Abstract
Background:
Oldest-old patients (≥85 years) constitute half the acute myocardial infarction hospitalizations among older adults and more commonly have atypical presentation, under-treatment and functional impairments. Yet this group has not been well characterized.
Objectives:
We characterized differences in presentation, functional impairments, treatments, health status, and mortality among middle-old (75–84 years) and oldest-old patients with myocardial infarction.
Methods:
We analyzed data from the ComprehenSIVe Evaluation of Risk Factors in Older Patients with AMI (SILVER-AMI) study that enrolled 3041 patients ≥75 years of age from 94 hospitals across the US between 2013–2016. We performed Cox proportional hazards regression to examine the association between the oldest-old (n=831) and middle-old (n=2210) age categories with post-discharge 6-month case fatality rate adjusting for socio-demographic and clinical variables, and mobility impairment.
Results:
The oldest-old were less likely to present with chest pain (52.7% vs. 57.7%) as their primary symptom or to receive coronary revascularization (58.1% vs. 71.8) (p<0.01 for both). The oldest-old were more likely to have functional impairments and had higher 6-month mortality compared with the middle-old patients (HR 1.78, 95% CI 1.39–2.28). This association was substantially attenuated after adjusting for mobility impairment (HR 1.29, 95% CI 0.99-1.68).
Conclusions:
There is considerable heterogeneity in presentation, treatment and outcomes among older patients with myocardial infarction. Mobility impairment, a marker for frailty, modifies the association between advanced age and treatments as well as outcomes.
Keywords: aged, myocardial infarction, frailty, health status, treatment outcome
INTRODUCTION
Oldest-old adults (i.e., those 85 years and older) represent approximately 13% of the older population (i.e., those 65 years and older) in the United States, yet account for half of all acute myocardial infarction hospital admissions among older adults.1 The number of people in the oldest-old age group is projected to grow from 5.9 million in 2012 to 8.9 million in 2030. Yet, acute myocardial infarction in the oldest-old is poorly understood. Prior studies either did not include them in sufficient numbers, or did not provide a comprehensive evaluation of functional, and quality of life measures that are essential for characterizing these patients and their outcomes.2-8 Notably, a recent scientific statement from the cardiology specialty societies highlighted the critical need for large population-based studies to better characterize the older population with regard to such factors.9
Accordingly, we analyzed data from the ComprehenSIVe Evaluation of Risk Factors in Older Patients with AMI (SILVER-AMI) study that recruited patients ≥75 years of age from 94 hospitals across the United States between 2013 and 2016. We divided the cohort into patients aged 75–84 years (middle-old) and ≥85 years (oldest-old) and stratified our analyses by mobility impairment, a marker for frailty, to determine its influence on the association of chronological age with treatments and outcomes after acute myocardial infarction in this population. The results of our study will be used to inform the development of systems of care for older patients with acute myocardial infarction.
METHODS
Study Population
We utilized data from the ComprehenSIVe Evaluation of Risk Factors in Older Patients with Acute Myocardial Infarction (SILVER-AMI) study, a prospective longitudinal study of adults aged 75 and older hospitalized with acute myocardial infarction. The methods for SILVER-AMI have been previously described.10 Briefly, among 5054 eligible patients, 3041 participants (1346 women, 1695 men) from 94 hospitals across the United States who met criteria for the Third Universal Definition of acute myocardial infarction11 were enrolled at the time of hospitalization and underwent a comprehensive structured interview and baseline physical assessment between January 2013 and October 2016. Patients were excluded if the myocardial infarction was the result of an inpatient procedure or surgery, they were unable to complete the baseline or follow-up interview, they were transferred >48 hours from an outside hospital, or they did not speak English or Spanish. The study protocol was approved by Institutional Review Boards at all study recruitment sites and the Yale Coordinating Center.
Data Collection
Site coordinators administered an interview and physical assessment during the hospitalization and collected information on demographics, symptoms, comorbid diseases, functional impairments, and conditions common with aging. Site coordinators and a research nurse at the Yale Coordinating Center abstracted medical record data from the baseline hospitalization regarding medical history, clinical characteristics, presenting symptoms, laboratory results, medications, cardiac procedures, in-hospital complications (i.e., bleeding and acute kidney injury) and discharge disposition. Physician investigators at the Yale Coordinating Center reviewed medical records and electrocardiograms to confirm eligibility and classify the index acute myocardial infarction as either ST segment elevation myocardial infarction or non-ST-segment elevation. A telephone interview was conducted by staff at the Yale Coordinating Center at 6 months after discharge from the index hospitalization to collect information about symptoms, quality of life, and physical function.
