Abstract
Purpose:
Antiretroviral therapy reduced infectious eye diseases (EDs) in HIV infected people. There is limited data on age-related EDs and visual impairment (VI) in people living with HIV. We report prevalence of VI and spectrum of EDs in HIV infected people in an ART era in Rakai, Uganda.
Methods:
A philanthropic campaign during 2009-2012 provided ophthalmic services to HIV+ patients in care. Unilateral presenting visual acuity (VA) was assessed by a trained staff in HIV clinics using a 6-meter Snellen chart. A slit lamp examination by an ophthalmologist evaluated eyes with impaired acuity. A retrospective chart review was later conducted retrieving data of patients participating the ophthalmic service. VI was defined referencing WHO’s ICD-11. Ophthalmic diagnosis was summarized by VI level. Logistic regressions estimated demographic associations with cataract diagnosis.
Results:
688 HIV+ patients were evaluated, median age was 44 (IQR: 37-50) years, 69% were female. 51% were on ART (median duration 4, IQR: 2-5 years). Crude prevalence of moderate/severe VI and blindness were both 2%. The main diagnoses were refractive error (55%), conjunctivitis (18%), cataract (15%), and pterygium (11%). Cataract prevalences were 10%, 12% and 26% among age groups of 19-34, 35-49 and ≥ 50 years, respectively. Cataract was found in 73% of the HIV+s with blindness and in 63% of those with moderate/severe VI. Older age and male sex were significantly associated with higher cataract prevalence.
Conclusion:
VI in HIV+ patients in Rakai was mainly due to refractive error and cataract. Cataract was common in all age groups.
Keywords: HIV, Cataract, Visual impairment, Africa, Accelerated Aging, Blindness, Toxoplasmosis
Introduction
Human immunodeficiency virus (HIV) infection can lead to acquired immunodeficiency syndrome (AIDS) affecting a broad spectrum of body organs.1 In 2017, 36.9 million people were living with HIV globally, in whom approximately 70% were in sub-Saharan Africa (SSA).2 In Uganda, 1.3 million people are HIV infected with a prevalence of 7.3% in 2016.3
Before antiretroviral therapy (ART) availability, most ocular diseases in HIV-infected patients were opportunistic infections which could lead to blindness in the absence of prompt treatment.4 Ocular manifestations secondary to HIV infection may involve different segments of the eye,1,5,6 and approximately 70% to 80% of ART naive HIV-infected patients experienced an HIV-associated eye disorder.7 However, the wide availability of ART has dramatically reduced the incidence of opportunistic infections and shifted the spectrum of ophthalmic disease among HIV infected patients.8,9 Immune recovery uveitis following ART initiation due to an immune response to cytomegalovirus antigen in the eye, is the most common form of immune reconstitution inflammatory syndrome in the early introduction of highly active antiretroviral therapy.10 ART has transformed HIV into a chronic disease, and ART treated HIV-infected patients may expect a normal or near normal lifespan.11 However, compared to HIV-negative people, ART treated HIV-infected patients are at higher risks of a myriad of age-related diseases, including cardiovascular disease,12 non-AIDS defining cancers,13 osteopenia/osteoporosis,14 liver disease,15 renal disease,16 and neurocognitive decline.17 Data on ocular functions and diseases in HIV-infected people in the ART era are mainly from developed countries18-20 where the dominant circulating strains are HIV subtypes B and C21. In SSA, limited data are available on the epidemiology of visual impairment and the spectrum of eye diseases in HIV-infected people in the ART era.
With philanthropic funds, the Uganda Rakai Health Sciences Program provided free ophthalmic services to HIV-infected patients who were under HIV care in 2009-2012 in Rakai district, Uganda. This retrospective chart review study aims to (i) describe the spectrum of eye diseases observed in the HIV positive patients who participated in the ophthalmic service camp, (ii) to report prevalence of visual impairment based on visual acuity (VA) and the pathologies associated with visual impairment, and (iii) to assess the associations of demographic variables and ART duration with prevalence of cataract diagnosis.
