ensure that fluorescence intensity measurements were consistent among all experiments. At least 200,000 PBMCs were acquired on a BD FACSCanto II flow cytometer. Gating on forward scatter and side scatter parameters was used to exclude cell debris from the analysis; the forward height and forward area were used to exclude doublets. Data analysis was performed using FlowJo 7.6.1 software version 10.4 (TreeStar, Ashland, OR, USA).
CD4+ T-cell count and viral load measurements
Routine blood CD4+ T-cell counts (cells/μL) were measured by four-color flow cytometry using the human CD45, CD3, CD4, and CD8 cell markers (BD Biosciences) in whole peripheral blood samples from each patient processed with a fluorescence activated cell sorter (FACS) lysing solution (BD Biosciences) according to the manufacturer's instructions. The plasma HIV-1 viral load (copies per milliliter of plasma) was quantified by real-time PCR (Abbott, Des Plaines, IL, USA). The sensitivity of viral RNA detection using this assay is 40 copies/mL of plasma (BD Biosciences).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 6.0 software (GraphPad Software, Inc., La Jolla, CA, USA). The statistical significance between the two groups was calculated using the Mann-Whitney U test, and correlations were determined by the Spearman rank correlation test, with r being the Spearman correlation coefficient. A P value < 0.05 was considered statistically significant.
Results
Demographic and clinical characteristics of the subjects enrolled in this study
We enrolled chronically HIV-1-infected patients undergoing ART for 4 years who were divided into two groups based on CD4+ T-cell recovery: a group of 32 INRs with <500 CD4+ T cells/μL and a group of 32 IRs with >500 CD4+ T cells/μL. Thirty-five age-matched HCs were also included in this study. The CD4+ T-cell counts of the IRs were higher than those of the INRs (P = 0.021 before ART; P < 0.0001 after ART). The CD8+ T-cell counts of the INRs were similar to those of the IRs before ART, but at the time of analysis after ART, the CD8+ T-cell counts in the IR group were higher than those in the INR group (P < 0.0001). There was no difference in the ratio of CD4/CD8 between the two groups before or after ART. The plasma HIV-1 viral load did not differ significantly between the IR and INR groups before ART. After 4 years of ART, all HIV-infected patients achieved viral suppression, and the plasma HIV-1 viral load could not be detected. No differences in demographics or treatment duration were present between the groups [Table 1].
INR patients have an increased proportion of CD56dimCD16dim/- NK cells
PBMCs were analyzed by flow cytometry with initial gating on CD3- peripheral blood lymphocytes (PBLs) and subsequent analysis based on CD56 and CD16 expression levels, identifying NK cell subsets as CD56brightCD16dim/-, CD56dimCD16dim/-, CD56dimCD16bright, and CD56-CD16bright (Figure 1A, presented in gates P1, P2, P3, and P4, respectively).
Figure 1.
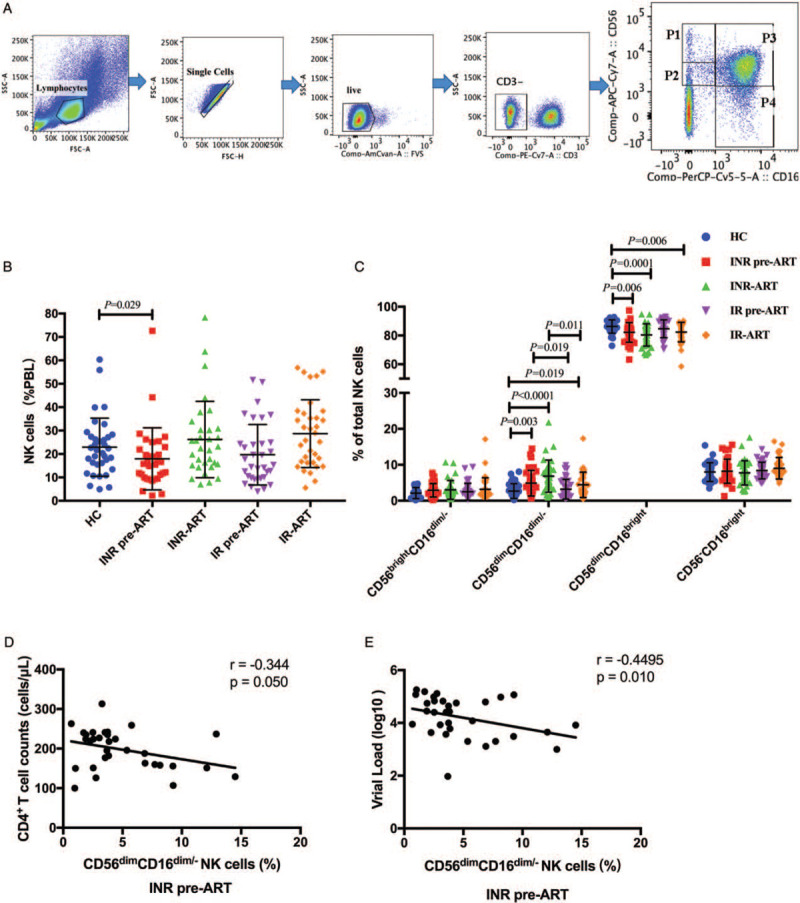
The CD56dimCD16dim/- NK cell subset is enlarged in INR patients. (A) The gating strategy used to identify NK cell subsets by flow cytometry and the frequencies of gated populations are shown for a representative HC. Lymphocytes were gated according to forward and side scatter dot plots. Single cells were gated according to forward height and side scatter forward area. Dead cells were excluded by staining with Live/Dead fixable viability stain 510. NK cells were defined within the CD3-gate on the basis of the expression of CD16 and CD56. NK cells were divided into four subsets: CD56brightCD16dim/- (P1), CD56dimCD16dim/- (P2), CD56dimCD16bright (P3) and CD56-CD16bright (P4). (B) Frequencies of NK cells derived from peripheral blood lymphocytes. (C) Percentages of the different natural killer (NK) cell subsets relative to the total NK cell population (100%). The frequency of CD56dimCD16dim\- cells in the total NK cell population was higher in INRs than that in IRs and HCs. (D) and (E) Correlation between CD56dimCD16dim/- NK cell subset with CD4+ T-cell counts and viral load in INR pre-ART group. The Mann-Whitney U test was used for statistical analysis. Horizontal lines indicate mean values, and error bars represent the mean ± standard error of mean. Correlations between two variables were analyzed in nonparametric Spearman's rank correlation tests, and p<0.05 was considered statistically significant. HCs: Health controls; INRs: Immunological non-responders; IR: Immunological responder; NK: Natural killer; PBL: Peripheral blood lymphocytes; VL: Viral load.