Functional Impairments and Conditions Common with Aging
Functional impairments (including those in cognition, vision, hearing, and mobility), and conditions common with aging (disability in activities of daily living and falls) were evaluated via self-report or structured objective assessments. During the baseline interview (participants reported on function 1 month before admission) and at 6 months, participants were asked how much help they needed from another person to bathe, dress, transfer (get in and out of a chair), and walk around their home. Response options were “no help,” “help,” and “unable to do.”12 Decline in activities of daily living was characterized as any decrease in ability to perform these tasks from baseline to 6 months after discharge. Cognitive function was assessed using the Telephone Interview of Cognitive Status, with scores <27 indicating cognitive impairment.13-15 Vision was evaluated using the Visual Functioning Questionnaire-25.16, 17 Hearing was assessed using the single question, “How much does your hearing interfere with your activities?” with response options of “not at all,” “a little,” “a moderate amount,” and “a lot.” We considered participants to be hearing impaired when they responded either “a moderate amount” or “a lot.”
The Timed-Up and Go (TUG) test was used to evaluate mobility impairment, with >25 seconds or unable to complete due to functional limitations used as a cutoff for slow gait speed.18, 19 Mobility impairment has been identified as a sensitive and specific marker of frailty among patients with cardiovascular disease.19, 20 Frailty has been linked with adverse outcomes, including higher risk of major bleeding in patients with myocardial infarction.20 We stratified analyses of interventions and complications by mobility impairment, in order to assess whether observed age-based differences were modified by frailty.
Outcomes
We compared the following clinical outcomes between age groups: major bleeding, acute kidney injury, discharge location, in-hospital case-fatality rate (CFR) and post-discharge all-cause CFR. We defined major bleeding using the Thrombolysis in Myocardial Infarction definition (any intracranial bleed, clinically overt bleeding with hemoglobin drop ≥5g/dL or hematocrit drop ≥15%, or fatal bleeding).21 The site coordinator ascertained these events based on medical record review. Acute kidney injury was based on laboratory values entered at the time of hospitalization, and defined using the Kidney Disease: Improving Global Outcomes criteria which included an increase in serum creatinine of either ≥0.3 mg/dL from baseline or ≥1.5 times baseline (baseline being creatinine at hospital admission).
Statistical Methods
We reported categorical variables as percentages and continuous variables as means. To compare differences between oldest-old and middle-old (baseline characteristics, functional impairments, treatment patterns, in-hospital complications), we used the chi-squared or Fisher’s Exact test for categorical variables and the t-test or Wilcoxon rank sum test for continuous variables. A 2-sided p value <0.05 was considered statistically significant. With the exception of TUG, missing in approximately 14% of patients, missingness in our data ranged from zero to approximately 3%. TUG was dichotomized in our analysis as abnormal versus normal (>25 seconds to complete or unable versus completion in ≤25 seconds). The remaining missing values for TUG and the small amount of missing data in the other candidate variables were multiply imputed based on an assumption of missing-at-random, as previously described.22
We performed multivariable Cox proportional hazards regression analyses to examine the association between the 2 age strata with post-discharge all-cause 6-month CFR adjusting for socio-demographic and clinical variables previously known to be associated with mortality23, 24 (gender, race, smoking history, education level, marital status, prior coronary artery disease, prior congestive heart failure, prior diabetes, prior chronic obstructive lung disease, prior chronic kidney disease, ST-elevation myocardial infarction, revascularization with percutaneous coronary revascularization (PCI), revascularization with coronary artery bypass grafting (CABG), hemoglobin) and further adjusting for mobility impairment.
All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
Baseline characteristics of our study population are presented in Table 1. The oldest-old were more frequently single, women, and white and reported lower levels of household income. The oldest-old were less likely to be obese, diabetic, and current or ever smokers compared with their middle-old counterparts. In contrast, they were more likely to have a history of atrial fibrillation and chronic kidney disease. The prevalence of other comorbidities was similar in both age groups.