Materials and methods
This study is a retrospective review of medical charts of HIV-positive patients who participated in an ophthalmic service camp held during 2009-2012 at HIV clinics in Rakai district, south-central Uganda. Rakai district is of a typical rural African setting, with a generalized heterosexual HIV epidemic.22,23 The circulating HIV subtypes are mainly HIV subtype A, D and A/D recombinants.24 In the population based survey of Rakai residents aged 15-49 years conducted during 2009-2011, HIV prevalence was 13% and 23% of HIV-infected people were on ART.23 The Rakai Health Sciences Program (RHSP) has conducted HIV-related surveillance and provided free HIV related prevention, care, and treatment services in Rakai communities since the 1990s.22 ART became available in Rakai in 2004, and the RHSP has been the main provider providing over 90% of HIV care and ART to Rakai residents. The criteria for ART initiation was CD4 < 250 cells/mm3 from 2004 to mid-2011 and changed to CD4 < 350 cells/mm3 in mid-2011. During 2009-2012, the program provided free ophthalmic exams and services to HIV-infected patients who were in HIV care or on ART treatment. Specifically, all patients seen in RHSP’s HIV clinics were invited to participate in visual acuity (VA) assessments and ophthalmic exams provided by trained clinical staff and an ophthalmologist in the RHSP’s central facility. If appropriate, treatments (e.g., antibiotic eye drops) were offered at no cost. Patients with cataract who would benefit from surgery were also scheduled for cataract surgery provided by the ophthalmologist at no charge.25
During the ophthalmic service camp, unilateral presenting VA was assessed by a trained clinical officer using a 6-meter Snellen chart in a dedicated clinic room. Ophthalmic diagnoses were made by the ophthalmologist using a slip lamp exam. Findings were recorded and this chart review was conducted in 2015 using a standard data abstraction form recording demographics, year of the ocular exam, presenting VAs of both eyes and ophthalmic diagnoses. Additionally, since all participants were under RHSP’s HIV care, their ART initiation year was also retrieved from their HIV care records.
This study was conducted according to the International Conference on Harmonization Good Clinical Practice Guidelines, the applicable regulatory requirements, the current Declaration of Helsinki, and the Health Insurance Portability and Accountability Act. Data for this study did not record identifiable information, and the study was considered as exempt from informed consent process by the Institutional Review Board of the University of Massachusetts-Amherst.
Data analysis
Based on the presenting VA, visual impairment (VI) was categorized in reference to World Health Organization International Classification of Disease-11 (WHO ICD-11) guidelines26 as 1) Normal/mild VI (VA better than 20/70); 2) moderate VI: VA equal to or worse than 20/70 and equal to or better than 20/200; 3) severe VI: VA worse than 20/200 and equal to or better than 20/400; and 4) blind: VA worse than 20/400 to no light perception. Logistic regression was used to assess the associations between the prevalence of common eye pathologies and demographic characteristics and ART treatment duration. All statistical analyses were performed using R 3.5.0. All P-values reported were two-sided, and a significance level of 0.05 was used.
Results
There were 688 HIV-infected patients under HIV care who participated in the ophthalmic service, including 6 patients aged < 18 years. Of the 688 patients (Table 1), 477 (69.3%) were female. Their ages ranged from 4 to 80 years with a median age of 44 years [interquartile range (IQR): 37–50]. 349 (50.7%) of the participants were on ART therapy with a median ART duration of 4 years (IQR: 2-5; range: 1-8). All patients were in HIV care, of whom the median duration of HIV care was 4 years (IQR: 2-5; range 0-8).
Table 1.
Demographic and clinical characteristics of HIV-infected individuals in an ophthalmic service camp conducted during 2009-2012* in Rakai, Uganda (N = 688)
| Variable | Number (%) |
|---|---|
| Age (median, IQR, Range) | 44, 37 - 50, 4 - 80 |
| Age by group | |
| 4-18 | 6 (0.87) |
| 19-34 | 125 (18.17) |
| 35-49 | 379 (55.09) |
| 50+ | 178 (25.87) |
| Gender* | |
| Female | 477 (69.30) |
| Male | 211 (30.70) |
| History of ART | |
| No | 339 (49.30) |
| Yes | 349 (50.70) |
| Duration of ART (years) (median, IQR, Range) | 4 , 2 - 5, 1 - 8 |
| Duration in HIV care (years) (median, IQR, Range) | 4 , 2 - 5, 0 - 8 |
IQR: interquartile range, ART: antiretroviral therapy
: The Rakai Health Sciences Program run population based surveys of residents aged 15-49 years in 45 Rakai communities22. There were 15258 residents surveyed in the survey round conducted during 2009-2011. There were 1908 confirmed HIV-positive cases and 13272 HIV-negative cases (HIV prevalence 12.6%). Among the 1908 HIV-positive cases, the median age was 34 (IQR 28-39, range 15-49) years, 1260 (66.0%) were female, 830 (43.5%) had engaged in HIV care, and 431 (22.6%) had initiated ART.
