Abstract
Antimicrobial peptides (AMPs) are small molecules produced by a myriad of cells and play important roles not only in protecting against infections and sustaining skin barrier homeostasis but also in contributing to immune dysregulation under pathological conditions. Recently, increasing evidence has indicated that AMPs, including cathelicidin (LL-37), human β-defensins, S100 proteins, lipocalin 2, and RNase 7, are highly expressed in psoriatic skin lesions. These peptides broadly regulate immunity by interacting with various immune cells and linking innate and adaptive immune responses during the progression of psoriasis. In this review, we summarize the recent findings regarding AMPs in the pathogenesis of psoriasis with a main focus on their immunomodulatory abilities.
Keywords: Antimicrobial peptides, Psoriasis, Immune response, Inflammation
Introduction
Antimicrobial peptides (AMPs) are an integral part of the first-line defense of a host against pathogens. AMPs are mainly amphipathic peptides with α-helical structures and β-sheets linked by disulfide bridges, extended loops, or cyclic configurations.[1] AMPs were initially discovered as substances with bactericidal effects derived from neutrophils and stored in secondary granules[2]; immune cells and epithelial cells in the skin, gut, and lungs can also secrete AMPs with various functions.[3] The majority of AMPs in human skin are expressed in a steady state and are induced in response to stimuli, including injury, tape stripping, and infections, such that the immune response, chemotaxis, wound healing, apoptosis, and angiogenesis are regulated.[4–6] Recently, the immunomodulatory effects of AMPs have been reported to be involved in many autoimmune diseases such as rheumatic arthritis,[7] Crohn disease,[8] and psoriasis.[9]
Psoriasis, a common skin disorder, is an autoimmune condition characterized by aberrant innate and adaptive immune responses, in which T cells, keratinocytes, and dendritic cells (DCs) play a central role.[10] Psoriasis can be triggered by injury, infections, and mechanical stimulation, especially in patients with a genetic predisposition.[11] In this context, many active immune substances, including AMPs, rapidly increase in concentration in the local skin and initiate the maturation and activation of DCs and T cells with excessive interleukin (IL)-17 expression, resulting in the infiltration of immune cells and an inflammatory cascade. The expression levels of AMPs, including cathelicidin (LL-37),[12] human β-defensins (hBDs),[13] S100 proteins,[14] lipocalin 2 (LCN2),[15] and RNase 7,[7] are higher in skin lesions and/or sera of psoriasis patients than in those of healthy participants. Thus, lethal infections are seldom observed in the skin lesions of patients with psoriasis. The immunoregulatory functions of AMPs in psoriasis have been highlighted in recent decades. AMPs activate keratinocytes and innate immune cells, including neutrophils, macrophages, and DCs, mainly in a pattern recognition receptor (PRR)-dependent manner; this activation leads to neutrophil and macrophage recruitment, neutrophil extracellular trap (NET) formation, and DC maturation.[16] In addition, AMPs modulate adaptive immune responses in psoriasis by directly interacting with T cells as autoantigens.[17] Studies have also reported positive associations between AMP expression and specific symptoms such as itch,[18] severity,[19] and genetic susceptibility[20] of psoriasis. In this review, we summarize the characteristics and pathogenic roles of AMPs in psoriasis [Figure 1] and further discuss their potential as therapeutic agents.
Figure 1.
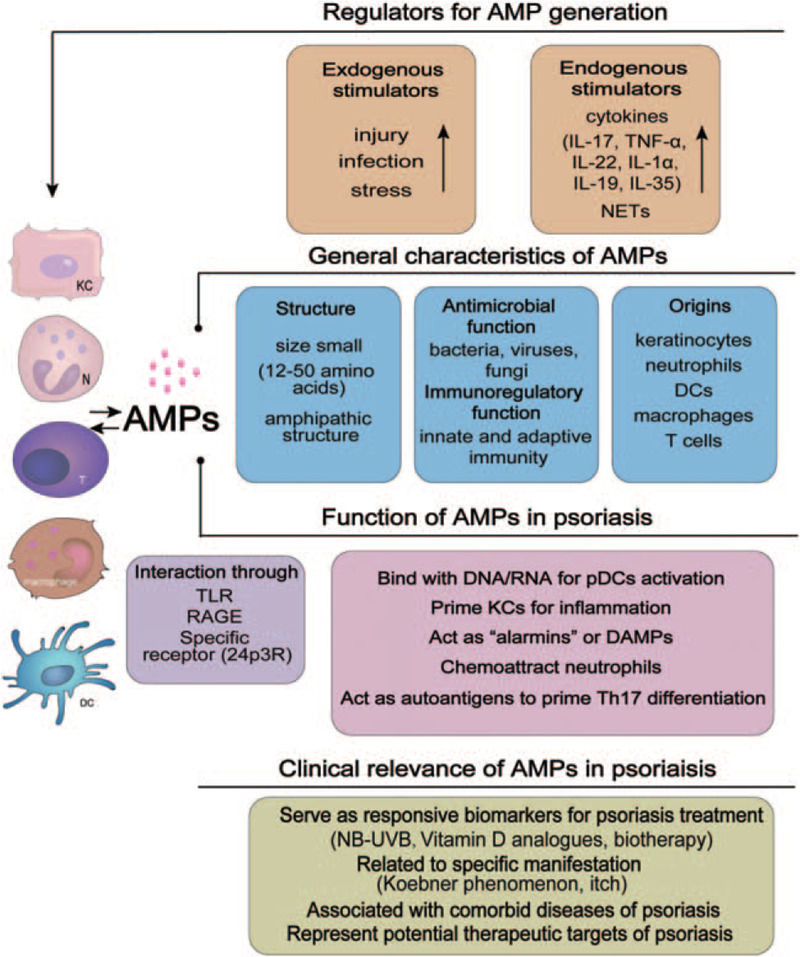
The main characteristics of antimicrobial peptides (AMPs) and their roles in the pathogenesis of psoriasis. AMPs are an integral part of the skin defense generated by keratinocytes and neutrophils. AMPs are induced by exogenous and endogenous stimuli and have antimicrobial functions. AMPs are mostly characterized as small amphipathic cationic peptides. In psoriasis, AMPs regulate both innate and adaptive immune responses. They act as “alarmins” or DAMPs to stimulate plasmacytoid dendritic cells and prime keratinocytes for inflammation through pattern recognition receptors and subsequently initiate the Th17-dominated adaptive immune response. AMPs also have close clinical relevance to psoriasis. DAMPs: Damage-associated molecular patterns; IL: Interleukin; NB-UVB: Narrowband ultraviolet B; NET: Neutrophil extracellular trap; pDCs: Plasmacytoid dendritic cells; RAGE: Receptor for advanced glycation end products; TLR: Toll-like receptor; TNF: Tumor necrosis factor.
LL-37
LL-37 is an α-helical amphipathic oligopeptide (37-monomer peptide) released from human cationic antimicrobial protein-18 and is the only human cathelicidin encoded by the gene CAMP.[21] These characteristics allow LL-37 to form pores on microbial membranes and limit microbial adhesion and proliferation, thus protecting against infections.[22] High LL-37 levels in psoriatic skin lesions have been positively correlated with disease activity.[23] In psoriasis, the expression of LL-37 in keratinocytes can be promoted by cytokines such as IL-17A and tumor necrosis factor (TNF)-α.[24] Given that LL-37 is rapidly induced following skin injury,[25] LL-37 may partially contribute to the Koebner phenomenon in which psoriatic lesions appear after physical trauma.
LL-37 binds and forms complexes with DNA and RNA structures released from injured cells.[26] The classic LL-37–nucleic acid complex in psoriasis has been widely studied and reported to stimulate plasmacytoid dendritic cells (pDCs) in a toll-like receptor (TLR) 9-dependent manner.[27] Stimulated pDCs then secrete a large amount of IFN-α to trigger the activation of myeloid dendritic cells (mDCs) and autoreactive T cells, thus inducing an adaptive immune cascade.[28] Moreover, the LL-37-DNA/RNA complex has been reported to directly activate mDCs[29] and keratinocytes[25] via TLR9/3 signaling. Recently, the source of the nucleic acids that bind to LL-37 has been further elucidated. In a recent study, it has been reported that complexes formed from LL-37 and RNA derived from NETs trigger the release of cytokines and NETs through the TLR8/TLR13 pathway, in which LL-37 is essential.[30] LL-37 can also interact with synthetic RNA oligonucleotides to promote the RNA aptamer internalization by keratinocytes and fibroblasts.[31] However, not all self-DNA can bind to LL-37, and it has been reported that LL-37 can bind to cellular DNA CpG sites but not to mitochondrial DNA.[32] These findings indicate that the structures of DNA and RNA may affect their binding mechanisms with LL-37 and the priming of inflammation. Notably, LL-37 can also bind to bacterial DNA present during chronic infections[33]; this bacterial DNA has not been detected in sterile inflammatory skin diseases. Nonetheless, further investigation that would provide key evidence that skin microbes are involved in psoriasis is warranted.
