Abstract
INTRODUCTION:
Irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD) are gastrointestinal pathologies affecting large numbers of the global population and incurring significant healthcare costs. Disruptions in the gut-brain axis occurring in these conditions can lead to increased inflammation, affecting gastrointestinal and autonomic nervous system function. Heart rate variability (HRV) is commonly used to assess the state of the sympathetic and parasympathetic function of the autonomic nervous system, but it remains unclear how HRV measures are associated with gastrointestinal pathologies. Here, we conduct a systematic review of the literature comparing HRV of subjects diagnosed with IBS or IBD to HRV in healthy controls (HC).
METHODS:
We searched PubMed, Cochrane Library, and CINAHL (EBSCO) for eligible studies up to 2018. We included any study comparing a recognized measure of HRV between a group of patients with either IBS or IBD to a group of matched HC before any intervention. Studies were screened, and data were extracted from included articles using predefined criteria. Random effects meta-analysis was performed for each outcome, with effect size reported as the standardized mean difference.
RESULTS:
There were significant differences between IBD and HC in time domain HRV and significant decreases in high-frequency power measures were also noted, in both IBS and IBD compared with HC.
DISCUSSION:
Parasympathetic nervous system activity, represented through high-frequency power, seems to be lower in people with IBS and IBD, but conclusions are limited by the small number of studies that provide usable data, methodological heterogeneity, and high risks of bias in primary study methods and measures.
INTRODUCTION
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder (FGID) consisting of 4 subtypes (constipation, diarrhea, mixed, and unclassified) in the absence of organic or structural etiologies and diagnosed with Rome or Manning criteria (1). Its global prevalence remains elusive because of the methodological heterogeneity, symptom perception, and reporting concerns (2). Its global prevalence rate has been estimated at 11.2% (95% confidence interval [CI], 9.8%–12.8%) and more recently at 8.8% (95% CI, 8.7–8.9%) (2,3). In North America, the prevalence is estimated at 11.8% (95% CI, 7.4–17.2) (3) with direct and indirect annual healthcare costs exceeding 20 billion dollars (4).
Unlike IBS, inflammatory bowel disease (IBD), comprising of Crohn's disease (CD) and ulcerative colitis (UC), is a chronic, potentially fatal illness diagnosed via gastrointestinal imaging and histological findings. Greater than 5 million individuals are affected globally (5,6) and its direct healthcare costs in the United States are estimated at 6.3 billion dollars, with a lack of data on indirect costs (7).
The ability to actively monitor or even predict flares before their occurrence may reduce costs and improve patient outcomes in these conditions. Such monitoring might be achieved through a set of noninvasive electrocardiography parameters, collectively referred to as heart rate variability (HRV), that provides an indirect measure of the autonomic nervous system (ANS) (8–14). Excessive sympathetic or parasympathetic activity in the ANS can lead to dysregulation within the gut-brain axis contributing to maladaptive gastrointestinal responses and resulting in symptomatic flares (8–12,14).
Despite suggestions that lowered HRV is associated with pathological processes and mortality, definitive reference ranges constituting normal or healthy HRV values remain unclear (13–15), and there remains a knowledge gap associating HRV values with incidence or severity of gastrointestinal disorders despite several published reviews pertaining to IBS and none assessing IBD studies (16–20). We aim to systematically review the literature comparing HRV of individuals diagnosed with IBS and IBD with HRV in healthy controls (HC) to determine whether these disorders are associated with low HRV.
METHODS
This study was registered through the International Prospective Register of Systematic Reviews (PROSPERO) registration: CRD4201800072.
Eligibility for inclusion
Any study that presented HRV data in both an IBS or IBD group and a healthy group, at a single point in time and before any intervention, was considered eligible for inclusion. No restrictions were placed on age, sex, or weight of participants; setting; or language of publication. HRV data in original studies must be available from continuous recordings collected between 5 minutes and 24 hours in a lying, seated, or ambulatory state. Studies using participants with cardiovascular diseases, central or peripheral nervous system comorbidities, diabetes, renal failure, alcoholism, or on beta-blockers, beta-adrenergic agonists, or calcium channel blockers were excluded because these conditions may influence HRV (14). FGIDs can coexist with other functional disorders such as dyspepsia, functional abdominal pain, or fibromyalgia, and studies in which participants with IBS were not separated from participants with other functional disorders were excluded, unless authors could be contacted for clarification or appropriate subgroup data.
Studies were included if IBS participants were diagnosed by a physician and/or met Manning or Rome I-IV criteria as assessed by study investigators. Participants with IBD had to be diagnosed by a physician or have evidence of IBD via imaging such as colonoscopy. Primary study authors were contacted by email 3 times for any missing or unclear information. If they did not respond after 3 attempts, those studies were excluded. If the same participants seemed to give data in multiple studies assessing HRV, authors were contacted for clarification and failure to respond resulted in earliest dated study used if otherwise eligible.
Search strategy
We searched PubMed, Cumulative Index to Nursing and Allied Health Literature (CINAHL EBSCO interface), and the Cochrane Database until June 2018. Reference lists of included studies, previous reviews, and meta-analyses were hand searched. ClinicalTrials.gov and the International Clinical Trials Registry Platform Search Portal were searched for ongoing or recently completed trials. PROSPERO was searched for similar ongoing or recently completed systematic reviews. A complete literature search strategy can be seen in Supplemental Digital Content 1 (see http://links.lww.com/CTG/A467).
Study selection, storage, and screening
Authors used Mendeley Desktop and DistillerSR for literature search results and management of screening results. Studies meeting inclusion criteria were entered into Review Manager (RevMan) version 5.3 software to assess studies, create comparison tables, examine and extract data from studies for meta-analysis, and present results in graphs if appropriate (21). The primary investigator (A.S.) screened titles and abstracts with coinvestigator CD, and level 2 screening with D.H. disagreement was settled via consensus between A.S. and D.H.
Data collection/data items
A.S. and D.H. conducted all data extraction after agreement, and data were recorded into a data extraction form and uploaded into RevMan5.3. Data items collected can be seen in Tables 1–3.
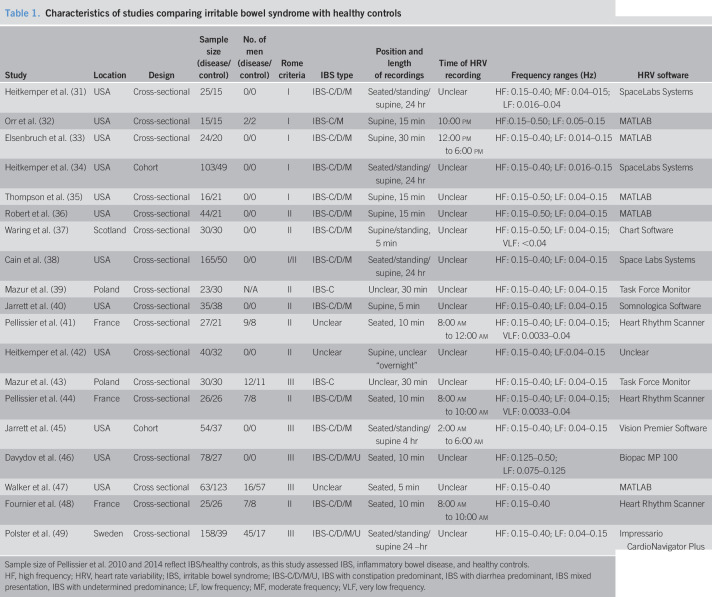
Table 1.
