Abstract
Background
Psoriasis is a common chronic inflammatory skin disease with 2% to 3% prevalence worldwide and a heavy social-psychological burden for patients and their families. As the exact pathogenesis of psoriasis is still unknown, the current treatment is far from satisfactory. Thus, there is an urgent need to find a more effective therapy for this disease. Keratin 17 (K17), a type I intermediate filament, is overexpressed in the psoriatic epidermis and plays a critical pathogenic role by stimulating T cells in psoriasis. Therefore, we hypothesized that inhibiting K17 may be a potential therapeutic approach for psoriasis. This study aimed to investigate the therapeutic effect of K17-specific small interfering RNA (siRNA) on mice with imiquimod (IMQ)-induced psoriasis-like dermatitis.
Methods
Eight-week-old female BALB/c mice were administered a 5% IMQ cream on both ears to produce psoriatic dermatitis. On day 3, K17 siRNA was mixed with an emulsion matrix and applied topically to the left ears of the mice after IMQ application every day for 7 days. The right ears of the mice were treated in parallel with negative control (NC) siRNA. Inflammation was evaluated by gross ear thickness, histopathology, the infiltration of inflammatory cells (CD3+ T cells and neutrophils) using immunofluorescence, and the expression of cytokine production using real-time quantitative polymerase chain reaction. The obtained data were statistically evaluated by unpaired t-tests and a one-way analysis of variance.
Results
The severity of IMQ-induced dermatitis on K17 siRNA-treated mice ears was significantly lower than that on NC siRNA-treated mice ears, as evidenced by the alleviated ear inflammation phenotype, including decreased ear thickness, infiltration of inflammatory cells (CD3+ T cells and neutrophils), and inflammatory cytokine/chemokine expression levels (interleukin 17 [IL-17], IL-22, IL-23, C-X-C motif chemokine ligand 1, and C-C motif chemokine ligand 20) (P < 0.05 vs. the Blank or NC siRNA groups). Compared to the NC siRNA treatment, the K17 siRNA treatment resulted in increased K1 and K10 expression, which are characteristic of keratinocyte differentiation (vs. NC siRNA, K17 siRNA1 group: K1, t = 4.782, P = 0.0050; K10, t = 3.365, P = 0.0120; K17 siRNA2 group: K1, t = 4.104, P = 0.0093; K10, t = 4.168, P = 0.0042; siRNA Mix group: K1, t = 3.065, P = 0.0221; K10, t = 10.83, P < 0.0001), and decreased K16 expression, which is characteristic of keratinocyte proliferation (vs. NC siRNA, K17 siRNA1 group: t = 4.156, P = 0.0043; K17 siRNA2 group: t = 2.834, P = 0.0253; siRNA Mix group: t = 2.734, P = 0.0250).
Conclusions
Inhibition of K17 expression by its specific siRNA significantly alleviated inflammation in mice with IMQ-induced psoriasis-like dermatitis. Thus, gene therapy targeting K17 may be a potential treatment approach for psoriasis.
Keywords: Psoriasis, Keratin 17, Small interfering RNA, Imiquimod, Inflammation
Introduction
Psoriasis is a common chronic autoinflammatory skin disease characterized by demarcated, red, and scaly plaques. It is widely believed that genetic and environmental factors work together to cause hyperproliferation and dysregulated differentiation of keratinocytes (parakeratosis), which leads to thickening of the epidermis (acanthosis).[1] Both innate and adaptive immune systems are involved in the dysregulated immune responses of psoriasis.[2] The inflammatory cells that infiltrate the lesions primarily consist of dendritic cells, T cells, and neutrophils.[2,3] As the etiology of psoriasis is still unclear, the current treatment is far from satisfactory, and there is an urgent need to identify a more effective therapy for the disease.
Keratin 17 (K17) is a type I intermediate filament that provides mechanical support for keratinocytes to maintain the functional integrity of the epidermis.[4,5] K17 expression is nearly undetectable in the normal epidermis but overexpressed in hyperproliferative states, such as psoriasis and wound healing.[6–8] Recent studies have reported that K17 is far more than a cytoskeletal protein, noting its involvement in various diseases from dermatoses to cancer.[9] Our research group has focused on the role of K17 in psoriasis for nearly two decades and determined that K17 plays an important role in the pathogenesis of psoriasis.[10,11] K17 has epitopes similar to those of the M6 Streptococcus protein in order to induce T cells to produce interferon gamma (IFN-γ) in psoriatic patients.[12] Under stress, keratinocytes are damaged or activated, and K17 epitopes are recognized and presented by dendritic cells or activated keratinocytes. The activated dendritic cells or keratinocytes containing K17 epitopes then induce T-cell proliferation as well as IFN-γ, interleukin 17A (IL-17A), and IL-22 production.[12–15] These cytokines in turn act on the activated keratinocytes and upregulate K17 gene expression. All of these factors constitute a K17/T cell/cytokine autoimmune loop that is involved in the pathogenesis of psoriasis.[12] Moreover, we found that K17 is the therapeutic target of several common drugs and strategies for treating psoriasis, including paeoniflorin, salvianolic acid B, and narrowband ultraviolet B irradiation.[16–18] Collectively, previous studies have fully elucidated that K17 plays a critical role in the pathogenesis of psoriasis, and targeting K17 may serve as a potential treatment mechanism for the disease. However, few studies have discussed treating psoriasis by directly inhibiting K17 expression via gene therapy.