Apart from the fact that LL-37 forms complexes with nucleic acids, LL-37 also directly regulates keratinocytes and immune cells in psoriasis, for example, LL-37 induces the production of cytokines and chemokines such as IFNs,[25,34] IL-36γ,[35] and C-X-C motif chemokine ligands (CXCLs)[36] in keratinocytes. Other regulatory effects of LL-37 on keratinocytes include the suppression of apoptosis[37] and enhancement of epidermal barrier function[38]; these effects indicate the involvement of LL-37 in psoriasis development. In addition, LL-37, as an autoantigen, directly activates T cells that tend to be involved in the maintenance of the IL-23/Th17 axis.[12] Moreover, patients with moderate-to-severe plaque psoriasis have been reported to develop LL-37-specific CD4+ and/or CD8+ T cells with skin-homing abilities.[17] These autoreactive T cells infiltrate either skin lesions or the blood of patients with psoriasis and produce IFN-γ and Th17 cytokines. Furthermore, it has been reported that the mouse homolog of LL-37 can serve as an autoantigen that promotes psoriasis-associated atherosclerosis.[39] Similarly, LL-37 has been identified as a novel autoantigen in psoriatic arthritis.[40] LL-37 can bind to the PSORS locus HLA-C∗06:02 allele and thereby form a complex that interacts with T cells via the T cell receptor.[20] In addition to T cells, LL-37 also regulates macrophages via TLR9 and induces mast cells to secrete IL-8.[29,41] LL-37 drives monocyte polarization toward the CD14highCD16+ subset in psoriasis guttate.[42] Thus, we speculate that LL-37 may be a key biomolecule that primes the innate or adaptive immune responses in the pathogenesis of psoriasis.
hBDs
Defensins are a group of small cationic polypeptides with potent antimicrobial activity. These polypeptides are usually structured as antiparallel β-sheets with abundant arginine and lysine residues stabilized by disulfide bonds.[43] Defensins are classified into two subfamilies, α- and β-defensins, according to their disulfide bond linkages. To date, six hBDs (hBD-1–6) have been identified, among which hBD-2 and hBD-3 have been extensively studied in psoriasis.[44] hBD-2[45] and hBD-3[46] have been found in most epithelial cells in the skin, respiratory tract, vagina, and gut. In the skin, these factors are expressed in the keratinocytes found in the uppermost layers of the epidermis and can be secreted into the intercellular space.[47,48] Many immune factors such as IL-17A, IL-22, and NETs modulate the expression of hBDs in keratinocytes.[43] hBD-2 is also found in Langerhans cells (LCs), whose changes are associated with skin aging.[49]
hBDs regulate a variety of cell types and facilitate psoriatic inflammation. Both hBD-2 and hBD-3 induce keratinocyte proliferation and the secretion of biomolecules such as IL-6, IL-10, IFN-γ-inducible proteins, monocyte chemoattractant protein-1, macrophage inflammatory protein (MIP)-3, and CC chemokine ligand 5.[50] hBD-3 has also been reported to induce the expression of IL-37,[51] an immunosuppressive cytokine, in human keratinocytes by interacting with CCR6.[52] hBD-3 has also been reported to exert a protective effect and improve the function of epithelial tight-junction barrier.[53] Therefore, it is worthwhile to elucidate the “double-edged sword” effects of hBD-3 in the pathogenesis of psoriasis. Furthermore, hBDs modulate not only keratinocytes but also immune cells. Similar to LL-37, hBD-2 and hBD-3 promote the uptake of self-DNA or CpG DNA by pDCs and enhance the production of IFN-α.[54] hBD-3 also activates mDCs in a TLR1/2-dependent manner.[55] In addition, both hBD-3 and its mouse ortholog murine β-defensin-14 induce the production of IL-23 by epidermal LCs and exacerbate psoriasis-like skin inflammation.[56] Moreover, hBDs promote type I interferon production in macrophages via different pathways such as hBD-2 through CCR2-mediated Nod2 transduction[57] and hBD-3 in a TLR-dependent manner.[58] hBD-3 also increases CD86 expression in monocytes by stimulating the ATP-gated channel P2X7[59]; as the P2X7 receptor is a key modulator of aerobic glycolysis,[60] this finding suggests that hBD-3 may be involved in glycolysis in the pathogenesis of psoriasis. In T cells, hBD-2 has been reported to exhibit a two-way regulatory effect; hBD-2 enhances IFN-γ and IL-10 production but suppresses IL-17 production in T cells by suppressing the transcriptional regulator STAT3.[61] Therefore, we suggest that hBDs may act as autocrine or paracrine signals in the pathogenesis of psoriasis; nonetheless, this requires further investigation.
Clinically, the high genomic copy number of β-defensin genes has been associated with an increased risk of psoriasis.[62,63] Additionally, HBDs can be secreted by commensal bacteria and can affect the homeostasis of the microbiome.[64] Hence, it is possible that dysregulated hBD expression also contributes to psoriatic inflammation in a microbiota-associated manner.
S100 proteins
S100 proteins are a family of calcium-binding molecules with small molecular weights.[65] The activation of S100 proteins, especially S100A8/A9 tetramers, requires Ca2+ binding, protein oligomerization, and the formation of homo- or heterodimers with the help of iron.[66] Many members of this family are encoded in the genes within the psoriasis susceptibility locus on chromosome 1q21; this indicates an association between S100 proteins and psoriasis.[67,68] Among these proteins, S100A4,[69] S100A7,[70,71] S100A8/A9,[72,73] S100A12,[19] and S100A15[71] are highly expressed in both the serum and skin of patients with psoriasis. S100 proteins can be secreted by keratinocytes,[74] neutrophils,[75] monocytes/macrophages,[76] and dermal DCs.[77] These proteins are stress-induced molecules and therefore can be rapidly up-regulated by injury,[78] proinflammatory cytokines such as IL-1α, TNF-α, IL-19, and IL-22,[79–81] or TLR-[82]/receptor for advanced glycation end products (RAGE) induced[83] signaling.
The major role of S100 proteins in psoriasis is their regulation of keratinocytes, for example, the mouse ortholog mS100a7a15 induces the expression of psoriasis-associated cytokines such as IL-1α, IL-23, and MIP-2 in keratinocytes in a RAGE-dependent manner.[67] S100A8/A9 stimulates the expression of IL-8, CXCL1, CXCL2, CCL20,[84] and complement component 3 in keratinocytes to mediate psoriatic inflammation.[85] Additionally, these proteins also promote the excessive proliferation[86] and abnormal differentiation[73,82] of keratinocytes that contribute to the development of hyperkeratosis and parakeratosis in psoriasis. Furthermore, S100 proteins can recruit and activate neutrophils[87,88]; this characteristic of S100 proteins may accelerate neutrophil infiltration in psoriatic skin lesions. Importantly, S100A8 and S100A9 promote the development of autoreactive CD8+ T cells, thereby suggesting that S100A8 and S100A9 are linked to inflammation and autoimmunity.[89] Intriguingly, S100A8 and S100A9 has been shown to inhibit self-activity by forming the (S100A8/S100A9)2 tetramer, which inhibits the binding of S100A8/S100A9 with TLR4/MD2 to restrict sterile inflammation.[90] The loss of this mechanism may free the binding sites from S100A8/S100A9 heterodimers and subsequently activate TLR4/MD2-mediated local inflammation. As this has not yet been fully elucidated, it requires further study.
S100 proteins have been linked to psoriasis-related comorbidities, including psoriatic arthritis, Crohn disease, metabolic syndrome, and cardiovascular disorders. For example, the concentrations of S100A8/S100A9 and S100A12 are markedly elevated in the serum of patients with psoriatic arthritis; S100A8/S100A9 and S100A12 act as potential biomarkers of disease severity.[76,91] Recently, the expression of S100 proteins was reportedly associated with lipid metabolism, which may be involved in metabolic syndrome and cardiovascular disease. Diet-induced obesity has also been demonstrated to induce aberrant S100 protein production and psoriasiform inflammation in clinical and murine model studies.[92–94] Additionally, S100A7/S100A15 and S100A8/S100A9 levels in serum are positively correlated with intima-media thickness[71] and aortic vascular inflammation,[95] respectively, in patients with psoriasis. Interestingly, the activation of the itch-associated genes transient receptor potential vanilloid type 1 (TRPV1)[96] and transient receptor potential ankyrin 1 (TRPA1)[97] positively regulates S100A8/S100A9 expression and promotes psoriatic inflammation, thereby indicating the potential role of S100 proteins in psoriatic pruritus.