Table 1:
Baseline Characteristics among Middle-old and Oldest-old patients Hospitalized with myocardial infarction
| Clinical Characteristics | Middle-old (75–84 yrs) (N=2210) Mean (SD) or N(%) |
Oldest-old (≥85 yrs) (N=831) Mean (SD) or N(%) |
p value |
|---|---|---|---|
| Demographics | |||
| Female Sex | 928 (42.0%) | 418 (50.3%) | <0.001 |
| Age (years), mean, SD | 79.2 (2.8) | 88.3 (3.0) | <0.001 |
| Nonwhite Race | 252 (11.4%) | 73 (8.8%) | 0.04 |
| Married/living as married or with partner | 1234 (55.8%) | 294 (35.4%) | <0.001 |
| Annual household income | <0.001 | ||
| <$10,000 | 111 (5.0%) | 42 (5.1%) | |
| $10,000–$29,999 | 590 (26.7%) | 238 (28.6%) | |
| $30,000–$49,999 | 404 (18.3%) | 133 (16.0%) | |
| $50,000–$69,999 | 246 (11.1%) | 66 (7.9%) | |
| $70,000–$99,999 | 166 (7.5%) | 40 (4.8%) | |
| ≥ $100,000 | 165 (7.5%) | 58 (7.0%) | |
| Refused | 313 (14.2%) | 140 (16.8%) | |
| Don't know | 215 (9.7%) | 114 (13.7%) | |
| Education Level | 0.22 | ||
| Less than high school | 277 (12.5%) | 116 (14.0%) | |
| High school | 963 (43.6%) | 367 (44.2%) | |
| 2-year or 4-year college degree | 640 (29.0%) | 217 (26.1%) | |
| Graduate or post-graduate degree | 315 (14.3%) | 120 (14.4%) | |
| Did not answer | 15 (0.7%) | 11 (1.3%) | |
| Medical History | |||
| Hypertension | 1894 (85.7%) | 701 (84.4%) | 0.35 |
| Dyslipidemia | 1408 (63.7%) | 511 (61.5%) | 0.26 |
| Tobacco use: current or ever Smoker | 1304 (59.0%) | 389 (46.8%) | <0.001 |
| Coronary artery disease | 1190 (53.8%) | 433 (52.1%) | 0.39 |
| Myocardial infarction | 612 (27.7%) | 217 (26.1%) | 0.38 |
| Atrial fibrillation | 349 (15.8%) | 206 (24.8%) | <0.001 |
| Heart failure | 400 (18.1%) | 172 (20.7%) | 0.10 |
| Peripheral vascular disease | 262 (11.9%) | 104 (12.5%) | 0.62 |
| Stroke | 234 (10.6%) | 100 (12.0%) | 0.52 |
| Diabetes mellitus | 887 (40.1%) | 241 (29.0%) | <0.001 |
| Chronic obstructive lung disease | 333 (15.1%) | 101 (12.2%) | 0.04 |
| Chronic kidney disease | 1243 (56.2%) | 588 (70.8%) | <0.001 |
| Percutaneous coronary intervention | 697 (31.5%) | 234 (28.2%) | 0.07 |
| Presentation Characteristics | |||
| ST-segment elevation myocardial infarction | 574 (26.0%) | 223 (26.8%) | 0.63 |
| Any symptom of chest pain | 1702 (77.0%) | 604 (72.7%) | 0.01 |
| Chest pain as primary symptom | 1276 (57.7%) | 438 (52.7%) | 0.01 |
| Any symptom of other discomfort | 1246 (56.4%) | 413 (49.7%) | 0.001 |
| Any other discomfort as primary symptom | 246 (11.1%) | 95 (11.4%) | 0.81 |
| Any respiratory symptom | 1076 (48.7%) | 372 (44.8%) | 0.05 |
| Respiratory as primary symptom | 284 (12.9%) | 106 (12.8%) | 0.94 |
| Any gastrointestinal symptom | 866 (39.2%) | 305 (36.7%) | 0.21 |
| Gastrointestinal as primary symptom | 140 (6.3%) | 61 (7.3%) | 0.32 |
| Any symptom of weakness | 608 (27.5%) | 233 (28.0%) | 0.77 |
| Weakness as primary symptom | 65 (2.9%) | 30 (3.6%) | 0.34 |
| Any symptom at presentation | 2145 (97.1%) | 799 (96.1%) | 0.31 |
| Hemoglobin (mg/dL) | 12..9 (2.09) | 12.5 (2.0) | <0.001 |
| Body mass index (kg/m2) | 28.1 (5.5) | 25.9 (4.67) | <0.001 |
| Decompensated heart failure | 278 (12.6%) | 120 (14.4%) | 0.18 |
| Killip class III/IV | 108 (4.9%) | 39 (4.7%) | 0.82 |
| First systolic blood pressure, mmHg | 145.7 (30.9) | 145.8 (30.8) | 0.91 |
| First diastolic blood pressure, mmHg | 78.4 (17.7) | 77.0 (17.6) | 0.06 |
| First heart rate, beats per minute | 83.7 (23.4) | 83.5 (20.6) | 0.81 |
| Glomerular filtration rate | 56.1 (20.4) | 50.1 (18.3) | <0.001 |
| Troponin >3 times upper limit of normal | 1994 (90.2%) | 773 (93.0%) | 0.02 |
| Cardiogenic shock | 42 (1.9%) | 8 (1.0%) | 0.07 |
At presentation, both age groups had similar blood pressure, heart rates, and rates of cardiogenic shock. The frequency of ST-segment elevation myocardial infarction was similar between both groups as well. The oldest-old were less likely to present with chest pain as the primary symptom (52.7% versus 57.7%) than the middle-old.