Characteristics of studies comparing irritable bowel syndrome with healthy controls
| Study | Location | Design | Sample size (disease/control) | No. of men (disease/control) | Rome criteria | IBS type | Position and length of recordings | Time of HRV recording | Frequency ranges (Hz) | HRV software |
| Heitkemper et al. (31) | USA | Cross-sectional | 25/15 | 0/0 | I | IBS-C/D/M | Seated/standing/supine, 24 hr | Unclear | HF: 0.15–0.40; MF: 0.04–015; LF: 0.016–0.04 | SpaceLabs Systems |
| Orr et al. (32) | USA | Cross-sectional | 15/15 | 2/2 | I | IBS-C/M | Supine, 15 min | 10:00 pm | HF:0.15–0.50; LF: 0.05–0.15 | MATLAB |
| Elsenbruch et al. (33) | USA | Cross-sectional | 24/20 | 0/0 | I | IBS-C/D/M | Supine, 30 min | 12:00 pm to 6:00 pm | HF: 0.15–0.40; LF: 0.014–0.15 | MATLAB |
| Heitkemper et al. (34) | USA | Cohort | 103/49 | 0/0 | I | IBS-C/D/M | Seated/standing/supine, 24 hr | Unclear | HF: 0.15–0.40; LF: 0.016–0.15 | SpaceLabs Systems |
| Thompson et al. (35) | USA | Cross-sectional | 16/21 | 0/0 | I | IBS-C/D/M | Supine, 15 min | Unclear | HF: 0.15–0.50; LF: 0.04–0.15 | MATLAB |
| Robert et al. (36) | USA | Cross-sectional | 44/21 | 0/0 | II | IBS-C/D/M | Supine, 15 min | Unclear | HF: 0.15–0.50; LF: 0.04–0.15 | MATLAB |
| Waring et al. (37) | Scotland | Cross-sectional | 30/30 | 0/0 | II | IBS-C/D/M | Supine/standing, 5 min | Unclear | HF: 0.15–0.50; LF: 0.04–0.15; VLF: <0.04 | Chart Software |
| Cain et al. (38) | USA | Cross-sectional | 165/50 | 0/0 | I/II | IBS-C/D/M | Seated/standing/supine, 24 hr | Unclear | HF: 0.15–0.40; LF: 0.04–0.15 | Space Labs Systems |
| Mazur et al. (39) | Poland | Cross-sectional | 23/30 | N/A | II | IBS-C | Unclear, 30 min | Unclear | HF: 0.15–0.40; LF: 0.04–0.15 | Task Force Monitor |
| Jarrett et al. (40) | USA | Cross-sectional | 35/38 | 0/0 | II | IBS-C/D/M | Supine, 5 min | Unclear | HF: 0.15–0.40; LF: 0.04–0.15 | Somnologica Software |
| Pellissier et al. (41) | France | Cross-sectional | 27/21 | 9/8 | II | Unclear | Seated, 10 min | 8:00 am to 12:00 am | HF: 0.15–0.40; LF: 0.04–0.15; VLF: 0.0033–0.04 | Heart Rhythm Scanner |
| Heitkemper et al. (42) | USA | Cross-sectional | 40/32 | 0/0 | II | Unclear | Supine, unclear “overnight” | Unclear | HF: 0.15–0.40; LF:0.04–0.15 | Unclear |
| Mazur et al. (43) | Poland | Cross-sectional | 30/30 | 12/11 | III | IBS-C | Unclear, 30 min | Unclear | HF: 0.15–0.40; LF: 0.04–0.15 | Task Force Monitor |
| Pellissier et al. (44) | France | Cross-sectional | 26/26 | 7/8 | II | IBS-C/D/M | Seated, 10 min | 8:00 am to 10:00 am | HF: 0.15–0.40; LF: 0.04–0.15; VLF: 0.0033–0.04 | Heart Rhythm Scanner |
| Jarrett et al. (45) | USA | Cohort | 54/37 | 0/0 | III | IBS-C/D/M | Seated/standing/supine 4 hr | 2:00 am to 6:00 am | HF: 0.15–0.40; LF: 0.04–0.15 | Vision Premier Software |
| Davydov et al. (46) | USA | Cross-sectional | 78/27 | 0/0 | III | IBS-C/D/M/U | Seated, 10 min | Unclear | HF: 0.125–0.50; LF: 0.075–0.125 | Biopac MP 100 |
| Walker et al. (47) | USA | Cross sectional | 63/123 | 16/57 | III | Unclear | Seated, 5 min | Unclear | HF: 0.15–0.40 | MATLAB |
| Fournier et al. (48) | France | Cross-sectional | 25/26 | 7/8 | II | IBS-C/D/M | Seated, 10 min | 8:00 am to 10:00 am | HF: 0.15–0.40 | Heart Rhythm Scanner |
| Polster et al. (49) | Sweden | Cross-sectional | 158/39 | 45/17 | III | IBS-C/D/M/U | Seated/standing/supine 24 –hr | Unclear | HF: 0.15–0.40; LF: 0.04–0.15 | Impressario CardioNavigator Plus |
Sample size of Pellissier et al. 2010 and 2014 reflect IBS/healthy controls, as this study assessed IBS, inflammatory bowel disease, and healthy controls.
HF, high frequency; HRV, heart rate variability; IBS, irritable bowel syndrome; IBS-C/D/M/U, IBS with constipation predominant, IBS with diarrhea predominant, IBS mixed presentation, IBS with undetermined predominance; LF, low frequency; MF, moderate frequency; VLF, very low frequency.
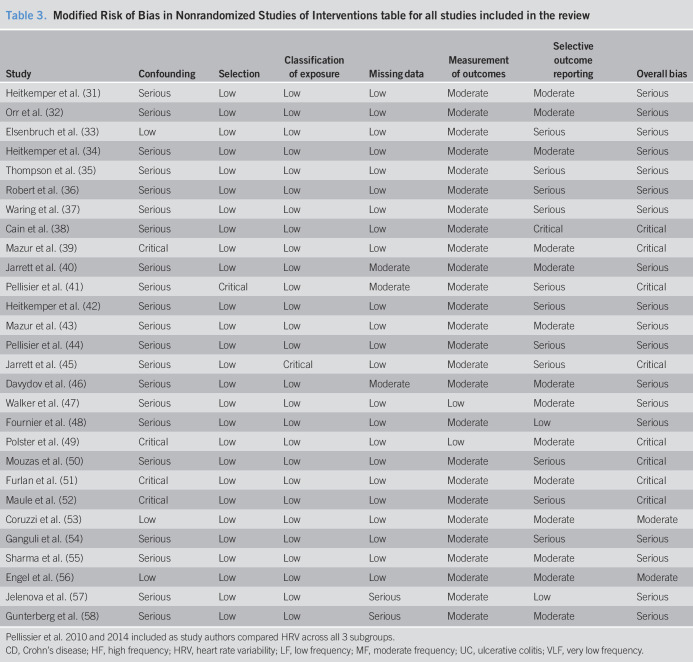
Table 3.
