Abstract
Background
Albuvirtide is a once-weekly injectable human immunodeficiency virus (HIV)-1 fusion inhibitor. We present interim data for a phase 3 trial assessing the safety and efficacy of albuvirtide plus lopinavir-ritonavir in HIV-1-infected adults already treated with antiretroviral drugs.
Methods
We carried out a 48-week, randomized, controlled, open-label non-inferiority trial at 12 sites in China. Adults on the World Health Organization (WHO)-recommended first-line treatment for >6 months with a plasma viral load >1000 copies/mL were enrolled and randomly assigned (1:1) to receive albuvirtide (once weekly) plus ritonavir-boosted lopinavir (ABT group) or the WHO-recommended second-line treatment (NRTI group). The primary endpoint was the proportion of patients with a plasma viral load below 50 copies/mL at 48 weeks. Non-inferiority was prespecified with a margin of 12%.
Results
At the time of analysis, week 24 data were available for 83 and 92 patients, and week 48 data were available for 46 and 50 patients in the albuvirtide and NRTI groups, respectively. At 48 weeks, 80.4% of patients in the ABT group and 66.0% of those in the NRTI group had HIV-1 RNA levels below 50 copies/mL, meeting the criteria for non-inferiority. For the per-protocol population, the superiority of albuvirtide over NRTI was demonstrated. The frequency of grade 3 to 4 adverse events was similar in the two groups; the most common adverse events were diarrhea, upper respiratory tract infections, and grade 3 to 4 increases in triglyceride concentration. Renal function was significantly more impaired at 12 weeks in the patients of the NRTI group who received tenofovir disoproxil fumarate than in those of the ABT group.
Conclusions
The TALENT study is the first phase 3 trial of an injectable long-acting HIV drug. This interim analysis indicates that once-weekly albuvirtide in combination with ritonavir-boosted lopinavir is well tolerated and non-inferior to the WHO-recommended second-line regimen in patients with first-line treatment failure.
Trial registration
ClinicalTrials.gov Identifier: NCT02369965; https://www.clinicaltrials.gov.
Chinese Clinical Trial Registry No. ChiCTR-TRC-14004276; http://www.chictr.org.cn/enindex.aspx
Keywords: HIV, Fusion inhibitor, Albuvirtide, LPV/r, Phase 3 clinical trial
Introduction
Antiretroviral therapy (ART) has been proven effective in reducing the viral loads in patients, the viral infectivity, and the morbidity and mortality of acquired immunodeficiency syndrome (AIDS)-related diseases.[1–3] In an effort to end the human immunodeficiency virus (HIV) epidemic by 2030, the World Health Organization (WHO) revised their ART guidelines in 2015, whereby people all over the world living with HIV/AIDS (PLWHs) were recommended for ART, regardless of the CD4 T-cell count or WHO clinical stage, to reduce the risk of disease progression, morbidity, and mortality among infected people and to prevent HIV transmission.[4–8] Among the benefits expected by WHO are significant increases in ART uptake and linkage to care, reduction in the time between HIV diagnosis and ART initiation regardless of the baseline CD4 T-cell count, and an increase in the median CD4 value at ART initiation.[4–6,9,10] The Joint United Nations Programme on HIV/AIDS (UNAIDS) has adopted 90-90-90 targets, which use the care cascade to track progress and end the AIDS epidemic.[2] The total number of PLWHs in China had reached 849,602 by the end of September 2018.[11,12] Through the National Free Antiretroviral Treatment Program (NFATP), China is continuing to expand treatment coverage for individuals infected with HIV.[1,13–16] The NFATP has been highly successful, but it faces serious challenges, including poor patient compliance, emerging drug resistance, drug intolerance, and adverse effects, and a lack of alternative regimens. Only seven antiretroviral drugs are used in most current first-line and second-line treatments, and the newest drugs introduced into these regimens are tenofovir disoproxil fumarate (TDF) and ritonavir-boosted lopinavir (LPV/r).[1,17,18] There is, therefore, an urgent need to develop new drugs or prevention strategies to address these issues.[19]
HIV-1 is an enveloped virus with 8 to 10 envelope “spike” complexes per virion, with two protein subunits, the surface subunit gp120 and the transmembrane subunit gp41, which mediate binding to receptors and viral entry by fusing cellular and viral membranes.[20,21] The N- and C-terminal heptad repeat regions of the gp41 ectodomain refold into a thermostable six-helix bundle structure (6-HB), representing a fusion-active conformation, and offer an attractive target for developing antiviral agents.[22,23] We have previously developed a novel HIV-1 fusion inhibitor, albuvirtide (ABT), a 3-Maleimidopropionic acid-modified peptide fusion inhibitor derived from the N-terminal sequence of HIV-1 gp41, which binds to the HIV-1 gp41 envelope protein.[24] ABT exhibits broad-spectrum anti-HIV-1 activity in vitro, with a half maximal inhibitory concentration (IC50) of 0.5 to 5.0 nmol/L. It has been shown to be active against 28 different clinical isolates of HIV-1 in China, with IC50 values ranging from 1.3 to 18.1 nmol/L.[25] ABT also has a much longer half-life than the only viral fusion inhibitor approved by the Food and Drug Administration, enfuvirtide (T20, Fuzeon), which must be administered by twice-daily injections.[26–28]
In phase 1 clinical studies, a single administration of ABT in HIV-1-infected adults was found to have a half-life of 10 to 12 days and to suppress viral replication for 6 to 10 days.[29,30] In phase 2 clinical study, ABT was administered once weekly via the intravenous route, together with LPV/r. Treatment with this nucleoside/nucleotide reverse transcriptase inhibitor (NRTI)-free, two-drug regimen for seven weeks was well tolerated.[29,31] The mean decrease in viral load in the 320 mg ABT group was 2.2 log10 copies/mL, and 56% of subjects had an HIV-1 RNA level below 50 copies/mL. In all previous preclinical and clinical studies, ABT was found to have a good safety profile. No ABT-related adverse effects, injection site reactions, or antibody generation were observed. Based on these results, we initiated a phase 3 pivotal trial to assess the safety, efficacy, and clinical practicality of a once-weekly ABT injection in treatment-experienced HIV-1-infected adults.
