Ovarian Cancer is the 5th leading cause of cancer deaths in the U.S. (Siegel et al., 2020). According to the National Cancer Institute, the 5-year survival rate for all stages of ovarian cancer is 48.6%. The rate, however, falls to 30.2% for those with distant metastases at the time of diagnosis (U.S. Cancer Statistics Working Group, 2019). Recurrence for advanced ovarian cancer patients after primary debulking surgery is very common, occurring for more than 80% of patients (Ghirardi et al., 2020). To reduce the rate of recurrence, the National Comprehensive Cancer Network recommends several maintenance therapy options for platinum-sensitive ovarian cancer after primary treatment including the anti-angiogenic bevacizumab and poly ADP ribose polymerase (PARP) inhibitors olaparib and niraparib, the latter, moreover, not dependent on BRCA-mutation (NCCN, 2020). The recent introduction of PARP inhibitors as standard of care therapy in frontline maintenance treatment has extended progression free rates for some patients, although long-term survival and safety data have not yet been demonstrated (Mirza et al., 2020). As such, a majority of advanced ovarian cancer patients still require a safe and effective treatment option that demonstrates long-term benefit.
As previously described in Maples et al., the immunotherapy Vigil is an autologous tumor cell vaccine constructed using harvested autologous tumor tissue in order to specifically target personal neoantigen display. Cells are transfected with a plasmid containing the GM-CSF gene and a bifunctional short hairpin RNA which targets furin. Successful furin knockdown is demonstrated by downstream inhibition of TGFβ1 and TGFβ2 which have been shown to promote immune response. By utilizing the patient’s own tumor cells, the immune system is thus educated to the relevant tumor associated and cancer neoantigens present so as to facilitate and augment a more effective immune response (Maples et al., 2010).
Previously, we conducted a Phase 2 open-label trial of Vigil in frontline maintenance therapy that enrolled 42 subjects with high-risk Stage III/IV ovarian cancer (Oh et al., 2016). The trial was opened to enrollment on March 4, 2011. The first patient consented to tissue procurement on March 8, 2011 and the last patient on September 29, 2014. The first patient enrolled onto the study June 23, 2011 and the last patient on April 6, 2015. The first patient to receive treatment was on July 5, 2011 and the last patient received treatment on January 12, 2016. Eligible patients achieved complete clinical response following primary surgical debulking and adjuvant chemotherapy with standard of care carboplatin and paclitaxel and were randomized 2:1 to receive Vigil (n = 31) or standard of care without maintenance therapy (n = 11). Vigil was administered at 1.0 × 10e7 cells/intradermal injection of gene transfected autologous tumor cells monthly for up to 12 doses. No reports of Vigil related Grade 3/4 adverse events were reported throughout the course of treatment.
Comparison of recurrence-free survival (RFS) from time of tissue procurement was 19.8 months for Vigil treated patients versus (vs.) 12.4 months for control patients (log-rank HR = 0.43, one-sided p-value 0.0165, data as of April 2016) (Oh et al., 2016). Using T-cell activity as a marker of immune reactivity to Vigil treatment, γ-IFN secretion was measured via ELISPOT assay. At baseline 40/41 patients (one was not evaluable) showed negative ELISPOT reactivity prior to Vigil. Following Vigil treatment, a positive ELISPOT response (as defined n > 10 γ-IFN spots/10e6 PBMCs) was observed in all 31 Vigil treated patients, but none of the 10 assessible control patients during treatment assessment.
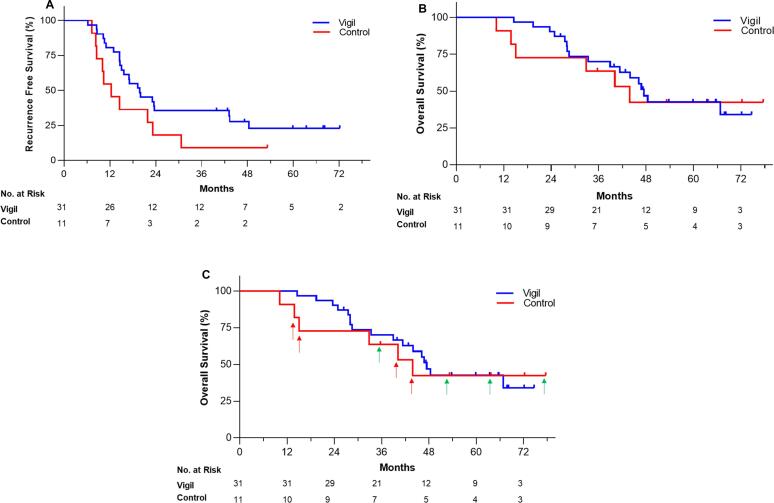
We now report the first follow-up analysis of RFS (median follow-up 43.4 months; range 10.1–77.6 months) which suggests that Vigil treatment continues to provide an advantage (Fig. 1A). A total of 241 Vigil vaccines were administered. No patients receiving Vigil required dose reduction or discontinuation as assessed during treatment and no long-term adverse effects have been reported. RFS was analyzed using GraphPad Prism version 8.3.0 (GraphPad Software, Inc., San Diego, CA) software of all patients and censoring was performed as previously described (Oh et al., 2016). The median RFS from time of tissue procurement in the Vigil group remains over 7 months longer than the control group (19.9 months vs. 12.4 months, log-rank HR = 0.52, one-sided p-value 0.0399). The 4-year RFS rate was 27.6% for Vigil group vs. 9.1% for control group from time of tissue procurement. By comparison, patients with complete response to platinum-based chemotherapy in the recent double-blind, Phase 3 trial of niraparib achieved progression free survival (PFS) of 16.4 months in the treatment group vs. 9.5 months in the placebo group although dose reductions were required for 70.9% of patients in the niraparib group and discontinuation of treatment due to adverse events for 12.0% of patients (2.5% in the placebo group) (González-Martín et al., 2019). Overall survival (OS) difference was not achieved with niraparib at analysis of PFS and is still undergoing observation. Comparing Vigil group to the control group, the median OS was 47.4 vs. 43.9 months from time of tissue procurement (Fig. 1B), however OS was not statistically evaluable since eight of the 11 patients entered into trial crossed over to receive Vigil after RFS endpoint was achieved. Interestingly, of the seven control patients who did not receive Vigil maintenance therapy but who received Vigil after the RFS endpoint was achieved, six of the seven survived nearly 36 months and four have remained alive beyond 48 months (Fig. 1C). Results of this Phase 2A trial of frontline maintenance Vigil vs. placebo were further verified by recent completion of a Phase 2B double-blind randomized placebo controlled trial involving 25 sites in the U.S.A. demonstrating RFS and OS advantage in Vigil treated patients with BRCA-wild type molecular profile (Rocconi et al., 2020).
