Abstract
All the above-ground organs of a plant are derived from stem cells that reside in shoot apical meristems (SAM). Over the last 25 years, the genetic pathways that control the proliferation of stem cells within the SAM, and the differentiation of their progenitors into lateral organs, have been described in great detail. However, longstanding questions regarding the importance of communication between cells within the SAM and lateral organs have, until recently, remained unanswered. In this review, we describe recent investigations into the extent, nature and significance of signaling both to and from the SAM.
Keywords: SAM, protein mobility, intercellular communication, developmental transitions, miRNA mobility
Introduction
The growth and architecture of a plant shoot depend on the activity of shoot apical meristems (SAMs). These structures are stably maintained by the precisely controlled balance between cell proliferation and differentiation, but are also capable of responding to endogenous and environmental cues that influence their growth and the types of organs they produce. The degree to which the SAM regulates shoot development autonomously, or acts in response to extrinsic factors that originate outside the SAM, is a classic question in plant biology. Half a century ago, Ian Sussex [1] asked: ‘are … meristems to be considered as organizer regions whose functional changes represent changes initiated within the meristem itself, or are they simply plastic regions in which new cells are molded into organs and tissues in response to stimuli proceeding from other sources?’ At the time, data from microsurgical, shoot culture, and hormone treatment studies suggested that the SAM was largely autonomous of neighboring tissues and organs. However, with the benefit of modern molecular tools, it has become apparent that apical meristems typically function as signaling integrators, coordinating cues from elsewhere in the plant and from the environment.
The structure and activity of the SAM, and the regulatory networks that determine these features, have been reviewed extensively recently [2,3]. SAMs possess several distinct functional domains (Figure 1). Stem cells—which divide to produce additional stem cells as well as cells that will differentiate—reside within the central zone (CZ). Cells derived from the CZ are displaced laterally to the peripheral zone (PZ), where they differentiate into lateral organs, or basally to the rib zone (RZ), where they differentiate into cells of the stem. Stem cell proliferation within the SAM is maintained by the activity of members of two distinct families of homeobox gene: WUSCHEL-LIKE (WOX) and KNOTTED-LIKE (KNOX). In Arabidopsis, WUSCHEL (WUS) is expressed in the ‘Organizing Center’ of the CZ (Figure 1), where it promotes the division of stem cells. The expression domain of WUS is restricted by the activity of the signaling peptide CLAVATA3 (CLV3), which regulates WUS in a negative feedback loop. Therefore loss-of CLV3 function leads to an expansion of the SAM, whereas loss-of WUS function leads to meristem termination. The Arabidopsis KNOX gene SHOOT MERISTEMLESS (STM) is expressed more broadly in the SAM, and acts to maintain stem cells in an undifferentiated state. Both WUS and STM are necessary for meristem maintenance throughout the ontogeny of individual meristems, and within different developmental contexts, i.e. SAMs in the vegetative and reproductive phases of a plant life cycle. In this review we detail how core developmental processes in the SAM are influenced by extrinsic genetic and metabolic factors, describe examples of environmental signals that are perceived both within and without the SAM, and provide an update on a longstanding hypothesis regarding extrinsic signaling by the SAM on lateral organs.
Figure 1. Functional domains of the SAM.
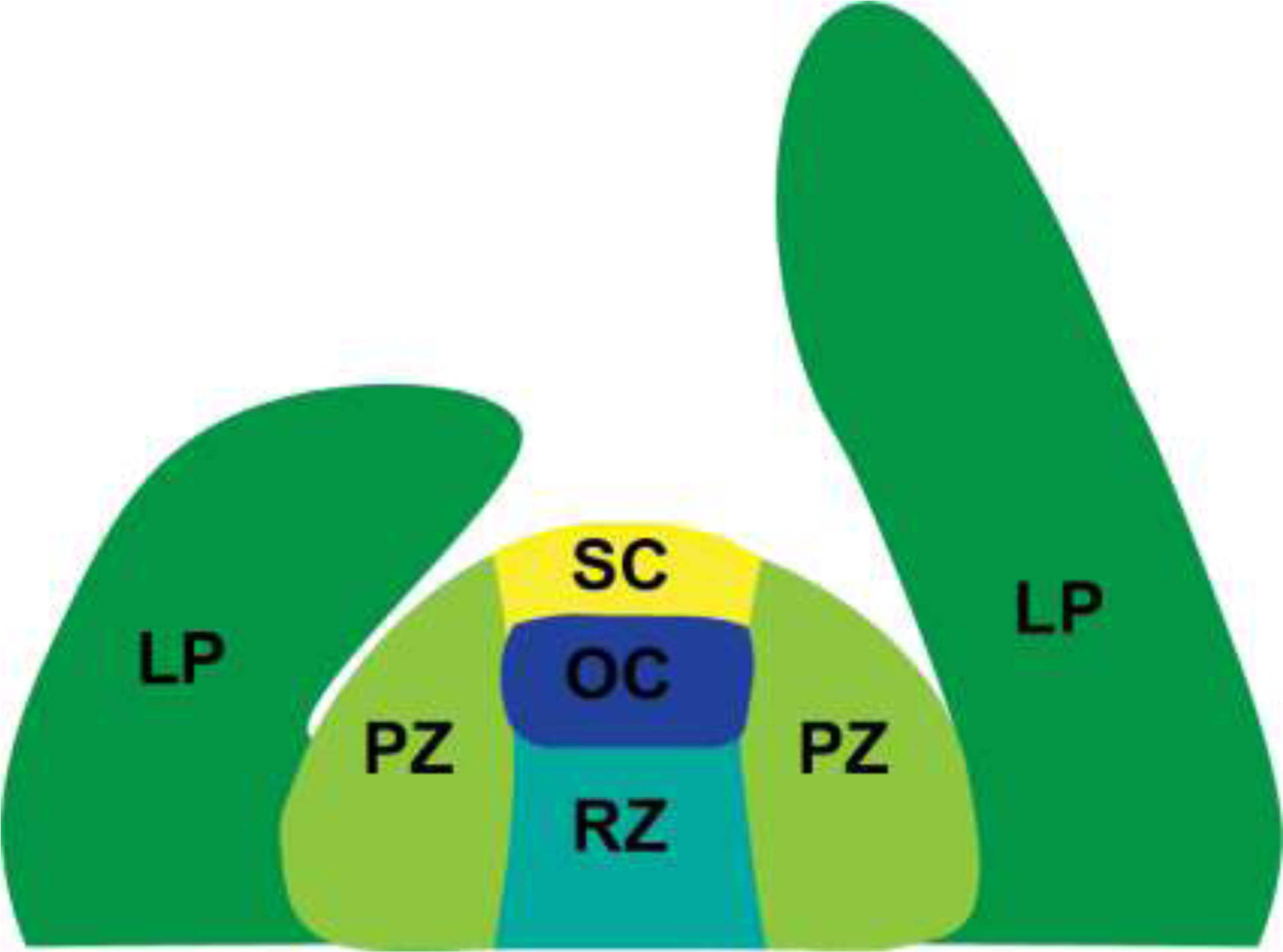
LP – leaf primordia, PZ – peripheral zone, RZ – rib zone, OC – organizing center, SC – stem cells. The SC and OC together define the SAM central zone (CZ). WOX gene family expression (e.g. WUS) is associated with the OC, whereas CLE family expression (e.g. CLV3) is associated with SC.