Methods
Ethics statement
The Institutional Ethics Committee at each site reviewed and approved the protocol (No. 2013-067) and the written informed consent form which was provided according to the Declaration of Helsinki. Each patient gave written informed consent before undergoing the study procedures. The methods were carried out in accordance with approved guidelines and regulations.
Study design and participants
TALENT is an ongoing phase 3, open-label, randomized, multicenter, parallel-group, non-inferiority study in treatment-experienced adults with HIV-1 at 12 centers in China. Adults (aged 16–60 years) infected with HIV-1 were eligible for inclusion if they had a plasma viral RNA load of at least 1000 copies/mL or higher and had received at least 6 months of treatment with two classes of antiretroviral agents (NRTIs and non-nucleoside/nucleotide reverse transcriptase inhibitors [NNRTIs]). The exclusion criteria included active AIDS-defining conditions, prior exposure to protease inhibitors and fusion inhibitors, active hepatitis, abnormal values in standard laboratory tests (hemoglobin concentration <9 g/dL, white blood cell count <2 × 109/L, neutrophil count <1 × 109/L, platelet count <75 × 109/L, aminopherase levels more than 3 times the upper limit of the normal range, a total bilirubin concentration more than twice the upper limit of the normal range, creatinine concentration above the upper limit of the normal range, a creatine phosphokinase levels more than twice the upper limit of the normal range), serious chronic disease, hemophilia A or B, alcohol and/or drug abuse, and pregnancy and/or breastfeeding.
Procedures
Clinical case report form data were recorded electronically for each patient and visit. Patients were randomly assigned (1:1) to either the ABT group or the NRTI group. The patients in the ABT group received 320 mg ABT daily for the first 3 days and then weekly by intravenous infusion, together with LPV/r (400 mg lopinavir, 100 mg ritonavir, twice daily). The patients in the NRTI group received LPV/r (same dose as above) plus two optimized NRTIs selected by the site investigator before randomization. For the NRTI group, if TDF had not been included in the first-line regimen, lamivudine (3TC, 300 mg daily) and TDF (300 mg, daily) were preferred. If TDF had been included in the first-line regimen, the acceptable alternative NRTIs were 3TC plus zidovudine (AZT, 300 mg, twice daily) or 3TC plus abacavir (ABC, 300 mg, twice daily) based on previous treatment experience and drug resistance.
Clinical examinations and laboratory analyses were conducted at the initial screening visit, at baseline (administration of study drugs), and at weeks 4, 12, 24, 36, and 48. CD4 T-cell count was measured at an accredited laboratory at each clinical site. The plasma HIV-1 RNA load was measured at a central laboratory in Beijing Youan Hospital, Capital Medical University. Genotype resistance was assessed at a laboratory in the National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention. Compliance with drug treatment was assessed at every visit. Adverse events were evaluated and graded according to the US NIH Division of AIDS toxicity scales.
An independent data and safety monitoring board (DSMB) periodically reviewed the safety and efficacy results and made recommendations regarding completion of enrollment, interim analysis, and continuation of the study.
Randomization and blinding
Patients were allocated (1:1) to the ABT plus LPV/r group or the LPV/r plus two optimized NRTIs group. The randomization sequence was generated centrally by a computer via an interactive system accessible via a website or telephone. We used a block size of four. Site investigators checked the inclusion and exclusion criteria to confirm eligibility before the online group assignment and had access to the participant's assignment details only once the checklists had been completed. The viral assay was performed in an accredited central laboratory, and the laboratory personnel were blind to the treatment group. The participants and other onsite personnel were not blind to the treatment group.
Outcomes
The primary objective of this study was to determine whether the efficacy of ABT plus LPV/r was non-inferior to standard second-line regimens containing LPV/r and two NRTIs. We performed a modified intention-to treat (mITT) analysis of efficacy, including data for all patients who received at least one dose of the study drug and underwent HIV-1 RNA load testing at least once after treatment.
The per-protocol analysis included patients who received the study drugs for 24 or 48 weeks, with no major protocol violation. The primary endpoint was the proportion of patients achieving plasma HIV-1 RNA levels of fewer than 50 copies/mL at week 48. For the primary analysis, the virological response was assessed with the snapshot algorithm defined by the US FDA. This algorithm scores efficacy based on a snapshot of HIV-1 RNA levels at particular weeks, with a missing-as-failure analysis (missing data for HIV-1 RNA considered to be >50 copies/mL). The secondary endpoints included the proportion of patients with plasma HIV-1 RNA levels below 400 copies/mL, changes from baseline in HIV-1 RNA log10 copies/mL, and CD4 T-cell counts.
The safety data were analyzed up to week 48 for all patients receiving at least one dose of the study drug. The main safety endpoints were the incidence and severity of adverse events and changes in laboratory parameters.