In the present study, we utilized BALB/c mice to establish imiquimod (IMQ)-induced psoriasis-like dermatitis and then evaluated the efficacy of K17 small interfering RNA (siRNA) in the treatment of psoriasis. The results indicated that, compared to the mice treated with negative control (NC) siRNA, the K17 siRNA application significantly alleviated IMQ-induced psoriatic dermatitis, as evidenced by decreased ear symptoms and inflammatory infiltration. Moreover, keratinocyte differentiation was improved while proliferation was inhibited after applying K17 siRNA. Our study demonstrated that K17 siRNA may be an effective treatment mechanism for psoriasis.
Methods
Synthesis of siRNAs
The siRNAs specific for mouse K17 were designed and chemically synthesized by GenePharma (Shanghai, China). The sequences were as follows: K17 siRNA1, sense 5′-GGGAGCAACAGUUAUUCCATT-3′, antisense 5′-UGGAAUAACUGUUGCUCCCTT-3′; K17 siRNA2, sense 5′-CUACAGCGCUUAUUACCAUTT-3′, antisense 5′-AUGGUAAUAAGCGCUGUAGTT-3′. The sequences for the scrambled NC siRNA were sense 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense 5′-ACGUGACACGUUCGGAGAATT-3′.
Mice and treatment
Eight-week-old female BALB/c mice were purchased from the Department of Laboratory Animal Medicine of Fourth Military Medical University (Xi’an, China). All experimental procedures involving mice were performed in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Review Committee for the Use of Animals of Fourth Military Medical University. For the generation of psoriatic dermatitis in mice, we applied a 5% IMQ cream (Aldara; 3M Health Care Ltd., Loughborough, UK) topically on both ears of the mice. To evaluate the effect of K17 inhibition on the development of psoriasis, 2.5 nmol K17 siRNA was mixed with 2 μL Lipofectamine 3000 (Invitrogen, Waltham, MA, USA) and 5 mmol/L emulsion matrix and applied topically to the left ear of the mice every day for 7 days after applying IMQ. The right ear of the mice was treated with NC siRNA.
Ear thickness measurement and histopathological analysis
Ear thickness was measured with a microcaliper (Type 0–12.7; YUEQING JINGCHENG, China) at a fixed location 3 mm from the edge of the ear, and an average numerical reading was recorded. On day 11, the ears of IMQ-treated mice were cut off after euthanization with CO2, fixed in 10% paraformaldehyde for 8 to 12 h, and embedded in paraffin. Six-micrometer-thick sections were prepared and stained with hematoxylin and eosin (H&E). The H&E-stained sections were scanned using the Hamamatsu Digital Pathology System, and epidermal thickness was measured using NDP.view software (Hamamatsu Photonics, Hamamatsu, Japan).
Immunofluorescence analysis
The embedded ear skin samples of the mice were cut into 4-μm sections and stained with anti-mouse K16 (Abcam, Cambridge, MA, USA), K17 (Abcam), K1 (Abcam), K10 (Abcam), CD3 (Abcam), and Ly6G (Abcam), followed by staining with an appropriate Cyanine 3-conjugated secondary antibody (Abcam). Then, the cell nuclei were counterstained with Hoechst 33258 solution (Abcam) for an additional 15 min at 37°C. Finally, the images were captured with LSM 880 (Zeiss, Oberkochen, Germany).
Real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA from ear skin tissues was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). RT-qPCR was conducted using SYBR Premix Ex Taq™ II (Takara, Japan) on a CFX384 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA). All primers were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). The β-actin was used as a reference transcript. The relative gene expression levels were calculated using the 2−ΔΔCt method. The primer sequences are listed below. All sequences were 5′-3′: IL-17A forward, CTCAGACTACCTCAACCGTTCC, reverse, ATGTGGTGGTCCAGCTTTCC; IL-22 forward, ATGAGTTTTTCCCTTATGGGGAC, reverse, GCTGGAAGTTGGACACCTCAA; IL-23p40 forward, GACCATCACTGTCAAAGAGTTTCTAGAT, reverse, AGGAAAGTCTTGTTTTTGAAATTTTTTAA; S100A9 forward, ACTCTTTAGCCTTGAAGAGCAAG, reverse, TTCTTGCTCAGGGTGTCAGG; CCL20 forward, CAGGCAGAAGCAAGCAACTAC, reverse, AGCTTCATCGGCCATCTGTC; CXCL1 forward, ACCCAAACCGAAGTCATAGC, reverse, ACAGGTGCCATCAGAGCAGT; β-actin forward, GTGACGTTGACATCCGTAAAGA, reverse, GCCGGACTCATCGTACTCC.
Statistical analysis
All data in our study were obtained from at least three independent experiments and are presented as mean ± standard error. Data between 2 groups were analyzed with unpaired statistical analyses using the Student's t-test, and comparisons between groups were performed using a one-way analysis of variance followed by Dunnett's multiple comparison test. A P < 0.05 was considered to be statistically significant. Statistical analysis was performed with Prism 8 software (GraphPad Software, La Jolla, California).