Regulation of developmental transitions by extrinsic factors
The best described example of an extrinsically regulated change in the activity of the SAM is floral induction (Figure 2A). It has been known for many years that inductive photoperiods trigger production of a mobile ‘florigen’ in leaves, which moves to the SAM to initiate flowering [4]. This signal is now known to be a small protein encoded by the FLOWERING LOCUS T (FT) gene. FT moves from leaves to the SAM via the phloem [5–8], where it interacts with the locally expressed bZIP transcription factor FLOWERING LOCUS D (FD) to induce the transition from a vegetative to an inflorescence meristem [9,10]. Recently it was shown that FT enhances, but is not necessary, for the binding of FD to its targets and that phosphorylation of FD, presumably by two calcium-dependent kinases, is required for this interaction [11,12]. It has also been suggested that leaves regulate floral induction at the SAM via the hormone, gibberellin (GA) [13,14], a hypothesis that is supported by the identification of GA-transporters and mobile forms of GA [15,16]. Although there is considerable evidence that GA regulates flowering time, whether GA acts in the leaves or in the SAM, as well as the functional significance of mobile GA, is still unclear because GA synthesized within the SAM can also affect flowering time [17].
Figure 2. Mobile regulators in the shoot apex.
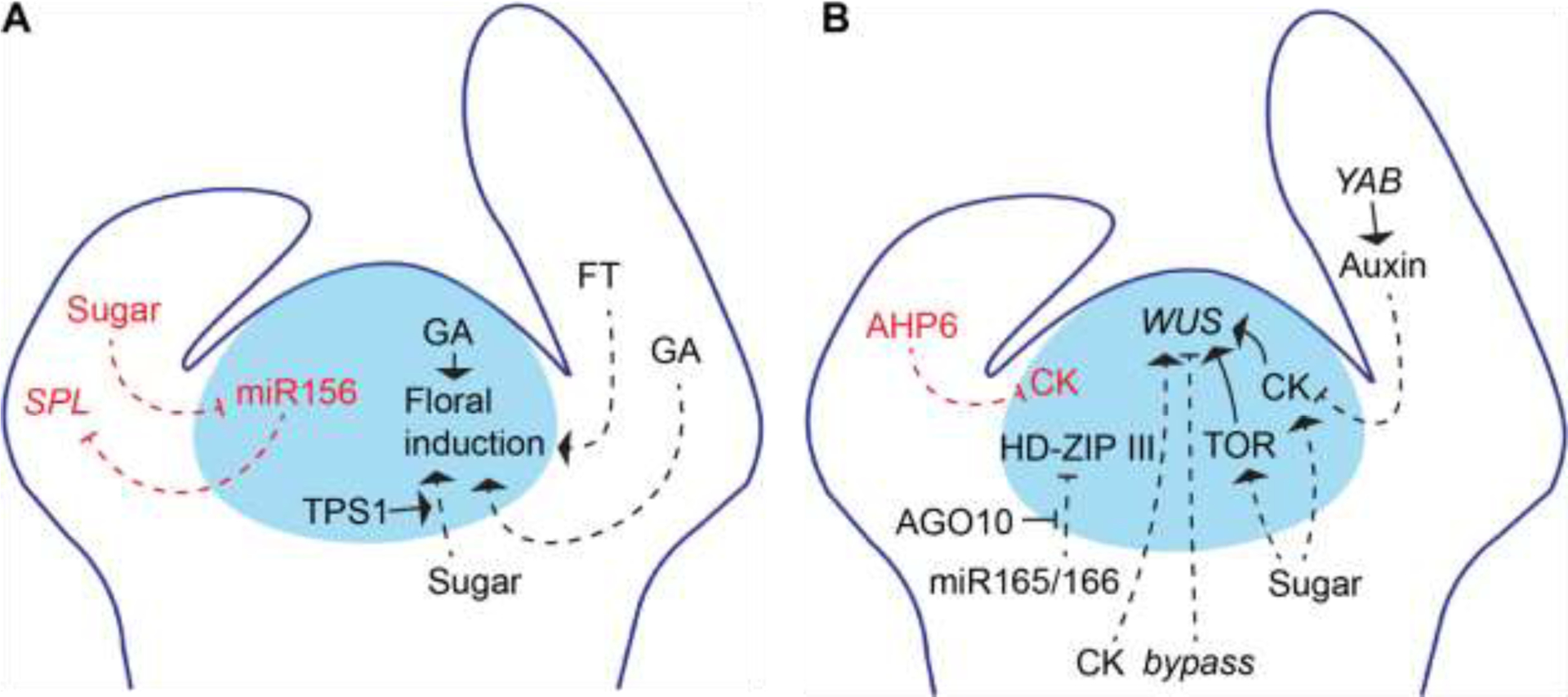
Simplified genetic networks regulating A) Vegetative phase change (red) and floral induction (black), B) Phyllotaxy (red) and meristem maintenance (black). Arrows represent positive regulation, flat lines represent negative regulation, blue circle represents the SAM (vegetative in A, vegetative or inflorescence in B), dashed lines depict movement into the SAM.
Communication between leaves and the SAM is also important for the regulation of the transition between the juvenile and adult phases of vegetative growth (vegetative phase change) [18]. Traditionally, it was thought that the phase identity of the vegetative shoot (i.e. juvenile versus adult) was determined by factors operating endogenously within the SAM [19]. However, more recent work has shown that this transition is promoted by signaling from pre-existing leaves, via repression of the master regulator of vegetative phase change, miR156 [20]. This leaf-derived signal consists, at least in part, of carbohydrates because exogenous application of glucose delays vegetative phase change, represses miR156 expression, and compensates for the loss of leaf primordia [21,22] (Figure 2A). Evidence that leaves regulate the activity of the SAM is also provided by the finding that a leaf-expressed miR156-resistant version of SPL9 is able to regulate the rate of leaf initiation in the SAM [23], although whether the endogenous SPL9 protein moves intercellularly within the shoot apex remains to be demonstrated.
Conversely, recent work has shown that the SAM is important for the specification of leaf identity early in shoot development [24**]. In Arabidopsis, constitutive expression of miR156 within the SAM regulates leaf identity and represses its direct targets in leaf primordia. These observations suggest that this is because miR156 is able to diffuse from the SAM into leaf primordia [24**]. These and the results of other region-specific gene expression experiments suggest that the control of vegetative identity shifts spatially from the SAM to leaves over time. More generally, while miRNA movement within the SAM regulates core genetic networks [25*,26], the extent of miRNA mobility across the shoot apex appears strictly controlled [27**].
