Abstract
Rationale and Objective:
Observational studies have reported a U-shaped association between pre-dialysis blood pressure (BP) and death. In contrast, a linear association between out-of-dialysis unit BP has been reported. Home BP may be a better target for treatment. To test the feasibility of this approach, we conducted a pilot trial of treating home vs. pre-dialysis BP in hemodialysis patients.
Study Design:
A 4-month parallel, randomized controlled trial.
Settings & Participants:
Fifty prevalent hemodialysis patients in San Francisco and Seattle. Participants were randomized using 1: 1 block randomization, stratified by site.
Interventions:
Target home systolic BP (SBP) vs. pre-dialysis SBP 140-100 mmHg. Home and pre-dialysis SBPs were ascertained every 2 weeks. Dry weight and BP medications were adjusted to reach the target SBP.
Outcomes:
Primary outcomes were feasibility, adherence, safety and tolerability.
Results:
Fifty out of seventy patients who were approached agreed to participate (71.4%). All enrollees completed the study except one who received a kidney transplant. In the home BP treatment group, adherence to obtaining/reporting home BP was 97.4% (and consistent over the 4 months). There was no increased frequency of high (defined as SBP>200 mmHg, 0.2% vs. 0%) or low (defined as <90 mmHg, 1.8% vs.1.2%) predialysis BP readings in the home vs. pre-dialysis treatment arms, respectively. However, participants in the home BP arm had higher frequency of fatigue (32% vs. 16%).
Limitations:
Small sample size.
Conclusions:
This pilot trial demonstrates feasibility and high adherence to home BP measurement and treatment in hemodialysis patients. Larger trials to test long-term feasibility, efficacy and safety of home BP treatment in hemodialysis patients should be conducted.
Funders:
National Institutes of Health, Satellite Healthcare and Northwest Kidney Centers
Trial Registration:
ClinicalTrials.gov Identifier: NCT03459807
Keywords: blood pressure, dialysis, home blood pressure
PLAIN-LANGUAGE SUMMARY
Home blood pressure may be a better target for treatment compared with blood pressure measured at the time of dialysis in hemodialysis patients. In this pilot clinical trial, we tested the feasibility of measuring and treating home blood pressure (compared with treating blood pressure at the time of hemodialysis) over 4 months in 50 participants at two sites. The enrollment rates were high in the trial and nearly all participants completed the study. We observed high rates of adherence to home blood pressure measures and promising safety signals. In conclusion, this trial demonstrates that measurement of home blood pressure is feasible in hemodialysis patients. Larger studies are needed to test the long-term effect and safety of treating home blood pressure in hemodialysis patients.
INTRODUCTION
Blood pressure (BP) is one of the most important, modifiable risk factors for cardiovascular events and death in the general population;1-4 and rates of these events are very high in patients with end-stage renal disease (ESRD) treated with maintenance hemodialysis.5-7 Yet, the management of BP in hemodialysis patients is a conundrum, largely due to paradoxical, U-shaped associations of systolic BP (SBP) measured at the dialysis unit prior to the start of the dialysis treatment ("pre-dialysis" SBP) with cardiovascular disease (CVD) and death reported in multiple observational studies.8-14 More specifically, hemodialysis patients with pre-dialysis SBP <140 mmHg experience higher risk of mortality than those with SBP >140 mmHg. Patients with pre-dialysis SBP of 150 to 179 mmHg seem to be at similar, if not lower, adjusted risk for all-cause mortality compared with those with pre-dialysis SBP of 140 to 149 mmHg, even accounting for case-mix. These data have led to uncertainty among providers and guideline committees on BP management.15
Observational studies have reported that while the association of pre-dialysis SBP and adverse clinical outcomes is U-shaped, the association between out-of-dialysis-unit SBP and risk of mortality and CVD is linear in the same patients.14, 16, 17 Many opinion leaders and practicing nephrologists, however, believe that measuring and targeting out-of-dialysis unit BP measurements may not be feasible.18 We hypothesized that there will more widespread measuring and targeting home BP (which will lead to better long-term outcomes19, 20) if studies demonstrate that this is a practical approach. Towards that end, we conducted this pilot clinical trial among in-center hemodialysis patients during to test the feasibility, adherence to, safety and tolerance of home BP measurement and treatment. (ClinicalTrials.gov identifier: NCT03459807).
METHODS
Study Design
This was a non-blinded 4-month, parallel group randomized controlled trial of 50 participants in the greater Seattle and San Francisco areas comparing a strategy of targeting home SBP<140 mmHg vs. pre-dialysis SBP <140 mmHg. We chose the same SBP targets in each treatment group to focus on the setting of BP measurement, rather than the BP target.
IRB approval was obtained at the University of Washington (UW) and the University of California, San Francisco (UCSF). The trial was registered at Clinicaltrials.gov (NCT03459807) and monitored by an external data safety monitoring board.
Study population
This trial was conducted among adult patients cared for by nephrology faculty members at UW and UCSF. Inclusion criteria included: undergoing in-center thrice weekly hemodialysis for treatment of ESRD; greater than 3 months since dialysis initiation; ability to obtain a brachial BP at dialysis and at home; and aged >18 years. Patients were excluded if they were pregnant or breastfeeding (or anticipated pregnancy); incarcerated or institutionalized which may prohibit measurement of home BP; or participating in another intervention study that may affect BP. Other exclusion criteria include unmeasurable SBP (e.g. those with left ventricular assist devices); chronic hypotension (defined as average pre-dialysis systolic BP <100 mmHg over last 2 weeks prior to screening off BP medications); life expectancy <4 months; or anticipated living donor kidney transplant within 4 months.
