Hepatitis B virus (HBV) infection, which affects 90 million people in China, remains a prominent cause of liver cancer and liver cirrhosis.[1] Chronic HBV infection has a complicated course, which is a dynamic process formed by the interaction between the virus and the immune system.[2,3] Hepatic fibrosis is the basis of liver cirrhosis and liver cancer.[4] In recent years, accumulating studies have indicated potential roles of intestinal microbiota, bile acids, and T helper (Th)17/ interleukin (IL)-17 axis in the process of HBV-related liver fibrosis. Gut microbiota actively communicates with bile acids and plays an important role in the pathogenesis of HBV-related liver fibrosis. In the following content, we are going to summarize current evidence of the role of intestinal microbiota, bile acids, and Th17/IL-17 axis in HBV-related liver fibrosis.
Age at the time of infection is an important factor affecting the immune response after HBV infection, and its mechanism may be related to the establishment of mature intestinal microbiota. In HBV transfected mouse models, virus clearance was substantially dependent on the formation of mature intestinal microbiota. Adult mice with mature and stable intestinal microbiota could completely clear the virus within 6 weeks after HBV plasmid injection, while young mice with immature intestinal microbiota could have the virus persistent after 6 weeks. Antibiotics treatment could delay the clearance of HBV in adult mice.[5] From another perspective, controlled clinical study of chronic HBV infection patients, the combination of nucleoside antiviral drugs with fecal microbiota transplantation could significantly reduce HBV viral load, increase HBV E antigen conversion, and improve liver function.[6] Taken together, it could be inferred that intestinal microbiota is involved in the immune response process of HBV virus clearance, while the specific mechanism remains unclear.
Th17 cells, as a subset of helper T cells, play an important role in chronic inflammation and immune-related diseases. Th17 cells could secrete inflammatory cytokines such as IL-17 and IL-22, which can activate hepatic stellate cells and Kupffer cells. This may promote the synthesis and accumulation of extracellular matrix, and further contribute to the process of liver fibrosis.[7] Chronic hepatic fibrosis diseases, such as alcoholic liver disease, autoimmune hepatitis, primary sclerosing cholangitis, primary biliary cirrhosis, biliary atresia, nonalcoholic fatty liver disease, and viral hepatitis, have been observed with increased peripheral Th17 cell count.[8] A number of studies have found that chronic HBV infected patients are accompanied by increased number of Th17 cells in peripheral circulation and liver tissue, together with alterations of related cytokines. In chronic HBV infected patients, the number of peripheral Th17 cells was significantly higher than that of the control group, and was positively correlated with the level of serum alanine aminotransferase (ALT).[9] The level of Th17 cell infiltration to liver tissues of chronic HBV infection patients is also positively correlated with the severity of liver inflammation and fibrosis.[10] After antiviral therapy with nucleoside analogs, the level of Th17 cells and IL-17 in peripheral blood of chronic HBV infection patients declines with viral load.[11]
Th17 cells in liver tissue might not differentiate in situ, but mainly migrate based on local signals in the liver parenchyma. HBV infection can induce Th17 cells to migrate from peripheral to hepatic tissue by upregulation of chemokines such as chemokine C-C motif ligand (CCL) 17, CCL20, and CCL22 in hepatocytes. In comparison with uninfected controls, the mRNA levels of hepatocellular chemokines CCL17 and CCL22 were significantly increased in patients with chronic HBV infection, as well as increased expression of chemokine receptors 6 (CCR6) and chemokine receptor 4 (CCR4) on Th17 cells. In vitro experiments also confirmed that HBV DNA could induce hepatocytes to express Th17 chemokines CCL17 and CCL22.[12]