Regulation of SAM size and maintenance by extrinsic factors
Short-range signaling
As described in the introduction, the SAM is initiated and maintained by transcription factors (WUS and STM) that are expressed exclusively within the SAM. The distribution and activity of these transcription factors is regulated by molecules that are produced locally within the SAM, and by molecules produced by surrounding organs and tissues. Several of these molecules act to repress the growth of the SAM. For example, although a basal level of auxin signaling is required for the maintenance of the SAM [28*], a combination of experimental approaches and mathematically modelling suggest that the growth of the meristem is repressed by auxin produced by lateral organs [28**] (Figure 2B). As lateral organs are stronger auxin sources than the SAM, it is predicted that basipetal auxin flow from leaves competitively inhibits the export of auxin from the SAM, and restricts meristem size [29**]. This model is supported by analyses of yabby (yab) mutants, which non-autonomously increase meristem size [30], possibly because they reduce the export of auxin from leaves [29**]. In maize, there is evidence that leaf primordia non-autonomously repress the growth of the SAM through their production of the CLV3-like peptide, FCP1. This conclusion is based on in situ data suggesting that FCP1 is expressed in leaf primordia, and the observation that expressing FCP1 specifically in leaf primordia reduces WUS expression and represses the growth of the SAM [31]. However, a transcriptomic survey of the maize SAM has found evidence for FCP1 expression within the CZ, indicating it likely functions at least in part SAM-autonomously [32*]. A third example is provided by the diffusible miRNAs, miR165/miR166, which repress the expression of a group of HD-ZIP III transcription factors essential for the specification of the apical domain of the embryo and the initiation and maintenance of the SAM [33,34]. miR165/miR166 are expressed in cells surrounding the SAM, but are capable of moving into the SAM and repressing HD-ZIP III expression in this domain [35,36]. They are prevented from doing so by ARGONAUTE10 (AGO10) [34], which is expressed in the SAM and provasculature [37,38] and specifically sequesters miR165/miR166 [39], causing them to become hyper-susceptible to degradation by SMALL RNA DEGRADING NUCLEASE family members [40]. Thus, meristem activity is exogenously maintained by the coordinated degradation of specific miRNAs.
In contrast to these examples of negative regulation of the SAM by lateral organs and tissues, a role for lateral organs in promoting meristem maintenance has recently been described in the liverwort Marchantia polymorpha [41**]. Naramoto et al identified the gene LATERAL ORGAN SUPRESSOR1 (MpLOS1) in a mutant screen for regulators of organogenesis. Mplos1 mutants produce unusual green outgrowths in place of transparent scales and, in addition, fail to maintain an apical meristem. MpLOS1 expression is restricted to lateral organs and the MpLOS1 protein does not appear to move into the apical meristem, suggesting that MpLOS1 promotes meristem maintenance non-cell autonomously. Whether it does so indirectly—by promoting the differentiation of lateral organs—or in a more direct fashion, remains to be determined. Interestingly, homologs of MpLOS1 in angiosperms are also important for the differentiation of lateral organs and, in eudicots at least, their expression domain is restricted to the flanks of the SAM [42–44]. Although it is beyond the scope of this review, it should be noted that meristem boundaries are critical for isolating proliferating stem cells from differentiation programs in lateral organs, and are required for SAM establishment and axillary meristem formation [reviewed by 45,46].
Long range signaling
The plant hormone cytokinin (CK) maintains the growth of the SAM by promoting the expression of the transcription factor, WUS, via CLV-dependent and independent mechanisms. Although mathematical models suggest that CK biosynthesis and signaling within the SAM are sufficient for its function [47–49], the transport of CK precursors from the root has also been shown to affect WUS expression and SAM size [50**,51] (Figure 2B). In Arabidopsis, acropetal transport of CK-precursors requires the activity of the ATP-binding cassette transporter ABCG14 in the root [52,53]. Root-derived trans-zeatin and trans-zeatin riboside both affect leaf development, but only trans-zeatin riboside is able to regulate the activity of the SAM [51]. Movement of CKs up the shoot is dependent on nitrate levels in the soil, providing a potential mechanism by which shoot growth can be regulated by nutrient availability [50**,54].
Additional evidence that root signals regulate the activity of the SAM is provided by mutations in the gene BYPASS1 (BPS1), which terminate the growth of the SAM by diminishing CK signaling in the SAM and repressing WUS expression [55]. The exact mechanism of this effect is still unknown, but it has been attributed to a mobile, root-synthesized signal [56] that is dependent on carotenoid biosynthesis [57]. Unknown seed-derived signals, in combination with an endogenous age-dependent pathway, also lead to the termination of apical growth through repression of WUS activity [58*,59].
Intrinsic and extrinsic environmental regulation of SAM activity
In nature, plants modify their growth and development to adapt to varying environmental conditions. Although many of these responses involve changes in the activity of the SAM (e.g. the timing of developmental transitions, the rate of leaf initiation, branching patterns), it is usually unclear if the environmental stimulus is perceived directly by the SAM, or perceived elsewhere in the plant and transmitted to the SAM. For example, cold-induced repression of the floral suppressor FLC occurs in both the SAM and leaves [60], while the mechanism for cold-induced SAM-termination in Brassica oleracea seedlings is unknown [61].
The best characterized examples of exogenous regulation of SAM activity concern the effects of light. The effects of light on the activity of the SAM can be partitioned into direct light-signaling effects and those mediated by the derivatives of photosynthesis [62]. The proliferation of the SAM, as well as changes in its pattern of differentiation, require both and—when deprived of light and sugar—meristematic growth, organogenesis, and developmental transitions are severely affected. As noted above, light (specifically, photoperiod) regulates flowering through its effect on the synthesis of FT in leaves. In addition, low red:far red light ratios, which recapitulate shade conditions, promote the expression of FT [63]. More generally light, and metabolic pathways dependent on light, regulate the activity of the SAM through their effects on cytokinin signaling and the TARGET OF RAP (TOR) kinase complex, which promote WUS expression [62,64–66] (Figure 2B). Light perception appears to take place in the leaves, rather than the SAM, as transgenic activation of light signaling, or exposure to high light in leaves (but not the SAM), promotes cell proliferation in the shoot apex [62,65]. However, the identity of the putative light-induced factor that non-cell autonomously regulates meristem development is unknown. In terms of sugar signaling, WUS activity requires metabolizable sugars, suggesting that sugars function as an energy source rather than as signaling molecules [62]. Although the SAM does not appear to be photosynthetically active [62,67], the sucrose-signaling regulator TREHALOSE-6-PHOSPHATE SYNTHASE1 is expressed within the SAM [68,69]. Therefore, despite depending on extrinsic sources of sugar, the SAM can regulate sugar-signaling networks intrinsically.