Results
Local inhibition of K17 expression alleviates the phenotype of IMQ-induced psoriasis-like dermatitis
To explore whether local direct inhibition of K17 expression by specific siRNAs can treat psoriasis, eight-week-old female BALB/c mice were administered a 5% IMQ cream on both ears to induce psoriatic dermatitis every day for 10 days. To optimize the efficacy of the application method, we applied two well-designed K17 siRNAs (K17 siRNA1 and 2) alone or in combination. The combination is hereafter referred to as siRNA Mix, which is a mixture of K17 siRNA1 and K17 siRNA2 in equal quantities. From day 3 to day 10 as indicated, K17 siRNA1, 2, or mixed siRNA were applied to the left ears of the mice while the right ears were treated in parallel with negative control (NC siRNA) every day after IMQ application [Figure 1A]. Compared to that of the Blank group, the phenotype of IMQ-treated mice was significantly exacerbated, suggesting that the IMQ-induced psoriasis mouse model was successfully constructed. Moreover, the erythema and scales of the mice treated with K17 siRNA alone or in combination were significantly decreased compared to those of the mice treated with NC siRNA on day 10 [Figure 1B]. RT-qPCR analysis was performed to detect the knockdown effect of K17 siRNA in locally treated skin, and the results showed that K17 expression was significantly decreased by more than 50% after treatment with K17 siRNA (vs. NC siRNA, K17 siRNA1 group: t = 4.035, P = 0.0157; K17 siRNA2 group: t = 4.035, P = 0.0060; siRNA Mix group: t = 6.506, P = 0.0074) [Figure 1C]. The dynamic ear thickness of the mice treated with K17 siRNA was significantly decreased compared to that of the mice treated with NC siRNA from day 6 to day 10 and peaked on day 10 (P < 0.05 vs. the Blank and NC siRNA groups). However, there was no difference in ear thickness from day 3 to day 6 [Figure 1D]. Skin biopsies were performed on day 10, and examination of the H&E-stained sections showed that inflammatory cell infiltration in the dermis of the K17 siRNA-treated psoriatic mice was significantly decreased [Figure 1B]. Epidermal thickness measured from the H&E-stained sections showed that epidermal hyperplasia in the K17 siRNA-treated mice was also significantly lower than that of the NC siRNA-treated group (vs. NC siRNA, K17 siRNA1 group: t = 12.75, P = 0.0002; K17 siRNA2 group: t = 15.20, P = 0.0001; siRNA Mix group: t = 11.08, P = 0.0004) [Figure 1E]. However, there was no significant difference between the single use and combined use K17 siRNA-treated groups, as evidenced by the psoriatic indicators. These results indicated that local inhibition of K17 expression with K17 siRNA could alleviate the symptoms of psoriasis-like dermatitis induced by IMQ.
Figure 1.
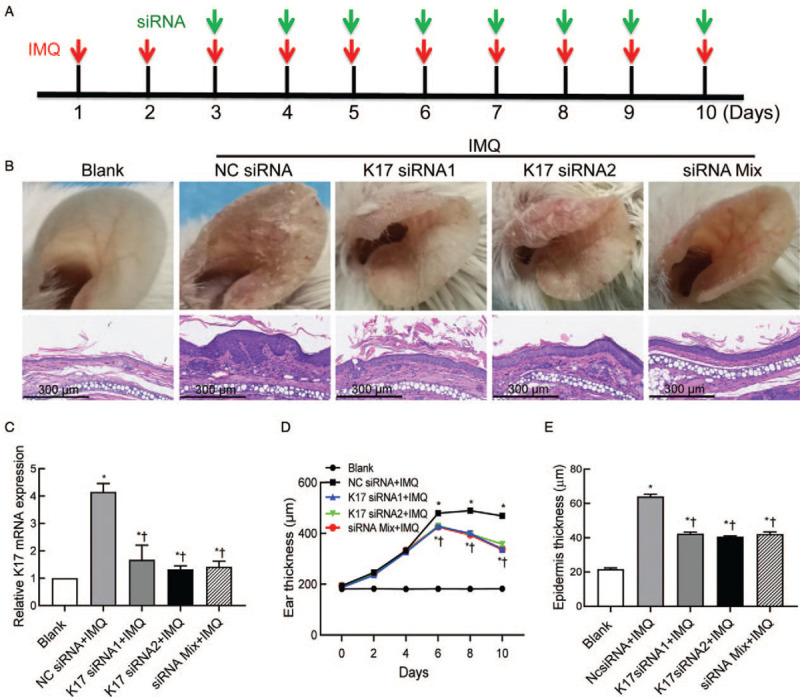
Suppressing K17 expression ameliorated the phenotype of psoriasis-like dermatitis induced by IMQ. IMQ and K17 siRNA or scrambled NC siRNA were topically applied every day to both ears of mice as indicated. Two specific K17 siRNAs (K17 siRNA1/2) alone or in combination were analyzed. Each group contained five mice. (A) Treatment strategy using K17 siRNA in IMQ-induced psoriasis-like mice. (B) Phenotypical presentation of mice ear skin (upper) and H&E staining of ear sections (lower; scale bar: 300 μm). (C) The knockdown efficiency of K17 siRNA. (D) Dynamic changes in gross ear thickness. (E) Epidermal thickness was measured from the H&E-stained sections. Ten randomly chosen fields of independent images per mouse were analyzed (n = 6). ∗P < 0.05 vs. Blank. †P < 0.05 vs. the NC siRNA + IMQ group. Blank: blank group of wild-type mice treated with an emulsion matrix; H&E: hematoxylin and eosin; IMQ: imiquimod; K: keratin; Mix: mixture of K17 siRNA1 and K17 siRNA2 in equal quantities; NC: negative control; siRNA: small interfering RNA.