To further delineate the potential role of NK cells in immune reconstitution, we analyzed their proportions according to the immune reconstitution status. In our study, before ART, the frequency of NK cells among PBLs in INRs was lower than that among HCs (Figure 1B, INR pre-ART vs. HC, P = 0.029), but it was restored by ART. No differences were observed in the frequency of NK cells between HCs and IRs.
Significant differences existed in the CD56dimCD16dim/- and CD56dimCD16bright subsets between groups, but there were no differences in the CD56brightCD16dim/- and CD56-CD16bright subsets [Figure 1C]. Interestingly, both before ART and after ART, the frequency of CD56dimCD16dim/- NK cells was significantly higher in INRs than that in IRs (INR pre-ART vs. IR pre-ART, P = 0.019; INR-ART vs. IR-ART, P = 0.011). Compared to that in the HC group, the frequency of CD56dimCD16dim/- NK cells in both the IR and INR groups was increased with HIV-1 infection (INR pre-ART vs. HC, P = 0.003; INR-ART vs. HC, P < 0.0001; IR-ART vs. HC, P = 0.019). With the prolongation of HIV infection, the frequency of CD56dimCD16dim/- NK cells was increased and not restored by ART (INR pre-ART vs. INR-ART, P = 0.008; IR pre-ART vs. IR-ART, P = 0.02). The CD56dimCD16bright subset in INRs was lower than that in HCs before ART (INR pre-ART vs. HC, P = 0.006). After 4 years of ART, the CD56dimCD16bright subset in both INRs and IRs was lower than that in HCs (INR-ART vs. HC, P = 0.0001; IR-ART vs. HC, P = 0.006), but there was no difference between the INRs and IRs. These results implied that NK cell subsets were only partially recovered after ART. Of note, in the INR pre-ART group, correlation analysis revealed that the increase in the CD56dimCD16dim/- NK cell subset was inversely correlated with CD4+ T-cell counts (r = –0.344, P = 0.050, Figure 1D) and also inversely correlated with viral load (r = −0.4495, P = 0.010, Figure 1E). We did not find any significant correlations in other groups.
INRs with an increase in the CD56dimCD16dim/- NK cell subset display higher immune activation
NK cell activation was confirmed by measuring CD69 upregulation in this study. We found that CD69 expression in the overall NK cell population was higher in HIV-infected patients than that in HCs (INR pre-ART vs. HC, P < 0.0001; INR-ART vs. HC, P = 0.012; IR pre-ART vs. HC, P = 0.001; Figure 2A). However, if HIV-infected patients were stratified into IRs and INRs, the INRs had a significantly higher frequency of CD69 expression than the IRs after ART and HCs (INR-ART vs. IR-ART, P = 0.038; Figure 2A). In the CD56dimCD16dim/- NK cell subset, the frequency of CD69 expression in the INR-ART group was still significantly higher than that in the IR-ART and HC groups (INR-ART vs. IR-ART, P = 0.018; INR-ART vs. HC, P = 0.003; Figure 2F). These results suggest that the CD56dimCD16dim/- NK cell subset is activated.
Figure 2.
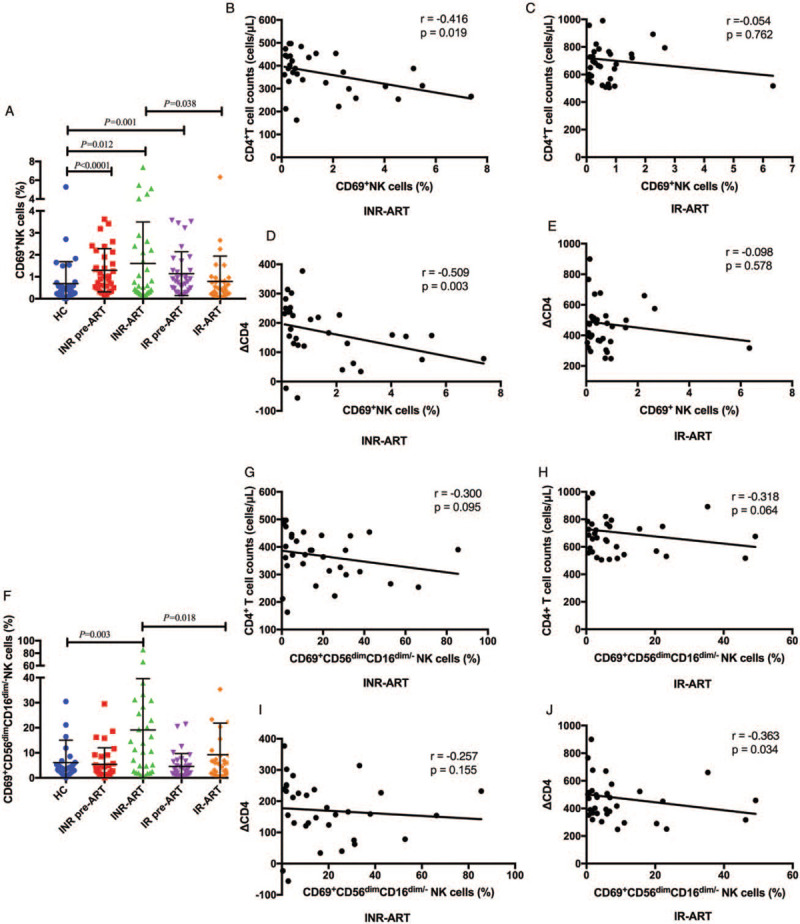
Expression of CD69 on NK cells. (A) The frequency of CD69+ NK cells. (B) Correlations between the percentages of CD69-expressing NK cells and CD4+ T cells in the INR-ART group and (C) the IR-ART group. (D) The number of CD4+ T cells was increased after 4 years of ART (ΔCD4) in the INR-ART group and (E) the IR-ART group. (F) The frequency of CD69-expressing CD56dimCD16dim/- NK cells. (G) Correlations between the percentages of CD69-expressing CD56dimCD16dim/-NK cells and CD4+ T cells in the INR-ART group and (H) the IR-ART group. (I) Correlations between the percentages of CD69-expressing CD56dimCD16dim/-NK cells and ΔCD4 in the INR-ART group and (J) the IR-ART group. The Mann-Whitney U test was used for statistical analysis. Horizontal lines indicate mean values, and error bars represent the mean ± standard error of mean. Correlations between two variables were analyzed in nonparametric Spearman rank correlation tests, and P < 0.05 was considered statistically significant. ΔCD4: Number of CD4 growth; ART: Antiretroviral therapy; INR: Immunological non-responder; IR: Immunological responder; NK: Natural killer; PBL: Peripheral blood lymphocytes; VL: Viral load.