LCN2
LCN2, also known as neutrophil gelatinase-associated lipocalin, is a glycoprotein encoded in a gene located at chromosome 9q34.11.[98] LCN2 is supposed to have two receptors, megalin and 24p3R[99]; however, the melanocortin-4 receptor was recently identified as another LCN2 receptor expressed on neurons in the hypothalamus and associated with appetite control.[100] LCN2 was originally identified as a component of neutrophil secondary granules with versatile functions in inflammatory processes.[101] LCN2 has also been regarded as an AMP that inhibits bacterial iron acquisition[102] and as an adipokine involved in obesity, insulin resistance, and metabolic diseases.[98,101] In a previous study, we have demonstrated that LCN2 is secreted by keratinocytes stimulated with IL-17A, IL-22, and TNF-α or by infiltrated neutrophils in psoriatic skin lesions.[15,103] Consistently, other studies have revealed that TNF-α induces LCN2 production in keratinocytes and granulocytes in hidradenitis suppurativa (HS).[104] LCN2 can also originate from CD4+ T cells,[105] macrophages,[106] DCs,[107] adipose tissues,[108] or hepatocytes[109,110] and contribute to systemic or local LCN2 levels.
We and others have found that LCN2 is overexpressed not only in the skin lesions of psoriasis vulgaris,[111] generalized pustular psoriasis, and palmoplantar pustular psoriasis, but also in imiquimod (IMQ)-induced psoriasis-like lesions in mice.[15] We further found that LCN2 induced chemotaxis and cytokine secretion in neutrophils via the 24p3R and downstream p38-MAPK and ERK-1/2 signaling pathways and that the neutralization of LCN2 significantly attenuated disease severity and inflammatory infiltration in IMQ-induced mice.[15,103] Although serum LCN2 levels have not been linked to psoriasis area and severity index, it has been positively linked to the degree of itch in patients with psoriasis.[18,112] Interestingly, recent studies highlight a mechanism by which LCN2 facilitates chronic itch in inflammatory skin diseases as a downstream effector of astrocytic STAT3 signaling, which directly enhances spinal gastrin-releasing peptide-induced scratching.[113,114]
Importantly, LCN2 has been reported to modulate lipid metabolism pathways, and LCN2 deficiency has been reported to protect mice from obesity-induced insulin resistance by inhibiting 12-lipoxygenase and TNF-α levels in adipose tissues.[115,116] Similarly, LCN2 also induces insulin resistance and inhibits autophagy in cardiomyocytes.[117] In view of these studies, LCN2 may contribute to the comorbid diseases of psoriasis such as metabolic syndrome, cardiovascular disease, Crohn disease, and non-alcoholic fatty liver disease.
RNase 7
RNase 7 is a member of the RNase A superfamily and a biomolecule with disulfide bonds; it is encoded on chromosome 14q11.2.[118] It was first identified as an AMP in healthy human skin in 2002[119] as it can protect against infections. In primary keratinocytes in the stratum corneum[119] and in bronchial[120] and kidney[121] epithelial cells, RNase 7 is reportedly abundant. The inflammatory cytokines IL-17A, IFN-γ, and IL-1β are positive regulators of RNase 7 expression.[122,123] Notably, RNase 7 has been reported to be more rapidly induced than hBD-2 and hBD-3 by skin injury with increased levels in skin lesions in both psoriasis and atopic dermatitis (AD).[124] This finding indicates the functions of RNase 7 in the acute phase of inflammation and refutes that the expression of AMPs in AD is decreased.[125] Notably, RNase 7 has been reported to promote the sensing of self-DNA by human pDCs and keratinocytes via TLR regulation[126,127]; this suggests that RNase 7 is another psoriasis trigger similar to LL-37 and hBDs. Interestingly, RNase 7 has been reported to suppress the production of Th2 cytokines (IL-13, IL-4, and IL-5) in human CD4+ T cells, which is independent of its ribonuclease activity.[128] Therefore, the functions of RNase 7, especially its regulatory roles in inflammatory skin disease, remain unclear and require further investigation [Table 1].
Table 1.
The origins and target cells of AMPs in the pathogenesis of psoriasis.
| AMPs | Origins | Target cells | Potential mechanism in the pathogenesis of psoriasis |
| Cathelicidin (LL-37) | Keratinocytes Neutrophils T cells Monocytes NK-cells Mast cells | Keratinocytes pDCs mDCs Monocytes/ macrophages Neutrophils CD4+ T cells CD8+ T cells | Forming complex with DNA/RNA to activate pDCs or mDCs through TLR dependent manner[27–30]; Priming keratinocytes for inflammation[25,34–36]; Serving as an autoantigen directly activating CD4+ and/or CD8+ T cells[17]; Driving monocyte polarization toward the CD14highCD16+ subset.[42] |
| Human β-defensin 2 | Keratinocytes LCs | Keratinocytes pDCs Macrophages | Inducing keratinocyte proliferation and secretion[50]; Promoting the uptake of self-DNA or CpG DNA by pDCs and enhance IFN-α production[54]; Promoting type I IFN production in macrophages.[57] |
| Human β-defensin 3 | Epithelial cells (keratinocytes) | Keratinocytes pDCs mDCs LCs Monocytes/ macrophages | Inducing keratinocyte proliferation and secretion[50]; Promoting the uptake of self-DNA or CpG DNA by pDCs and enhance IFN-α production[54]; Activating mDCs in via TLR1/2[55]; Promoting type I IFN production in macrophages[58]; Increasing CD86 expression on monocytes by stimulating the ATP-gated channel P2X7.[59] |
| Psoriasin (S100A7) | Keratinocytes Monocytes/ macrophages | Keratinocytes Monocytes/ macrophages Neutrophils | Inducing cytokines production of keratinocytes[67]; Chemoattractant.[87] |
| S100A8/A9 | Keratinocytes Monocytes/ macrophages Neutrophils mDCs Endothelial cells | Keratinocytes Neutrophils CD8+ T cells | Priming keratinocytes for inflammation[85]; Promoting excessive proliferation and abnormal differentiation of keratinocytes[73,82,86]; Chemoattracting and activating neutrophils[87,88]; Promoting the development of autoreactive CD8+ T cells[89]; Filling to inhibit self-activity by forming the (S100A8/S100A9)2 tetramer and activating TLR4/MD2-mediated local inflammation.[90] |
| S100A15 | Keratinocytes | Keratinocytes | Inducing cytokines production of keratinocytes.[67] |
| Lipocalin 2 | Neutrophils Keratinocytes mDCs Monocytes/ macrophages Aortic smooth muscle cells Adipose tissue Hepatocytes | Neutrophils | Inducing chemotaxis and cytokine secretion in neutrophils via the 24p3R and downstream p38-MAPK and ERK-1/2 signaling pathways.[15,103] |
| RNase 7 | Keratinocytes | Keratinocytes pDCs | Promoting the sensing of self-DNA by human pDCs and keratinocytes through TLR regulation[126,127] |
AMPs: Antimicrobial peptides; pDCs: Plasmacytoid dendritic cells; mDCs: Myeloid dendritic cells; LCs: Langerhans cells.
Perspectives and discussion
Increasing evidence has indicated that AMPs triggered by local tissue signals are essential for the initiation and development of inflammatory diseases such as psoriasis, rosacea, rheumatic diseases, and HS.[104,129] Here, we summarize the potential roles of AMPs in the pathogenesis of psoriasis. (1) AMPs act as chaperones to DNA/RNA and are recognized by pDCs, thereby triggering the activation of psoriatic inflammation. (2) AMPs are natural innate immune components triggered by local signaling that serve as alarmins or damage-associated molecular patterns and interact with PRRs such as TLRs and RAGE to induce uncontrolled inflammatory cascades in psoriasis. (3) AMPs (LL-37), as autoantigens, activate T cells to induce subsequent adaptive immune responses. This response is, in turn, regulated by cytokines and chemokines and establishes vicious feedback loops to maintain psoriatic inflammation.