Local inhibition of K17 expression suppresses K16 expression and promotes K1/K10 expression
K16 and K1/K10 are hyperproliferation- and differentiation-associated keratins, respectively, in keratinocytes.[6] To determine whether K17 siRNA treatment might affect the expression of K16, K1, and K10 in psoriasis, we detected K16/K1/K10 expression levels by immunofluorescence analysis. We found that, consistent with previous reports, K17 was not detectable in a steady state but was overexpressed in psoriatic ear skin lesions. After the treatment of psoriasis lesions with K17 siRNA, including K17 siRNA1/2 and Mix, K17 expression was suppressed by approximately 50%, further confirming the interference effect (vs. NC siRNA, K17 siRNA1 group: t = 4.971, P = 0.0016; K17 siRNA2 group: t = 6.066, P = 0.0005; siRNA Mix group: t = 3.911, P = 0.0113) [Figure 2A and B]. K16 expression, which is characteristic of proliferation, was also significantly inhibited in the K17 siRNA-treated group (vs. NC siRNA, K17 siRNA1 group: t = 4.156, P = 0.0043; K17 siRNA2 group: t = 2.834, P = 0.0253; siRNA Mix group: t = 2.734, P = 0.0250) [Figure 2A and C]. The expression levels of K1/K10 in keratinocytes, which are characteristic of differentiation, were significantly increased after K17 siRNA treatment compared to those of control mice (vs. NC siRNA, K17 siRNA1 group: K1, t = 4.782, P = 0.0050; K10, t = 3.365, P = 0.0120; K17 siRNA2 group: K1, t = 4.104, P = 0.0093; K10, t = 4.168, P = 0.0042; siRNA Mix group: K1, t = 3.065, P = 0.0221; K10, t = 10.83, P < 0.0001) [Figure 2A, D, and E]. No differences were found among the K17 siRNA1, K17 siRNA2, and Mix groups. These data suggest that K17 siRNA can suppress the proliferation but promote the differentiation of keratinocytes.
Figure 2.
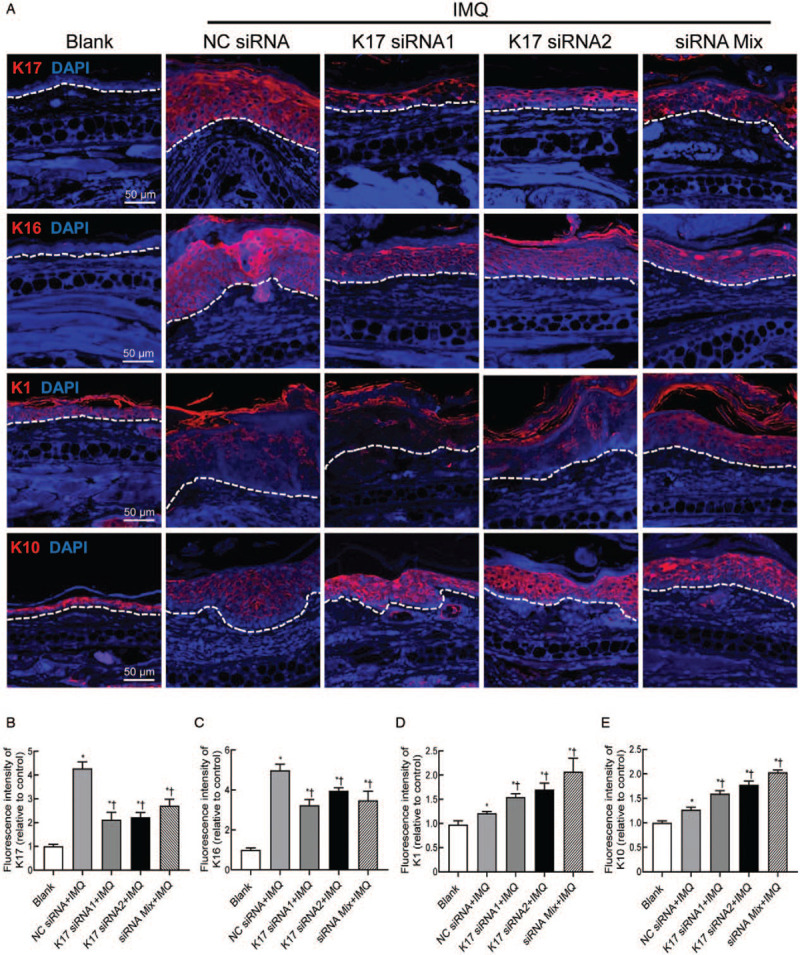
Suppressing K17 expression inhibited the proliferation and promoted the differentiation of keratinocytes. IMQ and K17 siRNA or scrambled NC siRNA were topically applied every day to the ears of mice as indicated. Skin biopsies were performed on day 11 and subjected to immunofluorescence staining. (A) Immunofluorescence staining of K16, K17, K1, and K10 (scale bar: 50 μm). (B) Fluorescence intensity of K16. (C) Fluorescence intensity of K17. (D) Fluorescence intensity of K1. (E) Fluorescence intensity of K10. Three independent images per mouse were analyzed (n = 3). One out of at least three experiments is depicted (mean ± standard error of n ≥ 3 mice per group). ∗P < 0.05 vs. Blank. †P < 0.05 vs. the NC siRNA + IMQ group. Blank: blank group of wild-type mice treated with an emulsion matrix; IMQ: imiquimod; K: keratin; Mix: mixture of K17 siRNA1 and K17 siRNA2 in equal quantities; NC: negative control; siRNA: small interfering RNA.