Modified Risk of Bias in Nonrandomized Studies of Interventions table for all studies included in the review
| Study | Confounding | Selection | Classification of exposure | Missing data | Measurement of outcomes | Selective outcome reporting | Overall bias |
| Heitkemper et al. (31) | Serious | Low | Low | Low | Moderate | Moderate | Serious |
| Orr et al. (32) | Serious | Low | Low | Low | Moderate | Moderate | Serious |
| Elsenbruch et al. (33) | Low | Low | Low | Low | Moderate | Serious | Serious |
| Heitkemper et al. (34) | Serious | Low | Low | Low | Moderate | Moderate | Serious |
| Thompson et al. (35) | Serious | Low | Low | Low | Moderate | Serious | Serious |
| Robert et al. (36) | Serious | Low | Low | Low | Moderate | Serious | Serious |
| Waring et al. (37) | Serious | Low | Low | Low | Moderate | Serious | Serious |
| Cain et al. (38) | Serious | Low | Low | Low | Moderate | Critical | Critical |
| Mazur et al. (39) | Critical | Low | Low | Low | Moderate | Moderate | Critical |
| Jarrett et al. (40) | Serious | Low | Low | Moderate | Moderate | Moderate | Serious |
| Pellisier et al. (41) | Serious | Critical | Low | Moderate | Moderate | Serious | Critical |
| Heitkemper et al. (42) | Serious | Low | Low | Low | Moderate | Serious | Serious |
| Mazur et al. (43) | Serious | Low | Low | Low | Moderate | Moderate | Serious |
| Pellisier et al. (44) | Serious | Low | Low | Low | Moderate | Serious | Serious |
| Jarrett et al. (45) | Serious | Low | Critical | Low | Moderate | Serious | Critical |
| Davydov et al. (46) | Serious | Low | Low | Moderate | Moderate | Moderate | Serious |
| Walker et al. (47) | Serious | Low | Low | Low | Low | Moderate | Serious |
| Fournier et al. (48) | Serious | Low | Low | Low | Moderate | Low | Serious |
| Polster et al. (49) | Critical | Low | Low | Low | Low | Moderate | Critical |
| Mouzas et al. (50) | Serious | Low | Low | Low | Moderate | Serious | Critical |
| Furlan et al. (51) | Critical | Low | Low | Low | Moderate | Moderate | Critical |
| Maule et al. (52) | Critical | Low | Low | Low | Moderate | Serious | Critical |
| Coruzzi et al. (53) | Low | Low | Low | Low | Moderate | Moderate | Moderate |
| Ganguli et al. (54) | Serious | Low | Low | Low | Moderate | Serious | Serious |
| Sharma et al. (55) | Serious | Low | Low | Low | Moderate | Moderate | Serious |
| Engel et al. (56) | Low | Low | Low | Low | Moderate | Moderate | Moderate |
| Jelenova et al. (57) | Serious | Low | Low | Serious | Moderate | Low | Serious |
| Gunterberg et al. (58) | Serious | Low | Low | Serious | Moderate | Moderate | Serious |
Pellissier et al. 2010 and 2014 included as study authors compared HRV across all 3 subgroups.
CD, Crohn's disease; HF, high frequency; HRV, heart rate variability; LF, low frequency; MF, moderate frequency; UC, ulcerative colitis; VLF, very low frequency.
Primary outcomes
Primary outcomes consist of the HRV time-domain measurements of standard deviation of the inter-beat-interval of normal sinus beats (SDNN), number of consecutive intervals differing from each other by more than 50 milliseconds (NN50), the NN50 represented as a percentage (pNN50), and the root mean square of successive differences between normal heartbeats (RMSSD) (13).
Secondary outcomes
Secondary outcomes consist of the HRV frequency-domain measurements of high-frequency (HF) band power ranging from 0.15 to 0.4 Hz, low-frequency (LF) band power ranging from 0.04 to 0.15 Hz, and the very low frequency (VLF) band power ranging from 0.003 to 0.04 Hz (22). HF and LF are sometimes expressed as a log-transformed measure (LnHF and LnLF) or as normalized units (HFnu and LFnu). Although these transformed measures are usually intended to represent the same characteristics of the ANS, log-transformed and untransformed data cannot be synthesized together in a meta-analysis (23).
Synthesis of results
Authors followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (24). Owing to the heterogeneity across study methodologies, a random-effects meta-analysis was used to derive pooled standardized mean difference (SMD) with 95% CIs using an inverse variance model (25). SMD expressed as a negative value indicates HC having greater mean values than IBS or IBD groups. The I2 statistic was used to assess heterogeneity between primary study results. I2 = 0%–24% is considered low heterogeneity, 25%–49% considered mild heterogeneity, 50%–74% considered high heterogeneity, and I2 > 75% considered extensive heterogeneity (16,26). We attempted to address high levels of heterogeneity with follow-up subanalyses. Evidence for potential publication bias was investigated visually using funnel plots.
Data for all primary and secondary HRV outcomes were compared with HC only because there was insufficient evidence for a comparison of IBS to IBD. Subanalysis was also performed for studies that used methods intended to match participants on the factors of age, sex, and body mass index (BMI). Pooling of data was based on length of HRV recording, separating studies according to short or long recording periods because these measures can represent different facets of ANS function (27,28). To obtain adequate data for meta-analysis of our primary outcome of interest, however, short and long RMSSD recordings were combined.
Quality appraisal
A modified Cochrane Collaboration's Risk of Bias in Non-Randomized Studies of Interventions was used to evaluate the risk of bias in selected studies as low, moderate, serious, and critical based on 6 domains (Table 3) (29,30). A modified tool was used because study authors were only interested in the initial HRV results and not the HRV results after an intervention. Remaining relevant domains were bias because of confounding, selection bias, classification of exposure, missing data, measurement of outcomes, and selective outcome reporting bias.
The overall risk of bias was graded as follows (29):
Low = Low risk of bias in all 6 domains
Moderate = Mostly low risk of bias or unclear risk of bias.
Serious = At least one domain at serious risk.
Critical = At least one domain at critical risk
No Information = No information on which to base a judgment about the risk of bias.
RESULTS
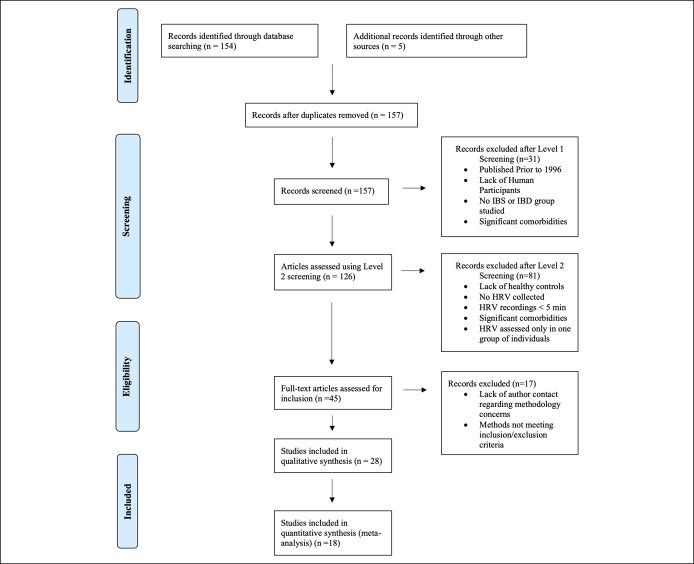
A total of 154 studies were identified; 27 were eligible for inclusion in the systematic review; an additional 5 were accessed through evaluation of references from included studies, of which one met the inclusion criteria. Eighteen of the 28 included studies provided suitable data for meta-analysis (Figure 1). No studies met inclusion criteria in ClinicalTrials.gov, and no relevant reviews were identified through PROSPERO.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram. IBD inflammatory bowel disease; IBS, irritable bowel syndrome; HRV, heart rate variability.