Discrepancies in AMP production among inflammatory skin diseases should be studied. Studies have indicated that AMP levels are increased in psoriasis but are decreased in AD.[130,131] In psoriasis, AMPs mainly exert proinflammatory effects, whereas beneficial effects of AMPs have been observed in AD, such as those relating to skin barrier maintenance and Th2 suppression. The distinct frequency of recurrent skin infections between the two diseases has been accepted to be partially due to this discrepancy. However, AMPs and several cytokines have been identified across the spectrum of these two diseases in recent decades. Harder et al[124] reported the overexpression of RNase 7, S100A7, and hBD-2 in skin lesions in patients with AD and psoriasis. Concurringly, increasing evidence shows overlaps in prominent IL-17 components between specific subtypes of AD (pediatric and Asian-origin AD) and psoriasis.[132,133] These results indicate that multiple immune pathways can be observed in one inflammatory milieu and that the imbalance in the interactions between T cell subsets may largely impact skin processes, including AMP production. Hence, continued research is warranted to understand the common and different immune pathways in psoriasis and AD. In recent decades, AMPs were reportedly highly expressed in other autoimmune skin diseases such as systemic lupus erythematosus[134] and HS.[75] It has been reported that S100 protein levels in the serum and urine of patients with systemic lupus erythematosus are higher than those of healthy participants and that the S100 protein may be a potential biomarker for lupus nephritis.[134,135]
Importantly, AMPs have clinical relevance to psoriasis [Figure 1] and may serve as biomarkers during treatment. For example, AMPs can be downregulated by psoriasis treatments, including narrowband ultraviolet B (NB-UVB) therapy and use of antipsoriatic vitamin D analogs.[136–138] Notably, biotherapies targeting the IL-23/IL-17 axis, including secukinumab, risankizumab, and ustekinumab, down-regulate the production of hBD-2 and LCN2 in psoriatic skin lesions.[45] Moreover, tasquinimod (an S100A9 inhibitor) has been successfully used in clinical trials of patients with metastatic castration-resistant prostate cancer, thereby indicating a promising strategy for targeting AMPs for psoriasis treatment.[139]
However, many unsolved problems regarding the roles of AMPs in psoriasis remain. LL-37 prevails during the development of psoriasis and has been identified as an autoantigen that amplifies psoriatic inflammation. Whether other AMPs, including LCN2 and S100 proteins, also have these properties, and the underlying distinction of LL-37 in psoriasis development are worth considering. Moreover, the differing effects of LL-37 in psoriasis cannot be overlooked. Whether the proinflammatory or protective effect of LL-37 is dominant in psoriasis and whether the effects of LL-37 are exerted only in the initial stage or throughout the entire process of psoriasis must be determined. Additionally, it is necessary to determine whether the overexpression of LL-37 is a constitutive factor that triggers the relapse of psoriasis. Furthermore, AMPs interact with PRRs or specific receptors to regulate different immune cells. The interplay among these biomolecules also deserves further investigation. Importantly, whether targeting AMPs is as sufficient as targeting TNF-α, IL-23, or IL-17 antagonists for psoriasis treatment is noteworthy to investigate. The dynamic bioactivity of AMPs warrants further elucidation in the context of the safety of clinical applications.
Conclusions
This review summarizes the roles of AMPs in the pathogenesis of psoriasis [Table 1] and suggests their roles as biomarkers and therapeutic targets in translational medicine. Nonetheless, we should closely observe their antimicrobial properties, which have potent impacts on immune functions. Additional studies that focus on the characteristics and interactions of AMPs may lead to breakthroughs in elucidating the pathogenesis of psoriasis and establishing promising therapeutic interventions.
Funding
This work was supported by a grant from the National Natural Science Foundation of China (No. 81703113).
Conflicts of interest
None.
Footnotes
How to cite this article: Ma JY, Shao S, Wang G. Antimicrobial peptides: bridging innate and adaptive immunity in the pathogenesis of psoriasis. Chin Med J 2020;133:2966–2975. doi: 10.1097/CM9.0000000000001240
References
- 1.Nguyen LT, Haney EF, Vogel HJ. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol 2011; 29:464–472. doi: 10.1016/j.tibtech.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Lehrer RI, Ganz T. Cathelicidins: a family of endogenous antimicrobial peptides. Curr Opin Hematol 2002; 9:18–22. doi: 10.1097/00062752-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Mangoni ML, McDermott AM, Zasloff M. Antimicrobial peptides and wound healing: biological and therapeutic considerations. Exp Dermatol 2016; 25:167–173. doi: 10.1111/exd.12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morizane S, Gallo RL. Antimicrobial peptides in the pathogenesis of psoriasis. J Dermatol 2012; 39:225–230. doi: 10.1111/j.1346-8138.2011.01483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Risso A. Leukocyte antimicrobial peptides: multifunctional effector molecules of innate immunity. J Leukoc Biol 2000; 68:785–792. doi: 10.1189/jlb.68.6.785. [PubMed] [Google Scholar]
- 6.Iannacone M. Platelet-mediated modulation of adaptive immunity. Semin Immunol 2016; 28:555–560. doi: 10.1016/j.smim.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Bierkarre H, Harder J, Cuthbert R, Emery P, Leuschner I, Mrowietz U, et al. Differential expression of antimicrobial peptides in psoriasis and psoriatic arthritis as a novel contributory mechanism for skin and joint disease heterogeneity. Scand J Rheumatol 2016; 45:188–196. doi: 10.3109/03009742.2015.1091497. [DOI] [PubMed] [Google Scholar]
- 8.Coretti L, Natale A, Cuomo M, Florio E, Keller S, Lembo F, et al. The interplay between defensins and microbiota in Crohn's disease. Mediators Inflamm 2017; 2017:8392523.doi: 10.1155/2017/8392523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niyonsaba F, Kiatsurayanon C, Chieosilapatham P, Ogawa H. Friends or Foes? Host defense (antimicrobial) peptides and proteins in human skin diseases. Exp Dermatol 2017; 26:989–998. doi: 10.1111/exd.13314. [DOI] [PubMed] [Google Scholar]
- 10.Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol 2017; 140:645–653. doi: 10.1016/j.jaci.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang WM, Jin HZ. Skin microbiome: an actor in the pathogenesis of psoriasis. Chin Med J 2018; 131:95–98. doi: 10.4103/0366-6999.221269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuentes-Duculan J, Bonifacio KM, Hawkes JE, Kunjravia N, Cueto I, Li X, et al. Autoantigens ADAMTSL5 and LL37 are significantly upregulated in active Psoriasis and localized with keratinocytes, dendritic cells and other leukocytes. Exp Dermatol 2017; 26:1075–1082. doi: 10.1111/exd.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolbinger F, Loesche C, Valentin MA, Jiang X, Cheng Y, Jarvis P, et al. β-Defensin 2 is a responsive biomarker of IL-17A-driven skin pathology in patients with psoriasis. J Allergy Clin Immunol 2017; 139:923–932.e8. doi: 10.1016/j.jaci.2016.06.038. [DOI] [PubMed] [Google Scholar]
- 14.D’Amico F, Skarmoutsou E, Granata M, Trovato C, Rossi GA, Mazzarino MC. S100A7: a rAMPing up AMP molecule in psoriasis. Cytokine Growth Factor Rev 2016; 32:97–104. doi: 10.1016/j.cytogfr.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Shao S, Cao T, Jin L, Li B, Fang H, Zhang J, et al. Increased lipocalin-2 contributes to the pathogenesis of psoriasis by modulating neutrophil chemotaxis and cytokine secretion. J Invest Dermatol 2016; 136:1418–1428. doi: 10.1016/j.jid.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Hwang ST, Nijsten T, Elder JT. Recent highlights in psoriasis research. J Invest Dermatol 2017; 137:550–556. doi: 10.1016/j.jid.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Lande R, Botti E, Jandus C, Dojcinovic D, Fanelli G, Conrad C, et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat Commun 2014; 5:5621.doi: 10.1038/ncomms6621. [DOI] [PubMed] [Google Scholar]