The most possible source of Th17 cells is the intestinal lamina propria, which contains a large number of CD4+ T cells, primarily Th17 cells and regulatory T cell (Treg) cells.[13] Intestinal microbiota maintains immune regulation function by changing the balance of Th17/Treg cells.[14] Th17 cells of specific pathogen-free (SPF) mouse were mainly observed in the ileum and colonic lamina, which also contain the highest density of bacteria. There are relatively few Th17 cells in the duodenum, jejunum, mesenteric lymph nodes, spleen, or liver.[15] In comparison with SPF mice, germ-free mice contained fewer Th17 cells in the intestinal lamina propria; moreover, after transplanting intestinal microbiota from normal mice to germ-free mice, the intestinal Th17 cell content increased accordingly.[16] Low-dose penicillin exposure early after birth can change the composition of intestinal microbiota, thus influencing the differentiation of Th17 cells and the expression of pro-inflammatory factor IL-17.[17] On the other hand, vancomycin added to drinking water significantly reduced Th17 cell levels in the lung.[18]
The differentiation of Th17 cells depends on the stimulation of symbiotic intestinal bacteria. However, whether the microbes interact with CD4+ T cells directly remains unclear. Some intestinal microbes have been found to be related to the differentiation of Th17 cells directly, such as segmented filamentous bacteria, Citrobacter Rodentium, and Escherichia coli O 157.[19] Besides, twenty mixed strains isolated from fecal flora could also stimulate the differentiation of intestinal Th17 cells. Then, further 16S rRNA sequencing analysis showed that the 20 strains were derived from Clostridium, Bifidobacterium, Ruminococcus, and Bacteroides.[20] Intestinal commensal Bacteroides species peptide derivatives could specifically stimulate the differentiation of intestinal Th17 cells and participate in the development of myocarditis.[21]
Although previous results have suggested a direct interaction between intestinal bacteria and Th17 cells, several lines of evidence have proposed that the stimulation might be mediated by the intestinal bile acids pool. Intestinal bacteria could produce choline dehydrogenase and steroid dehydrogenase, which can affect bile acids metabolism, covalent modification and isomerization, and participate in the formation of secondary bile acids.[22] Intestinal secondary bile acids can stimulate intestinal epithelial or lamina propria immune cells to produce inflammatory regulatory factors, which directly influence subsequent immune response.[23] Hang et al[24] screened more than 30 bile acid metabolites and identified two lithocholic acid derivatives, 3-oxo lithocholic acid (LCA) and isoallo LCA, which can reduce the differentiation of Th17 cells and increase the differentiation of Treg cells. The regulatory effect of intestinal bile acids pool on adaptive immunity was also observed in another study, which found that the composition of the intestinal bile acids pool can regulate the expression of the transcription factor retinoic acid receptor-related-orphan-receptor-C (RORγ) on colonic forkhead box protein P3+ (FOXP3+) Treg cells. The deletion of bile acids metabolic pathway significantly reduced the number of intestinal Treg cells, while the restoration of the intestinal bile acids pool showed increased the number of ROR+ Treg cells in the colon and improvement of the host colon inflammation.[25] Thus, it can be inferred that the intestinal bile acids pool may be involved in mediating the regulatory role of intestinal bacteria on Th17 cell differentiation.
Based on the evidence presented, we proposed a potential scientific hypothesis on the mechanism of HBV-related liver fibrosis [Figure 1]. Distinct gut microbiota in chronic HBV infection may lead to alterations of gut bile acids pool, then stimulate naive T cells to differentiate into Th17 cells, which then enter peripheral circulation and migrate to the liver via chemokine CCL20. Intrahepatic Th17 cells then secrete IL-17 and IL-22, which would activate hepatic stellate cells and Kupffer cells, and then lead to over-synthesis of the extracellular matrix. Although, there is still much to uncover for the specific mechanisms. With further research, the therapeutic potential of targeting the bile acids-intestinal microbiota-Th17/IL-17 axis will allow us to better discern the pathogenesis and treatment of HBV.
Figure 1.