Early activation of NK cells was negatively related to CD4+ T-cell counts
The immune activation of NK cells is responsible for the poor immune reconstitution of INRs.[29] To further determine the role of NK cell activation in HIV infection with different degrees of immune restoration after long-term ART and viral suppression, we analyzed the correlation between the expression of CD69 on NK cells and CD4+ T-cell counts. In the current study, there was a significantly negative association between the percentage of CD69+ NK cells and peripheral CD4+ T-cell counts in the INR-ART group (r = –0.416, P = 0.019; Figure 2B). The number of CD4+ T cells was increased after 4 years of ART (ΔCD4) among INRs and negatively correlated with the frequency of CD69+ NK cells (r = –0.509, P = 0.003; Figure 2D). However, a trend toward a negative relationship between the percentage of CD69+ NK cells and CD4+ T-cell counts, ΔCD4, was observed in the IR-ART group, but this relationship was not statistically significant (r = –0.054, P = 0.762; r = –0.098, P = 0.578; Figures 2C and 2E).
The correlation between the percentages of CD69-expressing CD56dimCD16dim/- NK cells and CD4+ T-cell counts in HIV-infected patients tended to negative; however, this correlation was not statistically significant [Figures 2G and 2H]. Notably, ΔCD4 in the IR-ART group was significantly inversely correlated with the percentages of CD69-expressing CD56dimCD16dim/- NK cells (r = –0.363, P = 0.034), but this relationship was not seen in the INR group [Figures 2I and 2J]. Thus, a strong CD56dimCD16dim/- NK cell activation capacity may have an adverse effect on INR CD4+ T-cell recovery after ART.
The frequency of CD56dimCD16dim/- NK cells in INRs was positively correlated with the early activation of NK cells
To further determine the contribution of the relatively high frequency of CD56dimCD16dim/- NK cells to NK cell activation, we analyzed the correlation between the frequencies of CD69+ NK cells and CD56dimCD16dim/- NK cells. In the current study, the frequency of CD56dimCD16dim/- NK cells was positively correlated with the early activation of NK cells in both INRs and IRs before and after ART (r = 0.360, P = 0.042, INR pre-ART; r = 0.381, P = 0.034, INR-ART; r = 0.602, P = 0.0002, IR pre-ART; r = 0.401, P = 0.018, IR-ART; Figures 3A–3D). These findings suggest that a higher level of CD69 expression on NK cells is correlated with a higher frequency of CD56dimCD16dim/- NK cells in HIV-infected patients. This also further confirms the negative role of CD56dimCD16dim/- NK cells in immune reconstitution.
Figure 3.
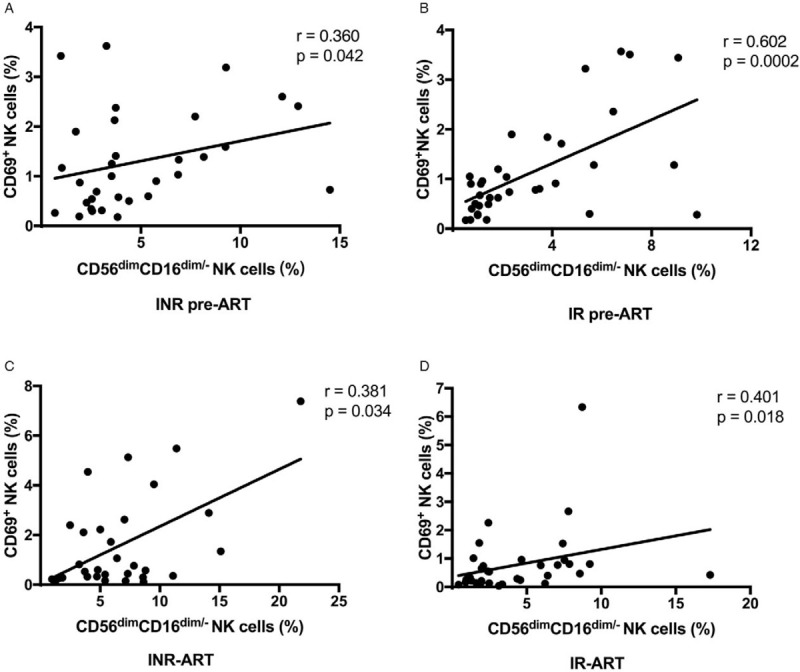
Correlations between the percentages of CD69-expressing NK cells and CD56dimCD16dim/- NK cells. (A) In the INR pre-ART group, (B) the INR-ART group, (C) the IR pre-ART group, and (D) the IR-ART group. Correlations between two variables were analyzed using nonparametric Spearman rank correlation tests, and P < 0.05 was considered statistically significant. ART: Antiretroviral therapy; INR: Immunological non-responder IR: Immunological responder; NK: Natural killer.
Expression of NK activating receptors (NKG2C, NKG2D, and NKp46) on the CD56dimCD16dim/- NK subset was higher in IRs than that in INRs after ART
NK cell functionality is coordinated by a dynamic balance between inhibitory and activating signals upon encountering a target cell.[11,30] Previous data have shown that abnormal expression of NK activating and inhibitory receptors is associated with impaired NK cell function.[31] We focused on the receptor spectrum in CD56dimCD16dim/- NK cells, including the inhibitory receptor NKG2A and activating receptors NKG2C, NKG2D, NKp30, NKp44, and NKp46 [Figure 4].
Figure 4.