It has recently been demonstrated that SAM-activity is dependent on the endogenous perception and regulation of its own oxygen environment. Oxygen is excluded from the CZ, which limits the oxygen-dependent proteolysis of the HD-ZIP III interactor LITTLE ZIPPER 2 [70**]. A general role for oxygen derivatives in the regulation of the SAM is supported by the mutant phenotypes of SAM-expressed redox regulators [71–73], and the non-uniform accumulation and effects of reactive oxygen species within the SAM [74]. Localized temperature manipulation experiments in cucumber suggest the SAM may also interpret temperature signals to regulate leaf initiation [75].
Extrinsic regulation by the SAM?
A classic hypothesis in plant biology is that the SAM non-cell autonomously regulates dorsoventral patterning in leaves [76]. Based on microsurgical experiments in potato and tomato, it has been proposed that a SAM-derived signal is required to correctly induce leaf dorsoventrality [76,77]. However, evidence from laser-ablation of cells in the SAM has recently led to an alternative interpretation of these microsurgical experiments [78**]. Caggiano et al demonstrated that wounding disrupts the adjacent expression domains of dorsoventral specificity factors REVOLUTA (REV) and KANADI1 (KAN) in the SAM, and the boundary of auxin signaling that separates them. In accordance with previous hypotheses [79,80], the authors propose that dorsoventral patterning of leaves does not require a mobile signal from the SAM, but instead maintenance of boundaries between key regulators during leaf initiation. It has also been suggested that dorsoventral patterning is dependent on the flow of auxin from the dorsal side of leaf primordia to the SAM [81,82]. Whether or not asymmetric auxin signaling establishes leaf polarity, and how to test this experimentally (particularly with regard to genetic auxin sensors), have recently been of some debate [83,84].
Conclusion and outlook
Although the function of the SAM depends on integrating extrinsic signals, the core regulatory networks that coordinate stem cell proliferation appear to operate with minimal external input. The relative independence of these networks is demonstrated by the limited range of signals to which they are susceptible (Table 1). Plant hormones, microRNAs, sugars and small proteins are all able to extrinsically regulate SAM activity, whereas there is little support for extrinsic regulation by larger proteins. The extent to which external factors can regulate the activity of the SAM may therefore depend on their size, which could determine their ability to enter the SAM. Smaller molecules may be able to travel symplastically from the vasculature to the SAM, but this may be impossible for larger proteins. On the other hand, transcription factors such as WUS and STM move readily within the SAM [85,86], suggesting that once inside the SAM intercellular movement of larger proteins is less restricted.
Table 1.
A summary of the plant-derived and environmental signals that regulate SAM growth. Unless specified, gene products encoded by Arabidopsis loci.
| Molecular signal | Direction | Effect | References |
|---|---|---|---|
| Protein FT | From leaves to the SAM | Induction of flowering | [5–8] |
| SPL9 | From leaves to the SAM | Repress leaf initiation | [23] |
| AHP6 | From leaves to the SAM | Reinforce phyllotactic patterning | [88] |
| MpLOSI | From lateral organs to the apical meristem | Promotes meristem maintenance | [41] |
| ZmFCPI | From lateral organs to the apical meristem | Limit SAM size | [30] |
|
miRNA
miR156 |
From the SAM to leaves | Repress adult identity | [24] |
| miR165/miR166 | From provasculature into the SAM | Repress SAM growth (butnormally excluded) | [35,36,39] |
| Hormone GA | From leaves to the SAM | Induction of flowering | [13,14] |
| Auxin | From leaves to the SAM | Induce dorsoventral patterning in leaves | [78] |
| From leaves to the SAM | Limit SAM size | [29] | |
| CK | From roots to the SAM | Promote SAM growth in response to nitrate | [50,51] |
|
Nutritional Sugar |
From leaves to the SAM | Induction of vegetative phase change | [21,22] |
| Into the SAM | Sugar is required for SAM proliferation | [62,65] | |
| Unknown bypass | From roots to the SAM | Represses SAM growth | [55,56] |
| Seed-derived | From seed to the SAM | Terminate flower production | [58,59] |
| AhPHAN- dependent | From leaves to the SAM |
Promote SAM maintenance | [89] |
| Environmental signal | Site of perception | Effect | References |
| Light (quantity) | Leaves | Low light delays production of adult traits | [90,91] |
| Leaves | Light is required for SAM proliferation |
[62,64,65] | |
| Light (quality) | Leaves | Shade promotes flowering and represses leaf initiation | [63,92,93] |
| Leaves | Shade promotes SAM size | [94] | |
| Temperature | Shoot apex | Leaf initiation rate increases with (sub-maximal) temperature | [75,95] |
| Unknown | Cold temperatures inhibit SAM growth | [61] | |
| Leaves and SAM | Induction of flowering by vernalization | [60] | |
| Oxygen | SAM | Low oxygen concentrations promote SAM activity | [70] |
| SAM | Reactive oxygen species regulate stem cell maintenance | [74] |
Regardless of how transport into the SAM is regulated, the exclusion of sRNAs and proteins that could lead to the mis-expression of SAM regulators is critical to ensuring balanced meristematic growth. Understanding the cellular and spatial mechanisms by which access to the SAM, and to subdomains within the SAM, is regulated is an important avenue of future research. Recent transcriptome [32*] and translatome [87*] maps of domains in the shoot apex make it easier to identify localized patterns of gene expression, and facilitate predictions about whether genes function non-cell autonomously across the shoot apex. These predictions can be tested using domain specific-expression approaches [24**,27**]. Such techniques will also be useful in elucidating the contribution of the SAM to the perception and interpretation of environmental signals.