Statistical analysis
The primary efficacy hypothesis was that ABT would display antiviral activity non-inferior to that of NRTI at week 48 if both treatments were administered in combination with LPV/r. The non-inferiority margin was prespecified as 12%, based on applicable HIV-1-specific and statistical guidelines. The treatment was considered non-inferior if the lower limit of the two-tailed 95% confidence interval (CI) for the difference in the proportion of patients with plasma HIV-1 RNA levels below 50 copies/mL at week 48 was –12% or greater. The aim was to test for superiority at a nominal significance level of 5% if non-inferiority was established in both the mITT and per-protocol analyses. Assuming a virological success rate of 80% in both groups and a dropout rate of 20%, we would need to include 420 patients to achieve 80% power in the two-tailed test, with an alpha risk of 5%, for the demonstration of non-inferiority for the primary endpoint.
We used SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) for all statistical analyses. An interim analysis was specified in advance in the study protocol and was performed in accordance with the recommendations of the DSMB.
Results
Between February 11, 2014, and December 5, 2015, we enrolled 347 participants. At the time of the interim analysis, 208 had completed the 24-week visit and the data for these patients are reported here. On July 1, 2015, one site was closed due to a serious protocol violation; consequently, nine patients from this site were excluded from the efficacy analysis [Figure 1].
Figure 1.
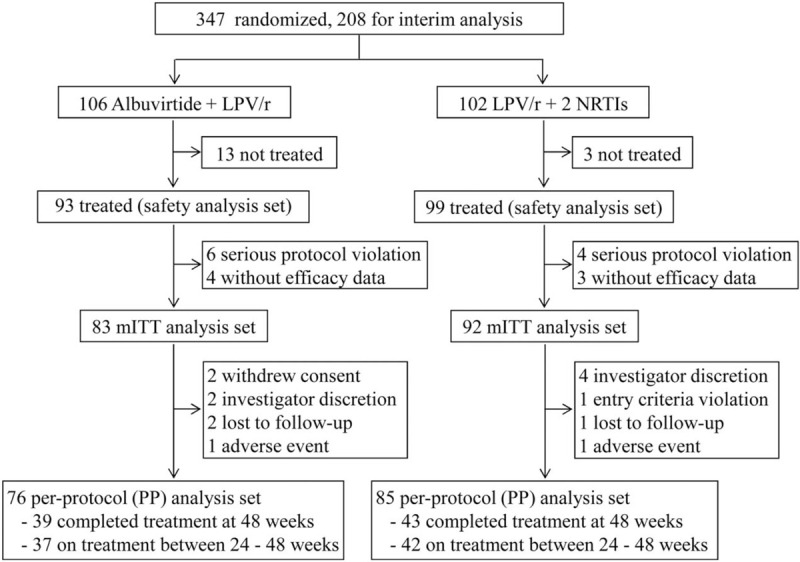
Trial profile at interim analysis. Screening, randomization, and follow-up of HIV-1-infected patients. HIV: Human immunodeficiency virus; LPV/r: Ritonavir-boosted lopinavir; mITT: Modified intention-to treat; NRTI: Nucleoside or nucleotide reverse transcriptase inhibitor.
The study treatments, baseline characteristics, previous treatment drugs, and genotypic resistance profile of the participants were distributed similarly between the two groups [Table 1]. Other than the baseline characteristics of HIV-1-infected patients summarized in Table 1, most of the patients were infected with HIV-1 subtype B (50.9%) viruses, with CRF01_AE viruses being the next most common (29.6%). In accordance with NFATP guidelines, 54.3% of the patients received AZT and 44.0% received TDF in their first-line treatment regimens. On entry into the study, the first-line nucleoside analog was switched from AZT to TDF, according to WHO guidelines for second-line treatment, or from other nucleoside drugs to TDF. Baseline genotypic resistance to at least one ART drug was observed in 81.7% of patients, with resistance to efavirenz (79.9%), nevirapine (79.9%), and lamivudine (67.5%) being the most frequently recorded (datatable not shown). Major protease inhibitor mutations were rare (1.2%), and none of those detected were expected to affect the susceptibility to LPV/r. In addition, using the baseline genotypic resistance data, we assessed the sensitivity of the viruses to the switched nucleoside inhibitor in the NRTI group; the virus was sensitive in 69.7% of cases and highly resistant in 12.4% (datatable not shown).
Table 1.
Baseline characteristics of HIV-1-infected patients.
| Parameters | ABT group (n = 83) | NRTI group (n = 92) |
| Age (years) | 40.1 ± 11.3 | 39.6 ± 11.1 |
| Men | 61 (73.5) | 67 (72.8) |
| Race | ||
| Han | 79 (95.2) | 88 (95.7) |
| Others | 4 (4.8) | 4 (4.3) |
| Plasma HIV-1 RNA (log10 copies/mL) | 3.8 ± 1.0 | 3.8 ± 1.0 |
| <100,000 | 74 (89.2) | 79 (85.9) |
| ≥100,000 | 9 (10.8) | 13 (14.1) |
| CD4 T-cell count (cells/μL) | 239.5 ± 184.6 | 233.7 ± 162.1 |
| <100 | 15 (18.1) | 14 (15.2) |
| ≥100 | 68 (81.9) | 78 (84.8) |
| Time on first-line regimen (months) | 25.9 (11.2–50.4) | 31.0 (10.7–66.1) |
| Baseline resistance mutations∗ | ||
| NRTI | 53 (66.3) | 67 (75.3) |
| NNRTI | 62 (77.5) | 73 (82) |
| Primary PI mutation | 1 (1.3) | 1 (1.1) |
| NRTIs selected for use at study entry | ||
| Tenofovir and lamivudine | – | 66 (71.7) |
| Zidovudine and lamivudine | – | 24 (26.1) |
| Abacavir and lamivudine | – | 1 (1.1) |
| Tenofovir, zidovudine and lamivudine | – | 1 (1.1) |
Data are median (Q1–Q3), n (%), and mean ± standard deviation. ∗n = 80 and 89 for ABT group and NRTI group respectively; –: No data. ABT: Albuvirtide; HIV: Human immunodeficiency virus; NNRTI: Non-nucleoside/nucleotide reverse transcriptase inhibitor; NRTI: Nucleoside or nucleotide reverse transcriptase inhibitor; PI: Protease inhibitor.