Fig. 1.
Kaplan Meier survival curves of (a) Regression Free Survival and (b) Overall Survival from the time of tissue procurement with (c) arrows indicate control group patients who crossed over to receive Vigil as second-line treatment; red = alive, green = dead. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In conclusion, Vigil is associated with minimal adverse effects both during treatment and throughout long-term follow-up. Moreover, correlative immune activation and durable long-term efficacy measured by RFS advantage was observed. These results provide long-term therapeutic safety observation and combined with Phase 2B results support further investigation of Vigil maintenance in advanced ovarian cancer.
Authors contributions
All authors made significant contributions to the manuscript and have read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to acknowledge Brenda Marr for her competent and knowledgeable assistance in the preparation of the manuscript.
References
- Ghirardi V., Moruzzi M.C., Bizzarri N., Vargiu V., D'Indinosante M., Garganese G., Pasciuto T., Loverro M., Scambia G., Fagotti A. Minimal residual disease at primary debulking surgery versus complete tumor resection at interval debulking surgery in advanced epithelial ovarian cancer: a survival analysis. Gynecol. Oncol. 2020;157(1):209–213. doi: 10.1016/j.ygyno.2020.01.010. [DOI] [PubMed] [Google Scholar]
- González-Martín A., Pothuri B., Vergote I., DePont Christensen R., Graybill W., Mirza M.R., McCormick C., Lorusso D., Hoskins P., Freyer G., Baumann K., Jardon K., Redondo A., Moore R.G., Vulsteke C., O’Cearbhaill R.E., Lund B., Backes F., Barretina-Ginesta P., Haggerty A.F., Rubio-Pérez M.J., Shahin M.S., Mangili G., Bradley W.H., Bruchim I., Sun K., Malinowska I.A., Li Y., Gupta D., Monk B.J. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl. J. Med. 2019;381(25):2391–2402. doi: 10.1056/NEJMoa1910962. [DOI] [PubMed] [Google Scholar]
- Maples P., Kumar P., Yu Y., Wang Z., Jay C., Pappen B., Rao D., Kuhn J., Nemunaitis J., Senzer N. FANG vaccine: autologous tumor cell vaccine genetically modified to express GM-CSF and block production of furin. BioProcess J. 2010;8(4):4–14. [Google Scholar]
- Mirza M.R., Coleman R.L., González-Martín A., Moore K.N., Colombo N., Ray-Coquard I., Pignata S. The forefront of ovarian cancer therapy: update on PARP inhibitors. Ann. Oncol. 2020;31(9):1148–1159. doi: 10.1016/j.annonc.2020.06.004. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network (NCCN), 2020. NCCN Clinical Practice Guidelines in Oncology. Ovarian Cancer. Version 1.2020. National Comprehensive Cancer Network. [DOI] [PubMed]
- Oh J. Phase II study of Vigil(R) DNA engineered immunotherapy as maintenance in advanced stage ovarian cancer. Gynecol. Oncol. 2016;143(3):504–510. doi: 10.1016/j.ygyno.2016.09.018. [DOI] [PubMed] [Google Scholar]
- Rocconi, R.P., et al. Randomized Double-Blind Placebo Controlled Trial of Primary Maintenance Vigil Immunotherapy (VITAL study) in Stage III/IV Ovarian Cancer: Efficacy Assessment in BRCA1/2-wt Patients (Late Breaking Oral Presentation). In: Society of Gynecologic Oncology Annual Meeting on Women's Cancer. 2020. Toronto, Canada.
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- U.S. Cancer Statistics Working Group, 2020. U.S. Cancer Statistics Data Visualizations Tool, based on 2019 submission data (1999-2017): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; www.cdc.gov/cancer/dataviz, released in June 2020.