Acknowledgements
We apologize to authors’ whose work we were unable to include due to size constraints and thank members of the Poethig lab for useful discussions. Work in the Poethig lab on developmental transitions is funded by National Institutes of Health grant R01-GM51893 to R.S.P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.Sussex IM: The permanence of meristems: Developmental organizers or reactors to exogenous stimuli? In Meristems and Differentiation. . Brookhaven National Laboratory; 1964:1–12. [Google Scholar]
- 2.Han H, Liu X, Zhou Y: Transcriptional circuits in control of shoot stem cell homeostasis. Curr Opin Plant Biol 2020, 53:50–56. [DOI] [PubMed] [Google Scholar]
- 3.Kitagawa M, Jackson D: Control of Meristem Size. Annu Rev Plant Biol 2019, 70:269–291. [DOI] [PubMed] [Google Scholar]
- 4.Zeevaart JAD: Physiology of Flower Formation. Annu Rev Plant Physiol 1976, 27:321–348. [Google Scholar]
- 5.Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, et al. : FT Protein Movement Contributes to Long-Distance Signaling in Floral Induction of Arabidopsis. Science 2007, 316:1030. [DOI] [PubMed] [Google Scholar]
- 6.Jaeger KE, Wigge PA: FT Protein Acts as a Long-Range Signal in Arabidopsis. Current Biology 2007, 17:1050–1054. [DOI] [PubMed] [Google Scholar]
- 7.Mathieu J, Warthmann N, Küttner F, Schmid M: Export of FT Protein from Phloem Companion Cells Is Sufficient for Floral Induction in Arabidopsis. Current Biology 2007, 17:1055–1060. [DOI] [PubMed] [Google Scholar]
- 8.Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K: Hd3a Protein Is a Mobile Flowering Signal in Rice. Science 2007, 316:1033–1036. [DOI] [PubMed] [Google Scholar]
- 9.Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T: FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 2005, 309:1052–1056. [DOI] [PubMed] [Google Scholar]
- 10.Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, Weigel D: Integration of spatial and temporal information during floral induction in Arabidopsis. Science 2005, 309:1056–1059. [DOI] [PubMed] [Google Scholar]
- 11.Collani S, Neumann M, Yant L, Schmid M: FT Modulates Genome-Wide DNA-Binding of the bZIP Transcription Factor FD. Plant Physiol 2019, 180:367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawamoto N, Sasabe M, Endo M, Machida Y, Araki T: Calcium-dependent protein kinases responsible for the phosphorylation of a bZIP transcription factor FD crucial for the florigen complex formation. Scientific Reports 2015, 5:8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eriksson S, Böhlenius H, Moritz T, Nilsson O: GA4 Is the Active Gibberellin in the Regulation of LEAFY Transcription and Arabidopsis Floral Initiation. Plant Cell 2006, 18:2172–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King RW, Moritz T, Evans LT, Junttila O, Herlt AJ: Long-Day Induction of Flowering in Lolium temulentumInvolves Sequential Increases in Specific Gibberellins at the Shoot Apex. Plant Physiol 2001, 127:624–632. [PMC free article] [PubMed] [Google Scholar]
- 15.Kanno Y, Oikawa T, Chiba Y, Ishimaru Y, Shimizu T, Sano N, Koshiba T, Kamiya Y, Ueda M, Seo M: AtSWEET13 and AtSWEET14 regulate gibberellin-mediated physiological processes. Nature Communications 2016, 7:13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regnault T, Davière J-M, Wild M, Sakvarelidze-Achard L, Heintz D, Carrera Bergua E, Lopez Diaz I, Gong F, Hedden P, Achard P: The gibberellin precursor GA12 acts as a long-distance growth signal in Arabidopsis. Nature Plants 2015, 1:15073. [DOI] [PubMed] [Google Scholar]
- 17.Andrés F, Porri A, Torti S, Mateos J, Romera-Branchat M, García-Martínez JL, Fornara F, Gregis V, Kater MM, Coupland G: SHORT VEGETATIVE PHASE reduces gibberellin biosynthesis at the Arabidopsis shoot apex to regulate the floral transition. Proc Natl Acad Sci USA 2014, 111:E2760–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poethig RS: Vegetative phase change and shoot maturation in plants. Curr Top Dev Biol 2013, 105:125–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wardlaw CW: The morphogenetic rôle of apical meristems: fundamental aspects (illustrated by means of the shoot apical meristem) In Differentiation and Development / Differenzierung und Entwicklung: Part 1 / Teil 1. Edited by Ruhland W. Springer Berlin; Heidelberg; 1965:443–451. [Google Scholar]
- 20.Yang L, Conway SR, Poethig RS: Vegetative phase change is mediated by a leaf-derived signal that represses the transcription of miR156. Development 2011, 138:245–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang L, Xu M, Koo Y, He J, Poethig RS: Sugar promotes vegetative phase change in Arabidopsis thaliana by repressing the expression of MIR156A and MIR156C. Elife 2013, 2:e00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu S, Cao L, Zhou CM, Zhang TQ, Lian H, Sun Y, Wu JQ, Huang JR, Wang GD, Wang JW: Sugar is an endogenous cue for juvenile-to-adult phase transition in plants. Elife 2013, 2:e00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang JW, Schwab R, Czech B, Mica E, Weigel D: Dual effects of miR156-targeted SPL genes and CYP78A5/KLUH on plastochron length and organ size in Arabidopsis thaliana. Plant Cell 2008, 20:1231–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fouracre JP, Poethig RS: Role for the shoot apical meristem in the specification of juvenile leaf identity in Arabidopsis. Proc Natl Acad Sci USA 2019, 116:10168–10177. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Analyses of meristem defective mutants and SAM localized expression of miR156 identifies a requirement for the SAM in the specification of juvenile leaf identity. The expression of miR156 within the SAM is able to both regulate the identity of early leaves, and suppress the expression SPL in leaf primordia, suggesting that miR156 functions non-cell autonomously across the shoot apex.
- 25.Han H, Yan A, Li L, Zhu Y, Feng B, Liu X, Zhou Y: A signal cascade originated from epidermis defines apical-basal patterning of Arabidopsis shoot apical meristems. Nature Communications 2020, 11:1214. [DOI] [PMC free article] [PubMed] [Google Scholar]; * The authors reveal that the expression gradient of HAM transcription factors within the SAM is determined by epidermal expression of miR171. The induction of miR171 within the epidermis by ATML1 limits the apical accumulation of HAMs, and reinforces apical-basal patterning within the SAM.
- 26.Knauer S, Holt AL, Rubio-Somoza I, Tucker EJ, Hinze A, Pisch M, Javelle M, Timmermans MC, Tucker MR, Laux T: A protodermal miR394 signal defines a region of stem cell competence in the Arabidopsis shoot meristem. Dev Cell 2013, 24:125–32. [DOI] [PubMed] [Google Scholar]
- 27.Skopelitis DS, Hill K, Klesen S, Marco CF, von Born P, Chitwood DH, Timmermans MCP: Gating of miRNA movement at defined cell-cell interfaces governs their impact as positional signals. Nat Commun 2018, 9:3107. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** By expressing a GFP reporter and an anti-GFP miRNA (miRGFP) in different functional domains of the SAM, the authors show that movement of the miRGFP is restricted between cells of the SAM. This restriction is independent of protein mobility, suggesting that inter-cellular movement of miRNAs is highly and specifically regulated.
- 28.Ma Y, Miotk A, Šutiković Z, Ermakova O, Wenzl C, Medzihradszky A, Gaillochet C, Forner J, Utan G, Brackmann K, et al. : WUSCHEL acts as an auxin response rheostat to maintain apical stem cells in Arabidopsis. Nature Communications 2019, 10:5093. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study demonstrates that a basal level of auxin signaling is required for stem cell maintenance, but that too much auxin signaling promotes stem cell differentiation. WUSCHEL functions to repress auxin signaling in the SAM largely via histone-deacytlation of its target genes.