At week 48, 37 (80.4%) of the 46 subjects in the ABT and 33 (66.0%) of the 50 subjects in the NRTI group had HIV-1 RNA levels of fewer than 50 copies/mL [Table 2 and Figure 2A]. There was a 14.4% difference between the treatments, with a 95% CI of –3.0 to 31.9, meeting the criteria for non-inferiority. We also concluded that ABT was non-inferior at week 24 when 66 (79.5%) of the 83 subjects in the ABT group and 72 (78.3%) of the 92 subjects in the NRTI group had HIV-1 RNA levels of fewer than 50 copies/mL (difference of 1.2%, 95% CI: –10.8% to 13.4%). At week 48, the response rate was higher in the per-protocol analysis than that in the mITT analysis. The percentage of subjects with HIV-1 RNA levels below 50 copies/mL was 94.9% for the ABT group and 74.4% for the NRTI group. Similar results were obtained for the proportion of patients with plasma HIV-1 RNA levels below 400 copies/mL [Figure 2B]. Statistical superiority was demonstrated for the ABT group (P = 0.01), with the difference between the treatments being driven primarily by virological outcomes.
Table 2.
Efficacy results of the interim analysis in the TALENT study.
| Parameters | ABT group | NRTI group | Difference (95% CI; P value) |
| Week 48 | n = 46 | n = 50 | |
| HIV-1 RNA <50 copies/mL (mITT) | 37/46 (80.4) | 33/50 (66) | 14.4 (–3.0 to 31.9) |
| HIV-1 RNA <50 copies/mL (PP) | 37/39 (94.9) | 32/43 (74.4) | 20.5 (5.7 to 35.2; P = 0.01) |
| HIV-1 RNA <400 copies/mL | 39 (84.8) | 37 (74) | 10.8 (–5.2 to 26.8) |
| HIV-1 RNA log10 copies/mL change | –2.27 ± 0.96 | –1.77 ± 1.33 | P = 0.015∗ |
| CD4 T-cell count change (cells/μL) | 120.5 (27.4–254.9) | 150.3 (72.3–244.5) | P = 0.557† |
| Week 24 | n = 83 | n = 92 | |
| HIV-1 RNA <50 copies/mL (mITT) | 66/83 (79.5) | 72/92 (78.3) | 1.2 (–10.8 to 13.4) |
| HIV-1 RNA <50 copies/mL (PP) | 64/76 (84.2) | 71/85 (83.5) | 0.7 (–10.7 to 12.1) |
| HIV-1 RNA <400 copies/mL | 74 (89.2) | 76 (82.6) | 6.6 (–3.7 to 16.8) |
| HIV-1 RNA log10 copies/mL change | –2.00 ± 1.01 | –1.85 ± 1.16 | P = 0.137∗ |
| CD4 T-cell count change (cells/μL) | 79.0 (35.2–156.5) | 86.0 (19.8–159.6) | P = 0.521† |
Data are n (%), n/N (%), mean ± standard deviation, median (Q1–Q3) or % (95% CI). ∗ANCOVA: Analysis of covariance, with the baseline as a covariate; †Wilcoxon test. ABT: Albuvirtide; CI: Confidence interval; HIV: Human immunodeficiency virus; NRTI: Nucleoside/Nucleotide reverse transcriptase inhibitor; mITT: modified intention-to treat; PP: Per-protocol.
Figure 2.
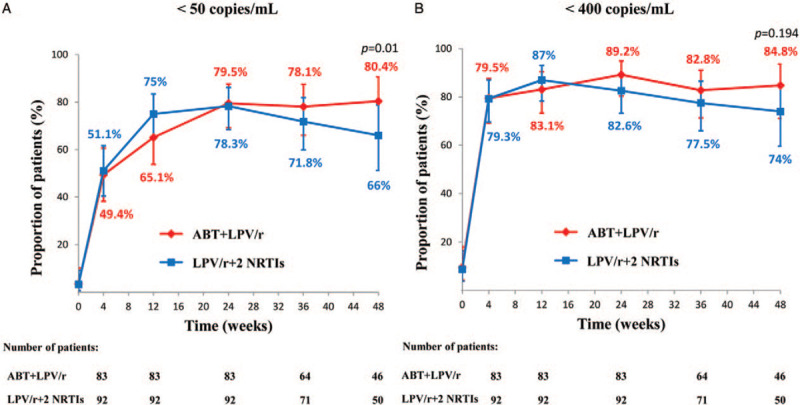
Randomized clinical trials analyzed the efficacy and clinical effectiveness between the ABT group and NRTI groups by the mITT approach. The proportion of patients achieving plasma HIV-1 RNA levels of fewer than 50 copies/mL (A) and the proportion of patients achieving plasma HIV-1 RNA levels of fewer than 400 copies/mL (B) are shown. Patients were allocated (1:1) to the ABT plus LPV/r group in red or to the LPV/r plus two optimized NRTIs group in blue. Error bars show 95% CIs, snapshot analysis (missing, switch, discontinuation = failure). ABT: Albuvirtide; CI: Confidence interval; HIV: Human immunodeficiency virus; LPV/r: Ritonavir-boosted lopinavir; mITT: Modified intention-to treat; NRTI: Nucleoside or nucleotide reverse transcriptase inhibitor.