- 18.Aizawa N, Ishiuji Y, Tominaga M, Sakata S, Takahashi N, Yanaba K, et al. Relationship between the degrees of itch and serum lipocalin-2 levels in patients with psoriasis. J Immunol Res 2019; 2019:8171373.doi: 10.1155/2019/8171373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilsmann-Theis D, Wagenpfeil J, Holzinger D, Roth J, Koch S, Schnautz S, et al. Among the S100 proteins, S100A12 is the most significant marker for psoriasis disease activity. J Eur Acad Dermatol Venereol 2016; 30:1165–1170. doi: 10.1111/jdv.13269. [DOI] [PubMed] [Google Scholar]
- 20.Mabuchi T, Hirayama N. Binding affinity and interaction of LL-37 with HLA-C∗06:02 in psoriasis. J Invest Dermatol 2016; 136:1901–1903. doi: 10.1016/j.jid.2016.04.033. [DOI] [PubMed] [Google Scholar]
- 21.Fabisiak A, Murawska N, Fichna J. LL-37: cathelicidin-related antimicrobial peptide with pleiotropic activity. Pharmacol Rep 2016; 68:802–808. doi: 10.1016/j.pharep.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Xhindoli D, Morgera F, Zinth U, Rizzo R, Pacor S, Tossi A. New aspects of the structure and mode of action of the human cathelicidin LL-37 revealed by the intrinsic probe p-cyanophenylalanine. Biochem J 2015; 465:443–457. doi: 10.1042/bj20141016. [DOI] [PubMed] [Google Scholar]
- 23.Hwang YJ, Jung HJ, Kim MJ, Roh NK, Jung JW, Lee YW, et al. Serum levels of LL-37 and inflammatory cytokines in plaque and guttate psoriasis. Mediators Inflamm 2014; 2014:268257.doi: 10.1155/2014/268257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schön MP. Adaptive and innate immunity in psoriasis and other inflammatory disorders. Front Immunol 2019; 10:1764.doi: 10.3389/fimmu.2019.01764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang LJ, Sen GL, Ward NL, Johnston A, Chun K, Chen Y, et al. Antimicrobial peptide LL37 and MAVS signaling drive interferon-β production by epidermal keratinocytes during skin injury. Immunity 2016; 45:119–130. doi: 10.1016/j.immuni.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang LJ. Type1 interferons potential initiating factors linking skin wounds with psoriasis pathogenesis. Front Immunol 2019; 10:1440.doi: 10.3389/fimmu.2019.01440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007; 449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 28.Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med 2005; 202:135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakagawa Y, Gallo RL. Endogenous intracellular cathelicidin enhances TLR9 activation in dendritic cells and macrophages. J Immunol 2015; 194:1274–1284. doi: 10.4049/jimmunol.1402388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herster F, Bittner Z, Archer NK, Dickhöfer S, Eisel D, Eigenbrod T, et al. Neutrophil extracellular trap-associated RNA and LL37 enable self-amplifying inflammation in psoriasis. Nat Commun 2020; 11:105.doi: 10.1038/s41467-019-13756-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macleod T, Ward J, Alase AA, Bridgewood C, Wittmann M, Stonehouse NJ. Antimicrobial peptide LL-37 facilitates intracellular uptake of RNA aptamer Apt 21-2 without inducing an inflammatory or interferon response. Front Immunol 2019; 10:857.doi: 10.3389/fimmu.2019.00857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ries M, Schuster P, Thomann S, Donhauser N, Vollmer J, Schmidt B. Identification of novel oligonucleotides from mitochondrial DNA that spontaneously induce plasmacytoid dendritic cell activation. J Leukoc Biol 2013; 94:123–135. doi: 10.1189/jlb.0612278. [DOI] [PubMed] [Google Scholar]
- 33.Limoli DH, Rockel AB, Host KM, Jha A, Kopp BT, Hollis T, et al. Cationic antimicrobial peptides promote microbial mutagenesis and pathoadaptation in chronic infections. PLoS Pathog 2014; 10:e1004083.doi: 10.1371/journal.ppat.1004083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morizane S, Yamasaki K, Mühleisen B, Kotol PF, Murakami M, Aoyama Y, et al. Cathelicidin antimicrobial peptide LL-37 in psoriasis enables keratinocyte reactivity against TLR9 ligands. J Invest Dermatol 2012; 132:135–143. doi: 10.1038/jid.2011.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li N, Yamasaki K, Saito R, Fukushi-Takahashi S, Shimada-Omori R, Asano M, et al. Alarmin function of cathelicidin antimicrobial peptide LL37 through IL-36γ induction in human epidermal keratinocytes. J Immunol 2014; 193:5140–5148. doi: 10.4049/jimmunol.1302574. [DOI] [PubMed] [Google Scholar]
- 36.Hemshekhar M, Choi KG, Mookherjee N. Host defense peptide LL-37-mediated chemoattractant properties, but not anti-inflammatory cytokine IL-1RA production, is selectively controlled by Cdc42 Rho GTPase via G protein-coupled receptors and JNK mitogen-activated protein kinase. Front Immunol 2018; 9:1871.doi: 10.3389/fimmu.2018.01871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chamorro CI, Weber G, Grönberg A, Pivarcsi A, Ståhle M. The human antimicrobial peptide LL-37 suppresses apoptosis in keratinocytes. J Invest Dermatol 2009; 129:937–944. doi: 10.1038/jid.2008.321. [DOI] [PubMed] [Google Scholar]
- 38.Akiyama T, Niyonsaba F, Kiatsurayanon C, Nguyen TT, Ushio H, Fujimura T, et al. The human cathelicidin LL-37 host defense peptide upregulates tight junction-related proteins and increases human epidermal keratinocyte barrier function. J Innate Immun 2014; 6:739–753. doi: 10.1159/000362789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mihailovic PM, Lio WM, Yano J, Zhao X, Zhou J, Chyu KY, et al. The cathelicidin protein CRAMP is a potential atherosclerosis self-antigen in ApoE(-/-) mice. PloS One 2017; 12:e0187432.doi: 10.1371/journal.pone.0187432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan Y, Qiu J, Lin ZT, Li W, Haley C, Mui UN, et al. Identification of novel autoantibodies associated with psoriatic arthritis. Arthritis Rheumatol 2019; 71:941–951. doi: 10.1002/art.40830. [DOI] [PubMed] [Google Scholar]
- 41.Yu Y, Zhang Y, Zhang Y, Lai Y, Chen W, Xiao Z, et al. LL-37-induced human mast cell activation through G protein-coupled receptor MrgX2. Int Immunopharmacol 2017; 49:6–12. doi: 10.1016/j.intimp.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 42.Qian L, Chen W, Sun W, Li M, Zheng R, Qian Q, et al. Antimicrobial peptide LL-37 along with peptidoglycan drive monocyte polarization toward CD14(high)CD16(+) subset and may play a crucial role in the pathogenesis of psoriasis guttata. Am J Transl Res 2015; 7:1081–1094. [PMC free article] [PubMed] [Google Scholar]
- 43.Fruitwala S, El-Naccache DW, Chang TL. Multifaceted immune functions of human defensins and underlying mechanisms. Semin Cell Dev Biol 2019; 88:163–172. doi: 10.1016/j.semcdb.2018.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang D, Biragyn A, Hoover DM, Lubkowski J, Oppenheim JJ. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu Rev Immunol 2004; 22:181–215. doi: 10.1146/annurev.immunol.22.012703.104603. [DOI] [PubMed] [Google Scholar]
- 45.Visvanathan S, Baum P, Vinisko R, Schmid R, Flack M, Lalovic B, et al. Psoriatic skin molecular and histopathologic profiles after treatment with risankizumab versus ustekinumab. J Allergy Clin Immunol 2019; 143:2158–2169. doi: 10.1016/j.jaci.2018.11.042. [DOI] [PubMed] [Google Scholar]
- 46.Wang F, Zhang X, Xia P, Zhang L, Zhang Z. Enhancement of mRNA expression of survivin and human beta-defensin-3 in lesions of psoriasis vulgaris. Eur J Dermatol 2016; 26:28–33. doi: 10.1684/ejd.2015.2698. [DOI] [PubMed] [Google Scholar]
- 47.Huh WK, Oono T, Shirafuji Y, Akiyama H, Arata J, Sakaguchi M, et al. Dynamic alteration of human beta-defensin 2 localization from cytoplasm to intercellular space in psoriatic skin. J Mol Med 2002; 80:678–684. doi: 10.1007/s00109-002-0373-z. [DOI] [PubMed] [Google Scholar]
- 48.Liu AY, Destoumieux D, Wong AV, Park CH, Valore EV, Liu L, et al. Human beta-defensin-2 production in keratinocytes is regulated by interleukin-1, bacteria, and the state of differentiation. J Invest Dermatol 2002; 118:275–281. doi: 10.1046/j.0022-202x.2001.01651.x. [DOI] [PubMed] [Google Scholar]