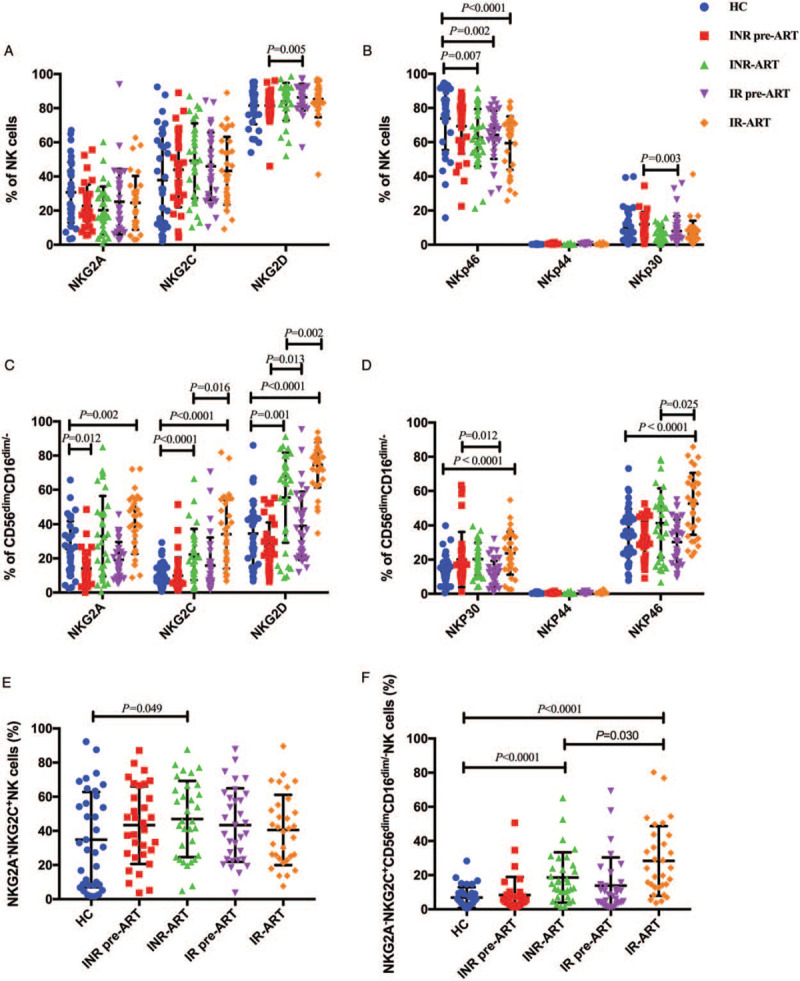
Expression of NK receptors on NK cell subsets. (A) The percentages of NKG2A-, NKG2C-, and NKG2D-expressing NK cells. (B) The percentages of NKp46-, NKp44-, and NKp30-expressing NK cells. (C) The percentages of NKG2A-, NKG2C-, and NKG2D-expressing CD56dimCD16dim/- NK cells. (D) The percentages of NKp46-, NKp44-, and NKp30-expressing CD56dimCD16dim/- NK cells. (E) The percentages of NKG2A-NKG2C+ NK cells. (F) The percentages of NKG2A-NKG2C+CD56dimCD16dim/- NK cells. The Mann-Whitney U test was used for statistical analysis. Horizontal lines indicate mean values, and error bars represent the mean ± standard error of mean. NK: Natural killer.
First, we analyzed receptor expression on total NK cells. Before ART, the frequency of NKG2D expression on NK cells was lower in INRs than IRs (INR pre-ART vs. IR pre-ART, P = 0.005; Figure 4A), and NKp30 expression on NK cells was higher in INRs (INR pre-ART vs. IR pre-ART, P = 0.003; Figure 4B). However, these differences disappeared after ART. Compared to that in HCs, the expression of NKp46 in IRs and INRs was lower, and this gap actually increased after ART (INR-ART vs. HCs, P = 0.007; IR pre-ART vs. HC, P = 0.002; IR-ART vs. HC, P < 0.0001; Figure 4B). The expression levels of NKp44 on the NK cells of HIV-1-infected patients were very low [Figure 4B] if not undetectable without in vitro stimulation.[32] There was no difference in NKG2A, NKG2C, and NKp44 expression on NK cells among the groups.
As shown in Figures 4C and 4D, we further investigated and analyzed the expression of NK cell receptors in the CD56dimCD16dim/- subset. A comparative evaluation of the phenotype of NK cells among INRs and IRs was performed. Before ART, the frequency of NKG2D-expressing CD56dimCD16dim/- NK cells was lower in the INRs (INR pre-ART vs. IR pre-ART, P = 0.013; Figure 4C), and NKp30 expression was higher in the INRs (INR pre-ART vs. IR pre-ART, P = 0.012; Figure 4D). After ART, the difference in NKp30 expression disappeared. However, the levels of NKG2C, NKG2D, and NKp46, the activating receptor of NK cells expressed in the CD56dimCD16dim/- subset, were all higher in the IR-ART group (INR-ART vs. IR-ART, P = 0.016, P = 0.002, and P = 0.025, respectively; Figures 4C and 4D). In addition, these activating receptor levels were all higher in HIV-infected patients after ART, both INRs and IRs, than those in HCs (INR-ART vs. HC, P < 0.0001 and P = 0.001; IR-ART vs. HC, P < 0.0001, P < 0.0001, and P < 0.0001, respectively; Figures 4C and 4D).
The expression of the inhibitory receptor NKG2A was lower in HIV-infected patients than that in HCs before ART (INR pre-ART vs. HC, P = 0.012; Figure 4C), and as the infection was sustained, the expression of NKG2A also increased despite after four years ART (IR-ART vs. HC, P = 0.002; Figure 4C). There was no difference between INRs and IRs.
The activating receptor NKG2C and the inhibitory receptor NKG2A recognize the same ligand, HLA-E, a non-classical HLA molecule characterized by limited polymorphism.[33,34] It has been reported that the proportion of NKG2A-NKG2C+ NK cells is a potential biomarker for predicting HIV disease progression.[35] In our results, NKG2A-NKG2C+ expression on total NK cells was increased in the INR-ART group compared to the HC group (INR-ART vs. HC, P = 0.049; Figure 4E). The frequency of NKG2A-NKG2C+ CD56dimCD16dim/- NK cells was higher in IR-ART than that in INR-ART (INR-ART vs. IR-ART, P = 0.03; Figure 4F). Compared to HCs, INR-ART, and IR-ART showed increased levels of NKG2A-NKG2C+ CD56dimCD16dim/- NK cells; however, were also increased in INR-ART and IR-ART, although there were no differences between HIV-infected patients and HCs at baseline (INR-ART vs. HC, P < 0.0001; IR-ART vs. HC, P < 0.0001; Figure 4F).
In summary, the expression of NK activating receptors (NKG2C, NKG2A-NKG2C+, NKG2D, and NKp46) on the CD56dimCD16dim/- NK subset was higher in IRs than that in INRs after 4 years of ART. Although we did not perform further analysis of NK cell inhibitory receptors due to logistical limitations, in the current study, the higher activating receptor expression may be reflective of the higher cytotoxicity against HIV infection and better immune reconstitution among IRs.
Correlations between CD69 and NKG2C, NKG2A-NKG2C+, NKG2D, and NKp46 expression on CD56dimCD16dim/- NK cells
Next, we analyzed the correlations between activation and functional markers on NK cells in HCs and HIV-infected subjects. Interestingly, we found significant negative correlations between NKG2C, NKG2A-NKG2C+, NKG2D, and NKp46 expression and CD69 expression on CD56dimCD16dim/- NK cells in the INR-ART group (r = –0.491, P = 0.004; r = –0.434, P = 0.013; r = –0.405, P = 0.021; r = –0.457, P = 0.008, respectively; Figures 5A–5D). In the IR-ART group, NKG2C, NKG2A-NKG2C+, and NKG2D expression on CD56dimCD16dim/- NK cells but not NKp46 expression was significantly negatively correlated with CD69-expressing CD56dimCD16dim/- NK cells (r = –0.416, P = 0.017; r = –0.424, P = 0.015; r = –0.435, P = 0.010, respectively; Figures 5E–5H). This evidence supports that increased activating receptor (NKp46, NKp30, and NKG2D) expression on the CD56dimCD16dim/- subset is accompanied by decreased CD69 expression. Altogether, this pattern indicates a relatively favorable immune reconstitution environment in IR patients. These results also suggest that NK cells may be activated to express these activation and functional markers by different mechanisms in HIV-infected patients.