The results of the secondary and primary efficacy analyses were consistent [Table 2]. CD4+ T-cell counts increased from baseline to week 48 in both groups (a median change of 120.5 cells/μL in the ABT group; 150.3 cells/μL in the NRTI group, P = 0.557).
We assessed the development of resistance mutations in patients whose viral load was >400 copies/mL at weeks 24 or 48: five patients from the ABT group and 13 patients from the NRTI group. NRTI resistance mutations (M41L and T215F) that had emerged since baseline were noted in one patient from the ABT group, whereas the M184V, M41LM, and K70KR mutations were observed in three patients from the NRTI group. NNRTI resistance mutations (Y181C and H221Y) emerged in only one patient from the NRTI group. One patient in the NRTI group developed both major (I50V and V82A) and minor protease resistance mutations (L33F, Q58E, and L10F). The minor protease resistance mutations L10I and A71AV were also detected in two patients from the ABT group, and the A71AT mutation was found in one patient from the NRTI group. None of the five patients in the ABT group developed resistance mutations of the gp41 gene after baseline.
Self-reported compliance/adherence to treatment was similar between the two groups. In the ABT group, 100% of patients had compliance levels of 90% to 110% for all study drugs over the treatment period. The corresponding value for the NRTI group was 96.7%.
Safety profiles were similar for the two groups, with similar rates of adverse events [Table 3], most of which were of mild to moderate intensity. Grade 3 to 4 adverse event frequencies were similar in the two groups (14.0% vs. 11.1%). The most common adverse events were diarrhea (8.6% vs. 14.1%, ABT vs. NRTI), upper respiratory tract infections (4.3% vs. 6.1%), and grade 3 to 4 increases in triglyceride concentration (6.5% vs. 4.0%).
Table 3.
Clinical adverse events and laboratory abnormalities.
| Parameters | ABT group (N = 93) | NRTI group (N = 99) |
| Grade 3–4 adverse events | 13 (14.0) | 11 (11.1) |
| Serious adverse events | 6 (6.5) | 3 (3.0) |
| Drug-related serious adverse events | 0 (0.0) | 1 (1.0) |
| Clinical adverse events in ≥2% of patients in either group | ||
| Pharyngitis | 3 (3.2) | 1 (1.0) |
| Tonsillitis | 2 (2.2) | 0 (0.0) |
| Upper respiratory tract infection | 4 (4.3) | 6 (6.1) |
| Pulmonary infection | 0 (0.0) | 2 (2.0) |
| Urethritis | 2 (2.2) | 1 (1.0) |
| Gastroenteritis | 0 (0.0) | 5 (5.1) |
| Enteritis | 2 (2.2) | 0 (0.0) |
| Diarrhea | 8 (8.6) | 14 (14.1) |
| Fever | 2 (2.2) | 3 (3.0) |
| Fatigue | 2 (2.2) | 0 (0.0) |
| Peripheral edema | 0 (0.0) | 2 (2.0) |
| Rash | 2 (2.2) | 2 (2.0) |
| Haematuria | 1 (1.1) | 4 (4.0) |
| Headache | 2 (2.2) | 0 (0.0) |
| Dizzy | 2 (2.2) | 0 (0.0) |
| Grade 3–4 laboratory abnormalities in ≥2% of patients in either group | ||
| High triglycerides | 6 (6.5) | 4 (4.0) |
| High total cholesterol | 1 (1.1) | 2 (2.0) |
| High hemobilirubin | 0 (0.0) | 2 (2.0) |
| Hepatic function disorder∗ | 2 (2.2) | 1 (1.0) |
Data are n (%). ∗Two or three items increased among aspartate aminotransferase, alanine aminotransferase, and gamma-glutamyl transferase. ABT: Albuvirtide; NRTI: Nucleoside/Nucleotide reverse transcriptase inhibitor.
Renal function was significantly more strongly impaired at 12 weeks in the patients of the NRTI group receiving TDF than in those of the ABT group (mean change in estimated glomerular filtration rate: –11.48 vs. –1.22 mL·min−1·1.73 m−2, P = 0.02) [Figure 3]. Safety events leading to treatment discontinuation (adverse events or stopping criteria) were infrequent in both treatment groups [Figure 1]. The rates and nature of the serious adverse events observed were also similar, and few patients developed a drug-related serious adverse event [Table 3]. No deaths occurred. The distribution and number of graded treatment-emergent laboratory toxicities were similar between groups [Table 3].
Figure 3.
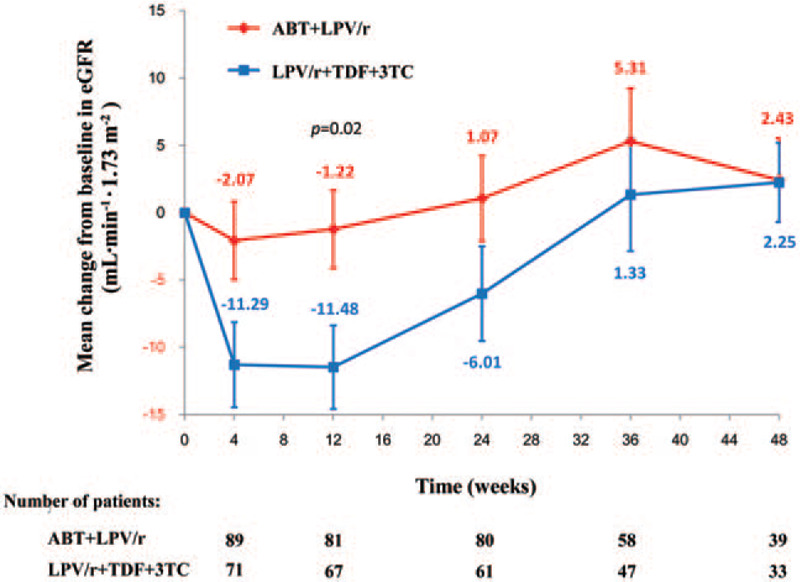
Mean change from baseline in eGFR over time. Renal function was evaluated by the eGFR. Error bars indicate the standard error. 3TC: Lamivudine; ABT: Albuvirtide; eGFR: Estimated glomerular filtration rate; LPV/r: Ritonavir-boosted lopinavir; TDF: Tenofovir Disoproxil Fumarate.