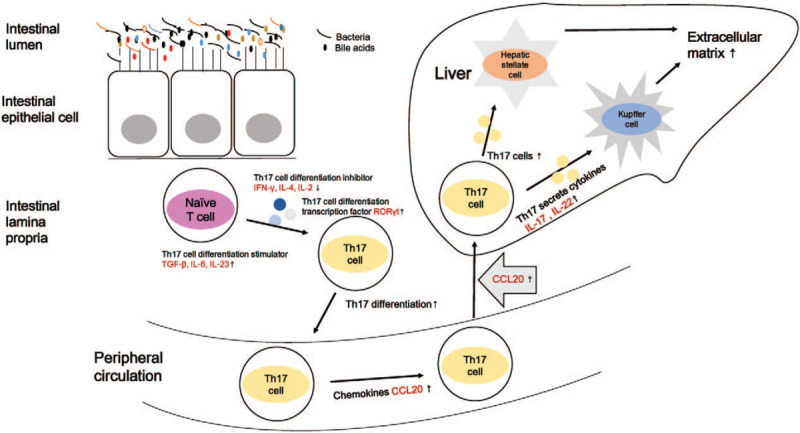
Possible relationship among intestinal microbiota, bile acids, and Th17/IL-17 axis in HBV-related liver fibrosis. Distinct gut microbiota in chronic HBV infection may lead to alterations of gut bile acids pool, then stimulate naive T cells to differentiate into Th17 cells, which then enter peripheral circulation and migrate to the liver via chemokine CCL20. Intrahepatic Th17 cells then secrete IL-17 and IL-22, which would activate hepatic stellate cells and Kupffer cells, lead to over-synthesis of the extracellular matrix. Th: T helper; IL: interleukin; HBV: Hepatitis B virus; CCL: C-C motif ligand.
Acknowledgements
We thank Maria Antony (University of Connecticut Health Center) for the help in language editing.
Conflicts of interest
None.
Footnotes
How to cite this article: Chen YF, Chen J, Li LJ. The role of intestinal microbiota, bile acids, and Th17/IL17 axis in hepatitis B virus-related liver fibrosis. Chin Med J 2020;133:2902–2904. doi: 10.1097/CM9.0000000000001199
References
- 1.Xiao J, Wang F, Wong NK, He J, Zhang R, Sun R, et al. Global liver disease burdens and research trends: analysis from a Chinese perspective. J Hepatol 2019; 71:212–221. doi: 10.1016/j.jhep.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Li MH, Lu Y, Zhang L, Wang XY, Ran CP, Hao HX, et al. Association of cytokines with alanine aminotransferase, hepatitis B virus surface antigen and hepatitis B envelope antigen levels in chronic hepatitis B. Chin Med J 2018; 131:1813–1818. doi: 10.4103/0366-6999.237394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang KM, Liu M. Chronic hepatitis B: immune pathogenesis and emerging immunotherapeutics. Curr Opin Pharmacol 2016; 30:93–105. doi: 10.1016/j.coph.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Dhar D, Baglieri J, Kisseleva T, Brenner DA. Mechanisms of liver fibrosis and its role in liver cancer. Exp Biol Med 2020; 245:96–108. doi: 10.1177/1535370219898141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chou HH, Chien WH, Wu LL, Cheng CH, Chung CH, Horng JH, et al. Age-related immune clearance of hepatitis B virus infection requires the establishment of gut microbiota. Proc Natl Acad Sci U S A 2015; 112:2175–2180. doi: 10.1073/pnas.1424775112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren YD, Ye ZS, Yang LZ, Jin LX, Wei WJ, Deng YY, et al. Fecal microbiota transplantation induces hepatitis B virus e-antigen (HBeAg) clearance in patients with positive HBeAg after long-term antiviral therapy. Hepatol 2017; 65:1765–1768. doi: 10.1002/hep.29008. [DOI] [PubMed] [Google Scholar]
- 7.Zhao J, Zhang Z, Luan Y, Zou Z, Sun Y, Li Y, et al. Pathological functions of interleukin-22 in chronic liver inflammation and fibrosis with hepatitis B virus infection by promoting T helper 17 cell recruitment. Hepatology 2014; 59:1331–1342. doi: 10.1002/hep.26916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paquissi FC. Immunity and fibrogenesis:The role of Th17/IL-17 axis in HBV and HCV-induced chronic hepatitis and progression to cirrhosis. Front Immunol 2017; 8:1195.doi: 10.3389/fimmu.2017.01195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ge J, Wang K, Meng QH, Qi ZX, Meng FL, Fan YC. Implication of Th17 and Th1 cells in patients with chronic active hepatitis B. J Clin Immunol 2010; 30:60–67. doi: 10.1007/s10875-009-9328-2. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Su Y, Hua X, Xie C, Liu J, Huang Y, et al. Levels of hepatic Th17 cells and regulatory T cells upregulated by hepatic stellate cells in advanced HBV-related liver fibrosis. J Transl Med 2017; 15:75.doi: 10.1186/s12967-017-1167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Ying H, Wei L, Hong LJ. Effect of nucleoside analogues in the treatment of hepatitis B cirrhosis and its effect on Th17 cell. Eur Rev Med Pharmacol Sci 2017; 21:416–420. [PubMed] [Google Scholar]