Study characteristics
Study characteristics can be seen in Tables 1 and 2. Nineteen studies compared IBS with HC (31–49), 9 compared IBD with HC (50–58), and 2 compared HRV across all 3 comparators (41,44).
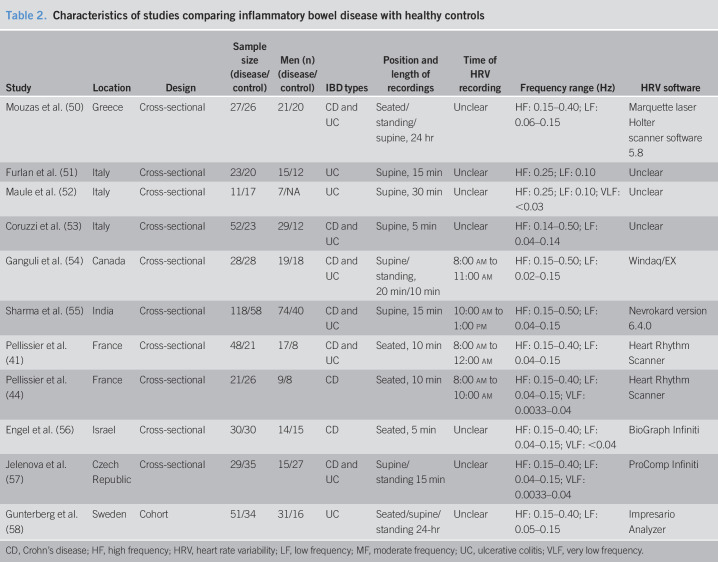
Table 2.
Characteristics of studies comparing inflammatory bowel disease with healthy controls
| Study | Location | Design | Sample size (disease/control) | Men (n) (disease/control) | IBD types | Position and length of recordings | Time of HRV recording | Frequency range (Hz) | HRV software |
| Mouzas et al. (50) | Greece | Cross-sectional | 27/26 | 21/20 | CD and UC | Seated/standing/supine, 24 hr | Unclear | HF: 0.15–0.40; LF: 0.06–0.15 | Marquette laser Holter scanner software 5.8 |
| Furlan et al. (51) | Italy | Cross-sectional | 23/20 | 15/12 | UC | Supine, 15 min | Unclear | HF: 0.25; LF: 0.10 | Unclear |
| Maule et al. (52) | Italy | Cross-sectional | 11/17 | 7/NA | UC | Supine, 30 min | Unclear | HF: 0.25; LF: 0.10; VLF: <0.03 | Unclear |
| Coruzzi et al. (53) | Italy | Cross-sectional | 52/23 | 29/12 | CD and UC | Supine, 5 min | Unclear | HF: 0.14–0.50; LF: 0.04–0.14 | Unclear |
| Ganguli et al. (54) | Canada | Cross-sectional | 28/28 | 19/18 | CD and UC | Supine/standing, 20 min/10 min | 8:00 am to 11:00 am | HF: 0.15–0.50; LF: 0.02–0.15 | Windaq/EX |
| Sharma et al. (55) | India | Cross-sectional | 118/58 | 74/40 | CD and UC | Supine, 15 min | 10:00 am to 1:00 pm | HF: 0.15–0.50; LF: 0.04–0.15 | Nevrokard version 6.4.0 |
| Pellissier et al. (41) | France | Cross-sectional | 48/21 | 17/8 | CD and UC | Seated, 10 min | 8:00 am to 12:00 am | HF: 0.15–0.40; LF: 0.04–0.15 | Heart Rhythm Scanner |
| Pellissier et al. (44) | France | Cross-sectional | 21/26 | 9/8 | CD | Seated, 10 min | 8:00 am to 10:00 am | HF: 0.15–0.40; LF: 0.04–0.15; VLF: 0.0033–0.04 | Heart Rhythm Scanner |
| Engel et al. (56) | Israel | Cross-sectional | 30/30 | 14/15 | CD | Seated, 5 min | Unclear | HF: 0.15–0.40; LF: 0.04–0.15; VLF: <0.04 | BioGraph Infiniti |
| Jelenova et al. (57) | Czech Republic | Cross-sectional | 29/35 | 15/27 | CD and UC | Supine/standing 15 min | Unclear | HF: 0.15–0.40; LF: 0.04–0.15; VLF: 0.0033–0.04 | ProComp Infiniti |
| Gunterberg et al. (58) | Sweden | Cohort | 51/34 | 31/16 | UC | Seated/supine/standing 24-hr | Unclear | HF: 0.15–0.40; LF: 0.05–0.15 | Impresario Analyzer |
CD, Crohn's disease; HF, high frequency; HRV, heart rate variability; LF, low frequency; MF, moderate frequency; UC, ulcerative colitis; VLF, very low frequency.
Specific to IBS studies, one combined IBS with diarrhea predominant (IBS-D) and IBS mixed presentation (IBS-M) into one group (32), one combined IBS with constipation predominant (IBS-C) and IBS-M into one group (45), 2 recruited only IBS-C participants (39,43), and 3 did not report subtype (41,42,47); although one reported data on a combined group of FGIDs including IBS and functional abdominal pain (47), data for IBS-only participants were available after contacting the primary author. In addition, because 2 studies (44,48) used the same participants in successive appraisals, we excluded data from the latter study in meta-analysis; both, however, were included in the narrative review.
Specific to IBD studies, 3 compared UC with HC (51,52,58), 2 compared CD only with HC (44,56), and 4 presented data on both subgroups (41,53–55).
Diagnosis.
All participants with IBS were diagnosed using the Rome criteria. Five studies used Rome I (31–35), 8 used Rome II (36,39–42,44,48), 5 used Rome III (43,45–47,49), none used Rome IV, one used both Rome I and II (38), and one used both Manning and Rome II criteria(39). Eight studies (35,36,41,44,46–49) used physician diagnosis in addition to Rome criteria during enrolment; the remainder relied on a previous medical diagnosis and confirmed with Rome criteria in a study interview. All IBD participants were recruited with an already established diagnosis of CD or UC via imaging.
Short duration laboratory collection of HRV.
Studies conducted HRV in a seated (41,44,46–48,56), supine (32,33,35,36,40,51–53,55), and both a supine and standing position (37,54,57). Two studies were unclear in which position short recordings were conducted (39,43).
Long duration collection of HRV.
Six studies conducted 24-hour HRV recordings (31,34,38,49,50,58) and one conducted 12-hour recordings (45); 4 were conducted while sleeping (32,35,36,45), of which 3 separated data into sleep stages (32,35,40).
Specific HRV software was unclear in 4 studies (42,51–53); MATLAB software was the most commonly reported (n = 5) (32,33,35,36,47). Frequency ranges were not consistent across all studies, without sufficient reasoning for deviations from the 1996 European Task Force guidelines (22). Studies not using recommended reference ranges were still included for review and meta-analysis, provided that the reference ranges used seemed qualitatively similar to the guideline ranges. HF was recorded in all studies, LF was recorded in 26 (96.8%) (31–47,50–58), and VLF was recorded in 6 (21.4%) (37,41,44,52,56,57). HF was mostly calculated over the frequency range of 0.15–0.40 Hz (n = 18) (31,33,34,38–45,47–50,56–58); LF was usually calculated over 0.04–0.15 Hz (n = 15) (35–45,49,55–57); VLF was most commonly calculated over 0.0033–0.04 Hz (n = 3) (41,44,57).