Figure 5.
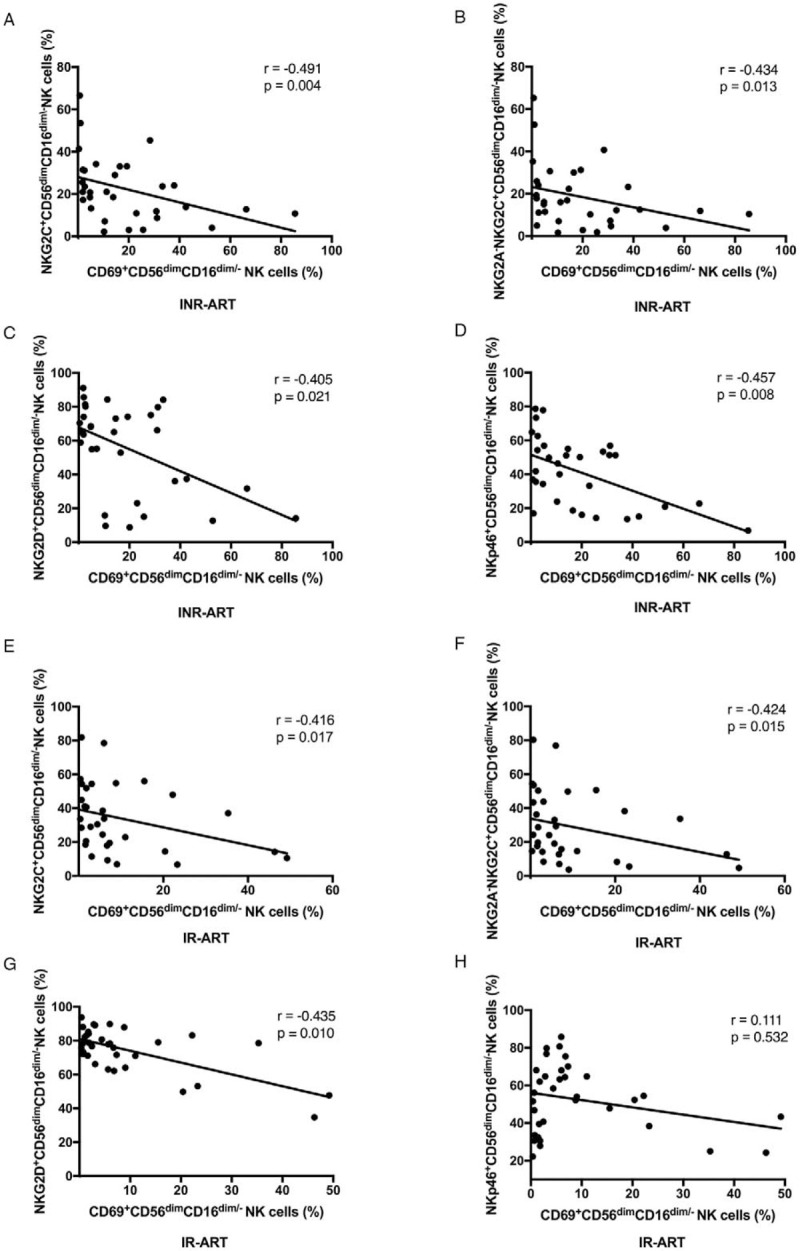
Correlations between CD69 expression and NKG2C, NKG2A-NKG2C+, NKG2D, and NKp46 expression on CD56dimCD16dim/-NK cells. (A) NKG2C in INR-ART, (B) NKG2A-NKG2C+ in INR-ART, (C) NKG2D in INR-ART, (D) NKp46 in INR-ART, (E) NKG2C in IR-ART, (F) NKG2A-NKG2C+ in IR-ART, (G) NKG2D in IR-ART, and (H) NKp46 in IR-ART. Correlations between two variables were analyzed with non-parametric Spearman rank correlation tests, and P < 0.05 was considered statistically significant. ART: Antiretroviral therapy; INR: Immunological non-responder; IR: Immunological responder; NK: Natural killer.
Discussion
This study was performed to investigate the role of NK cells in the immune reconstitution of HIV-1-infected individuals. In INRs, the frequency of CD56dimCD16dim/- NK cells was higher than that in IRs both before ART and after four years of ART. The increase in CD56dimCD16dim/- NK cells was inversely related to CD4+ T-cell counts in the INR pre-ART group. In addition, INRs displayed higher levels of early activation than IRs after ART, and the early activation of NK cells was negatively correlated with CD4+ T-cell counts in HIV-infected individuals. Furthermore, CD56dimCD16dim/- NK cells were positively correlated with the early activation of NK cells. Accordingly, CD56dimCD16dim/- NK cells may be involved in the poor immune reconstitution of INRs.
It has been demonstrated that in the acute phase of HIV-1 infection, NK cells expand in the peripheral blood to inhibit HIV replication and can mediate in vivo immune pressure in infected individuals, resulting in the viral escape.[36] NK cell features are affected by high and chronic viremia, including the frequencies, phenotypes, and functions.[37] The number of circulating NK cells appears to be restored as soon as HIV-1 infection enters its chronic stage, but the distribution of the different subsets undergoes pathological redistribution.[38] Several studies have shown that HIV-1 pathologically changes NK cell homeostasis and hampers NK cell antiviral effector functions. These pathogenic events are associated with a pathological redistribution of NK cell subsets that includes the expansion of anergic CD56-CD16bright NK cells with an aberrant repertoire of activating and inhibitory receptors.[39] Recently, the existence of a population of CD56dimCD16dim NK cells was detected but found to be globally reduced in a longitudinal cohort of HIV-1-infected individuals. On CD16 versus CD56 flow cytometry dot plots of fresh human PBMCs, the five usual NK cell subpopulations appeared: CD56brightCD16-, CD56brightCD16dim, CD56dimCD16-, CD56dimCD16bright, and CD56-CD16bright. From a functional point of view, in HCs and HIV-1-infected patients, the most proficient degranulating subset was CD56dimCD16- cells and sorted CD56dimCD16dim cells degranulated more than CD56dimCD16bright cells but less than CD56dimCD16- NK cells. The CD56dimCD16- population was similarly the subset that most effectively produced IFN-γ, followed by the CD56dimCD16dim subset.[40] Therefore, it can be speculated that the CD56dimCD16dim/- subset has strong cytotoxicity and the ability to produce IFN-γ. Due to the clinical interest in NK cells, it is therefore highly relevant to precisely identify NK cell populations with specific or pronounced functions. In particular, multiparametric cytometry allows in-depth investigation of human immune cells, and clear, univocal identification on the basis of phenotypic traits determined using flow cytometry can help us understand the role of NK cells in disease.[41]
Our data are consistent with previous reports indicating that CD56dimCD16bright NK cells are the largest population of NK cells in PBLs and that there is a decrease in the cytotoxic CD56dimCD16bright NK cell level despite four years of ART.[38,42,43] CD56brightCD16dim/- NK cells are described as the progenitors of CD56dimCD16bright cells. In an African adult HIV treatment cohort, researchers found high proinflammatory CD56brightCD16dim/- NK cell levels among sub-optimal IRs despite four years of suppressive ART.[44] However, we did not find a difference in the CD56brightCD16dim/- subset between INRs and IRs.