Functional Impairments and Conditions Common with Aging
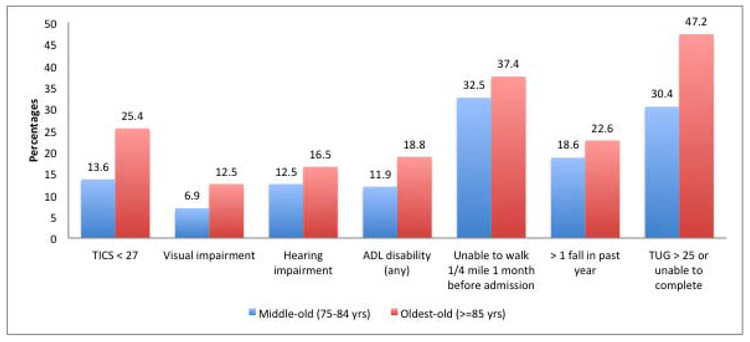
We noted several differences in baseline functional impairments and conditions of aging between the age groups (Figure 1). The oldest-old patients had significantly higher prevalence of cognitive impairment as well as hearing and visual impairment. Mobility impairment was more common among the oldest old compared with the middle-old (47.2% versus 30.4%). A greater proportion of the oldest-old were unable to walk a quarter mile in the month before hospitalization compared with the middle-old. They also had higher rates of disability of activities of daily living and falls in the month and year (respectively) before the myocardial infarction.
Figure 1. Functional impairments among the middle-old and oldest-old patients with myocardial infarction *.
*p-value <0.05 for all impairments
In-hospital Treatments
The treatment characteristics and outcomes of oldest-old and middle-old patients are shown in Table 2. Table 3 demonstrates the treatment characteristics and outcomes in these groups stratified by mobility impairment. Crude rates of medical therapies for myocardial infarction, including aspirin, thienopyridines, anticoagulants, and beta-blockers were similar between the groups. Among patients who were mobility-impaired, angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers and statins were less likely to be administered to oldest-old patients compared with the middle-old, but rates were similar for the 2 groups without impairment. Oldest-old patients were less likely to receive coronary angiography (73.0% versus 88.8%) and PCI (53.5% versus 58.5%) compared with middle-old patients. When stratified by mobility impairment, however, the association of age with PCI was no longer significant. Revascularization with CABG was less likely to be performed in the oldest-old patients even when stratified by mobility impairment.
Table 2.
Treatment Characteristics of Middle-old and Oldest-old patients Hospitalized with myocardial infarction: Medical Therapies, Intervention and In-hospital Outcomes
| Treatment Characteristics | Middle-old (75–84 yrs) (N=2210) N(%) |
Oldest-old (≥85 yrs) (N=831) N(%) |
p value |
|---|---|---|---|
| Medical therapies on admission | |||
| Aspirin | 1641 (74.3%) | 591 (71.1%) | 0.08 |
| Theinopyridines | 1376 (62.3%) | 513 (61.7%) | 0.79 |
| Anticoagulant use (intravenous or subcutaneous) | 1836 (83.1%) | 667 (80.3%) | 0.07 |
| Statin | 1288 (58.3%) | 423 (50.9%) | <0.001 |
| Beta-blockers | 1160 (52.5%) | 420 (50.5%) | 0.37 |
| Angiotensinogen-converting enzyme inhibitors/angiotensin-receptor blockers | 1108 (50.1%) | 365 (43.9%) | <0.01 |
| Interventions | |||
| Coronary angiography | 1963 (88.8%) | 607 (73.0%) | <0.0001 |
| PCI | 1293 (58.5%) | 445 (53.5%) | 0.01 |
| CABG | 315 (14.3%) | 47 (5.7%) | <0.001 |
| Revascularization (PCI or CABG) | 1587 (71.8%) | 483 (58.1%) | <0.001 |
| In-hospital outcomes | |||
| Major bleeding event | 222 (10.0%) | 52 (6.3%) | 0.001 |
| Heart Failure | 280 (12.7%) | 148 (17.8%) | <0.001) |
| Cardiogenic shock | 92 (4.2%) | 23 (2.8%) | 0.07 |
| Arrhythmia | 397 (18.0%) | 155 (18.7%) | 0.66 |
| Acute kidney injury | 527 (23.8%) | 190 (22.9%) | 0.56 |
| In-hospital mortality | 22 (1.0%) | 13 (1.6%) | 0.19 |
PCI- percutaneous coronary intervention, CABG- coronary artery bypass grafting
Table 3:
Treatments and Complications Among Middle-Old and Oldest-Old Patients with myocardial infarction in Strata defined by Mobility Impairment*
| Mobility-impaired | Not mobility-impaired | |||||
|---|---|---|---|---|---|---|
| Patient Presentation Characteristics | Middle-old (N=672) N(%) |
Oldest-old (N=392) N(%) |
p value | Middle-old (N=1184) N(%) |
Oldest-old (N=309) N(%) |
p value |
| Medical Therapies on Admission | ||||||
| Aspirin | 489 (72.8%) | 265 (67.6%) | 0.06 | 883 (74.6%) | 229 (74.1%) | 1.0 |
| Theinopyridines | 376 (56.0%) | 227 (57.9%) | 0.53 | 786 (66.4%) | 206 (66.7%) | 0.93 |
| Anticoagulant use (intravenous or subcutaneous) | 535 (79.6%) | 294 (75.0%) | 0.08 | 1026 (86.7%) | 263 (85.1%) | 0.48 |
| Statin | 431 (64.1%) | 187 (47.7%) | <0.001 | 626 (52.9%) | 165 (53.4%) | 0.78 |
| Beta-blockers | 390 (58.0%) | 207 (52.8%) | 0.10 | 574 (48.5%) | 145 (46.9%) | 0.71 |
| Angiotensinogen-converting enzyme inhibitors/angiotensin-receptor blockers | 358 (53.3%) | 170 (43.4%) | <0.01 | 571 (48.2%) | 132 (42.7%) | 0.09 |