Data presented as medium frequency represent LF data and low frequency represent VLF in meta-analysis for Heitkemper 1998 (31) because those respective ranges are consistent with the task force guidelines.
Participant characteristics
A total of n = 2,314 participants across 28 studies were included in the review (n = 1,447 participants across 18 studies were able to be included in meta-analysis.) The review sample contained a total of n = 956 IBS, n = 438 IBD, and n = 895 HC participants. 27.3% (n = 628) of study participants were men, and the mean ages of HC, IBS, and IBD participants were 31.6, 33.3, and 37.9 years, respectively. Mean BMI of participants was 23.7, 24.3, and 22.5 for HC, IBS, and IB, respectively. Baseline demographics according to groups were not reported in several studies (31,40–42,50–52,54,57).
Outcome characteristics
Of the primary outcomes of interest, SDNN (34,42,49,50,55,56), the natural log of SDNN (42), RMSSD (34,42,48–50,53,55,56,58), and pNN50 (34,49,50,53,55) were presented. NN50 was not presented in any study. One study used HRV methods before the 1996 guidelines and was not included in the meta-analysis (54).
HF and LF outcomes were presented in absolute units (31,34,38,39,43,45–47,51,53,55–57), normalized units (37,39,41,43,44,48,49,51,52,55), natural log form (31,34,39,40,42,46,50), and as frequency percentages (32,35,54). VLF was presented in absolute units (31,37,43,44,56) and as the natural log only (31,41). Three studies only represented data graphically, and no adequate response was provided from authors, when contacted to request numerical values (32,33,35). One study did not include adequate data regarding IBS-M in their analysis despite comparing results between all 3 IBS subgroups and HC (38).
Meta-analysis
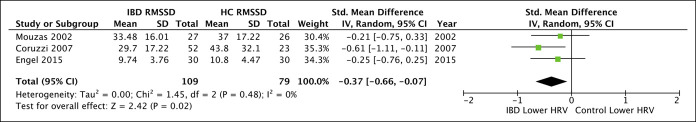
The results of meta-analyses can be seen in Figures 2–5. Comparison of RMSSD between IBD and HC was the only primary outcome with adequate data for meta-analysis, with significantly lower RMSSD in IBD relative to HC (3 studies; pooled SMD = −0.37 [−0.66, −0.07], P = 0.02, I2 = 0%).
Figure 2.
Forest plot for primary outcome comparing inflammatory bowel disease (IBD) with healthy controls (HC). CI, confidence interval; RMSSD, root mean square of successive RR interval differences.
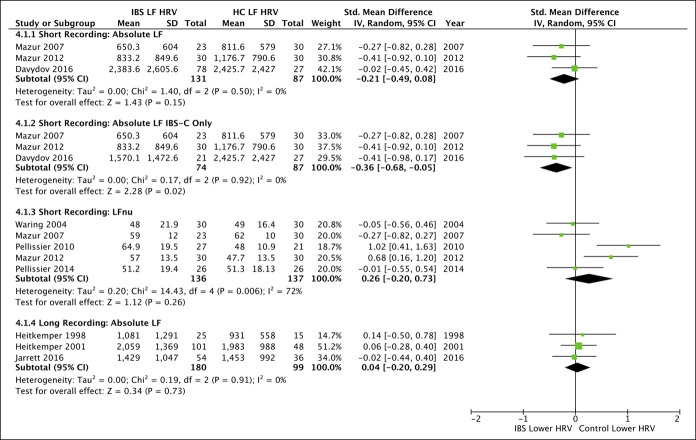
Figure 5.
Forest plots for all secondary outcomes available for meta-analysis comparing low-frequency (LF) domain heart rate variability (HRV) between irritable bowel syndrome (IBS) and healthy controls (HC). Studies were separated by short and long duration recordings, with subanalysis available for IBS with constipation predominant (IBS-C). LF represented in normalized units (LFnu). CI, confidence interval.
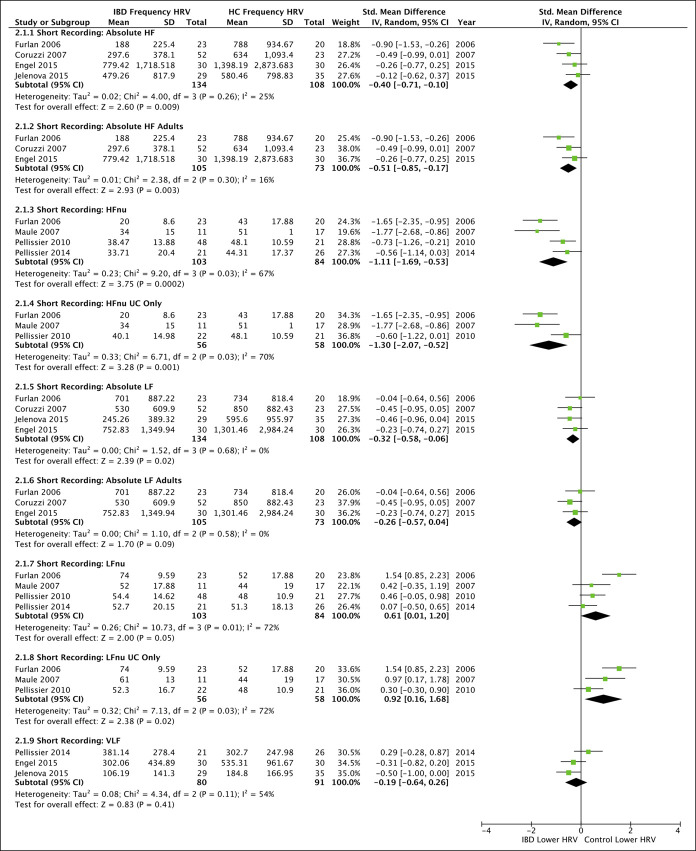
Absolute HF (Figure 3) in short recordings remained significantly lower in IBD compared with HC even after subanalysis in adults only (3 studies; pooled SMD = −0.51 [−0.85, −0.17], P = 0.003, I2 = 16%). There was a lack of adequate absolute HF data to investigate CD or UC subgroups, during short or long recordings. HFnu was significantly lower in IBD compared with HC (P = 0.0002), and the difference remained significant when analyzing UC only (P = 0.001).
Figure 3.
Forest plots for all secondary outcomes available for meta-analysis comparing frequency domain heart rate variability (HRV) between inflammatory bowel disease (IBD) and healthy controls (HC). All studies were conducted over short recording lengths. Subanalyses were only possible for age and ulcerative colitis (UC) where indicated. High-frequency (HF), HF represented in normalized units (HFnu), low frequency (LF), LF represented in normalized units (LFnu), and very low frequency (VLF). CI, confidence interval.