CD56dimCD16bright cells sequentially progress from an immature population via characteristic loss of inhibitory receptors, such as NKG2A, and gain of CD16, KIR, and CD57 receptors.[45] CD56dimCD16bright NK cells also lose expression of CD16 through metalloprotease-mediated shedding and become CD56dimCD16-, and the latter population demonstrates a higher degranulation ability.[46] Under these conditions, CD56dimCD16dim/- NK cells are likely a mixture of relatively immature cells and previously CD56dimCD16bright cells that are on their way to losing CD16 expression due to activation-induced shedding. Recently, a study from France confirmed that the NK cell maturation status of primary infected patients should be considered a relevant marker of an immune process contributing to the early outcome of ART that could help in the management of HIV-infected patients. In that study, HIV-infected individuals were grouped into those with predominantly immature/early differentiated NK cells and those with predominantly mature NK cells. In conclusion, a better early response to ART is observed in patients whose NK cell profile is skewed to maturation at inclusion.[47] According to Mathieu's investigation, CD56dimCD16dim/- NK cells may be an intermediate stage between CD56dimCD16bright and CD56brightCD16dim NK cells.[40] In this study, the higher proportion of immature/early differentiated CD56dimCD16dim/- NK cells in INRs might contribute to a poor response to ART and be disadvantageous for CD4+ T-cell recovery. Phenotypic immaturity does not necessarily correlate with the absence of functionality, and it has been demonstrated that sorted CD56dimCD16dim/- cells more effectively degranulate and produce IFN-γ than CD56dimCD16bright cells.[40] Previous studies have confirmed that CD56brightCD16dim/- NK cells from INRs show relatively high cytotoxicity against autologous activated CD4+ T cells.[24] A limitation of the study is the lack of NK cell functional assays. We could not determine whether the CD56dimCD16dim/- NK cells have the same cytotoxicity against autologous activated CD4+ T cells, but based on the above evidence, we speculate that the CD56dimCD16dim/- NK sub-group plays a detrimental role in the recovery of CD4+ T cells.
The pathogenic and clinical relevance of this persistent NK cell activation during the course of immune recovery is still unclear. NK cell activation has been correlated with microbial translocation, a factor associated with poor CD4+ T-cell restoration after long-term virus-suppressive ART.[48] Increased NK cell activation in INRs is inversely correlated with CD4+ T-cell recovery after ART.[29] In this study, we analyzed early NK cell activation by monitoring the expression of CD69. Increased NK cell activation was found in HIV-infected individuals. The percentage of CD69-expressing NK cells among INR-ART was inversely correlated with CD4+ T-cell counts. In the CD56dimCD16dim/- subset, CD69 expression in the INR-ART group was also higher than that in the IR-ART and HC groups, and CD56dimCD16dim/- NK cells exhibited elevated early activation, which was inversely correlated with CD4+ T-cell counts. In addition, in INRs, CD56dimCD16dim/- NK cells were positively correlated with the early activation of NK cells. Overall, the increase in CD56dimCD16dim/- NK cells may be a driver of NK cell activation in INRs with virus-suppressed HIV.
Due to different functional and migratory behaviors, the phenotypes of the various NK cell sub-populations in terms of repertoires of activating and inhibitory receptors are not the same.[49] In a study on poor CD4+ T-cell reconstitution evaluating the cell-surface expression of CD56brightCD16dim/- NK cell-activating receptors (NKp30, NKp44, NKp46, and NKG2D), only slightly higher NKp46 expression was observed in INRs than IRs.[50] However, we did not find a difference in the expression of NKp46 or NKG2A, NKG2C, NKG2D, NKp30, and NKp44 on total NK cells between INRs and IRs after ART. With regard to the CD56dimCD16dim/- subset, we were surprised to find that NK cell functional marker (NKG2C, NKG2A-NKG2A+, NKG2D, and NKp46) expression in the IR-ART group was higher than that in the INR-ART group. The increased expression of activating receptors may be reflective of the comprehensive functions against HIV infection and better immune reconstitution in IRs. In addition, we found a negative correlation between NKG2C, NKG2A-NKG2C+, NKG2D, and NKp46 expression and CD69 expression on CD56dimCD16dim/-NK cells in the INR-ART and IR-ART groups. Consistently, the sustained activation of the CD56dimCD16dim/-subset in INRs might play an inhibitory role in immune reconstitution in this patient population.
Our results imply that after four years of suppressive ART, HIV-associated NK cell dysfunction is only partially rescued, with a high CD56dimCD16dim/- subset characterized by relatively high activation in INRs both before and after ART. The increase in the CD56dimCD16dim/- NK cell level was inversely related to CD4+ T-cell counts in the INR pre-ART group. Increased CD56dimCD16dim/-NK cell activation in INRs was also inversely correlated with CD4+ T cells. These findings reveal a novel mechanism by which the alterations in NK cell populations may play an adverse role in CD4+ T-cell recovery in INRs. Further analysis of the function of the CD56dimCD16dim/-subset in INRs is urgently needed to inform targeted interventions to optimize immune recovery.
Acknowledgements
The authors thank Drs. Wei Xia, and Yue-Fang Zhou for patients recruiting, blood, and information collecting; Yun-Xia Ji, Xiao-Xue Tian for cell counting and viral load detecting.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 81772165, 81974303, 81571973, and 82072271), the NSFC-NIH Biomedical collaborative research program (No. 81761128001), the National 13th Five-Year Grand Program on Key Infectious Disease Control (Nos. 2017ZX10202102-005-003 and 2017ZX10202101-004-001), the Beijing Municipal of Science and Technology Major Project (No. D161100000416003), the Beijing Key Laboratory for HIV/AIDS Research (No. BZ0089), and the Key Project of Tianjin Second People's Hospital (No. YS0001).
Conflicts of interest
None.