- 29.Shi B, Guo X, Wang Y, Xiong Y, Wang J, Hayashi K, Lei J, Zhang L, Jiao Y: Feedback from Lateral Organs Controls Shoot Apical Meristem Growth by Modulating Auxin Transport. Developmental Cell 2018, 44:204–216.e6. [DOI] [PubMed] [Google Scholar]; ** Modeling and experimental analyses show that lateral organs and the SAM form a competitive auxin transport network. Increased flow of auxin from lateral organs limits the export of auxin from the SAM, limiting its size. Auxin transport from lateral organs is dependent on the activity of YABBY genes.
- 30.Goldshmidt A, Alvarez JP, Bowman JL, Eshed Y: Signals Derived from YABBY Gene Activities in Organ Primordia Regulate Growth and Partitioning of Arabidopsis Shoot Apical Meristems. Plant Cell 2008, 20:1217–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Je BI, Gruel J, Lee YK, Bommert P, Arevalo ED, Eveland AL, Wu Q, Goldshmidt A, Meeley R, Bartlett M, et al. : Signaling from maize organ primordia via FASCIATED EAR3 regulates stem cell proliferation and yield traits. Nature Genetics 2016, 48:785–791. [DOI] [PubMed] [Google Scholar]
- 32.Knauer S, Javelle M, Li L, Li X, Ma X, Wimalanathan K, Kumari S, Johnston R, Leiboff S, Meeley R, et al. : A high-resolution gene expression atlas links dedicated meristem genes to key architectural traits. Genome Research 2019, 29:1962–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]; * By isolating domains of the maize SAM using laser capture microdissection, the authors use RNA-seq to describe the spatial distribution of genetic networks. These networks reveal differences in the regulation of the SAM between maize and Arabidopsis, and positive correlations with agriculturally important SNPs
- 33.McConnell JR, Emery J, Eshed Y, Bao N, Bowman J, Barton MK: Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature 2001, 411:709–713. [DOI] [PubMed] [Google Scholar]
- 34.Prigge MJ, Otsuga D, Alonso JM, Ecker JR, Drews GN, Clark SE: Class III homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. Plant Cell 2005, 17:61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Q, Yao X, Pi L, Wang H, Cui X, Huang H: The ARGONAUTE10 gene modulates shoot apical meristem maintenance and establishment of leaf polarity by repressing miR165/166 in Arabidopsis. Plant J 2009, 58:27–40. [DOI] [PubMed] [Google Scholar]
- 36.Miyashima S, Honda M, Hashimoto K, Tatematsu K, Hashimoto T, Sato-Nara K, Okada K, Nakajima K: A comprehensive expression analysis of the Arabidopsis MICRORNA165/6 gene family during embryogenesis reveals a conserved role in meristem specification and a non-cell-autonomous function. Plant Cell Physiol 2013, 54:375–84. [DOI] [PubMed] [Google Scholar]
- 37.Lynn K, Fernandez A, Aida M, Sedbrook J, Tasaka M, Masson P, Barton MK: The PINHEAD/ZWILLE gene acts pleiotropically in Arabidopsis development and has overlapping functions with the ARGONAUTE1 gene. Development 1999, 126:469–481. [DOI] [PubMed] [Google Scholar]
- 38.Tucker MR, Hinze A, Tucker EJ, Takada S, Jurgens G, Laux T: Vascular signalling mediated by ZWILLE potentiates WUSCHEL function during shoot meristem stem cell development in the Arabidopsis embryo. Development 2008, 135:2839–2843. [DOI] [PubMed] [Google Scholar]
- 39.Zhu H, Hu F, Wang R, Zhou X, Sze SH, Liou LW, Barefoot A, Dickman M, Zhang X: Arabidopsis Argonaute10 specifically sequesters miR166/165 to regulate shoot apical meristem development. Cell 2011, 145:242–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu Y, Ji L, Le BH, Zhai J, Chen J, Luscher E, Gao L, Liu C, Cao X, Mo B, et al. : ARGONAUTE10 promotes the degradation of miR165/6 through the SDN1 and SDN2 exonucleases in Arabidopsis. PLOS Biology 2017, 15:e2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naramoto S, Jones VAS, Trozzi N, Sato M, Toyooka K, Shimamura M, Ishida S, Nishitani K, Ishizaki K, Nishihama R, et al. : A conserved regulatory mechanism mediates the convergent evolution of plant shoot lateral organs. PLOS Biology 2019, 17:e3000560. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Using a mutant screen the authors identify a member of the ALOG gene family that regulates lateral organ outgrowth in liverworts – MpLOS1. In addition to regulating lateral organ development, MpLOS1 non-cell autonomously promotes apical meristem maintenance. ALOG function appears to be conserved through land plant evolution, as MpLOS1 is able to rescue loss-of G1 in rice.
- 42.MacAlister CA, Park SJ, Jiang K, Marcel F, Bendahmane A, Izkovich Y, Eshed Y, Lippman ZB: Synchronization of the flowering transition by the tomato TERMINATING FLOWER gene. Nature Genetics 2012, 44:1393–1398. [DOI] [PubMed] [Google Scholar]
- 43.Takeda S, Hanano K, Kariya A, Shimizu S, Zhao L, Matsui M, Tasaka M, Aida M: CUP-SHAPED COTYLEDON1 transcription factor activates the expression of LSH4 and LSH3, two members of the ALOG gene family, in shoot organ boundary cells. The Plant Journal 2011, 66:1066–1077. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida A, Sasao M, Yasuno N, Takagi K, Daimon Y, Chen R, Yamazaki R, Tokunaga H, Kitaguchi Y, Sato Y, et al. : TAWAWA1, a regulator of rice inflorescence architecture, functions through the suppression of meristem phase transition. Proc Natl Acad Sci USA 2013, 110:767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hepworth SR, Pautot VA: Beyond the Divide: Boundaries for Patterning and Stem Cell Regulation in Plants. Frontiers in Plant Science 2015, 6:1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Q, Hasson A, Rossmann S, Theres K: Divide et impera: boundaries shape the plant body and initiate new meristems. New Phytologist 2016, 209:485–498. [DOI] [PubMed] [Google Scholar]
- 47.Chickarmane VS, Gordon SP, Tarr PT, Heisler MG, Meyerowitz EM: Cytokinin signaling as a positional cue for patterning the apical–basal axis of the growing Arabidopsis shoot meristem. Proc Natl Acad Sci USA 2012, 109:4002–4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gordon SP, Chickarmane VS, Ohno C, Meyerowitz EM: Multiple feedback loops through cytokinin signaling control stem cell number within the Arabidopsis shoot meristem. Proc Natl Acad Sci U S A 2009, 106:16529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gruel J, Landrein B, Tarr P, Schuster C, Refahi Y, Sampathkumar A, Hamant O, Meyerowitz EM, Jönsson H: An epidermis-driven mechanism positions and scales stem cell niches in plants. Sci Adv 2016, 2:e1500989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Landrein B, Formosa-Jordan P, Malivert A, Schuster C, Melnyk CW, Yang W, Turnbull C, Meyerowitz EM, Locke JCW, Jönsson H: Nitrate modulates stem cell dynamics in Arabidopsis shoot meristems through cytokinins. Proc Natl Acad Sci USA 2018, 115:1382–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** It has previously been demonstrated that root-to-shoot cytokinin signaling regulates SAM size. This study demonstrates an interaction between mobile CKs and WUS expression in the shoot. Furthermore, it shows that WUS expression is modulated by root nitrate conditions, providing a mechanism by which nutrient conditions regulate shoot growth.