The four most common pathologies diagnosed were refractive errors (N=366, 54.8%), conjunctivitis (N=120, 18.0%), cataract (N=101, 15.01%), and pterygium (N=71, 10.7%) (Table 2). Toxoplasmosis retinitis was found in three patients (0.45%). The full spectrum of pathologies are presented in Table 2.
Table 2.
Prevalence of ocular diagnosis for all participants of the ophthalmic service camp offered during 2009-2012 in Rakai, Uganda
| Diagnosis* | Frequency (n) | Percent (%)* |
|---|---|---|
| Refractive error | 366 | 54.80 |
| Allergic Conjunctivitis | 120 | 18.00 |
| Cataract | 101 | 15.10 |
| Pterygium | 71 | 10.70 |
| Corneal (scar and/or ulcer) | 12 | 1.80 |
| Uveitis(Post uveitis) | 7 | 1.00 |
| Squamous cell carcinoma | 6 | 0.90 |
| Foreign body | 4 | 0.60 |
| Toxoplasmosis retinitis | 3 | 0.45 |
| Vitreous opacity | 2 | 0.30 |
| Ectropion | 2 | 0.30 |
| Chalazion | 1 | 0.15 |
| Divergent squints | 1 | 0.15 |
| Post CMV retinitis | 1 | 0.15 |
| Post-operative complication | 1 | 0.15 |
| Zoster ophthalamia | 1 | 0.15 |
| Glaucoma | 1 | 0.15 |
| Trachoma | 1 | 0.15 |
Diagnosis record was retrieved from 688 patients.
When VI was defined by the better-seeing eye, 96.0% participants had a normal vision or mild VI (i.e., VA –better than 20/70) (Table 3); 2.1% participants had moderate VI (i.e., VA: 20/70 - 20/200); no participants had severe VI (i.e., VA worse than 20/200 – 20/400), and 11 (1.9%) participants had blindness (i.e., VA worse than 20/400). Regarding VI at the eye level, 92.0% eyes had no or mild VI, 2.7% with moderate VI, and 5.3% with blindness (Table 3). Table 3 also presents the crude prevalence estimates by age groups of 19-49 and ≥50 years.
Table 3.
Crude prevalence of visual impairment for the better/worse seeing-eyes and all eyes* and by age group
| Visual impairment | Better-seeing eye, N (%) | All eyes, N (%) |
|---|---|---|
| Normal/Mild: better than 20/70 | 563/586 (96.00) | 1077/1171 (92.00) |
| Among age 19-49 years | 412/421 (97.86) | 794/841 (94.44) |
| Among age ≥50 years | 150/164 (91.46) | 281/328 (85.67) |
| Moderate: equal to or worse than 20/70 and equal to or better than 20/200 | 12/586 (2.10) | 32/1171 (2.70) |
| Among age 19-49 years | 4/421 (0.95) | 15/841 (1.55) |
| Among age ≥50 years | 8/164 (4.88) | 19/328 (5.79) |
| Severe: worse than 20/200 and equal to or better than 20/400 | 0 | 0 |
| Among age 19-49 years | 0 | 0 |
| Among age ≥50 years | 0 | 0 |
| Blind: worse than 20/400 to no light perception | 11/586 (1.90) | 62/1171 (5.30) |
| Among age 19-49 years | 5/421 (1.19) | 34/841 (4.04) |
| Among age ≥50 years | 6/164 (3.66) | 28/328 (8.54) |
Visual acuity was available for 1171 eyes of 586 patients of whom 585 patients aged≥18 years
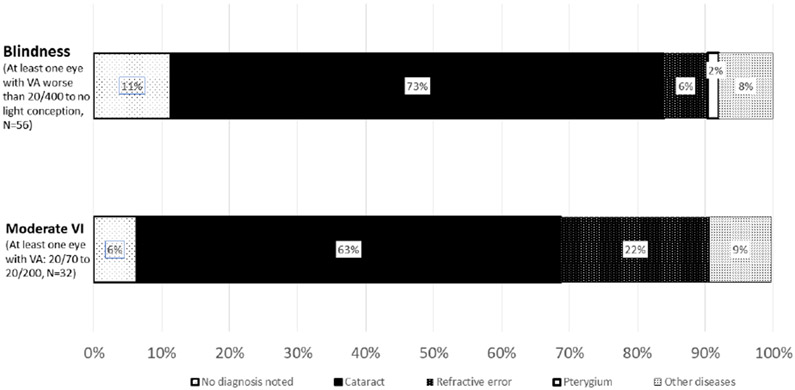
To examine the pathologies associated with VI, Figure 1 shows the distribution of pathologies in participants with blindness or moderate VI. In participants with at least one eye blind (i.e. VA worse than 20/400 or no light conception) (N=56), 73.0% had a diagnosis of cataract, and 6.0% had a diagnosis of refractive error. In participants with at least one eye with moderate VI (i.e. VA between 20/70 to 20/200) (N=32), 63.0% had a cataract diagnosed, and 22.0% had a refractive error diagnosed.