Discussion
Our findings from 12 hospitals and health centers in China show that 80.4% of the HIV-1-treated patients in the ABT group and 66.0% of those in the NRTI group had HIV-1 RNA levels below 50 copies/mL at week 48 in this TALENT study, thereby meeting the criteria for non-inferiority. Moreover, for the per-protocol population, the superiority of ABT over NRTI was demonstrated. These results highlight a clear contribution of ABT to the plasma HIV-1 RNA reduction and safety of the clinical two-drug combination regimen.
The T20 peptide is the first US FDA-approved HIV entry inhibitor; however, its clinical application is limited by the twice-daily dosage of 90 mg per dose, leading to the high cost and serious local injection reactions with a short in vivo half-life.[26,27,32] It was previously demonstrated that ABT possesses potent and broad inhibitory activity against diverse HIV-1 subtypes and variants in vitro with an even higher inhibitory effect than T20 through a mechanism of blocking fusion-active 6-HB formation and membrane fusion.[25] In our previous phase 2 clinical trial, the mean decline of HIV-1 RNA from baseline was 2.2 log10 copies/mL, and the viral suppression of HIV-1 RNA to below 50 copies/mL was achieved in 55.6% of patients when ABT was taken in combination with LPV/r in week 7.[29] LPV/r is an orally administered co-formulated ritonavir-boosted protease inhibitor as a part of combination therapy with other antiretroviral agents and is a well-established and effective treatment for both ART-naive and HIV-1-infected patients.[17,33] Therefore, we chose LPV/r for the ART regimen in this phase 3 clinical trial, that is, a 48-week, randomized, controlled, multicenter study to investigate the safety, efficacy, and clinical practicality of once-weekly ABT injection in treatment-experienced HIV-1-infected adults, which may have potential as a next-generation HIV fusion inhibitor in clinical trials and clinical practice.
The hydrophobic pocket of gp41 is highly conserved; therefore, antiviral drugs targeting the pocket are expected to have potent and broad anti-HIV-1 activity against diverse HIV-1 strains and a high genetic barrier for drug resistance.[22] A previous study showed that treatment resistance has emerged in patients who fail T20-containing regimens.[34] However, an in vitro study demonstrated that ABT has potent and broad anti-HIV-1 activity not only against currently circulating subtypes worldwide but also against the induced variants that are resistant to T20.[25] Of note, in our TALENT study, although five patients whose viral load was >400 copies/mL at weeks 24 and 48 in the ABT group were assessed for the development of resistance mutations, none of them developed resistance mutations of gp41 after baseline. These findings suggest that the novel fusion inhibitor ABT has a higher potency than T20 and exhibits a higher genetic barrier for drug resistance.
A strength of ABT is that it can irreversibly conjugate with serum albumin, thus prolonging the half-life of 10 to 12 days, indicating that it could be suitable for once-weekly and less frequent dosing intervals.[30] Therefore, this long half-life of ABT has the potential to improve adherence to therapy, allow a more forgiving time window of drug administration, and even significantly reduce the cost of treatment.
In the previous phase 2 study, ABT was well tolerated by patients, and there were no serious adverse events during the 47 days of treatment.[29] In the present study, we found that the profile and frequency of grade 3 to 4 adverse events with long-term ABT treatment were similar to those of the NRTI group [Table 3]. The most common adverse events were diarrhea, upper respiratory tract infections, and grade 3 to 4 increases in triglyceride concentration. Moreover, renal function was significantly less impaired at 12 weeks in the patients in the ABT group than those in the NRTI group who received TDF [Figure 3]. Thus, long-term treatment (48 weeks) with ABT/LPV/r showed potent efficacy in HIV-1-infected patients, without an increased rate of adverse events.
In conclusion, this phase 3 clinical trial showed that the injectable long-acting HIV-1 drug ABT combined with LPV/r is both safe and effective. Compared with regimens of 3 to 4 drugs, the two-drug regimen offers a simplified therapy with better safety, less drug-drug interaction, and fewer patients who develop a drug-related serious adverse event. This interim analysis indicates that once-weekly ABT in combination with LPV/r is well tolerated and non-inferior to the WHO-recommended second-line regimen in patients with first-line treatment failure. Although investigational long-acting ART may allow HIV-1-infected patients who have difficulty with daily oral therapy to maintain viral suppression, with long-acting therapies, these patients and their retention in care should be closely monitored to avoid risk for the emergence of resistance to treatment.
Acknowledgements
The authors thank all members of the TALENT team; they were responsible for study oversight and played important roles in the study at their sites; cared for our patients before, during, and following this study; and provided input as well as intellectual and other valuable contributions. The authors greatly appreciate and acknowledge their patient described herein for volunteering his time and for donating numerous samples that allowed the detailed analyses to be performed. In addition, the authors are grateful to Dr. Christine Katlama (Hospital Pitié Salpétrière, Paris, France) and Dr. Christiane Moog (INSERM U1109, Strasbourg, France) for the helpful discussions and critical evaluation of this manuscript.