Suppressing K17 expression decreases inflammatory cell infiltration in IMQ-treated mice
As psoriasis lesions are characterized by T cell and neutrophil infiltration, we further detected CD3+ T cell and Ly6G+ neutrophil counts by immunofluorescence analysis. The results showed that the numbers of CD3+ T cells and Ly6G+ neutrophils were significantly increased in the IMQ- and NC siRNA-treated groups compared to the Blank group (vs. Blank, K17 siRNA1 group: CD3+ T cells, t = 5.839, P = 0.0011 and Ly6G+ neutrophils, t = 6.625, P = 0.0012; K17 siRNA2 group: CD3+ T cells, t = 3.767, P = 0.0093 and Ly6G+ neutrophils, t = 7.353, P = 0.0003; siRNA Mix group: CD3+ T cells, t = 3.232, P = 0.0231 and Ly6G+ neutrophils, t = 4.994, P = 0.0025). However, after K17 siRNA treatment, the numbers of both CD3+ T cells and Ly6G+ neutrophils in psoriatic lesions were significantly decreased compared to those in the group treated with NC siRNA (vs. the NC siRNA group, K17 siRNA1 group: CD3+ T cells, t = 9.549, P < 0.0001 and Ly6G+ neutrophils, t = 5.976, P = 0.0010; K17 siRNA2 group: CD3+ T cells, t = 8.079, P < 0.0001 and Ly6G+ neutrophils, t = 6.036, P = 0.0005; siRNA Mix group: CD3+ T cells, t = 6.651, P = 0.0002 and Ly6G+ neutrophils, t = 5.071, P = 0.0014) [Figure 3A–D]. These results confirmed that suppressing K17 expression with K17 siRNA can decrease the infiltration of inflammatory cells and subsequently inhibit skin inflammatory responses upon IMQ application.
Figure 3.
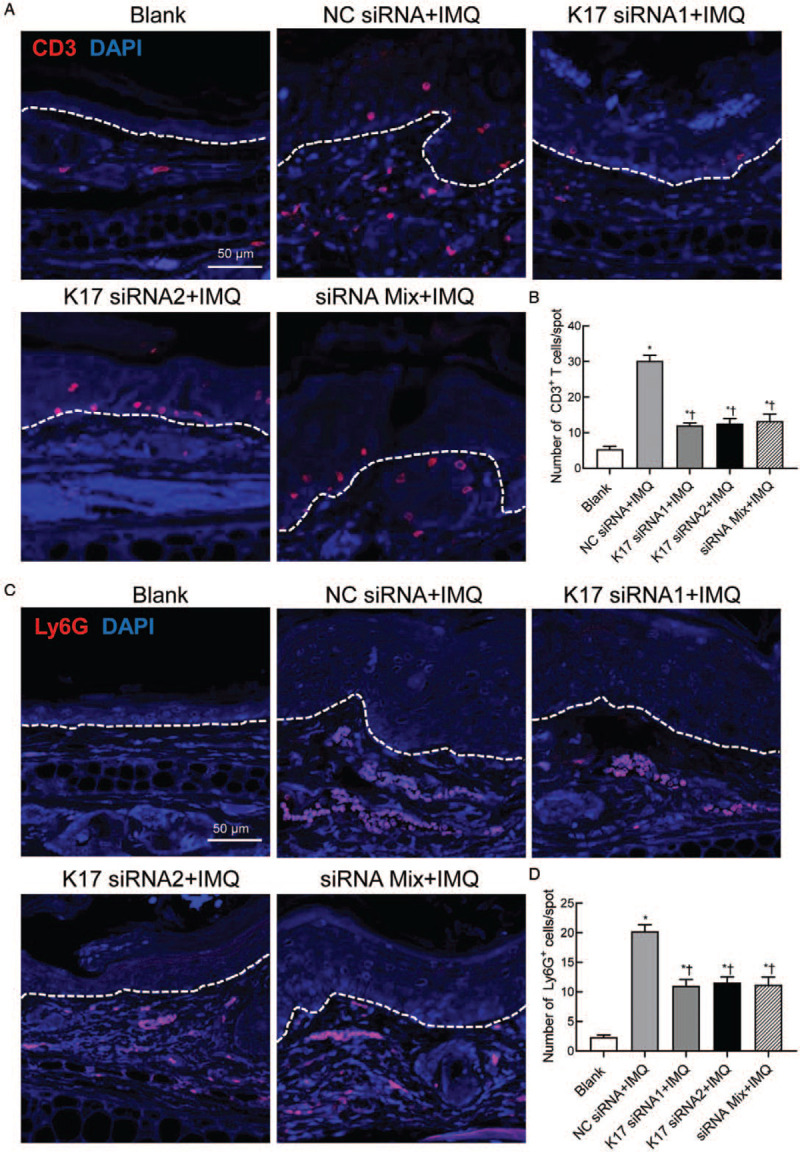
Suppressing K17 expression decreased inflammatory cell infiltration in IMQ-treated mice. IMQ and K17 siRNA or scrambled NC siRNA were topically applied every day to the ears of mice treated with IMQ as indicated. Skin biopsies were performed on day 11 and subjected to immunofluorescence staining. (A) Immunofluorescence staining of CD3+ T cells (scale bar: 50 μm). (B) Absolute number of CD3+ T cells per spot. (C) Immunofluorescence staining of Ly6G+ neutrophils (scale bar: 50 μm). (D) Absolute number of Ly6G+ neutrophils per spot. Three independent images per mouse were analyzed (n = 3). One out of at least three experiments is depicted (mean ± standard error of n ≥ 3 mice per group). ∗P < 0.05 vs. Blank. †P < 0.05 vs. the NC siRNA + IMQ group. Blank: blank group of wild-type mice treated with an emulsion matrix; IMQ: imiquimod; K: keratin; Mix: mixture of K17 siRNA1 and K17 siRNA2 in equal quantities; NC: negative control; siRNA: small interfering RNA.