Figure 1:
Distribution of ocular pathological diagnosis among HIV+ participants with blindness or moderate visual impairment (VI). There were no eyes with acuity of severe visual impairment.
To explore factors associated with cataract diagnosis, Table 4 presents the unadjusted and adjusted prevalence odds ratio (POR) for cataract. The crude and adjusted logistic models showed comparable results. Males had higher odds of cataract compared to females [adjusted POR, 1.66, 95% confidence interval (CI) 1.05-2.59]. When adjusting for sex and ART duration, each 5 years increased in age was associated with significantly higher odds of having cataract (adjusted POR, 5.25, 95% CI 5.15-5.40); categorized adults ≥ 50 years old compared with 19-34 years old was significantly associated with higher odds of having cataract (adjusted POR, 2.63, 95% CI 1.34-5.49). When adjusting for age and sex, each 5 years increase in ART duration was significantly associated with lower odds of cataract diagnosis (adjusted POR, 0.52, 95% CI 0.30-0.88); categorized received ART therapy ≥ 5 years compared with non-ART therapy was significantly associated with lower odds of diagnosis of cataract (adjusted POR, 0.36, 95% CI 0.15-0.75).
Table 4.
Logistic regression of cataracts and demographic characteristics and ART duration among HIV+ participants
| N (%) | Crude prevalence OR, 95% CI |
P-value | Adjusted prevalence OR, 95% CI* |
P-value | |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 56/459 (12.2%) | REF | Ref | Ref | Ref |
| Male | 45/203 (22.2%) | 2.05 (1.33,3.16) | <0.01 | 1.66 (1.05, 2.59) | 0.03 |
| Age (continuous) | 1.06 (1.04,1.09) | <0.01 | 1.05 (1.03,1.08) | <0.01 | |
| Age (categorical) | |||||
| 19-34 | 12/118 (10.2%) | Ref | Ref | Ref | Ref |
| 35-49 | 44/369 (11.9%) | 1.20 (0.63,2.45) | 0.6 | 1.13 (0.59,2.31) | 0.72 |
| ≥ 50 | 45/175 (25.7%) | 3.06 (1.58,6.32) | <0.01 | 2.63 (1.34,5.49) | <0.01 |
| ART duration (continuous)** | 0.56 (0.33,0.94) | 0.03 | 0.52 (0.30,0.88) | 0.02 | |
| ART duration (categorical) | |||||
| Non-ART | 54/327(16.5%) | Ref | Ref | Ref | Ref |
| 1-2 years | 19/106 (17.9%) | 1.10 (0.61, 1.94) | 0.73 | 1.19 (0.64,2.13) | 0.57 |
| 3-4 years | 20/118 (16.9%) | 1.03 (0.58, 1.79) | 0.91 | 0.97 (0.53,1.71) | 0.91 |
| ≥ 5 years | 8/111 (7.2%) | 0.39 (0.17, 0.81) | 0.02 | 0.36 (0.15,0.75) | 0.01 |
ART, antiretroviral therapy; HIV, human immunodeficiency virus; HLT, Holsmer-Lemeshow test; OR, odds ratio; CI, confidence interval
adjusted age and sex
report OR associated with every 5 years of more ART use
Discussion
The study reported ocular manifestations among HIV positive patients in an ART era (2009-2012) in Rakai, Uganda. We observed that refractive error, conjunctivitis, and cataract were the main pathological diagnosis in this rural Ugandan population. Older age and male sex were associated with diagnosis of cataracts, and longer ART duration was associated with lower cataract prevalence.