- 49.Pilkington SM, Dearman RJ, Kimber I, Griffiths CEM. Langerhans cells express human β-defensin 3: relevance for immunity during skin ageing. Br J Dermatol 2018; 179:1170–1171. doi: 10.1111/bjd.16770. [DOI] [PubMed] [Google Scholar]
- 50.Niyonsaba F, Ushio H, Nakano N, Ng W, Sayama K, Hashimoto K, et al. Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J Invest Dermatol 2007; 127:594–604. doi: 10.1038/sj.jid.5700599. [DOI] [PubMed] [Google Scholar]
- 51.Cavalli G, Dinarello CA. Suppression of inflammation and acquired immunity by IL-37. Immunol Rev 2018; 281:179–190. doi: 10.1111/imr.12605. [DOI] [PubMed] [Google Scholar]
- 52.Smithrithee R, Niyonsaba F, Kiatsurayanon C, Ushio H, Ikeda S, Okumura K, et al. Human β-defensin-3 increases the expression of interleukin-37 through CCR6 in human keratinocytes. J Dermatol Sci 2015; 77:46–53. doi: 10.1016/j.jdermsci.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 53.Kiatsurayanon C, Niyonsaba F, Smithrithee R, Akiyama T, Ushio H, Hara M, et al. Host defense (antimicrobial) peptide, human β-defensin-3, improves the function of the epithelial tight-junction barrier in human keratinocytes. J Invest Dermatol 2014; 134:2163–2173. doi: 10.1038/jid.2014.143. [DOI] [PubMed] [Google Scholar]
- 54.Tewary P, de la Rosa G, Sharma N, Rodriguez LG, Tarasov SG, Howard OM, et al. β-Defensin 2 and 3 promote the uptake of self or CpG DNA, enhance IFN-α production by human plasmacytoid dendritic cells, and promote inflammation. J Immunol 2013; 191:865–874. doi: 10.4049/jimmunol.1201648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Funderburg N, Lederman MM, Feng Z, Drage MG, Jadlowsky J, Harding CV, et al. Human -defensin-3 activates professional antigen-presenting cells via Toll-like receptors 1 and 2. Proc Natl Acad Sci U S A 2007; 104:18631–18635. doi: 10.1073/pnas.0702130104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sweeney CM, Russell SE, Malara A, Kelly G, Hughes R, Tobin AM, et al. Human ß-defensin 3 and its mouse ortholog murine ß-defensin 14 activate langerhans cells and exacerbate psoriasis-like skin inflammation in mice. J Invest Dermatol 2016; 136:723–727. doi: 10.1016/j.jid.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 57.Kim J, Yang YL, Jang YS. Human β-defensin 2 is involved in CCR2-mediated Nod2 signal transduction, leading to activation of the innate immune response in macrophages. Immunobiology 2019; 224:502–510. doi: 10.1016/j.imbio.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Semple F, MacPherson H, Webb S, Kilanowski F, Lettice L, McGlasson SL, et al. Human β-defensin 3 exacerbates MDA5 but suppresses TLR3 responses to the viral molecular pattern mimic polyinosinic:polycytidylic acid. PLoS Genetics 2015; 11:e1005673.doi: 10.1371/journal.pgen.1005673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lioi AB, Ferrari BM, Dubyak GR, Weinberg A, Sieg SF. Human β defensin-3 increases CD86 expression on monocytes by activating the ATP-gated channel P2X7. J Immunol 2015; 195:4438–4445. doi: 10.4049/jimmunol.1401319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amoroso F, Falzoni S, Adinolfi E, Ferrari D, Di Virgilio F. The P2X7 receptor is a key modulator of aerobic glycolysis. Cell Death Dis 2012; 3:e370.doi: 10.1038/cddis.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kanda N, Kamata M, Tada Y, Ishikawa T, Sato S, Watanabe S. Human β-defensin-2 enhances IFN-γ and IL-10 production and suppresses IL-17 production in T cells. J Leukoc Biol 2011; 89:935–944. doi: 10.1189/jlb.0111004. [DOI] [PubMed] [Google Scholar]
- 62.Hollox EJ, Huffmeier U, Zeeuwen PL, Palla R, Lascorz J, Rodijk-Olthuis D, et al. Psoriasis is associated with increased beta-defensin genomic copy number. Nat Genet 2008; 40:23–25. doi: 10.1038/ng.2007.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stuart PE, Hüffmeier U, Nair RP, Palla R, Tejasvi T, Schalkwijk J, et al. Association of β-defensin copy number and psoriasis in three cohorts of European origin. J Invest Dermatol 2012; 132:2407–2413. doi: 10.1038/jid.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meade KG, O’Farrelly C. β-Defensins: farming the microbiome for homeostasis and health. Front Immunol 2018; 9:3072.doi: 10.3389/fimmu.2018.03072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Holzinger D, Tenbrock K, Roth J. Alarmins of the S100-family in juvenile autoimmune and auto-inflammatory diseases. Front Immunol 2019; 10:182.doi: 10.3389/fimmu.2019.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chazin WJ. Relating form and function of EF-hand calcium binding proteins. Acc Chem Res 2011; 44:171–179. doi: 10.1021/ar100110d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wolf R, Mascia F, Dharamsi A, Howard OM, Cataisson C, Bliskovski V, et al. Gene from a psoriasis susceptibility locus primes the skin for inflammation. Sci Transl Med 2010; 2:61ra90.doi: 10.1126/scitranslmed.3001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kypriotou M, Huber M, Hohl D. The human epidermal differentiation complex: cornified envelope precursors, S100 proteins and the ’fused genes’ family. Exp Dermatol 2012; 21:643–649. doi: 10.1111/j.1600-0625.2012.01472.x. [DOI] [PubMed] [Google Scholar]
- 69.Zibert JR, Skov L, Thyssen JP, Jacobsen GK, Grigorian M. Significance of the S100A4 protein in psoriasis. J Invest Dermatol 2010; 130:150–160. doi: 10.1038/jid.2009.206. [DOI] [PubMed] [Google Scholar]
- 70.Ekman AK, Vegfors J, Eding CB, Enerbäck C. Overexpression of psoriasin (S100A7) contributes to dysregulated differentiation in psoriasis. Acta Derm Venereol 2017; 97:441–448. doi: 10.2340/00015555-2596. [DOI] [PubMed] [Google Scholar]
- 71.Awad SM, Attallah DA, Salama RH, Mahran AM, Abu El-Hamed E. Serum levels of psoriasin (S100A7) and koebnerisin (S100A15) as potential markers of atherosclerosis in patients with psoriasis. Clin Exp Dermatol 2018; 43:262–267. doi: 10.1111/ced.13370. [DOI] [PubMed] [Google Scholar]
- 72.Lee Y, Jang S, Min JK, Lee K, Sohn KC, Lim JS, et al. S100A8 and S100A9 are messengers in the crosstalk between epidermis and dermis modulating a psoriatic milieu in human skin. Biochem Biophys Res Commun 2012; 423:647–653. doi: 10.1016/j.bbrc.2012.05.162. [DOI] [PubMed] [Google Scholar]
- 73.Benoit S, Toksoy A, Ahlmann M, Schmidt M, Sunderkötter C, Foell D, et al. Elevated serum levels of calcium-binding S100 proteins A8 and A9 reflect disease activity and abnormal differentiation of keratinocytes in psoriasis. Br J Dermatol 2006; 155:62–66. doi: 10.1111/j.1365-2133.2006.07198.x. [DOI] [PubMed] [Google Scholar]
- 74.Qiao M, Li R, Zhao X, Yan J, Sun Q. Up-regulated lncRNA-MSX2P1 promotes the growth of IL-22-stimulated keratinocytes by inhibiting miR-6731-5p and activating S100A7. Exp Cell Res 2018; 363:243–254. doi: 10.1016/j.yexcr.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 75.Lima AL, Karl I, Giner T, Poppe H, Schmidt M, Presser D, et al. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br J Dermatol 2016; 174:514–521. doi: 10.1111/bjd.14214. [DOI] [PubMed] [Google Scholar]
- 76.Aochi S, Tsuji K, Sakaguchi M, Huh N, Tsuda T, Yamanishi K, et al. Markedly elevated serum levels of calcium-binding S100A8/A9 proteins in psoriatic arthritis are due to activated monocytes/macrophages. J Am Acad Dermatol 2011; 64:879–887. doi: 10.1016/j.jaad.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 77.Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, Johnson-Huang LM, Nograles KE, White TR, et al. Identification of TNF-related apoptosis-inducing ligand and other molecules that distinguish inflammatory from resident dendritic cells in patients with psoriasis. J Allergy Clin Immunol 2010; 125:1261–1268.e9. doi: 10.1016/j.jaci.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Taverna D, Pollins AC, Sindona G, Caprioli RM, Nanney LB. Imaging mass spectrometry for assessing cutaneous wound healing: analysis of pressure ulcers. J Proteome Res 2015; 14:986–996. doi: 10.1021/pr5010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guilloteau K, Paris I, Pedretti N, Boniface K, Juchaux F, Huguier V, et al. Skin inflammation induced by the synergistic action of IL-17A, IL-22, oncostatin M, IL-1{alpha}, and TNF-{alpha} recapitulates some features of psoriasis. J Immunol 2010; 184:5263–5270. doi: 10.4049/jimmunol.0902464. [DOI] [PubMed] [Google Scholar]