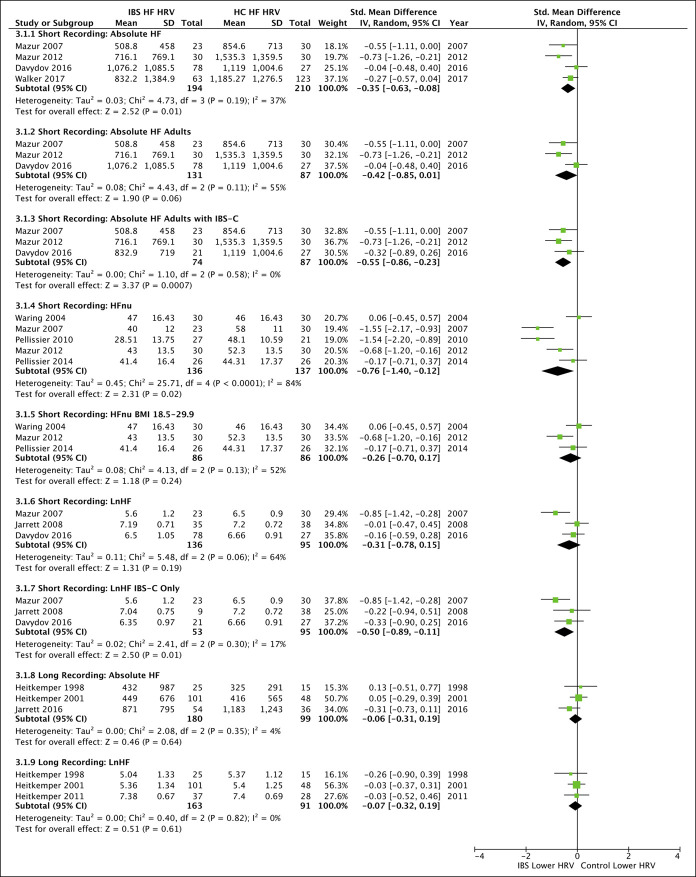
Absolute frequency data for short HF recordings (4 studies; pooled SMD = −0.35 [−0.63, −0.08], P = 0.01, I2 = 37%) but not long HF recordings (3 studies; pooled SMD = −0.06 [−0.31, 0.19], P = 0.64, I2 = 4%) were significantly lower in IBS than HC (Figure 4). The results remained significant in IBS-C (3 studies; pooled SMD = −0.55 [−0.86, −0.23], P = 0.0007, I2 = 0%) with a lack of adequate data to investigate IBS-D, IBS-M, or IBS with undetermined predominance subgroups during any recording length.
Figure 4.
Forest plots for all secondary outcomes available for meta-analysis comparing high-frequency (HF) domain heart rate variability (HRV) between irritable bowel syndrome (IBS) and healthy controls (HC). Studies were separated by short and long duration recordings, with subanalyses for age, IBS subtype, and body mass index (BMI) where indicated. IBS with constipation predominant (IBS-C), HF represented in normalized units (HFnu), HF represented in the natural log (lnHF), low frequency (LF), LF represented in normalized units (LFnu). CI, confidence interval.
Publication bias
Only HFnu and LFnu in studies comparing IBS with HC pooled from more than 4 primary studies, and as such funnel plots are not shown; however, visual inspection showed no signs of publication bias for any analysis.
Risk of bias in individual studies
Most studies had a moderate or serious amount of bias (Table 3). Confounding bias was largely because of a lack of matching between disease and healthy groups, or sufficiently controlling for one or more factors including age, sex, BMI, smoking status, and medication use. We did not treat anxiety or depression as confounding comorbidities because these are common in, and could even result from, IBS and IBD. Only 2 studies confirmed the use of blinded HRV assessment (47,49), resulting in substantial potential for bias in measurement of outcomes, and there was substantial variation in selection of HRV outcome measures to report. Studies further differed in HRV collection methods and time and length of recordings.
DISCUSSION
Twenty-eight studies compared HRV measurements in individuals with either IBS or IBD with HC at rest, showing some evidence for an association of HRV with gastrointestinal disorders, evidenced by decreased RMSSD and HF relative to HC. It is unknown whether decreases in HF may be indicative of ANS dysregulation via parasympathetic withdrawal or of sympathetic dominance.
Quality of evidence assessing HRV in IBS and IBD remains low, as seen in the paucity of included studies and the high risks of bias in individual studies. There is limited evidence to associate HRV parameters with health outcomes in individuals with gastrointestinal pathology, and what should be considered healthy or unhealthy HRV in gastrointestinal pathologies can only be speculative at this time. Caution is strongly advised when using metrics developed to measure neuronal functions of the heart to noncardiac health outcomes (28).
Strengths
The strengths of this review include the incorporation of both IBS and IBD as compared to HC, an assessment of overall initial HRV measures across groups, and methods guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist. Both time and frequency domain outcomes were assessed and limiting data collection to initial collection prevented confounding from interventions.
Limitations
The main limitations consisted of including studies of small sample sizes, significant amounts of heterogeneity in primary study methods and measures, and studies at high risk of bias. There was inconsistent reporting of HRV measures, recording software, timing of HRV collection, and lack of HRV assessor blinding. There were inconsistencies regarding disease severity (34,35,38,41,44–46,49,50,52,53,55,56,58), association of disease severity with HRV parameters, and lack of sufficient data to make any conclusions regarding associations with severity within diseased populations.
Implications/recommendations for research
HRV research on IBS and IBD should follow the 1996 guidelines in the absence of an update in addition to the recommendations by Tak et al. (22,59). We currently recommend future studies report all initial HRV parameters only using either 24-hour measures or short recordings over 5 min to strengthen the consistency in methods used to collect HRV. We do not recommend the use of the LF/HF ratio, given the significant evidence against its use, and because the usefulness of VLF remains unknown, we recommend increased reporting to determine its relationship to gastrointestinal pathologies (14,27,28,60–63). As all outcomes can be computed from the same interbeat-interval data, we recommend that studies report on all common measures, even when transforming skewed data. Future studies comparing HRV of distinct populations should match participants for age, sex, BMI, smoking status, comorbidities, and medication use, and should explicitly blind HRV assessors. To reduce heterogeneity across studies, future research should conduct repeated measures to produce more reliable measures and assess intrasubject variability. To our knowledge, reliability has not been assessed in the IBS or IBD patient populations, and there are inconsistencies regarding the reproducibility of HRV measures in a variety of unrelated populations (64–66). In addition, we recommend focusing research on a specific disease subtype (such as IBS-C, IBS-D, CD, or UC), or presenting results separately for these subgroups, whenever possible, because HRV patterns could vary dramatically between different forms of the conditions.
If cross-sectional associations of IBS/IBD with altered HRV continues to be seen, investigators should conduct longitudinal study to determine whether HRV measures are responsive to changes in symptoms or severity (whether occurring spontaneously or because of treatment). In particular, they should attempt to identify whether improvements in HRV precede improved disease course or whether improvements in the disease course are accompanied by improvements in HRV. Only a limited number of clinical trials in IBS have evaluated associations between improvements in the disease course and changes in HRV outcomes, with conflicting results; no such trials have been conducted for IBD (67,68). In addition, the role of inflammation should be assessed to determine whether it is a driving factor in HRV outcomes, especially because inflammation is characteristic of IBD but not always present in IBS. When Pellissier et al. used HFnu to categorize subjects into high or low parasympathetic activity across CD, IBS, and HC groups, individuals in the CD group with low parasympathetic activity had significantly greater tumor necrosis factor-α levels compared with IBS and HC groups. When categorized into high parasympathetic activity; however, there were no differences in tumor necrosis factor-α levels between CD, IBS, or HC groups (44). Increased evidence for the direct comparison of IBS to IBD could help to elucidate the role of inflammation.