In the current era of lifelong ART therapy, limited data are available on the epidemiology of ocular diseases among HIV-positive patients in SSA. ART therapy has extended the life expectancy of HIV infected patients and thus increasing risks of eye lesions related to aging and metabolic disorders ( i.e., cataract, glaucoma, diabetic and hypertensive retinopathy).9 The US-based longitudinal Study of Ocular Complications of AIDS (SOCA) cohort reported a higher risk of cataract and a ~4-fold increase in prevalence and ~1.8-fold increase in incidence of intermediate stage AMD (age-related macular degeneration) in AIDS patients compared to population-based HIV-negative controls18-20. There is also growing evidence that ART is associated with an “accelerated aging” phenotype;27 however, the ocular comorbidities in ART treated HIV-positive people, especially age-related eye diseases and visual function, have not been fully investigated in SSA. There is large difference in life style and environmental factors between SSA and developed countries. The dominant circulating HIV subtypes in SSA are also different from developed countries, and different HIV subtypes, particularly subtype D has faster rates of progression and mortality.28,29 Therefore, the spectrum and prevalences of eye diseases in people living with HIV in SSA can differ from developed countries.
The spectrum of eye disease in our study population changed significantly from findings in the pre-ART era:4,7,9 we observed a low prevalence of infectious eye diseases, i.e., toxoplasmosis retinitis, post CMV retinitis, and zoster ophthalmia, but a higher prevalence of noninfectious cataracts and pterygium. Different pathological mechanisms have been reported associated with susceptible eye diseases in the ART era. One study from rural South Africa showed that increased lens density was associated with shorter telomere length which is a biomarker of aging, conforming to the accelerated aging phenotype in HIV-infected individuals.30 The microvascular changes of lacrimal glands, higher fibrinogen levels, and degeneration of pericytes resulting from HIV immune activation and lymphocytic infiltrations could lead to the development of pterygium.31 An Ethiopian study also found pterygium and cataract formation were the main ocular diseases in HIV-infected participants in the ART era.32
It was reassuring to observe in our data that older age was significantly associated with higher odds of cataract diagnosis. 33 Nevertheless, in our study population, even in the young and middle aged groups, cataract prevalence was higher than 10% (Table 4). This conforms to the prior observation of accelerated formation of lens density associated with HIV 30 and may suggest an ophthalmic reflection of the accelerated aging phenotype in people living with HIV 27,34. In this study, we found individuals with long duration of ART (i.e., ≥ 5 years) had a lower prevalence of cataracts compared to non-ART users, contrary to other studies.9,30,32,35 One study by Accorinti et al., in Italy9 reported that patients on ART had a significantly higher frequency of cataracts and glaucoma than ART-naïve patients. A chart review in an outpatient HIV clinic showed higher cataracts among HIV-infected persons receiving ART than the general US population.36 However, interpretations of these findings are difficult given that factors such as duration of HIV infection, set-point viral load and viral load at ART initiation were not considered.
We found that HIV+ males had higher cataract prevalence than females when adjusting for age and ART duration. Other studies reported female sex was a risk factor for cataracts in an HIV uninfected population.33,37-39 . Most of these studies were based on data from developed countries. It is unknown whether the epidemiology of cataract is different in SSA compared to developed countries, or, the association of sex and cataract in HIV-positive people is different from the association in uninfected populations. There was also the possibility of differential self-selection to receive the ocular services in our data where HIV positive men were more likely to participate the ocular services than HIV positive women. Further population-based studies with both HIV-positives and HIV-negatives are essential to understand the epidemiology of eye diseases in the modern ART era in SSA.