Suppressing K17 expression decreases proinflammatory cytokine expression in the psoriatic lesions of IMQ-treated mice
IL-17, IL-22, IL-23, and chemokines, including CXCL1 and CCL20, are signature inflammatory cytokines in psoriatic lesions that have been shown to be involved in the excessive immune response in psoriasis.[1,19] Therefore, the ear skin lesions of mice were harvested for RT-qPCR analysis to determine cytokine expression levels. The results showed that the expression levels of IL-17, IL-22, and IL-23p40 were significantly lower in K17 siRNA-treated mice than in NC siRNA-treated mice (P < 0.05 vs. the NC siRNA group) [Figure 4A–C]. We also analyzed the expression of S100A9, which is an important antimicrobial peptide overexpressed in psoriatic skin that has been suggested to play critical roles in psoriasis,[20] and found that S100A9 mRNA expression was significantly decreased after K17 siRNA treatment (vs. the NC siRNA group, K17 siRNA1 group: t = 9.169, P = 0.0008; K17 siRNA2 group: t = 3.464, P = 0.0405; siRNA Mix group: t = 11.21, P = 0.0004) [Figure 4D]. Chemokines, including CCL20 and CXCL1, are important for the onset and maintenance of psoriasis. The results showed that the expression levels of CCL20 and CXCL1 were significantly decreased in K17 siRNA-treated mice compared to the control mice treated with NC siRNA (CCL20, K17 siRNA1/2 or Mix group: P < 0.0001; CXCL1, K17 siRNA1 group: t = 2.862, P = 0.0353; K17 siRNA2 group: t = 3.009, P = 0.0396; siRNA Mix group: t = 2.454, P = 0.0495) [Figure 4E and F]. These data indicated that K17 siRNA application can ameliorate the inflammation of IMQ-induced psoriasis by inhibiting the production of inflammatory cytokines and chemokines, resulting in the suppression of inflammatory responses.
Figure 4.
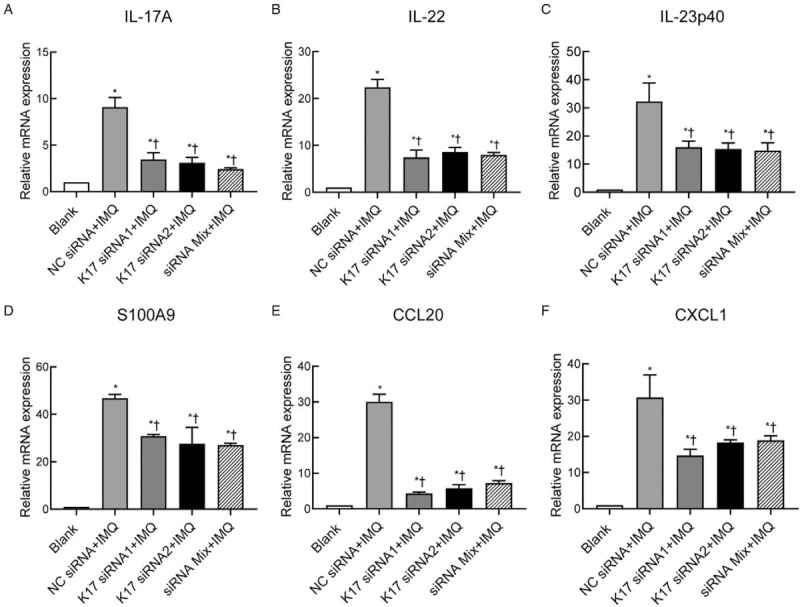
Suppressing K17 expression decreased the expression of proinflammatory cytokines in the psoriatic lesions of IMQ-treated mice. IMQ and K17 siRNA or scrambled NC siRNA were topically applied every day to the ears of mice as indicated. Skin biopsies were performed on day 11 and subjected to RT-qPCR analysis. The ratio is the relative mRNA level of WT mice treated with IMQ and/or K17 siRNA to that of the Blank group of WT mice treated with an emulsion matrix. Data are representative of at least three independent experiments, and each group consisted of three mice (n = 3). The results are presented as the mean ± standard error. ∗P < 0.05 vs. Blank. †P < 0.05 vs. the NC siRNA + IMQ group. IMQ: imiquimod; K: keratin; Mix: mixture of K17 siRNA1 and K17 siRNA2 in equal quantities; mRNA: messenger RNA; NC: negative control; RT-qPCR: real-time quantitative polymerase chain reaction; siRNA: small interfering RNA; WT: wild type.
Discussion
Psoriasis is a chronic autoimmune disease that is prone to clinical relapse. For some patients, it is a lifelong condition with serious physical and mental health consequences for themselves and their families.[1,21] The etiology of psoriasis is very complex and involves various factors, including heredity, immune dysfunction, endocrine disorder, and environmental factors.[2] Thus, there is an urgent need to develop a more effective treatment for psoriasis. Current research has identified an effective gene therapy targeting K17 for psoriasis. In this study, by applying K17 siRNA or NC siRNA to mice with IMQ-induced psoriasis-like dermatitis, we analyzed the characteristic indicators of psoriasis to evaluate inflammation severity. We discovered that topical K17 siRNA application can improve psoriatic inflammation. Upon treatment with K17 siRNA, the physical symptoms, epidermal hyperplasia, and inflammatory cell infiltration of the affected mice were significantly decreased compared to those of the control mice.
K17 is a keratin family member, and its expression in keratinocytes represents a highly activated and proliferative stage under pathological conditions like those of psoriasis.[10] Increasing studies have demonstrated that K17 plays a critical role in the psoriasis maintenance, aggravation, and relapse. Our group has been investigating the associations between K17 and psoriasis and recently proposed a K17/T cell/cytokine positive feedback regulatory loop in psoriasis based on the latest findings.[12] The K17 loop implies that K17 links aberrant keratinocyte proliferation, the production of psoriatic-related cytokines, and autoreactive T cells in this disease, which are key events in the pathogenesis of psoriasis. Therefore, K17 may be an attractive target for treating psoriasis. The findings of this study showed that, as expected, inhibition of K17 expression significantly alleviated psoriatic inflammation.