Footnotes
How to cite this article: Zhang QY, Zhang X, Su B, Liu LF, Yang XD, Tang B, Xia H, Ma P, Zhang T, Wu H. Increased early activation of CD56dimCD16dim/- natural killer cells in immunological non-responders correlates with CD4+ T-cell recovery. Chin Med J 2020;133:2928–2939. doi: 10.1097/CM9.0000000000001262
References
- 1.Yang X, Su B, Zhang X, Liu Y, Wu H, Zhang T. Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: challenges of immunological non-responders. J Leukoc Biol 2020; 107:597–612. doi: 10.1002/jlb.4mr1019-189r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zoufaly A, Cozzi-Lepri A, Reekie J, Kirk O, Lundgren J, Reiss P, et al. Immuno-virological discordance and the risk of non-AIDS and AIDS events in a large observational cohort of HIV-Patients in Europe. Plos One 2014; 9:e87160.doi: 10.1371/journal.pone.0087160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corbeau P, Reynes J. Immune reconstitution under antiretroviral therapy: the new challenge in HIV-1 infection. Blood 2011; 117:5582–5590. doi: 10.1182/blood-2010-12-322453. [DOI] [PubMed] [Google Scholar]
- 4.Bandera A, Mangioni D, Incontri A, Perseghin P, Gori A. Characterization of immune failure by monocyte activation phenotypes in HIV-infected patients receiving antiretroviral therapy. J Infect Dis 2015; 212:839–U169. doi: 10.1093/infdis/jiv166. [DOI] [PubMed] [Google Scholar]
- 5.Pacheco YM, Jarrin I, Rosado I, Campins AA, Berenguer J, Iribarren JA, et al. Increased risk of non-AIDS-related events in HIV subjects with persistent low CD4 counts despite cART in the CoRIS cohort. Antiviral Res 2015; 117:69–74. doi: 10.1016/j.antiviral.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Rb-Silva R, Nobrega C, Azevedo C, Athayde E, Canto-Gomes J, Ferreira I, et al. Thymic function as a predictor of immune recovery in chronically HIV-infected patients initiating antiretroviral therapy. Front Immunol 2019; 10:25.doi: 10.3389/fimmu.2019.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez-Gallego E, Gomez J, Pacheco YM, Peraire J, Vilades C, Beltran-Debon R, et al. A baseline metabolomic signature is associated with immunological CD4(+) T-cell recovery after 36 months of antiretroviral therapy in HIV-infected patients. Aids 2018; 32:565–573. doi: 10.1097/qad.0000000000001730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaardbo JC, Hartling HJ, Gerstoft J, Nielsen SD. Incomplete immune recovery in HIV infection: mechanisms, relevance for clinical care, and possible solutions. Clin Dev Immunol 2012; 2012:670957.doi: 10.1155/2012/670957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol 2001; 22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 10.Björkström NK, Ljunggren HG, Sandberg JK. CD56 negative NK cells: origin, function, and role in chronic viral disease. Trends Immunol 2010; 31:401–406. doi: 10.1016/j.it.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Sivori S, Vacca P, Del Zotto G, Munari E, Mingari MC, Moretta L. Human NK cells: surface receptors, inhibitory checkpoints, and translational applications. Cell Mol Immunol 2019; 16:430–441. doi: 10.1038/s41423-019-0206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Zotto G, Marcenaro E, Vacca P, Sivori S, Pende D, Della Chiesa M, et al. Markers and function of human NK cells in normal and pathological conditions. Cytometry B Clin Cytom 2017; 92:100–114. doi: 10.1002/cyto.b.21508. [DOI] [PubMed] [Google Scholar]
- 13.Strauss-Albee DM, Fukuyama J, Liang EC, Yao Y, Jarrell JA, Drake AL, et al. Human NK cell repertoire diversity reflects immune experience and correlates with viral susceptibility. Sci Transl Med 2015; 7:297ra115.doi: 10.1126/scitranslmed.aac5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Maria A, Fogli M, Costa P, Murdaca G, Puppo F, Mavilio D, et al. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44). Eur J Immunol 2003; 33:2410–2418. doi: 10.1002/eji.200324141. [DOI] [PubMed] [Google Scholar]
- 15.Horton RE, McLaren PJ, Fowke K, Kimani J, Ball TB. Cohorts for the study of HIV-1-exposed but uninfected individuals: benefits and limitations. J Infect Dis 2010; 202: Suppl 3: S377–S381. doi: 10.1086/655971. [DOI] [PubMed] [Google Scholar]
- 16.Johansson SE, Rollman E, Chung AW, Center RJ, Hejdeman B, Stratov I, et al. NK cell function and antibodies mediating ADCC in HIV-1-infected viremic and controller patients. Viral Immunol 2011; 24:359–368. doi: 10.1089/vim.2011.0025. [DOI] [PubMed] [Google Scholar]
- 17.Gasper MA, Kunwar P, Itaya G, Lejarcegui N, Bosire R, Maleche-Obimbo E, et al. Natural killer cell and T-cell subset distributions and activation influence susceptibility to perinatal HIV-1 infection. Aids 2014; 28:1115–1124. doi: 10.1097/qad.0000000000000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richard J, Sindhu S, Pham TNQ, Belzile J-P, Cohen EA. HIV-1 Vpr up-regulates expression of ligands for the activating NKG2D receptor and promotes NK cell-mediated killing. Blood 2010; 115:1354–1363. doi: 10.1182/blood-2009-08-237370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desimio MG, Giuliani E, Doria M. The histone deacetylase inhibitor SAHA simultaneously reactivates HIV-1 from latency and up-regulates NKG2D ligands sensitizing for natural killer cell cytotoxicity. Virology 2017; 510:9–21. doi: 10.1016/j.virol.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 20.Desimio MG, Covino DA, Doria M. Potential of the NKG2D/NKG2DL axis in NK cell-mediated clearance of the HIV-1 reservoir. Int J Mol Sci 2019; 20:4490.doi: 10.3390/ijms20184490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sennepin A, Baychelier F, Guihot A, Nel I, Fang RHT, Calin R, et al. NKp44L expression on CD4(+) T cells is associated with impaired immunological recovery in HIV-infected patients under highly active antiretroviral therapy. Aids 2013; 27:1857–1866. doi: 10.1097/QAD.0b013e328361a3fe. [DOI] [PubMed] [Google Scholar]