| Interventions | ||||||
| Coronary angiography | 552 (82.1%) | 255 (65.1%) | <0.001 | 1094 (92.4%) | 254 (82.2%) | <0.001 |
| PCI | 307 (45.7%) | 176 (44.9%) | 0.80 | 780 (65.9%) | 199 (64.4%) | 0.63 |
| CABG | 127 (18.9%) | 26 (6.6%) | <0.0001 | 131 (11.1%) | 16 (5.2%) | <0.01 |
| Revascularization (PCI or CABG) | 428 (63.7%) | 197 (50.3%) | <0.0001 | 902 (76.2%) | 211 (68.3%) | <0.01 |
| In-hospital outcomes | ||||||
| Major bleeding event | 85 (12.6%) | 37 (9.4%) | 0.11 | 98 (8.3%) | 7 (2.3%) | <0.001 |
| Acute kidney injury | 221 (32.9%) | 119 (30.4%) | 0.39 | 208 (17.6%) | 39 (12.6%) | 0.04 |
| Discharge to home | 394 (58.6%) | 186 (47.4%) | <0.001 | 1105 (93.3%) | 280 (90.6%) | 0.10 |
Mobility impairment - TUG >25 seconds or unable to complete
PCI- percutaneous coronary intervention, CABG- coronary artery bypass grafting
In-hospital Outcomes
Major bleeding and acute kidney injury were observed at similar rates among the oldest-old and middle-old who were mobility-impaired. Among patients without mobility impairment, however, major bleeding and acute kidney injury were observed less frequently among the oldest-old. The oldest-old with mobility impairment were less likely to be discharged home (47.4% versus 58.6%) and more likely to be discharged to a short-term rehabilitation facility or an extended-care facility than the middle-old. Among patients without mobility impairment, rates of discharge to home were similar in the 2 age-groups (90.6% versus 93.3%). In-hospital CFR was similar in the two age subgroups (1.6% in the oldest-old and 1.0% in the middle-old).
Post-Discharge 6-Month Case-Fatality Rate
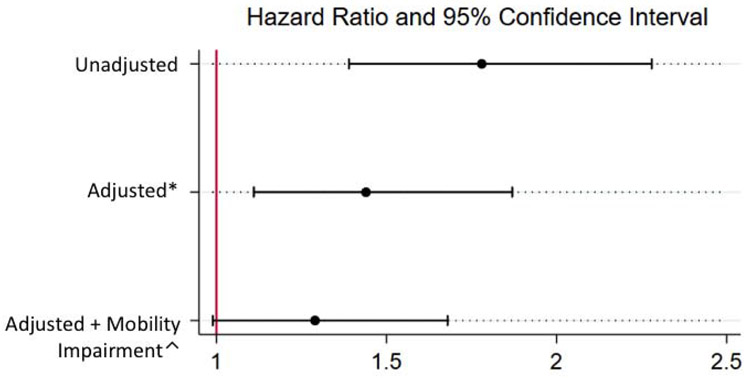
In crude analysis, the oldest-old patients were likely to have higher all-cause 6-month CFR compared with the middle-old patients (HR 1.78, 95% CI 1.39–2.28). In multivariable analyses, when adjusted for socio-demographic and clinical variables, the oldest-old patients had 44% higher odds of mortality at 6 months than middle-old patients (HR 1.44, 95% CI 1.11–1.87). When further adjusted for mobility impairment, the odds of death at 6 months were 29% higher among the oldest-old patients compared with middle-old, but these results were not statistically significant (HR 1.29, 95% CI 0.99–1.68) (Figure 2).
Figure 2. Cox proportional hazards regression analyses showing association between oldest-old patients and mortality at 6 months.
Reference: middle-old
Abbreviations: HR – Hazard ratio, LCL – lower confidence limit, UCL – upper confidence limit
*Model adjusted for gender, race, smoking history, education level, marital status, prior CAD, prior CHF, prior diabetes, prior COPD, prior CKD, type of MI (STEMI), revascularization with PCI, revascularization with CABG, hemoglobin
^Model adjusted for mobility impairment in addition to gender, race, smoking history, education level, marital status, prior CAD, prior CHF, prior diabetes, prior COPD, prior CKD, type of MI (STEMI), revascularization with PCI, revascularization with CABG, hemoglobin
DISCUSSION
To our knowledge, this is the first large-scale study characterizing heterogeneity in medical comorbidities and clinical outcomes, as well as functional status among older adults with myocardial infarction. Our findings are notable in several key respects. First, we observed important differences in baseline and presentation characteristics between the oldest-old and middle-old patients with myocardial infarction. For example, the oldest-old were less likely to present with symptoms of chest pain. Second, functional impairments and conditions common with aging were more common amongst the oldest-old. Third, evidence-based medical therapies for myocardial infarction were administered similarly between the 2 groups overall, but some differences were observed that appeared to be driven by mobility impairment, a marker of frailty. For example, frail oldest-old were less likely to receive statins and angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers than frail middle-old. The oldest-old were also less likely to receive revascularization with PCI and CABG, although the association of age with PCI was no longer present after adjusting for mobility impairment. Finally, the mortality gap between the oldest-old and middle-old patients appeared to be modified by mobility impairment.