- 12.Zhang K, Liu Y, Yang X, Sun H, Shu X, Zhang Y, et al. HBV promotes the recruitment of IL-17 secreting T cells via chemokines CCL22 and CCL17. Liver Int 2020; 40:1327–1338. doi: 10.1111/liv.14438. [DOI] [PubMed] [Google Scholar]
- 13.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006; 441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 14.Cheng H, Guan X, Chen D, Ma W. The Th17/Treg cell balance: a gut microbiota-modulated story. Microorganisms 2019; 7.doi: 10.3390/microorganisms7120583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niess J, Leithäuser F, Adler G, Reimann J. Commensal gut flora drives the expansion of proinflammatory CD4 T cells in the colonic lamina propria under normal and inflammatory conditions. J Immunol 2008; 180:559–568. doi: 10.4049/jimmunol.180.1.559. [DOI] [PubMed] [Google Scholar]
- 16.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 2009; 9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin S, Zhao D, Cai C, Song D, Shen J, Xu A, et al. Low-dose penicillin exposure in early life decreases Th17 and the susceptibility to DSS colitis in mice through gut microbiota modification. Sci Rep 2017; 7:43662.doi: 10.1038/srep43662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAleer JP, Nguyen NLH, Chen K, Kumar P, Ricks DM, Binnie M, et al. Pulmonary Th17 antifungal immunity is regulated by the gut microbiome. J Immunol 2016; 197:97–107. doi: 10.4049/jimmunol.1502566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivanov II, Frutos RdeL, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 2008; 4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atarashi K, Tanoue T, Ando M, Kamada N, Nagano Y, Narushima S, et al. Th17 Cell induction by adhesion of microbes to intestinal epithelial cells. Cell 2015; 163:367–380. doi: 10.1016/j.cell.2015.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gil-Cruz C, Perez-Shibayama C, De Martin A, Ronchi F, van der Borght K, Niederer R, et al. Microbiota-derived peptide mimics drive lethal inflammatory cardiomyopathy. Science 2019; 366:881–886. doi: 10.1126/science.aav3487. [DOI] [PubMed] [Google Scholar]
- 22.Di Ciaula A, Garruti G, Lunardi Baccetto R, Molina-Molina E, Bonfrate L, Wang DQ-H, et al. Bile Acid Physiology. Ann Hepatol 2017; 16:s4–s14. doi: 10.5604/01.3001.0010.5493. [DOI] [PubMed] [Google Scholar]
- 23.Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol 2018; 15:111–128. doi: 10.1038/nrgastro.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hang S, Paik D, Yao L, Kim E, Trinath J, Lu J, et al. Bile acid metabolites control TH17 and Treg cell differentiation. Nature 2019; 576:143–148. doi: 10.1038/s41586-019-1785-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song X, Sun X, Oh SF, Wu M, Zhang Y, Zheng W, et al. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature 2020; 577:410–415. doi: 10.1038/s41586-019-1865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