- 80.Witte E, Kokolakis G, Witte K, Philipp S, Doecke WD, Babel N, et al. IL-19 is a component of the pathogenetic IL-23/IL-17 cascade in psoriasis. J Invest Dermatol 2014; 134:2757–2767. doi: 10.1038/jid.2014.308. [DOI] [PubMed] [Google Scholar]
- 81.Muromoto R, Hirao T, Tawa K, Hirashima K, Kon S, Kitai Y, et al. IL-17A plays a central role in the expression of psoriasis signature genes through the induction of IκB-ζ in keratinocytes. Int Immunol 2016; 28:443–452. doi: 10.1093/intimm/dxw011. [DOI] [PubMed] [Google Scholar]
- 82.Lei H, Wang Y, Zhang T, Chang L, Wu Y, Lai Y. TLR3 activation induces S100A7 to regulate keratinocyte differentiation after skin injury. Sci China Life Sci 2017; 60:158–167. doi: 10.1007/s11427-016-0027-2. [DOI] [PubMed] [Google Scholar]
- 83.Sakaguchi M, Murata H, Aoyama Y, Hibino T, Putranto EW, Ruma IM, et al. DNAX-activating protein 10 (DAP10) membrane adaptor associates with receptor for advanced glycation end products (RAGE) and modulates the RAGE-triggered signaling pathway in human keratinocytes. J Biol Chem 2014; 289:23389–23402. doi: 10.1074/jbc.M114.573071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nukui T, Ehama R, Sakaguchi M, Sonegawa H, Katagiri C, Hibino T, et al. S100A8/A9, a key mediator for positive feedback growth stimulation of normal human keratinocytes. J Cell Biochem 2008; 104:453–464. doi: 10.1002/jcb.21639. [DOI] [PubMed] [Google Scholar]
- 85.Schonthaler HB, Guinea-Viniegra J, Wculek SK, Ruppen I, Ximénez-Embún P, Guío-Carrión A, et al. S100A8-S100A9 protein complex mediates psoriasis by regulating the expression of complement factor C3. Immunity 2013; 39:1171–1181. doi: 10.1016/j.immuni.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 86.Granata M, Skarmoutsou E, Gangemi P, Mazzarino MC, D’Amico F. S100A7, Jab1, and p27(kip1) expression in psoriasis and S100A7 CRISPR-activated human keratinocyte cell line. J Cell Biochem 2019; 120:3384–3392. doi: 10.1002/jcb.27609. [DOI] [PubMed] [Google Scholar]
- 87.Zheng Y, Niyonsaba F, Ushio H, Ikeda S, Nagaoka I, Okumura K, et al. Microbicidal protein psoriasin is a multifunctional modulator of neutrophil activation. Immunology 2008; 124:357–367. doi: 10.1111/j.1365-2567.2007.02782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ryckman C, Vandal K, Rouleau P, Talbot M, Tessier PA. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J Immunol 2003; 170:3233–3242. doi: 10.4049/jimmunol.170.6.3233. [DOI] [PubMed] [Google Scholar]
- 89.Loser K, Vogl T, Voskort M, Lueken A, Kupas V, Nacken W, et al. The toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells. Nat Med 2010; 16:713–717. doi: 10.1038/nm.2150. [DOI] [PubMed] [Google Scholar]
- 90.Vogl T, Stratis A, Wixler V, Völler T, Thurainayagam S, Jorch SK, et al. Autoinhibitory regulation of S100A8/S100A9 alarmin activity locally restricts sterile inflammation. J Clin Investig 2018; 128:1852–1866. doi: 10.1172/jci89867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ademowo OS, Hernandez B, Collins E, Rooney C, Fearon U, van Kuijk AW, et al. Discovery and confirmation of a protein biomarker panel with potential to predict response to biological therapy in psoriatic arthritis. Ann Rheum Dis 2016; 75:234–241. doi: 10.1136/annrheumdis-2014-205417. [DOI] [PubMed] [Google Scholar]
- 92.Yu S, Wu X, Shi Z, Huynh M, Jena PK, Sheng L, et al. Diet-induced obesity exacerbates imiquimod-mediated psoriasiform dermatitis in anti-PD-1 antibody-treated mice: Implications for patients being treated with checkpoint inhibitors for cancer. J Dermatol Sci 2020; 97:194–200. doi: 10.1016/j.jdermsci.2020.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jena PK, Sheng L, McNeil K, Chau TQ, Yu S, Kiuru M, et al. Long-term Western diet intake leads to dysregulated bile acid signaling and dermatitis with Th2 and Th17 pathway features in mice. J Dermatol Sci 2019; 95:13–20. doi: 10.1016/j.jdermsci.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Salama RH, Al-Shobaili HA, Al Robaee AA, Alzolibani AA. Psoriasin: a novel marker linked obesity with psoriasis. Dis Markers 2013; 34:33–39. doi: 10.3233/dma-2012-120945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Naik HB, Natarajan B, Stansky E, Ahlman MA, Teague H, Salahuddin T, et al. Severity of psoriasis associates with aortic vascular inflammation detected by FDG PET/CT and neutrophil activation in a prospective observational study. Arterioscler Thromb Vasc Biol 2015; 35:2667–2676. doi: 10.1161/atvbaha.115.306460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhou Y, Follansbee T, Wu X, Han D, Yu S, Domocos DT, et al. TRPV1 mediates inflammation and hyperplasia in imiquimod (IMQ)-induced psoriasiform dermatitis (PsD) in mice. J Dermatol Sci 2018; 92:264–271. doi: 10.1016/j.jdermsci.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou Y, Han D, Follansbee T, Wu X, Yu S, Wang B, et al. Transient receptor potential ankyrin 1 (TRPA1) positively regulates imiquimod-induced, psoriasiform dermal inflammation in mice. J Cell Mol Med 2019; 23:4819–4828. doi: 10.1111/jcmm.14392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Francisco V, Ruiz-Fernández C, Pino J, Mera A, González-Gay MA, Gómez R, et al. Adipokines: linking metabolic syndrome, the immune system, and arthritic diseases. Biochem Pharmacol 2019; 165:196–206. doi: 10.1016/j.bcp.2019.03.030. [DOI] [PubMed] [Google Scholar]
- 99.Villalvilla A, García-Martín A, Largo R, Gualillo O, Herrero-Beaumont G, Gómez R. The adipokine lipocalin-2 in the context of the osteoarthritic osteochondral junction. Sci Rep 2016; 6:29243.doi: 10.1038/srep29243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mosialou I, Shikhel S, Liu JM, Maurizi A, Luo N, He Z, et al. MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature 2017; 543:385–390. doi: 10.1038/nature21697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moschen AR, Adolph TE, Gerner RR, Wieser V, Tilg H. Lipocalin-2: a master mediator of intestinal and metabolic inflammation. Trends Endocrinol Metab 2017; 28:388–397. doi: 10.1016/j.tem.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 102.Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell 2002; 10:1033–1043. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- 103.Shao S, Fang H, Dang E, Xue K, Zhang J, Li B, et al. Neutrophil extracellular traps promote inflammatory responses in psoriasis via activating epidermal TLR4/IL-36R crosstalk. Front Immunol 2019; 10:746.doi: 10.3389/fimmu.2019.00746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wolk K, Wenzel J, Tsaousi A, Witte-Händel E, Babel N, Zelenak C, et al. Lipocalin-2 is expressed by activated granulocytes and keratinocytes in affected skin and reflects disease activity in acne inversa/hidradenitis suppurativa. Br J Dermatol 2017; 177:1385–1393. doi: 10.1111/bjd.15424. [DOI] [PubMed] [Google Scholar]
- 105.Lee SA, Noel S, Kurzhagen JT, Sadasivam M, Pierorazio PM, Arend LJ, et al. CD4(+) T cell-derived NGAL modifies the outcome of ischemic acute kidney injury. J Immunol 2020; 204:586–595. doi: 10.4049/jimmunol.1900677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eilenberg W, Stojkovic S, Piechota-Polanczyk A, Kaun C, Rauscher S, Gröger M, et al. Neutrophil gelatinase-associated lipocalin (NGAL) is associated with symptomatic carotid atherosclerosis and drives pro-inflammatory state in vitro. Eur J Vasc Endovasc Surg 2016; 51:623–631. doi: 10.1016/j.ejvs.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 107.Floderer M, Prchal-Murphy M, Vizzardelli C. Dendritic cell-secreted lipocalin2 induces CD8+ T-cell apoptosis, contributes to T-cell priming and leads to a TH1 phenotype. PloS One 2014; 9:e101881.doi: 10.1371/journal.pone.0101881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sun WY, Bai B, Luo C, Yang K, Li D, Wu D, et al. Lipocalin-2 derived from adipose tissue mediates aldosterone-induced renal injury. JCI Insight 2018; 3:e120196.doi: 10.1172/jci.insight.120196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wieser V, Tymoszuk P, Adolph TE, Grander C, Grabherr F, Enrich B, et al. Lipocalin 2 drives neutrophilic inflammation in alcoholic liver disease. J Hepatol 2016; 64:872–880. doi: 10.1016/j.jhep.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 110.Xu MJ, Feng D, Wu H, Wang H, Chan Y, Kolls J, et al. Liver is the major source of elevated serum lipocalin-2 levels after bacterial infection or partial hepatectomy: a critical role for IL-6/STAT3. Hepatology 2015; 61:692–702. doi: 10.1002/hep.27447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.El-Hadidi H, Samir N, Shaker OG, Otb S. Estimation of tissue and serum lipocalin-2 in psoriasis vulgaris and its relation to metabolic syndrome. Arch Dermatol Res 2014; 306:239–245. doi: 10.1007/s00403-013-1414-x. [DOI] [PubMed] [Google Scholar]