Functional Impairments and Conditions Associated with Aging
The prototypical patient with myocardial infarction is an older adult, and management is fundamentally linked to the frailty and multimorbities associated with advanced age.25 We report a high prevalence of several functional impairments, including those in cognition, vision, hearing, and grip strength, as well as falls, collectively found in more than half of all patients. Moreover, these were more commonly seen among the oldest-old patients compared with their middle-old counterparts. Our findings are in concordance with a meta-analysis of 9 studies encompassing 54,250 older patients with a mean weighted follow-up of 6.2 years.26 Among patients with documented severe coronary artery disease, prevalence of frailty was 50–54%. In these community-dwelling older adults, cardiovascular disease was associated with an odds ratio of 2.7–4.1 for prevalent frailty. Gait velocity (a measure of frailty) was associated with an odds ratio of 1.6 for incident cardiovascular disease in this study, suggesting that functional impairments, like frailty, may not only coexist in older adults with myocardial infarction, but might lend them to physiological changes and lifestyles that contribute to incident coronary artery disease and myocardial infarction.
Treatments
The impact of functional impairments on the management of myocardial infarction is poorly understood. While less aggressive medical and invasive therapies of myocardial infarction in the oldest-old have previously been reported,7, 27 our findings provide insights into these disparities in the context of these impairments. We found that the oldest-old received evidence-based medical therapies at similar rates as the middle-old, although statins were less frequently prescribed in the oldest-old. When we stratified our analyses by mobility impairment, however, we found that age-related differences in statin prescription persisted only in frail patients. In a pragmatic randomized clinical trial by Kutner et al., statin discontinuation in patients with limited life expectancy was not only safe, but was also associated with improvement in quality of life.28 Lower rates of statin prescription for frail patients in our study reflects the empiric ‘less is more’ approach to care of these older adults for whom the risk-benefit ratio likely does not favor prescription of statins. Moreover, while the majority of patients in our study received diagnostic coronary angiography and coronary revascularization, the oldest-old were less likely to receive these therapies. Interestingly, the association of age with PCI was no longer significant after adjusting for mobility impairment, suggesting that age-related differences in rates of PCI are primarily driven by frailty. The lower rates of PCI in frail patients may have been justified, given that frailty increases the odds of mortality up to 4-fold after a PCI.29
Mortality
Finally, the frail oldest-old patients had higher CFR at 6 months than their middle-old counterparts. When adjusted for mobility impairment, in addition to other clinical and sociodemographic factors, the increased odds of 6-month CFR of the oldest-old relative to middle-old decreased from 47% to 29%, and the association was no longer statistically significant. This suggests that age-associated impairments may modify the impact of chronological age on adverse outcomes after myocardial infarction.
Strengths and Limitations
This study extends the previous literature in several important ways. No previous study has provided such a comprehensive evaluation of the oldest-old population with myocardial infarction, particularly in regards to several age-related conditions and functional impairments. The sparse literature that focuses on the oldest-old includes studies that are either claims-based,5, 6 retrospective,3, 7 or focused on none or only some of these functional measures.4, 8, 30, 31 Understanding the heterogeneity in presentation, treatment, and outcomes after myocardial infarction necessitates examining a wide range of these heath indicators in this vulnerable population. Finally, we were able to show that the associations of chronological age with receipt of treatments like PCI, as well outcomes such as 6-month mortality, are partially explained by mobility impairment or frailty.
Our study has several limitations. First, there was a shift in the interview mode from in-person interviews at baseline to telephone interviews during follow-up. Although this change in interview mode may have influenced patient responses to questions, trained interviewers administered all interviews, and interview modes were consistent across all patients at each time point. Any changes in patients’ responses resulting from interview mode should be the same for all patients regardless of age. Second, performing a longitudinal study with patient interviews requires patient consent and participation. As occurs in these studies, some patients were lost to follow-up, and some patients did not respond to requests for a follow-up interview. Among 2950 oldest-old and 6098 middle-old patients screened, 828 (28.1%) and 2213 (36.3%), respectively, were ultimately enrolled in our study. Third, because this was an observational study, the differences in mortality between oldest-old and middle-old patients may be attributable to residual confounding. However, our detailed data collection allowed us to examine an extensive range of patient-level factors that are typically not included in myocardial infarction research. Finally, the specific rationale used by each healthcare provider in SILVER-AMI was not captured, preventing conclusive ascertainment of the forces underlying clinical decision-making.