- 51.Osugi A, Kojima M, Takebayashi Y, Ueda N, Kiba T, Sakakibara H: Systemic transport of trans-zeatin and its precursor have differing roles in Arabidopsis shoots. Nature Plants 2017, 3:17112. [DOI] [PubMed] [Google Scholar]
- 52.Ko D, Kang J, Kiba T, Park J, Kojima M, Do J, Kim KY, Kwon M, Endler A, Song W-Y, et al. : Arabidopsis ABCG14 is essential for the root-to-shoot translocation of cytokinin. Proc Natl Acad Sci USA 2014, 111:7150–7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang K, Novak O, Wei Z, Gou M, Zhang X, Yu Y, Yang H, Cai Y, Strnad M, Liu C-J: Arabidopsis ABCG14 protein controls the acropetal translocation of root-synthesized cytokinins. Nature Communications 2014, 5:3274. [DOI] [PubMed] [Google Scholar]
- 54.Takei K, Sakakibara H, Taniguchi M, Sugiyama T: Nitrogen-Dependent Accumulation of Cytokinins in Root and the Translocation to Leaf: Implication of Cytokinin Species that Induces Gene Expression of Maize Response Regulator. Plant and Cell Physiology 2001, 42:85–93. [DOI] [PubMed] [Google Scholar]
- 55.Lee D-K, Parrott DL, Adhikari E, Fraser N, Sieburth LE: The Mobile bypass Signal Arrests Shoot Growth by Disrupting Shoot Apical Meristem Maintenance, Cytokinin Signaling, and WUS Transcription Factor Expression. Plant Physiol 2016, 171:2178–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Norman JM, Frederick RL, Sieburth LE: BYPASS1 Negatively Regulates a Root-Derived Signal that Controls Plant Architecture. Current Biology 2004, 14:1739–1746. [DOI] [PubMed] [Google Scholar]
- 57.Van Norman JM, Sieburth LE: Dissecting the biosynthetic pathway for the bypass1 root-derived signal. The Plant Journal 2007, 49:619–628. [DOI] [PubMed] [Google Scholar]
- 58.Balanzà V, Martinez-Fernandez I, Sato S, Yanofsky MF, Kaufmann K, Angenent GC, Bemer M, Ferrandiz C: Genetic control of meristem arrest and life span in Arabidopsis by a FRUITFULL-APETALA2 pathway. Nat Commun 2018, 9:565. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Reproductive growth in monocarpic plants is terminated when WUS expression ceases in the inflorescence SAM. This paper identifies a genetic network that determines the duration of WUS expression during reproductive growth. It shows that FRUITFULL coordinates an age-dependent mechanism that represses WUS expression, in parallel to additional repressive signals from seed.
- 59.Balanzà V, Martínez-Fernández I, Sato S, Yanofsky MF, Ferrándiz C: Inflorescence Meristem Fate Is Dependent on Seed Development and FRUITFULL in Arabidopsis thaliana. Frontiers in Plant Science 2019, 10:1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Searle I, He Y, Turck F, Vincent C, Fornara F, Kröber S, Amasino RA, Coupland G: The transcription factor FLC confers a flowering response to vernalization by repressing meristem competence and systemic signaling in Arabidopsis. Genes & Development 2006, 20:898–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Jonge J, Kodde J, Severing EI, Bonnema G, Angenent GC, Immink RGH, Groot SPC: Low Temperature Affects Stem Cell Maintenance in Brassica oleracea Seedlings. Frontiers in Plant Science 2016, 7:800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pfeiffer A, Janocha D, Dong Y, Medzihradszky A, Schöne S, Daum G, Suzaki T, Forner J, Langenecker T, Rempel E, et al. : Integration of light and metabolic signals for stem cell activation at the shoot apical meristem. eLife 2016, 5:e17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Galvāo VC, Fiorucci A-S, Trevisan M, Franco-Zorilla JM, Goyal A, Schmid-Siegert E, Solano R, Fankhauser C: PIF transcription factors link a neighbor threat cue to accelerated reproduction in Arabidopsis. Nature Communications 2019, 10:4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li X, Cai W, Liu Y, Li H, Fu L, Liu Z, Xu L, Liu H, Xu T, Xiong Y: Differential TOR activation and cell proliferation in Arabidopsis root and shoot apexes. Proc Natl Acad Sci USA 2017, 114:2765–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mohammed B, Bilooei SF, Dóczi R, Grove E, Railo S, Palme K, Ditengou FA, Bögre L, López-Juez E: Converging Light, Energy and Hormonal Signaling Control Meristem Activity, Leaf Initiation, and Growth. Plant Physiol 2018, 176:1365–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoshida S, Mandel T, Kuhlemeier C: Stem cell activation by light guides plant organogenesis. Genes & Development 2011, 25:1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fleming A: Metabolic aspects of organogenesis in the shoot apical meristem. Journal of Experimental Botany 2006, 57:1863–1870. [DOI] [PubMed] [Google Scholar]
- 68.Fichtner F, Olas JJ, Feil R, Watanabe M, Krause U, Hoefgen R, Stitt M, Lunn JE: Functional Features of TREHALOSE-6-PHOSPHATE SYNTHASE1, an Essential Enzyme in Arabidopsis. Plant Cell 2020, 32:1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wahl V, Ponnu J, Schlereth A, Arrivault S, Langenecker T, Franke A, Feil R, Lunn JE, Stitt M, Schmid M: Regulation of Flowering by Trehalose-6-Phosphate Signaling in Arabidopsis thaliana. Science 2013, 339:704. [DOI] [PubMed] [Google Scholar]
- 70.Weits DA, Kunkowska AB, Kamps NCW, Portz KMS, Packbier NK, Nemec Venza Z, Gaillochet C, Lohmann JU, Pedersen O, van Dongen JT, et al. : An apical hypoxic niche sets the pace of shoot meristem activity. Nature 2019, 569:714–717. [DOI] [PubMed] [Google Scholar]; ** This study demonstrates that, similar to animal stem cell niches, the SAM CZ is a hypoxic environment. Increased oxygen inhibits leaf initiation, in part through oxygen-dependent proteolysis of the LITTLE ZIPPER2 (ZPR2) protein. ZPR2 is expressed within the SAM, where it regulates the activity of specific members of the HD-ZIP III family.