Implications for clinical practice
The use of HRV to clinically monitor symptoms in IBS and IBD cannot currently be made and further research is required. Validation of HRV regarding established markers of disease severity, as well as enhanced standardization of HRV recording processes, are needed. If HRV differs between diseased states and healthy, it is still unclear how HRV correlates to disease severity, within diseased populations, or if changes in HRV are associated with successful treatment.
CONCLUSIONS
This is the first review that study authors are aware of evaluating initial differences in HRV between both IBS and IBD as compared to HC. There is evidence to suggest that individuals with these conditions have reduced HF variability, relative to HC. Despite some significant findings, these results need to be interpreted with great caution, and further studies are warranted, especially with improvements in study quality and increased homogeneity across study methods and data collection. In addition, future studies should rely on updated Rome Criteria for diagnosis of IBS because the most recent guidelines suggest that IBS subgroups exist on a spectrum and not as distinct entities (69).
CONFLICTS OF INTEREST
Guarantor of the article: Adam Sadowski, ND, MS.
Specific author contributions: A.S. and D.H.: planned the study, collected and interpreted data, and wrote all manuscript drafts. A.S.: approved the final draft submitted. C.D.: aided in the planning of the study and data collection. A.L.: aided in the planning of the study.
Financial support: Helfgott Research Institute provided organizational support, however, played no role in the study design, collection, analysis, and interpretation of the data.
Potential competing interests: None to report.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A467
REFERENCES
- 1.Ford AC, Lacy BE, Talley NJ. Irritable bowel syndrome. N Engl J Med 2017;376(26):2566–78. [DOI] [PubMed] [Google Scholar]
- 2.Sperber AD, Dumitrascu D, Fukudo S, et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: A Rome Foundation working team literature review. Gut 2017;66(6):1075–82. [DOI] [PubMed] [Google Scholar]
- 3.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin Gastroenterol Hepatol 2012;10(7):712–21.e4. [DOI] [PubMed] [Google Scholar]
- 4.Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: A clinical review. JAMA 2015;313(9):949–58. [DOI] [PubMed] [Google Scholar]
- 5.Sairenji T, Collins KL, Evans DV. An update on inflammatory bowel disease. Prim Care 2017;44(4):673–92. [DOI] [PubMed] [Google Scholar]
- 6.Burisch J, Munkholm P. The epidemiology of inflammatory bowel disease. Scand J Gastroenterol 2015;50(8):942–51. [DOI] [PubMed] [Google Scholar]
- 7.Kappelman MD, Rifas-Shiman SL, Porter CQ, et al. Direct health care costs of Crohn's disease and ulcerative colitis in US children and adults. Gastroenterology 2008;135(6):1907–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carabotti M, Scirocco A, Maselli MA, et al. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 2015;28(2):203–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Holtmann GJ, Ford AC, Talley NJ. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol Hepatol 2016;1(2):133–46. [DOI] [PubMed] [Google Scholar]
- 10.Drossman DA, Hasler WL. Rome IV-functional GI disorders: Disorders of gut-brain interaction. Gastroenterology 2016;150(6):1257–61. [DOI] [PubMed] [Google Scholar]
- 11.Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology 2013;144(1):36–49. [DOI] [PubMed] [Google Scholar]
- 12.Bonaz B, Sinniger V, Pellissier S. The vagus nerve in the neuro-immune axis: Implications in the pathology of the gastrointestinal tract. Front Immunol 2017;8:1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health 2017;5:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajendra Acharya U, Paul JK, Kannathal N, et al. Heart rate variability: A review. Med Biol Eng Comput 2006;44(12):1031–51. [DOI] [PubMed] [Google Scholar]
- 15.Nunan D, Sandercock GR, Brodie DA. A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing Clin Electrophysiol 2010;33(11):1407–17. [DOI] [PubMed] [Google Scholar]
- 16.Liu Q, Wang EM, Yan XJ, et al. Autonomic functioning in irritable bowel syndrome measured by heart rate variability: A meta-analysis. J Dig Dis 2013;14(12):638–46. [DOI] [PubMed] [Google Scholar]
- 17.Burr RL, Motzer SA, Chen W, et al. Heart rate variability and 24-hour minimum heart rate. Biol Res Nurs 2006;7(4):256–67. [DOI] [PubMed] [Google Scholar]
- 18.Park HJ. Heart rate variability as a measure of disease state in irritable bowel syndrome. Asian Nurs Res (Korean Soc Nurs Sci) 2008;2(1):5–16. [DOI] [PubMed] [Google Scholar]
- 19.Mazurak N, Seredyuk N, Sauer H, et al. Heart rate variability in the irritable bowel syndrome: A review of the literature. Neurogastroenterol Motil 2012;24(3):206–16. [DOI] [PubMed] [Google Scholar]
- 20.Martínez-Martínez LA, Mora T, Vargas A, et al. Sympathetic nervous system dysfunction in fibromyalgia, chronic fatigue syndrome, irritable bowel syndrome, and interstitial cystitis: A review of case-control studies. J Clin Rheumatol 2014;20(3):146–50. [DOI] [PubMed] [Google Scholar]
- 21.Review Manager (RevMan) [Computer program]. Version 5.3. The Nordic Cochrane Centre, The Cochrane Collaboration: Copenhagen, Denmark, 2014. [Google Scholar]
- 22.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J 1996;17(3):354–81. [PubMed] [Google Scholar]
- 23.Higgins JPT, Thomas J, Chandler J, et al. (eds). Cochrane Handbook for Systematic Reviews of Interventions: Version 6.0. Cochrane: Chichester, UK, 2019. (www.training.cochrane.org/handbook). Accessed December 2019. [Google Scholar]
- 24.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials 2015;45(pt A):139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berntson GG, Bigger JT, Eckberg DL, et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology 1997;34(6):623–48. [DOI] [PubMed] [Google Scholar]
- 28.Hayano J, Yuda E. Pitfalls of assessment of autonomic function by heart rate variability. J Physiol Anthropol 2019;38(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schünemann HJ, Cuello C, Akl EA, et al. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. J Clin Epidemiol 2019;111:105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heitkemper M, Burr RL, Jarrett M, et al. Evidence for autonomic nervous system imbalance in women with irritable bowel syndrome. Dig Dis Sci 1998;43(9):2093–8. [DOI] [PubMed] [Google Scholar]
- 32.Orr WC, Elsenbruch S, Harnish MJ. Autonomic regulation of cardiac function during sleep in patients with irritable bowel syndrome. Am J Gastroenterol 2000;95(10):2865–71. [DOI] [PubMed] [Google Scholar]
- 33.Elsenbruch S, Orr WC. Diarrhea- and constipation-predominant IBS patients differ in postprandial autonomic and cortisol responses. Am J Gastroenterol 2001;96(2):460–6. [DOI] [PubMed] [Google Scholar]
- 34.Heitkemper M, Jarrett M, Cain KC, et al. Autonomic nervous system function in women with irritable bowel syndrome. Dig Dis Sci 2001;46(6):1276–84. [DOI] [PubMed] [Google Scholar]
- 35.Thompson JJ, Elsenbruch S, Harnish MJ, et al. Autonomic functioning during REM sleep differentiates IBS symptom subgroups. Am J Gastroenterol 2002;97(12):3147–53. [DOI] [PubMed] [Google Scholar]