Funding
This work was supported by the Frontier Biotechnologies Inc., Ministry of Science and Technology of China (Nos. 2013ZX09101001 and 2017ZX09201007), the Beijing Municipal of Science and Technology Major Project (Nos. D141100000314005, D141100000314002, and D161100000416003), the National Natural Science Foundation of China (Nos. 81772165, 81974303, and 81571973), and Beijing Key Laboratory for HIV/AIDS Research (No. BZ0089).
Conflicts of interest
The authors of this manuscript have read the journal's policy and have the following competing interests: CY, RJL, JHH, and DX have received salary support from Frontier Biotechnologies Inc. All authors had full access to all study data and analyses and approved the final report. All other authors have declared that no competing interests exist.
Footnotes
How to cite this article: Su B, Yao C, Zhao QX, Cai WP, Wang M, Lu HZ, Chen YY, Liu L, Wang H, He Y, Zheng YH, Li LH, Chen JF, Yu JH, Zhu B, Zhao M, Sun YT, Lun WH, Xia W, Sun LJ, Dai LL, Jiang TY, Wang MX, Zheng QS, Peng HY, Wang Y, Lu RJ, Hu JH, Xing H, Shao YM, Xie D, Zhang T, Zhang FJ, Wu H. Efficacy and safety of the long-acting fusion inhibitor albuvirtide in antiretroviral-experienced adults with human immunodeficiency virus-1: interim analysis of the randomized, controlled, phase 3, non-inferiority TALENT study. Chin Med J 2020;133:2919–2927. doi: 10.1097/CM9.0000000000001273
Bin Su, Cheng Yao, Qing-Xia Zhao, Wei-Ping Cai, Min Wang, and Hong-Zhou Lu contributed equally to this work.
References
- 1.AIDS and Hepatitis C Professional Group, Society of Infectious Diseases, Chinese Medical Association, Chinese Center for Disease Control and Prevention. Chinese guidelines for diagnosis and treatment of HIV/AIDS (2018). Chin J Intern Med 2018; 57:1–18. doi: 10.3760/cma.j.issn.0578-1426.2018.12.002. [Google Scholar]
- 2. Joint United Nations Programme on HIV/AIDS (UNAIDS), Switzerland. 90-90-90 An ambitious treatment target to help end the AIDS epidemic; October 2014. Available from: https://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf. [Google Scholar]
- 3.Thompson MA, Mugavero MJ, Amico KR, Cargill VA, Chang LW, Gross R, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med 2012; 156:817–833. doi: 10.7326/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Joint United Nations Programme on HIV/AIDS (UNAIDS), UNAIDS data 2020, 2020. Available from: https://www.unaids.org/sites/default/files/media_asset/2020_aids-data-book_en.pdf. [PubMed] [Google Scholar]
- 5.World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection Recommendations for a Public Health Approach, Second edition. Geneva, Switzerland: World Health Organization; 2016. [PubMed] [Google Scholar]
- 6. World Health Organization. Guideline on When to Start Antiretroviral Therapy and on Pre-Exposure Prophylaxis for HIV. WHO Guidelines Approved by the Guidelines Review Committee 2015. [PubMed] [Google Scholar]
- 7.Gunthard HF, Saag MS, Benson CA, del Rio C, Eron JJ, Gallant JE, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the international antiviral society-USA panel. JAMA 2016; 316:191–210. doi: 10.1001/jama.2016.8900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joint United Nations Programme on HIV/AIDS (UNAIDS), Global HIV & AIDS statistics — 2020 fact sheet, 2020. Available from: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf. [Google Scholar]
- 9. Developed by the DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV; July 10, 2019. Available from: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. [Google Scholar]
- 10.Prabhu S, Harwell JI, Kumarasamy N. Advanced HIV: diagnosis, treatment, and prevention. Lancet HIV 2019; 6:e540–e551. doi: 10.1016/S2352-3018(19)30189-4. [DOI] [PubMed] [Google Scholar]
- 11. Joint United Nations Programme on HIV/AIDS (UNAIDS). The Global HIV/AIDS Epidemic; 2019. Available from: https://www.unaids.org/en/resources/fact-sheet. [Google Scholar]
- 12.NCAIDS, NCSTD, China CDC. Update on the AIDS/STD epidemic in China the third quarter of 2018. Chin J AIDS STD 2018; 24:1075.doi: 10.13419/j.cnki.aids.2018.11.01. [Google Scholar]
- 13.Liu P, Tang Z, Lan G, Zhu Q, Chen H, You Y, et al. Early antiretroviral therapy on reducing HIV transmission in China: strengths, weaknesses and next focus of the program. Sci Rep 2018; 8:3431.doi: 10.1038/s41598-018-21791-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang F, Dou Z, Ma Y, Zhang Y, Zhao Y, Zhao D, et al. Effect of earlier initiation of antiretroviral treatment and increased treatment coverage on HIV-related mortality in China: a national observational cohort study. Lancet Infect Dis 2011; 11:516–524. doi: 10.1016/S1473-3099(11)70097-4. [DOI] [PubMed] [Google Scholar]
- 15.Zhang F, Haberer JE, Wang Y, Zhao Y, Ma Y, Zhao D, et al. The Chinese free antiretroviral treatment program: challenges and responses. AIDS 2007; 21 Suppl 8:S143–S148. doi: 10.1097/01.aids.0000304710.10036.2b. [DOI] [PubMed] [Google Scholar]