Cataract is the leading cause of blindness in the general populations in SSA.40,41 Conforming to this knowledge, our data showed that in HIV-infected patients, cataract was common and was diagnosed in 73% of people with blindness. We also found that refractive error was common. Both refractive error and cataract are treatable. Therefore, HIV care programs integrating basic eye care, such as provision of corrective lenses and referral to cataract assessment, may provide a venue to improve quality of life and daily functioning of ART-treated HIV infection people.
This study has important limitations. First, the study was based on clinic data, and patients participating in the ophthalmology service were self-selected, so it was likely people with eye complaints were more likely to participate in the ocular exams, resulting in over estimation of the prevalences of eye diseases among HIV-infected people who were under HIV care. During 2009-2012, there were ~4,776 HIV-infected patients seen in RHSP’s HIV care clinics. Lower bounds (LB) of the prevalence estimates were calculated by assuming all HIV+ patients with VI presented themselves to the ophthalmology service camp. The LBs for the crude prevalence of refractive error and cataract would be 7.7% and 2.1%, respectively; and the LBs for moderate/severe VI and blindness would be 0.25% and 0.23%, respectively. On the other hand, the ophthalmic service was provided in HIV clinics to HIV+ patients who were on care, and based on Rakai’s population based surveillance of 15-49 years old residents at that time, ~57% of HIV-infected people had not engaged in HIV care (Table 1 footnote). It was possible that VI was higher among HIV+ people who were not enrolled in HIV care and thus the prevalences of eye diseases in HIV+ patients engaged in care can be under estimating of the prevalences in all HIV+ people in Rakai. Nonetheless, these two sources of selection bias were likely to have minimal impact on the causes of VI and blindness. A second limitation is that this study did not include HIV-negative people; therefore, we could not compare the occurrence and severity of ocular diseases for HIV-infected patients to those of HIV-uninfected people. Third, we did not study the association between ocular diseases and specific ART drugs because of limited individual ART regimens available. Fourth, other studies have explored the relationship between high CD4 count and reduced risk of cataract diagnosis;35 however, our study could not systematically retrieve data on CD4 counts due to limited resources. Fifth, as aforementioned, we did not have information on the duration of HIV infection.
In summary, the prevalence of visual impairment was high among HIV-infected patients who were under HIV care and presented themselves for eye evaluation in Rakai. The spectrum of ocular diseases showed that opportunistic ocular diseases were rare in the ART era. Cataract and refractive errors were the main causes of visual impairment, and in addition to cataract burden in patients aged ≥50 years, cataract was also seen in young and middle aged HIV infected patients. Incorporating ocular services such as visual acuity screening, provisions of eye glasses and referrals to cataract evaluation in HIV clinics may provide a venue to improve the general functioning of people living with HIV in Rakai and in similar resource limited settings.
Acknowledgements
We thank Dr. Steven Reynolds for his input on the study conceptualization.
Footnotes
Disclosure statement
None of the authors have any proprietary interests or conflicts of interest related to this submission
Financial disclosure
None
Reference
- 1.Douek DC, Roederer M, Koup RA. Emerging concepts in the immunopathogenesis of AIDS. Annu Rev Med. 2009;60:471–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Joint United Nations Programme on HIV/AIDS. Fact sheet 2018, Global Statistics - 2018 http://www.unaids.org/en/resources/fact-sheet. 2018.
- 3.The USAID Strengthening Uganda’s Systems for Treating AIDS Nationally. Uganda (2017) Improving systems for delivery of quality tuberculosis control services. .