Conclusions
Collectively, our evidence demonstrates that there is considerable heterogeneity in baseline characteristics including functional status, presentation, treatment, and outcomes among older patients with myocardial infarction. Mobility impairment, a marker of frailty appears to partially modify the association of advanced age with rates of important myocardial infarction treatment decisions as well as outcomes. Despite the glaring pattern of age-related complexity, and the precarious course of the disease, the current paradigm of care for older patients with myocardial infarction is mostly an extrapolation from conventional evidence-based cardiovascular guidelines. In addition to their impact on patients’ day-to-day experience, we have shown that functional impairments and conditions common with aging have a substantial impact on the management and outcomes of older patients. There is a need to routinely incorporate the assessment of functional impairments not only in clinical practice, but also in myocardial infarction registries and studies in order to facilitate better characterization of these patients and their outcomes.
CLINICAL SIGNIFICANCE.
Compared with middle-old, oldest-old patients with acute myocardial infarction are less likely to present with chest pain, have higher burden of functional impairments, are less likely to receive coronary revascularization, and have worse mortality.
Age-associated differences in treatments and outcomes of myocardial infarction are driven by frailty.
Assessment of functional impairments should be incorporated in routine clinical practice and future registries for better characterization of older patients and their outcomes.
Acknowledgments
Funding: This research was supported by the National Heart, Lung and Blood Institute of the National Institutes of Health (R01HL115295). This work was conducted at the Yale Program on Aging/Claude D. Pepper Older Americans Independence Center (P30AG021342). The project described used REDCap which is supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH), through grant UL1 TR00000. Dr. Gupta is supported by NIH training grant T32 HL007854. Dr. Nanna is supported by NIH training grant 5T32-HL069749-15. Dr. Dodson receives support from a Patient Oriented Career Development Award (K23 AG052463) from the National Institutes of Health/National Institute on Aging.
Disclosures: Dr. Krumholz was a recipient of a research grant, through Yale, from Medtronic and the U.S. Food and Drug Administration to develop methods for post-market surveillance of medical devices; is a recipient of research agreements with Medtronic and Johnson & Johnson (Janssen), through Yale, to develop methods of clinical trial data sharing; chairs a Cardiac Scientific Advisory Board for UnitedHealth; is a participant/participant representative of the IBM Watson Health Life Sciences Board; is a member of the Advisory Boards for Element Science and for Facebook; is a member of the Physician Advisory Board for Aetna; is the founder of Hugo, a personal health information platform; and receives funding from contracts through Yale from the Centers for Medicare & Medicaid Services to develop and maintain performance measures that are used for public reporting. Drs. Krumholz and Gupta received payment from the Arnold & Porter Law Firm for work related to the Sanofi clopidogrel litigation and from the Ben C. Martin Law Firm for work related to the Cook inferior vena cava filter litigation. Dr. Gupta holds equity in a healthcare telecardiology startup, Heartbeat Health, Inc. The other co-authors report no potential competing interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krumholz HM, Normand S-LT, Wang Y. Twenty-Year Trends in Outcomes for Older Adults With Acute Myocardial Infarction in the United States. JAMA Network Open. 2019;2:e191938–e191938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldberg RJ, McCormick D, Gurwitz JH, Yarzebski J, Lessard D, Gore JM. Age-related trends in short-and long-term survival after acute myocardial infarction: a 20-year population-based perspective (1975–1995). The American Journal of Cardiology. 1998;82:1311–1317. [DOI] [PubMed] [Google Scholar]
- 3.Mehta RH, Rathore SS, Radford MJ, Wang Y, Wang Y, Krumholz HM. Acute myocardial infarction in the elderly: differences by age. J Am Coll Cardiol. 2001;38:736–741. [DOI] [PubMed] [Google Scholar]
- 4.Forman DE, Chen AY, Wiviott SD, Wang TY, Magid DJ, Alexander KP. Comparison of outcomes in patients aged< 75, 75 to 84, and≥ 85 years with ST-elevation myocardial infarction (from the ACTION Registry-GWTG). The American Journal of Cardiology. 2010;106:1382–1388. [DOI] [PubMed] [Google Scholar]
- 5.Khera S, Kolte D, Palaniswamy C, et al. ST-elevation myocardial infarction in the elderly—temporal trends in incidence, utilization of percutaneous coronary intervention and outcomes in the United States. Int J Cardiol. 2013;168:3683–3690. [DOI] [PubMed] [Google Scholar]