- 112.Abdel Hay R, Samir N, Safwat M, Rashed L, Soliman M. Tissue lipocalin-2 in psoriasis: is it a marker of metabolic disturbance or a possible marker of therapeutic efficacy after narrow band ultraviolet B? J Dermatolog Treat 2020; 31:519–523. doi: 10.1080/09546634.2019.1605141. [DOI] [PubMed] [Google Scholar]
- 113.Shiratori-Hayashi M, Koga K, Tozaki-Saitoh H, Kohro Y, Toyonaga H, Yamaguchi C, et al. STAT3-dependent reactive astrogliosis in the spinal dorsal horn underlies chronic itch. Nat Med 2015; 21:927–931. doi: 10.1038/nm.3912. [DOI] [PubMed] [Google Scholar]
- 114.Koga K, Yamagata R, Kohno K, Yamane T, Shiratori-Hayashi M, Kohro Y, et al. Sensitization of spinal itch transmission neurons in a mouse model of chronic itch requires an astrocytic factor. J Allergy Clin Immunol 2020; 145:183–191.e10. doi: 10.1016/j.jaci.2019.09.034. [DOI] [PubMed] [Google Scholar]
- 115.Law IK, Xu A, Lam KS, Berger T, Mak TW, Vanhoutte PM, et al. Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes 2010; 59:872–882. doi: 10.2337/db09-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guo H, Jin D, Zhang Y, Wright W, Bazuine M, Brockman DA, et al. Lipocalin-2 deficiency impairs thermogenesis and potentiates diet-induced insulin resistance in mice. Diabetes 2010; 59:1376–1385. doi: 10.2337/db09-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chan YK, Sung HK, Jahng JW, Kim GH, Han M, Sweeney G. Lipocalin-2 inhibits autophagy and induces insulin resistance in H9c2 cells. Mol Cellu Endocrinol 2016; 430:68–76. doi: 10.1016/j.mce.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 118.Rosenberg HF. RNase A ribonucleases and host defense: an evolving story. J Leukoc Biol 2008; 83:1079–1087. doi: 10.1189/jlb.1107725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Harder J, Schroder JM. RNase 7, a novel innate immune defense antimicrobial protein of healthy human skin. J Biol Chem 2002; 277:46779–46784. doi: 10.1074/jbc.M207587200. [DOI] [PubMed] [Google Scholar]
- 120.Amatngalim GD, van Wijck Y, de Mooij-Eijk Y, Verhoosel RM, Harder J, Lekkerkerker AN, et al. Basal cells contribute to innate immunity of the airway epithelium through production of the antimicrobial protein RNase 7. J Immunol 2015; 194:3340–3350. doi: 10.4049/jimmunol.1402169. [DOI] [PubMed] [Google Scholar]
- 121.Becknell B, Ching C, Spencer JD. The responses of the ribonuclease A superfamily to urinary tract infection. Front Immunol 2019; 10:2786.doi: 10.3389/fimmu.2019.02786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Simanski M, Rademacher F, Schröder L, Schumacher HM, Gläser R, Harder J. IL-17A and IFN-γ synergistically induce RNase 7 expression via STAT3 in primary keratinocytes. PloS One 2013; 8:e59531.doi: 10.1371/journal.pone.0059531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mohammed I, Yeung A, Abedin A, Hopkinson A, Dua HS. Signalling pathways involved in ribonuclease-7 expression. Cell Mol Life Sci 2011; 68:1941–1952. doi: 10.1007/s00018-010-0540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Harder J, Dressel S, Wittersheim M, Cordes J, Meyer-Hoffert U, Mrowietz U, et al. Enhanced expression and secretion of antimicrobial peptides in atopic dermatitis and after superficial skin injury. J Invest Dermatol 2010; 130:1355–1364. doi: 10.1038/jid.2009.432. [DOI] [PubMed] [Google Scholar]
- 125.Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med 2017; 9:eaah4680.doi: 10.1126/scitranslmed.aah4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kopfnagel V, Wagenknecht S, Harder J, Hofmann K, Kleine M, Buch A, et al. RNase 7 strongly promotes TLR9-mediated DNA sensing by human plasmacytoid dendritic cells. J Invest Dermatol 2018; 138:872–881. doi: 10.1016/j.jid.2017.09.052. [DOI] [PubMed] [Google Scholar]
- 127.Kopfnagel V, Dreyer S, Baumert K, Stark M, Harder J, Hofmann K, et al. RNase 7 promotes sensing of self-DNA by human keratinocytes and activates an antiviral immune response. J Invest Dermatol 2020; 140:1589–1598.e3. doi: 10.1016/j.jid.2019.09.029. [DOI] [PubMed] [Google Scholar]
- 128.Kopfnagel V, Wagenknecht S, Brand L, Zeitvogel J, Harder J, Hofmann K, et al. RNase 7 downregulates TH2 cytokine production by activated human T cells. Allergy 2017; 72:1694–1703. doi: 10.1111/all.13173. [DOI] [PubMed] [Google Scholar]
- 129.Umnyakova ES, Zharkova MS, Berlov MN, Shamova OV, Kokryakov VN. Human antimicrobial peptides in autoimmunity. Autoimmunity 2020; 53:137–147. doi: 10.1080/08916934.2020.1711517. [DOI] [PubMed] [Google Scholar]
- 130.Clausen ML, Slotved HC, Krogfelt KA, Andersen PS, Agner T. In vivo expression of antimicrobial peptides in atopic dermatitis. Exp Dermatol 2016; 25:3–9. doi: 10.1111/exd.12831. [DOI] [PubMed] [Google Scholar]
- 131.Ogawa E, Sato Y, Minagawa A, Okuyama R. Pathogenesis of psoriasis and development of treatment. J Dermatol 2018; 45:264–272. doi: 10.1111/1346-8138.14139. [DOI] [PubMed] [Google Scholar]
- 132.Esaki H, Brunner PM, Renert-Yuval Y, Czarnowicki T, Huynh T, Tran G, et al. Early-onset pediatric atopic dermatitis is T(H)2 but also T(H)17 polarized in skin. J Allergy Clin Immunol 2016; 138:1639–1651. doi: 10.1016/j.jaci.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 133.Guttman-Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol 2017; 48:68–73. doi: 10.1016/j.coi.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 134.Donohue SJ, Midgley A, Davies JC, Wright RD, Bruce I, Beresford MW, et al. Differential analysis of serum and urine S100 proteins in juvenile-onset systemic lupus erythematosus (jSLE). Clin Immunol 2020; 214:108375.doi: 10.1016/j.clim.2020.108375. [DOI] [PubMed] [Google Scholar]
- 135.Turnier JL, Fall N, Thornton S, Witte D, Bennett MR, Appenzeller S, et al. Urine S100 proteins as potential biomarkers of lupus nephritis activity. Arthritis Res Ther 2017; 19:242.doi: 10.1186/s13075-017-1444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vähävihu K, Ala-Houhala M, Peric M, Karisola P, Kautiainen H, Hasan T, et al. Narrowband ultraviolet B treatment improves vitamin D balance and alters antimicrobial peptide expression in skin lesions of psoriasis and atopic dermatitis. Br J Dermatol 2010; 163:321–328. doi: 10.1111/j.1365-2133.2010.09767.x. [DOI] [PubMed] [Google Scholar]
- 137.Abdel Hay R, Samir N, Safwat M, Rashed L, Soliman M. Tissue lipocalin-2 in psoriasis: is it a marker of metabolic disturbance or a possible marker of therapeutic efficacy after narrow band ultraviolet B? J Dermatol Treat 2020; 31:519–523. doi: 10.1080/09546634.2019.1605141. [DOI] [PubMed] [Google Scholar]
- 138.Hegyi Z, Zwicker S, Bureik D, Peric M, Koglin S, Batycka-Baran A, et al. Vitamin D analog calcipotriol suppresses the Th17 cytokine-induced proinflammatory S100 “alarmins” psoriasin (S100A7) and koebnerisin (S100A15) in psoriasis. J Invest Dermatol 2012; 132:1416–1424. doi: 10.1038/jid.2011.486. [DOI] [PubMed] [Google Scholar]
- 139.Armstrong AJ, Häggman M, Stadler WM, Gingrich JR, Assikis V, Polikoff J, et al. Long-term survival and biomarker correlates of tasquinimod efficacy in a multicenter randomized study of men with minimally symptomatic metastatic castration-resistant prostate cancer. Clin Cancer Res 2013; 19:6891–6901. doi: 10.1158/1078-0432.Ccr-13-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]