- 4.Robinson MR, Ross ML, Whitcup SM. Ocular manifestations of HIV infection. Curr Opin Ophthalmol. 1999. December;10(6):431–7. [DOI] [PubMed] [Google Scholar]
- 5.Bekele S, Gelaw Y, Tessema F. Ocular manifestation of HIV/AIDS and correlation with CD4+ cells count among adult HIV/AIDS patients in Jimma town, Ethiopia: a cross sectional study. BMC Ophthalmol. 2013. May 27;13:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan CC, Zhang M. [Ocular manifestations in HIV/AIDS]. Zhonghua Yan Ke Za Zhi. 2005. June;41(6):563–71. [PubMed] [Google Scholar]
- 7.Cunningham ET Jr., Margolis TP. Ocular manifestations of HIV infection. N Engl J Med. 1998. July 23;339(4):236–44. [DOI] [PubMed] [Google Scholar]
- 8.Salzberger B, Hartmann P, Hanses F, et al. Incidence and prognosis of CMV disease in HIV-infected patients before and after introduction of combination antiretroviral therapy. Infection. 2005. October;33(5–6):345–9. [DOI] [PubMed] [Google Scholar]
- 9.Accorinti M, Pirraglia MP, Corradi R, et al. Changing patterns of ocular manifestations in HIV seropositive patients treated with HAART. Eur J Ophthalmol. 2006. Sep-Oct;16(5):728–32. [DOI] [PubMed] [Google Scholar]
- 10.Otiti-Sengeri J, Meenken C, van den Horn GJ, et al. Ocular immune reconstitution inflammatory syndromes. Curr Opin HIV AIDS. 2008;3(4):432–437. [DOI] [PubMed] [Google Scholar]
- 11.Wandeler G, Johnson LF, Egger M. Trends in life expectancy of HIV-positive adults on antiretroviral therapy across the globe: comparisons with general population. Curr Opin HIV AIDS. 2016. September;11(5):492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ballocca F, D'Ascenzo F, Gili S, et al. Cardiovascular disease in patients with HIV. Trends Cardiovasc Med. 2017. November;27(8):558–563. [DOI] [PubMed] [Google Scholar]
- 13.Silverberg MJ, Chao C, Leyden WA, et al. HIV infection and the risk of cancers with and without a known infectious cause. AIDS. 2009. November 13;23(17):2337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Womack JA, Goulet JL, Gibert C, et al. Increased risk of fragility fractures among HIV infected compared to uninfected male veterans. PLoS One. 2011. February 16;6(2):e17217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sulkowski MS, Thomas DL. Hepatitis C in the HIV-Infected Person. Ann Intern Med. 2003. February 4;138(3):197–207. [DOI] [PubMed] [Google Scholar]
- 16.Lucas GM, Mehta SH, Atta MG, et al. End-stage renal disease and chronic kidney disease in a cohort of African-American HIV-infected and at-risk HIV-seronegative participants followed between 1988 and 2004. AIDS. 2007. November 30;21(18):2435–43. [DOI] [PubMed] [Google Scholar]
- 17.Saylor D, Dickens AM, Sacktor N, et al. HIV-associated neurocognitive disorder--pathogenesis and prospects for treatment. Nat Rev Neurol. 2016. April;12(4):234–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jabs DA, Van Natta ML, Sezgin E, et al. Prevalence of intermediate-stage age-related macular degeneration in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 2015. June;159(6):1115–1122 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jabs DA, Van Natta ML, Pak JW, et al. Incidence of Intermediate-stage Age-related Macular Degeneration in Patients With Acquired Immunodeficiency Syndrome. Am J Ophthalmol. 2017. July;179:151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kempen JH, Sugar EA, Varma R, et al. Risk of cataract among subjects with acquired immune deficiency syndrome free of ocular opportunistic infections. Ophthalmology. 2014. December;121(12):2317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamalet C, Tissot-Dupont H, Motte A, et al. Emergence of uncommon HIV-1 non-B subtypes and circulating recombinant forms and trends in transmission of antiretroviral drug resistance in patients with primary infection during the 2013-2015 period in Marseille, Southeastern France. J Med Virol. 2018. October;90(10):1559–1567. [DOI] [PubMed] [Google Scholar]