Consistent with our proposed K17 loop, K17 links aberrant keratinocyte proliferation with the production of psoriasis-related cytokines in psoriasis. Our results also indicated that inhibition of K17 expression can suppress the hyperproliferation of keratinocytes, as evidenced by a reduction in K16 expression. We also found that the expression levels of chemokines CXCL1 and CCL20, which mainly recruit neutrophils and dendritic cells to local lesions, respectively, were both significantly decreased after inhibiting K17 expression. Recent accumulating evidence has clarified that K17 is not only a cytoskeletal protein but also a regulatory protein.[9] Thus, we hypothesized that the mechanism of suppressing K17 expression to improve inflammation might involve reducing the recruitment of neutrophils or dendritic cells to local lesions by decreasing the production of associated chemokines from keratinocytes. Further studies are needed to explore this concept.
Over the past decade, RNA interference (RNAi) has emerged as a powerful tool for therapeutic gene silencing, as it offers the possibility to silence virtually any known pathology-causing gene with high selectivity and efficiency.[22] RNAi technology has previously been explored as a treatment option for psoriasis.[23,24] Studies have reported that tumor necrosis factor alpha (TNF-α) small hairpin RNA, anti-signal transducer and activator of transcription 3, anti-TNF-α siRNA, anti-IL-6, and anti-defensin beta 4 have been evaluated in a psoriasis xenograft transplantation model or the IMQ mouse model.[23,25–27] In the current study, we used K17 siRNA to treat IMQ-induced psoriatic dermatitis and observed a significant improvement in inflammation. Considering the specific expression of K17 in keratinocytes rather than circulatory distribution, siRNA targeting K17 may have a better effect in the treatment of psoriasis.
The efficacy of gene therapy for skin diseases depends on the use of effective pathways, delivery methods, and skin structural characteristics. In the current study, given the consideration of reducing the likelihood of local and systemic toxicity, we gave preference to topical application. To enhance the delivery of siRNA, we fully mixed liposomes (Lipofectamine 3000) and an emulsion matrix with K17 siRNA before application. The findings indicated that K17 siRNA1 and 2 alone or in combination significantly decreased the expression of K17, resulting in a marked improvement of IMQ-induced dermatitis, evidenced by significant alleviation of erythema and scales as well as reduction in epidermal thickness. The results are consistent with our previous study reporting topical treatment with liposome-encapsulated K17 siRNA in a psoriasis xenograft transplantation model.[28] Although we tested different psoriasis research models, we reached the same conclusion that K17 siRNA can relieve psoriatic inflammation, which further confirmed the potential of K17 siRNA to treat psoriasis. Compared to the previous study, we designed and screened two different K17 siRNAs with positive therapeutic effects.
Collectively, the results of the present study further confirm the essential role of K17 in the pathogenesis of psoriasis. Anti-K17 gene therapy using siRNA can significantly ameliorate psoriatic inflammation. Therefore, targeting K17 may become a potential mechanism for the treatment of psoriasis in the near future.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81803125 and 81903192).
Conflicts of interest
None.
Footnotes
How to cite this article: Xiao CY, Zhu ZL, Zhang C, Fu M, Qiao HJ, Wang G, Dang EL. Small interfering RNA targeting of keratin 17 reduces inflammation in imiquimod-induced psoriasis-like dermatitis. Chin Med J 2020;133:2910–2918. doi: 10.1097/CM9.0000000000001197
References
- 1.Rendon A, Schakel K. Psoriasis pathogenesis and treatment. Int J Mol Sci 2019; 20:1475.doi: 10.3390/ijms20061475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamiya K, Kishimoto M, Sugai J, Komine M, Ohtsuki M. Risk factors for the development of psoriasis. Int J Mol Sci 2019; 20:4347.doi: 10.3390/ijms20184347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang WM, Jin HZ. Role of neutrophils in psoriasis. J Immunol Res 2020; 2020:3709749.doi: 10.1155/2020/3709749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Windoffer R, Beil M, Magin TM, Leube RE. Cytoskeleton in motion: the dynamics of keratin intermediate filaments in epithelia. J Cell Biol 2011; 194:669–678. doi: 10.1083/jcb.201008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehrlich F, Fischer H, Langbein L, Praetzel-Wunder S, Ebner B, Figlak K, et al. Differential evolution of the epidermal keratin cytoskeleton in terrestrial and aquatic mammals. Mol Biol Evol 2019; 36:328–340. doi: 10.1093/molbev/msy214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leigh IM, Navsaria H, Purkis PE, McKay IA, Bowden PE, Riddle PN. Keratins (K16 and K17) as markers of keratinocyte hyperproliferation in psoriasis in vivo and in vitro. Br J Dermatol 1995; 133:501–511. doi: 10.1111/j.1365-2133.1995.tb02696.x. [DOI] [PubMed] [Google Scholar]