- 71.Dolzblasz A, Smakowska E, Gola EM, Sokołowska K, Kicia M, Janska H: The mitochondrial protease AtFTSH4 safeguards Arabidopsis shoot apical meristem function. Scientific Reports 2016, 6:28315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kitagawa M, Balkunde R, Bui H, Jackson D: An Aminoacyl tRNA Synthetase, OKI1, Is Required for Proper Shoot Meristem Size in Arabidopsis. Plant and Cell Physiology 2019, 60:2597–2608. [DOI] [PubMed] [Google Scholar]
- 73.Yang F, Bui HT, Pautler M, Llaca V, Johnston R, Lee BH, Kolbe A, Sakai H, Jackson D: A maize glutaredoxin gene, abphyl2, regulates shoot meristem size and phyllotaxy. Plant Cell 2015, 27:121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zeng J, Dong Z, Wu H, Tian Z, Zhao Z: Redox regulation of plant stem cell fate. The EMBO Journal 2017, 36:2844–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Savvides A, Dieleman JA, van Ieperen W, Marcelis LFM: A unique approach to demonstrating that apical bud temperature specifically determines leaf initiation rate in the dicot Cucumis sativus. Planta 2016, 243:1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sussex IM: Experiments on the Cause of Dorsiventrality in Leaves. Nature 1951, 167:651–652. [DOI] [PubMed] [Google Scholar]
- 77.Reinhardt D, Frenz M, Mandel T, Kuhlemeier C: Microsurgical and laser ablation analysis of leaf positioning and dorsoventral patterning in tomato. Development 2005, 132:15–26. [DOI] [PubMed] [Google Scholar]
- 78.Caggiano MP, Yu XL, Bhatia N, Larsson A, Ram H, Ohno C, Sappl P, Meyerowitz EM, Jonsson H, Heisler MG: Cell type boundaries organize plant development. Elife 2017, 6:e27421. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** The authors extend previous work on the expression domains of abaxial and adaxial regulators of leaf polarity to show that dorsoventrality is pre-patterned by the expression domains of REV and KAN1 in the SAM, and that these domains are separated and reinforced by auxin activity. Disruption of these domains by wounding explains the results of the classic Sussex experiments into dorsoventrality.
- 79.Husbands AY, Chitwood DH, Plavskin Y, Timmermans MC: Signals and prepatterns: new insights into organ polarity in plants. Genes Dev 2009, 23:1986–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kerstetter RA, Bollman K, Taylor RA, Bomblies K, Poethig RS: KANADI regulates organ polarity in Arabidopsis. Nature 2001, 411:706–709. [DOI] [PubMed] [Google Scholar]
- 81.Guan C, Wu B, Yu T, Wang Q, Krogan NT, Liu X, Jiao Y: Spatial Auxin Signaling Controls Leaf Flattening in Arabidopsis. Current Biology 2017, 27:2940–2950.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qi J, Wang Y, Yu T, Cunha A, Wu B, Vernoux T, Meyerowitz E, Jiao Y: Auxin depletion from leaf primordia contributes to organ patterning. Proc Natl Acad Sci U S A 2014, 111:18769–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bhatia N, Åhl H, Jönsson H, Heisler MG: Quantitative analysis of auxin sensing in leaf primordia argues against proposed role in regulating leaf dorsoventrality. eLife 2019, 8:e39298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guan C, Du F, Xiong Y, Jiao Y: The 35S promoter-driven mDII auxin control sensor is uniformly distributed in leaf primordia. Journal of Integrative Plant Biology 2019, 61:1114–1120. [DOI] [PubMed] [Google Scholar]
- 85.Balkunde R, Kitagawa M, Xu XM, Wang J, Jackson D: SHOOT MERISTEMLESS trafficking controls axillary meristem formation, meristem size and organ boundaries in Arabidopsis. The Plant Journal 2017, 90:435–446. [DOI] [PubMed] [Google Scholar]
- 86.Yadav RK, Perales M, Gruel J, Girke T, Jonsson H, Reddy GV: WUSCHEL protein movement mediates stem cell homeostasis in the Arabidopsis shoot apex. Genes Dev 2011, 25:2025–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tian C, Wang Y, Yu H, He J, Wang J, Shi B, Du Q, Provart NJ, Meyerowitz EM, Jiao Y: A gene expression map of shoot domains reveals regulatory mechanisms. Nature Communications 2019, 10:141. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Employing promoters specific to a number of distinct domains of the SAM and young leaves, the authors use TRAP-seq to define a translatome map across the Arabidopsis shoot apex. This map revealed novel roles for previously characterized transcription factors in the regulation of axillary meristem formation.
- 88.Besnard F, Refahi Y, Morin V, Marteaux B, Brunoud G, Chambrier P, Rozier F, Mirabet V, Legrand J, Laine S, et al. : Cytokinin signalling inhibitory fields provide robustness to phyllotaxis. Nature 2014, 505:417–21. [DOI] [PubMed] [Google Scholar]
- 89.Waites R, Selvadurai HRN, Oliver IR, Hudson A: The PHANTASTICA Gene Encodes a MYB Transcription Factor Involved in Growth and Dorsoventrality of Lateral Organs in Antirrhinum. Cell 1998, 93:779–789. [DOI] [PubMed] [Google Scholar]
- 90.Leichty AR, Poethig RS: Development and evolution of age-dependent defenses in ant-acacias. Proc Natl Acad Sci USA 2019, 116:15596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Willmann MR, Poethig RS: The effect of the floral repressor FLC on the timing and progression of vegetative phase change in Arabidopsis. Development 2011, 138:677–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xie Y, Liu Y, Wang H, Ma X, Wang B, Wu G, Wang H: Phytochrome-interacting factors directly suppress MIR156 expression to enhance shade-avoidance syndrome in Arabidopsis. Nature Communications 2017, 8:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xie Y, Zhou Q, Zhao Y, Li Q, Liu Y, Ma M, Wang B, Shen R, Zheng Z, Wang H: FHY3 and FAR1 Integrate Light Signals with the miR156-SPL Module-Mediated Aging Pathway to Regulate Arabidopsis Flowering. Molecular Plant 2020, 13:483–498. [DOI] [PubMed] [Google Scholar]
- 94.Chitwood DH, Kumar R, Ranjan A, Pelletier JM, Townsley BT, Ichihashi Y, Martinez CC, Zumstein K, Harada JJ, Maloof JN, et al. : Light-Induced Indeterminacy Alters Shade-Avoiding Tomato Leaf Morphology. Plant Physiol 2015, 169:2030–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Granier C, Massonnet C, Turc O, Muller B, Chenu K, Tardieu F: Individual Leaf Development in Arabidopsis thaliana: a Stable Thermal-time-based Programme. Annals of Botany 2002, 89:595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]