- 36.Robert JJ, Orr WC, Elsenbruch S. Modulation of sleep quality and autonomic functioning by symptoms of depression in women with irritable bowel syndrome. Dig Dis Sci 2004;49(7–8):1250–8. [DOI] [PubMed] [Google Scholar]
- 37.Waring WS, Chui M, Japp A, et al. Autonomic cardiovascular responses are impaired in women with irritable bowel syndrome. J Clin Gastroenterol 2004;38(8):658–63. [DOI] [PubMed] [Google Scholar]
- 38.Cain KC, Jarrett ME, Burr RL, et al. Heart rate variability is related to pain severity and predominant bowel pattern in women with irritable bowel syndrome. Neurogastroenterol Motil 2007;19(2):110–8. [DOI] [PubMed] [Google Scholar]
- 39.Mazur M, Furgała A, Jabłoński K, et al. Dysfunction of the autonomic nervous system activity is responsible for gastric myoelectric disturbances in the irritable bowel syndrome patients. J Physiol Pharmacol 2007;58(Suppl 3):131–9. [PubMed] [Google Scholar]
- 40.Jarrett ME, Burr RL, Cain KC, et al. Autonomic nervous system function during sleep among women with irritable bowel syndrome. Dig Dis Sci 2008;53(3):694–703. [DOI] [PubMed] [Google Scholar]
- 41.Pellissier S, Dantzer C, Canini F, et al. Psychological adjustment and autonomic disturbances in inflammatory bowel diseases and irritable bowel syndrome. Psychoneuroendocrinology 2010;35(5):653–62. [DOI] [PubMed] [Google Scholar]
- 42.Heitkemper MM, Cain KC, Burr RL, et al. Is childhood abuse or neglect associated with symptom reports and physiological measures in women with irritable bowel syndrome? Biol Res Nurs 2011;13(4):399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazur M, Furgała A, Jabłoński K, et al. Autonomic nervous system activity in constipation-predominant irritable bowel syndrome patients. Med Sci Monit 2012;18(8):CR493–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pellissier S, Dantzer C, Mondillon L, et al. Relationship between vagal tone, cortisol, TNF-alpha, epinephrine and negative affects in Crohn's disease and irritable bowel syndrome. PLoS One 2014;9(9):e105328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jarrett ME, Han CJ, Cain KC, et al. Relationships of abdominal pain, reports to visceral and temperature pain sensitivity, conditioned pain modulation, and heart rate variability in irritable bowel syndrome. Neurogastroenterol Motil 2016;28(7):1094–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davydov DM, Naliboff B, Shahabi L, et al. Baroreflex mechanisms in irritable bowel syndrome: Part I. Traditional indices. Physiol Behav 2016;157:102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walker LS, Stone AL, Smith CA, et al. Interacting influences of gender and chronic pain status on parasympathetically mediated heart rate variability in adolescents and young adults. Pain 2017;158(8):1509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fournier A, Mondillon L, Dantzer C, et al. Emotional overactivity in patients with irritable bowel syndrome. Neurogastroenterol Motil 2018;30(10):e13387. [DOI] [PubMed] [Google Scholar]
- 49.Polster A, Friberg P, Gunterberg V, et al. Heart rate variability characteristics of patients with irritable bowel syndrome and associations with symptoms. Neurogastroenterol Motil 2018;30(7):e13320. [DOI] [PubMed] [Google Scholar]
- 50.Mouzas IA, Pallis AG, Kochiadakis GE, et al. Autonomic imbalance during the day in patients with inflammatory bowel disease in remission. Evidence from spectral analysis of heart rate variability over 24 hours. Dig Liver Dis 2002;34(11):775–80. [DOI] [PubMed] [Google Scholar]
- 51.Furlan R, Ardizzone S, Palazzolo L, et al. Sympathetic overactivity in active ulcerative colitis: Effects of clonidine. Am J Physiol Regul Integr Comp Physiol 2006;290(1):R224–32. [DOI] [PubMed] [Google Scholar]
- 52.Maule S, Pierangeli G, Cevoli S, et al. Sympathetic hyperactivity in patients with ulcerative colitis. Clin Auton Res 2007;17(4):217–20. [DOI] [PubMed] [Google Scholar]
- 53.Coruzzi P, Castiglioni P, Parati G, et al. Autonomic cardiovascular regulation in quiescent ulcerative colitis and Crohn's disease. Eur J Clin Invest 2007;37(12):964–70. [DOI] [PubMed] [Google Scholar]
- 54.Ganguli SC, Kamath MV, Redmond K, et al. A comparison of autonomic function in patients with inflammatory bowel disease and in healthy controls. Neurogastroenterol Motil 2007;19(12):961–7. [DOI] [PubMed] [Google Scholar]
- 55.Sharma P, Makharia GK, Ahuja V, et al. Autonomic dysfunctions in patients with inflammatory bowel disease in clinical remission. Dig Dis Sci 2009;54(4):853–61. [DOI] [PubMed] [Google Scholar]
- 56.Engel T, Ben-horin S, Beer-gabel M. Autonomic dysfunction correlates with clinical and inflammatory activity in patients with Crohn's disease. Inflamm Bowel Dis 2015;21(10):2320–6. [DOI] [PubMed] [Google Scholar]
- 57.Jelenova D, Ociskova M, Prasko J, et al. Heart rate variability in children with inflammatory bowel diseases. Neuro Endocrinol Lett 2015;36(1):72–9. [PubMed] [Google Scholar]
- 58.Gunterberg V, Simrén M, Öhman L, et al. Autonomic nervous system function predicts the inflammatory response over three years in newly diagnosed ulcerative colitis patients. Neurogastroenterol Motil 2016;28(11):1655–62. [DOI] [PubMed] [Google Scholar]
- 59.Tak LM, Riese H, De bock GH, et al. As good as it gets? A meta-analysis and systematic review of methodological quality of heart rate variability studies in functional somatic disorders. Biol Psychol 2009;82(2):101–10. [DOI] [PubMed] [Google Scholar]
- 60.Billman GE. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front Physiol 2013;4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reyes del Paso GA, Langewitz W, Mulder LJ, et al. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: A review with emphasis on a reanalysis of previous studies. Psychophysiology 2013;50(5):477–87. [DOI] [PubMed] [Google Scholar]
- 62.Ernst G. Hidden signals-the history and methods of heart rate variability. Front Public Health 2017;5:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heathers JA. Everything hertz: Methodological issues in short-term frequency-domain HRV. Front Physiol 2014;5:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sandercock GR, Bromley PD, Brodie DA. The reliability of short-term measurements of heart rate variability. Int J Cardiol 2005;103(3):238–47. [DOI] [PubMed] [Google Scholar]
- 65.Bjelakovic B, Ilic D, Lukic S, et al. Reproducibility of 24-h heart rate variability in children. Clin Auton Res 2017;27(4):273–8. [DOI] [PubMed] [Google Scholar]
- 66.Plaza-florido A, Alcantara JMA, Migueles JH, et al. Inter- and intra-researcher reproducibility of heart rate variability parameters in three human cohorts. Sci Rep 2020;10(1):11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jarrett ME, Cain KC, Barney PG, et al. Balance of autonomic nervous system predicts who benefits from a self-management intervention program for irritable bowel syndrome. J Neurogastroenterol Motil 2016;22(1):102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jang A, Hwang SK, Padhye NS, et al. Effects of cognitive behavior therapy on heart rate variability in young females with constipation-predominant irritable bowel syndrome: A parallel-group trial. J Neurogastroenterol Motil 2017;23(3):435–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schmulson MJ, Drossman DA. What is new in Rome IV. J Neurogastroenterol Motil 2017;23(2):151–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.