- 16.Zhang FJ, Pan J, Yu L, Wen Y, Zhao Y. Current progress of China's free ART program. Cell Res 2005; 15:877–882. doi: 10.1038/sj.cr.7290362. [DOI] [PubMed] [Google Scholar]
- 17.Su B, Wang Y, Zhou R, Jiang T, Zhang H, Li Z, et al. Efficacy and tolerability of lopinavir/ritonavir- and efavirenz-based initial antiretroviral therapy in HIV-1-infected patients in a tertiary care hospital in Beijing, China. Front Pharmacol 2019; 10:1472.doi: 10.3389/fphar.2019.01472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AIDS Professional Group, Society of Infectious Diseases, Chinese Medical Association. Third Edition of the Guidelines for Diagnosis and Treatment of HIV/AIDS. Chinese Journal of Clinical Infectious Diseases 2015; 5:385–401. doi: 10.3760/cma.j.issn.1674-2397.2015.05.001. [Google Scholar]
- 19.Wu ZY, Scott SR. Human immunodeficiency virus prevention strategies in China. Chin Med J 2020; 133:318–325. doi: 10.1097/CM9.0000000000000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu P, Chertova E, Bess J, Jr, Lifson JD, Arthur LO, Liu J, et al. Electron tomography analysis of envelope glycoprotein trimers on HIV and simian immunodeficiency virus virions. Proc Natl Acad Sci U S A 2003; 100:15812–15817. doi: 10.1073/pnas.2634931100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan DC, Kim PS. HIV entry and its inhibition. Cell 1998; 93:681–684. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- 22.Lu L, Yu F, Cai L, Debnath AK, Jiang S. Development of small-molecule HIV entry inhibitors specifically targeting gp120 or gp41. Curr Top Med Chem 2016; 16:1074–1090. doi: 10.2174/1568026615666150901114527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan DC, Chutkowski CT, Kim PS. Evidence that a prominent cavity in the coiled coil of HIV type 1 gp41 is an attractive drug target. Proc Natl Acad Sci U S A 1998; 95:15613–15617. doi: 10.1073/pnas.95.26.15613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie D, Yao C, Wang L, Min W, Xu J, Xiao J, et al. An albumin-conjugated peptide exhibits potent anti-HIV activity and long in vivo half-life. Antimicrob Agents Chemother 2010; 54:191–196. doi: 10.1128/AAC.00976-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chong H, Yao X, Zhang C, Cai L, Cui S, Wang Y, et al. Biophysical property and broad anti-HIV activity of albuvirtide, a 3-maleimimidopropionic acid-modified peptide fusion inhibitor. PLoS One 2012; 7:e32599.doi: 10.1371/journal.pone.0032599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazzarin A, Clotet B, Cooper D, Reynes J, Arasteh K, Nelson M, et al. Efficacy of enfuvirtide in patients infected with drug-resistant HIV-1 in Europe and Australia. N Engl J Med 2003; 348:2186–2195. doi: 10.1056/NEJMoa035211. [DOI] [PubMed] [Google Scholar]
- 27.Lalezari JP, Henry K, O’Hearn M, Montaner JS, Piliero PJ, Trottier B, et al. Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. N Engl J Med 2003; 348:2175–2185. doi: 10.1056/NEJMoa035026. [DOI] [PubMed] [Google Scholar]
- 28.Jiang S, Lin K, Strick N, Neurath AR. HIV-1 inhibition by a peptide. Nature 1993; 365:113.doi: 10.1038/365113a0. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, Jin R, Yao C, Zhang T, Wang M, Xia W, et al. Combination of long-acting HIV fusion inhibitor albuvirtide and LPV/r showed potent efficacy in HIV-1 patients. AIDS Res Ther 2016; 13:8.doi: 10.1186/s12981-016-0091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu H, Yao C, Lu RJ, Zhang T, Wang MX, Zhao HX, et al. Albuvirtide, the First Long-acting HIV Fusion Inhibitor, Suppressed Viral Replicaiton in HIV-Infected Adults. 52nd Interscience Conference on Antimicrobials and Chemotherapy (ICAAC) 2012, Abstract H-554. [Google Scholar]
- 31.Yang W, Xiao Q, Wang D, Yao C, Yang J. Evaluation of pharmacokinetic interactions between long-acting HIV-1 fusion inhibitor albuvirtide and lopinavir/ritonavir, in HIV-infected subjects, combined with clinical study and simulation results. Xenobiotica 2017; 47:133–143. doi: 10.3109/00498254.2016.1166532. [DOI] [PubMed] [Google Scholar]
- 32.Patel IH, Zhang X, Nieforth K, Salgo M, Buss N. Pharmacokinetics, pharmacodynamics and drug interaction potential of enfuvirtide. Clin Pharmacokinet 2005; 44:175–186. doi: 10.2165/00003088-200544020-00003. [DOI] [PubMed] [Google Scholar]
- 33.Rabi SA, Laird GM, Durand CM, Laskey S, Shan L, Bailey JR, et al. Multi-step inhibition explains HIV-1 protease inhibitor pharmacodynamics and resistance. J Clin Invest 2013; 123:3848–3860. doi: 10.1172/JCI67399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charpentier C, Jenabian MA, Piketty C, Karmochkine M, Tisserand P, Laureillard D, et al. Dynamics of enfuvirtide resistance mutations in enfuvirtide-experienced patients remaining in virological failure under salvage therapy. Scand J Infect Dis 2011; 43:373–379. doi: 10.3109/00365548.2011.552520. [DOI] [PubMed] [Google Scholar]