- 7.Luo S, Yufit T, Carson P, Fiore D, Falanga J, Lin X, et al. Differential keratin expression during epiboly in a wound model of bioengineered skin and in human chronic wounds. Int J Low Extrem Wounds 2011; 10:122–129. doi: 10.1177/1534734611418157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Yin M, Zhang LJ. Keratin 6, 16 and 17-critical barrier alarmin molecules in skin wounds and psoriasis. Cells 2019; 8:807–821. doi: 10.3390/cells8080807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang L, Zhang S, Wang G. Keratin 17 in disease pathogenesis: from cancer to dermatoses. J Pathol 2019; 247:158–165. doi: 10.1002/path.5178. [DOI] [PubMed] [Google Scholar]
- 10.Jin L, Wang G. Keratin 17: a critical player in the pathogenesis of psoriasis. Med Res Rev 2014; 34:438–454. doi: 10.1002/med.21291. [DOI] [PubMed] [Google Scholar]
- 11.Fu M, Wang G. Keratin 17 as a therapeutic target for the treatment of psoriasis. J Dermatol Sci 2012; 67:161–165. doi: 10.1016/j.jdermsci.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Shen Z, Chen L, Liu YF, Gao TW, Wang G, Fan XL, et al. Altered keratin 17 peptide ligands inhibit in vitro proliferation of keratinocytes and T cells isolated from patients with psoriasis. J Am Acad Dermatol 2006; 54:992–1002. doi: 10.1016/j.jaad.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 13.Bonnekoh B, Huerkamp C, Wevers A, Geisel J, Sebok B, Bange FC, et al. Up-regulation of keratin 17 expression in human HaCaT keratinocytes by interferon-gamma. J Invest Dermatol 1995; 104:58–61. doi: 10.1111/1523-1747.ep12613492. [DOI] [PubMed] [Google Scholar]
- 14.Shi X, Jin L, Dang E, Chang T, Feng Z, Liu Y, et al. IL-17A upregulates keratin 17 expression in keratinocytes through STAT1- and STAT3-dependent mechanisms. J Invest Dermatol 2011; 131:2401–2408. doi: 10.1038/jid.2011.222. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Dang E, Shi X, Jin L, Feng Z, Hu L, et al. The pro-inflammatory cytokine IL-22 up-regulates keratin 17 expression in keratinocytes via STAT3 and ERK1/2. PLoS One 2012; 7:e40797.doi: 10.1371/journal.pone.0040797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bai X, Yu C, Yang L, Luo Y, Zhi D, Wang G, et al. Anti-psoriatic properties of paeoniflorin: suppression of the NF-kappaB pathway and Keratin 17. Eur J Dermatol 2020; 30:243–250. doi: 10.1684/ejd.2020.3770. [DOI] [PubMed] [Google Scholar]
- 17.Wang S, Zhu L, Xu Y, Qin Z, Xu A. Salvianolic acid B ameliorates psoriatic changes in imiquimod-induced psoriasis on BALB/c mice by inhibiting inflammatory and keratin markers via altering phosphatidylinositol-3-kinase/protein kinase B signaling pathway. Korean J Physiol Pharmacol 2020; 24:213–221. doi: 10.4196/kjpp.2020.24.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhuang Y, Han C, Li B, Jin L, Dang E, Fang H, et al. NB-UVB irradiation downregulates keratin-17 expression in keratinocytes by inhibiting the ERK1/2 and STAT3 signaling pathways. Arch Dermatol Res 2018; 310:147–156. doi: 10.1007/s00403-018-1812-1. [DOI] [PubMed] [Google Scholar]
- 19.Furue K, Ito T, Tsuji G, Nakahara T, Furue M. The CCL20 and CCR6 axis in psoriasis. Scand J Immunol 2020; 91:e12846.doi: 10.1111/sji.12846. [DOI] [PubMed] [Google Scholar]
- 20.Lande R, Botti E, Jandus C, Dojcinovic D, Fanelli G, Conrad C, et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat Commun 2014; 5:5621.doi: 10.1038/ncomms6621. [DOI] [PubMed] [Google Scholar]
- 21.Lowes MA, Suarez-Farinas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol 2014; 32:227–255. doi: 10.1146/annurev-immunol-032713-120225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bracke S, Desmet E, Guerrero-Aspizua S, Tjabringa SG, Schalkwijk J, Van Gele M, et al. Identifying targets for topical RNAi therapeutics in psoriasis: assessment of a new in vitro psoriasis model. Arch Dermatol Res 2013; 305:501–512. doi: 10.1007/s00403-013-1379-9. [DOI] [PubMed] [Google Scholar]
- 23.Desmet E, Bracke S, Forier K, Taevernier L, Stuart MC, De Spiegeleer B, et al. An elastic liposomal formulation for RNAi-based topical treatment of skin disorders: Proof-of-concept in the treatment of psoriasis. Int J Pharm 2016; 500:268–274. doi: 10.1016/j.ijpharm.2016.01.042. [DOI] [PubMed] [Google Scholar]
- 24.Fan T, Wang S, Yu L, Yi H, Liu R, Geng W, et al. Treating psoriasis by targeting its susceptibility gene Rel. Clin Immunol 2016; 165:47–54. doi: 10.1016/j.clim.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Depieri LV, Borgheti-Cardoso LN, Campos PM, Otaguiri KK, Vicentini FT, Lopes LB, et al. RNAi mediated IL-6 in vitro knockdown in psoriasis skin model with topical siRNA delivery system based on liquid crystalline phase. Eur J Pharm Biopharm 2016; 105:50–58. doi: 10.1016/j.ejpb.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Jakobsen M, Stenderup K, Rosada C, Moldt B, Kamp S, Dam TN, et al. Amelioration of psoriasis by anti-TNF-alpha RNAi in the xenograft transplantation model. Mol Ther 2009; 17:1743–1753. doi: 10.1038/mt.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desai PR, Marepally S, Patel AR, Voshavar C, Chaudhuri A, Singh M. Topical delivery of anti-TNF-alpha siRNA and capsaicin via novel lipid-polymer hybrid nanoparticles efficiently inhibits skin inflammation in vivo. J Control Release 2013; 170:51–63. doi: 10.1016/j.jconrel.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang T, Sun L, Wang Y, Wang D, Li W, Li C, et al. Inhibition of keratin 17 expression with antisense and RNAi strategies: exploring novel therapy for psoriasis. Exp Dermatol 2011; 20:555–560. doi: 10.1111/j.1600-0625.2010.01235.x. [DOI] [PubMed] [Google Scholar]