- 6.Rao SV, Schulman KA, Curtis LH, Gersh BJ, Jollis JG. Socioeconomic status and outcome following acute myocardial infarction in elderly patients. Arch Intern Med. 2004;164:1128–1133. [DOI] [PubMed] [Google Scholar]
- 7.Rathore SS, Mehta RH, Wang Y, Radford MJ, Krumholz HM. Effects of age on the quality of care provided to older patients with acute myocardial infarction. The American Journal of Medicine. 2003;114:307–315. [DOI] [PubMed] [Google Scholar]
- 8.Gurwitz JH, Goldberg RJ, Chen Z, Gore JM, Alpert JS. Recent trends in hospital mortality of acute myocardial infarction—the Worcester Heart Attack Study: have improvements been realized for all age groups? Arch Intern Med. 1994;154:2202–2208. [PubMed] [Google Scholar]
- 9.Rich MW, Chyun DA, Skolnick AH, et al. Knowledge gaps in cardiovascular care of the older adult population: a scientific statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society. J Am Coll Cardiol. 2016;67:2419–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodson JA, Geda M, Krumholz HM, et al. Design and rationale of the comprehensive evaluation of risk factors in older patients with AMI (SILVER-AMI) study. BMC Health Serv Res. 2014;14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. [DOI] [PubMed] [Google Scholar]
- 12.Katz S Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31:721–727. [DOI] [PubMed] [Google Scholar]
- 13.MAGRUDERHABIB K, BREITNER J, WELSH K. PERFORMANCE-CHARACTERISTICS OF THE TELEPHONE INTERVIEW FOR COGNITIVE STATUS AMERICAN JOURNAL OF EPIDEMIOLOGY. Vol 132: AMER J EPIDEMIOLOGY 624 N BROADWAY RM 225, BALTIMORE, MD: 21205; 1990:788–788. [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 15.Fong TG, Fearing MA, Jones RN, et al. Telephone interview for cognitive status: Creating a crosswalk with the Mini-Mental State Examination. Alzheimer's & Dementia. 2009;5:492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25-list-item national eye institute visual function questionnaire. Arch Ophthalmol. 2001;119:1050–1058. [DOI] [PubMed] [Google Scholar]
- 17.Owen CG, Rudnicka AR, Smeeth L, Evans JR, Wormald RP, Fletcher AE. Is the NEI-VFQ-25 a useful tool in identifying visual impairment in an elderly population? BMC Ophthalmol. 2006;6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donoghue OA, Savva GM, Cronin H, Kenny RA, Horgan NF. Using timed up and go and usual gait speed to predict incident disability in daily activities among community-dwelling adults aged 65 and older. Arch Phys Med Rehabil. 2014;95:1954–1961. [DOI] [PubMed] [Google Scholar]
- 19.Savva GM, Donoghue OA, Horgan F, O’regan C, Cronin H, Kenny RA. Using timed up-and-go to identify frail members of the older population. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2012;68:441–446. [DOI] [PubMed] [Google Scholar]
- 20.Dodson JA, Hochman JS, Roe MT, et al. The association of frailty with in-hospital bleeding among older adults with acute myocardial infarction: insights from the ACTION Registry. JACC Cardiovasc Interv. 2018;11:2287–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. [DOI] [PubMed] [Google Scholar]
- 22.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. [DOI] [PubMed] [Google Scholar]
- 23.Brennan JM, Curtis JP, Dai D, et al. Enhanced mortality risk prediction with a focus on high-risk percutaneous coronary intervention: results from 1,208,137 procedures in the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc Interv. 2013;6:790–799. [DOI] [PubMed] [Google Scholar]
- 24.Roe MT, Chen AY, Thomas L, et al. Predicting long-term mortality in older patients after non–ST-segment elevation myocardial infarction: the CRUSADE long-term mortality model and risk score. Am Heart J. 2011;162:875–883. e871. [DOI] [PubMed] [Google Scholar]
- 25.Forman DE, Rich MW, Alexander KP, et al. Cardiac care for older adults: time for a new paradigm. J Am Coll Cardiol. 2011;57:1801–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. The American Journal of Cardiology. 2009;103:1616–1621. [DOI] [PubMed] [Google Scholar]
- 27.Stone PH, Thompson B, Anderson HV, et al. Influence of race, sex, and age on management of unstable angina and non—Q-wave myocardial infarction: The TIMI III Registry. JAMA. 1996;275:1104–1112. [PubMed] [Google Scholar]
- 28.Kutner JS, Blatchford PJ, Taylor DH, et al. Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: a randomized clinical trial. JAMA Internal Medicine. 2015;175:691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh M, Rihal CS, Lennon RJ, Spertus JA, Nair KS, Roger VL. Influence of frailty and health status on outcomes in patients with coronary disease undergoing percutaneous revascularization. Circ Cardiovasc Qual Outcomes. 2011;4:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah P, Najafi AH, Panza JA, Cooper HA. Outcomes and quality of life in patients≥ 85 years of age with ST-elevation myocardial infarction. The American Journal of Cardiology. 2009;103:170–174. [DOI] [PubMed] [Google Scholar]
- 31.Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non–ST-segment elevation myocardial infarction. Circulation. 2011;124:2397–2404. [DOI] [PubMed] [Google Scholar]