- 22.Kong X, Kigozi G, Ssekasanvu J, et al. Association of Medical Male Circumcision and Antiretroviral Therapy Scale-up With Community HIV Incidence in Rakai, Uganda. JAMA. 2016. July 12;316(2):182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grabowski MK, Serwadda DM, Gray RH, et al. HIV Prevention Efforts and Incidence of HIV in Uganda. N Engl J Med. 2017. November 30;377(22):2154–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conroy SA, Laeyendecker O, Redd AD, et al. Changes in the distribution of HIV type 1 subtypes D and A in Rakai District, Uganda between 1994 and 2002. AIDS Res Hum Retroviruses. 2010. October;26(10):1087–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otiti-Sengeri J, Colebunders R, Reynolds SJ, et al. Elevated inflammatory cytokines in aqueous cytokine profile in HIV-1 infected patients with cataracts in Uganda. BMC Ophthalmol. 2018. January 19;18(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. World report on vision. Switzerland: 2019. [Google Scholar]
- 27.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sacktor N, Saylor D, Nakigozi G, et al. Effect of HIV Subtype and Antiretroviral therapy on HIV-associated neurocognitive disorder (HAND) stage in Rakai, Uganda. J Acquir Immune Defic Syndr. 2019. February 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiwanuka N, Robb M, Laeyendecker O, et al. HIV-1 viral subtype differences in the rate of CD4+ T-cell decline among HIV seroincident antiretroviral naive persons in Rakai district, Uganda. J Acquir Immune Defic Syndr. 2010. June;54(2):180–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pathai S, Lawn SD, Weiss HA, et al. Increased ocular lens density in HIV-infected individuals with low nadir CD4 counts in South Africa: evidence of accelerated aging. J Acquir Immune Defic Syndr. 2013. July 1;63(3):307–14. [DOI] [PubMed] [Google Scholar]
- 31.Engstrom RE Jr., Holland GN, Hardy WD, et al. Hemorheologic abnormalities in patients with human immunodeficiency virus infection and ophthalmic microvasculopathy. Am J Ophthalmol. 1990. February 15;109(2):153–61. [DOI] [PubMed] [Google Scholar]
- 32.Schaftenaar E, Khosa NS, Baarsma GS, et al. HIV-infected individuals on long-term antiretroviral therapy are at higher risk for ocular disease. Epidemiol Infect. 2017. September;145(12):2520–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zetterberg M, Celojevic D. Gender and cataract--the role of estrogen. Curr Eye Res. 2015. February;40(2):176–90. [DOI] [PubMed] [Google Scholar]
- 34.De Francesco D, Wit FW, Burkle A, et al. Do people living with HIV experience greater age advancement than their HIV-negative counterparts? AIDS. 2019. February 1;33(2):259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rasmussen LD, Kessel L, Molander LD, et al. Risk of cataract surgery in HIV-infected individuals: a Danish Nationwide Population-based cohort study. Clin Infect Dis. 2011. December;53(11):1156–63. [DOI] [PubMed] [Google Scholar]
- 36.Morales M, Nabha L, Toussant B, et al. Risk Factors for Premature Nuclear Cataract Formation in Human Immunodeficiency Virus (HIV) Infected Individuals Receiving Antiretroviral Therapy. Vol. CRHA-108 2017. [Google Scholar]
- 37.Klein BE, Klein R, Linton KL. Prevalence of age-related lens opacities in a population. The Beaver Dam Eye Study. Ophthalmology. 1992. April;99(4):546–52. [DOI] [PubMed] [Google Scholar]
- 38.Lundstrom M, Stenevi U, Thorburn W. Gender and cataract surgery in Sweden 1992-1997. A retrospective observational study based on the Swedish National Cataract Register. Acta Ophthalmol Scand. 1999. April;77(2):204–8. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell P, Cumming RG, Attebo K, et al. Prevalence of cataract in Australia: the Blue Mountains eye study. Ophthalmology. 1997. April;104(4):581–8. [DOI] [PubMed] [Google Scholar]
- 40.Johnson GJ, Minassian DC, Weale R. The Epidemiology of Eye Disease. Community Eye Health. 1999;12(29):10–10. [PMC free article] [PubMed] [Google Scholar]
- 41.Naidoo K, Kempen JH, Gichuhi S, et al. Prevalence and causes of vision loss in sub-Saharan Africa in 2015: magnitude, temporal trends and projections. Br J Ophthalmol. 2020. March 30. [DOI] [PubMed] [Google Scholar]