- 22.Vieillard V, Strominger JL, Debre P. NK cytotoxicity against CD4(+) T cells during HIV-1 infection: a gp41 peptide induces the expression of an NKp44 ligand. Proc Natl Acad Sci U S A 2005; 102:10981–10986. doi: 10.1073/pnas.0504315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fausther-Bovendo H, Vieillard V, Sagan S, Bismuth G, Debre P. HIV gp41 engages gC1qR on CD4+ T cells to induce the expression of an NK ligand through the PIP3/H2O2 pathway. PLoS Pathog 2010; 6:e1000975.doi: 10.1371/journal.ppat.1000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giuliani E, Vassena L, Di Cesare S, Malagnino V, Desimio MG, Andreoni M, et al. NK cells of HIV-1-infected patients with poor CD4(+) T-cell reconstitution despite suppressive HAART show reduced IFN-γ production and high frequency of autoreactive CD56(bright) cells. Immunol Lett 2017; 190:185–193. doi: 10.1016/j.imlet.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 25.Mavilio D, Benjamin J, Daucher M, Lombardo G, Kottilil S, Planta MA, et al. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc Natl Acad Sci U S A 2003; 100:15011–15016. doi: 10.1073/pnas.2336091100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Florez-Alvarez L, Hernandez JC, Zapata W. NK cells in HIV-1 infection: from basic science to vaccine strategies. Front Immunol 2018; 9:2290.doi: 10.3389/fimmu.2018.02290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mikulak J, Oriolo F, Zaghi E, Di Vito C, Mavilio D. Natural killer cells in HIV-1 infection and therapy. Aids 2017; 31:2317–2330. doi: 10.1097/qad.0000000000001645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fauci AS, Mavilio D, Kottilil S. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat Rev Immunol 2005; 5:835–843. doi: 10.1038/nri1711. [DOI] [PubMed] [Google Scholar]
- 29.Luo Z, Li Z, Martin L, Hu Z, Wu H, Wan Z, et al. Increased natural killer cell activation in HIV-infected immunologic non-responders correlates with CD4+T cell recovery after antiretroviral therapy and viral suppression. Plos One 2017; 12:e0167640.doi: 10.1371/journal.pone.0167640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol 2008; 9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mavilio D, Benjamin J, Daucher M, Lombardo G, Kottilil S, Planta MA, et al. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc Natl Acad Sci U S A 2004; 101:6326–6326. doi: 10.1073/pnas.0401560101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marras F, Nicco E, Bozzano F, Di Biagio A, Dentone C, Pontali E, et al. Natural killer cells in HIV controller patients express an activated effector phenotype and do not up-regulate NKp44 on IL-2 stimulation. Proc Natl Acad Sci U S A 2013; 110:11970–11975. doi: 10.1073/pnas.1302090110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braud VM, Allan DS, O’Callaghan CA, Söderström K, D’Andrea A, Ogg GS, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 1998; 391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 34.Brooks AG, Boyington JC, Sun PD. Natural killer cell recognition of HLA class I molecules. Rev Immunogenet 2000; 2:433–448. [PubMed] [Google Scholar]
- 35.Ma M, Wang Z, Chen X, Tao A, He L, Fu S, et al. NKG2C(+)NKG2A(-) natural killer cells are associated with a lower viral set point and may predict disease progression in individuals with primary HIV infection. Front Immunol 2017; 8:1176.doi: 10.3389/fimmu.2017.01176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elemans M, Boelen L, Rasmussen M, Buus S, Asquith B. HIV-1 adaptation to NK cell-mediated immune pressure. PLoS Pathog 2017; 13:e1006361.doi: 10.1371/journal.ppat.1006361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hens J, Jennes W, Kestens L. The role of NK cells in HIV-1 protection: autologous, allogeneic or both? AIDS Res Ther 2016; 13:15.doi: 10.1186/s12981-016-0099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brunetta E, Hudspeth KL, Mavilio D. Pathologic natural killer cell subset redistribution in HIV-1 infection: new insights in pathophysiology and clinical outcomes. J Leukoc Biol 2010; 88:1119–1130. doi: 10.1189/jlb.0410225. [DOI] [PubMed] [Google Scholar]
- 39.Bjorkstrom NK, Ljunggren H-G, Sandberg JK. CD56 negative NK cells: origin, function, and role in chronic viral disease. Trends Immunol 2010; 31:401–406. doi: 10.1016/j.it.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Amand M, Iserentant G, Poli A, Sleiman M, Fievez V, Pilar Sanchez I, et al. Human CD56(dim)CD16(dim) cells as an individualized natural killer cell subset. Front Immunol 2017; 8:699.doi: 10.3389/fimmu.2017.00699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Del Zotto G, Antonini F, Pesce S, Moretta F, Moretta L, Marcenaro E. Comprehensive phenotyping of human PB NK cells by flow cytometry. Cytometry A 2020; 97:891–899. doi: 10.1002/cyto.a.24001. [DOI] [PubMed] [Google Scholar]
- 42.Mavilio D, Lombardo G, Benjamin J, Kim D, Follman D, Marcenaro E, et al. Characterization of CD56(-)/CD16(+) natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci U S A 2005; 102:2886–2891. doi: 10.1073/pnas.0409872102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lugli E, Marcenaro E, Mavilio D. NK cell subset redistribution during the course of viral infections. Front Immunol 2014; 5: doi: 10.3389/fimmu.2014.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bayigga L, Nabatanzi R, Sekiziyivu PN, Mayanja-Kizza H, Kamya MR, Kambugu A, et al. High CD56(++)CD16(-) natural killer (NK) cells among suboptimal immune responders after four years of suppressive antiretroviral therapy in an African adult HIV treatment cohort. BMC Immunol 2014; 15: doi: 10.1186/1471-2172-15-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moretta L. Dissecting CD56(dim) human NK cells. Blood 2010; 116:3689–3691. doi: 10.1182/blood-2010-09-303057. [DOI] [PubMed] [Google Scholar]
- 46.Romee R, Foley B, Lenvik T, Wang Y, Zhang B, Ankarlo D, et al. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17). Blood 2013; 121:3599–3608. doi: 10.1182/blood-2012-04-425397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gondois-Rey F, Cheret A, Mallet F, Bidaut G, Granjeaud S, Lecuroux C, et al. A mature NK profile at the time of HIV primary infection is associated with an early response to cART. Front Immunol 2017; 8:54.doi: 10.3389/fimmu.2017.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuri-Cervantes L, de Oca GS, Avila-Ríos S, Hernández-Juan R, Reyes-Terán G. Activation of NK cells is associated with HIV-1 disease progression. J Leukoc Biol 2014; 96:7–16. doi: 10.1189/jlb.0913514. [DOI] [PubMed] [Google Scholar]
- 49.Carrega P, Ferlazzo G. Natural killer cell distribution and trafficking in human tissues. Front Immunol 2012; 3:347.doi: 10.3389/fimmu.2012.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giuliani E, Vassena L, Di Cesare S, Malagnino V, Desimio MG, Andreoni M, et al. NK cells of HIV-1-infected patients with poor CD4(+) T-cell reconstitution despite suppressive HAART show reduced IFN-gamma production and high frequency of autoreactive CD56(bright) cells. Immunol Lett 2017; 190:185–193. doi: 10.1016/j.imlet.2017.08.014. [DOI] [PubMed] [Google Scholar]