Recruitment and randomization
Recruitment started in March 2018 and ended August 2018, with the date of last follow-up in January 2019. We used the electronic medical record and canvassed nephrologists at each site to identify eligible participants (“pre-screen”). Once a potentially eligible patient was identified through the pre-screen, he/she was approached during their regularly scheduled hemodialysis treatment session, when eligibility was confirmed. If eligible, the participant was invited to participate. Once informed consent was obtained, participants had a baseline study visit at which time participants were randomized 1:1 to: (1) target home SBP <140 mmHg or (2) target pre-dialysis SBP <140 mmHg. Randomization was done by a computer algorithm, in random size blocks (e.g. 2, 4, or 6) stratified by recruitment site (See study protocol).
Study visits
All study visits occurred during the patients’ regularly scheduled hemodialysis session. At the baseline visit, medical history, dialysis history/prescription and medication (both BP and non-BP medications) were reviewed and recorded. Additionally, clinical laboratory values that were performed for routine testing in hemodialysis patients were also recorded. Optional 44-hour ambulatory blood pressure monitoring (ABPM) was offered at this visit as well (See study protocol) since ABPM is considered the reference standard for BP measurement.21
Follow-up visits occurred approximately every 2 weeks. Study visits may have been delayed up to 7 days if the participant missed dialysis for hospitalizations, vacations, or other reasons. At these follow-up visits, BPs measured at home (if randomized to the home BP treatment arm) and during dialysis were reviewed. At these study visits, adjustments in dry weight or BP medications were made according to the clinical trial BP treatment algorithm (see below).
At the final study visit (4 months after randomization), for participants randomized to the home BP treatment arm, a survey on the home BP experience was administered. For the subset of participants who agreed to the 44-hour ABPM at baseline, they were offered the opportunity to repeat this study at the final study visit.
Blood pressure measurement in both treatment groups
Participants were randomized to either the home SBP or pre-dialysis SBP treatment arms and followed in parallel. The goal SBP was 100-139 mmHg for both treatment groups. We chose a target of SBP <140 mmHg to be consistent with practice guidelines such as K/DOQI.22-28 Current guidelines do not specify different SBP targets based on the timing or setting of BP measurement in hemodialysis patients. The lower bound of SBP 100 mmHg was chosen based on clinical judgement.
Home BP treatment arm:
Every 2 weeks, participants randomized to the home BP treatment arm measured their home BPs at 2 sittings--one in the morning and one in the evening—which were averaged by the study team. Because of BP variability throughout the week in hemodialysis patients,22, 29-32 participants were instructed to take their home BP the day after the dialysis session (ideally mid-week to avoid the longer inter-dialytic period). Participants were asked to only take 2 BP readings over a 2 week period to facilitate a pragmatic approach that would not be burdensome to the participants. Participants were trained by research staff on proper techniques for home BP measurement22 The device that was used was the Microlife Watch Home A BT33, 34 which has been validated in hemodialysis patients.35 The home BP device was programmed to take 3 BP measurements consecutively at 1-minute intervals at each sitting and report out the mean. Participants received in-person visits at their hemodialysis sessions or phone calls by the local study team at least weekly to remind them to take their home BPs. Participants had several options to share the home BP readings with the study team, including text messaging, phone call, in-person or paper log. For quality control, we manually compared the daily mean SBP recorded on the home BP devise to that reported by 11 study participants. We found that overall 94% of the daily SBP measures reported by study participants matched those recorded by the home BP device.
Pre-dialysis BP treatment arm.
For those randomized to the pre-dialysis BP treatment arm, the mean of all sitting SBP readings taken immediately prior to the start of each hemodialysis treatment over 2 weeks was used to define pre- dialysis SBP. There would typically be 6 readings if there were no missed or extra hemodialysis sessions. Pre- dialysis SBP was taken by dialysis unit staff using standard dialysis unit equipment per usual protocol and was recorded in the dialysis unit electronic medical record.
Treatment algorithm in both study arms
The same BP treatment algorithm was used in both treatment groups. All adjustments in BP treatment were done in close collaboration with the primary nephrologist. The BP treatment algorithm included: dry weight adjustment (with specific counseling on dietary sodium and fluid intake as needed); and adjustment of standard anti-hypertensive medications. At the study visit, if the SBP (home or pre-dialysis, depending on treatment arm) was 100-139 mmHg, no adjustments in dry weight or medications were made. If the SBP ≥140 mmHg, the first step was to adjust the dry weight (usually by 0.5-1 kg increments, or as clinically appropriate). This step was repeated at each study visit until the patient was deemed euvolemic or had intolerance/adverse effects. Once the dry weight was optimized as much as possible, the next step if the SBP ≥140mmHg was to increase doses of the patient’s existing medications in the following order (if possible), based on input from the patient’s primary nephrologist and other clinical parameters (e.g. heart rate): ACE-I/ARB; β-blockers; calcium channel blockers; alpha blockers; vasodilators; anti-adrenergic agents. If the current medications were at maximum dose, new BP medications were started, also in the same order of preference as above. If the SBP <100 mmHg at a study visit, we first titrated down/off the patient’s existing medications (following the reverse algorithm outlined above). After these changes, if the SBP was still <100 mmHg, the dry weight target was increased. All medications were clinically indicated for treatment of hypertension at approved doses, and thus were covered by the patient's insurance.
For patients who were hospitalized, we resumed study procedures after discharge and after obtaining clearance from the primary treating nephrologist (no participant stopped study interventions due to this).
Outcomes
The primary outcomes of the study were feasibility, adherence, safety and tolerability. Feasibility was quantified by how many eligible patients agreed to participate in the study after pre-screening (approach to enroll ratio). Adherence was defined as the percentage of participants in the home BP arm who were able to successfully perform home BP readings and transmit readings to the research team. Adherence was also measured by the percentage of participants who dropped out of the study. Safety was defined by the frequency of excessively low or high BP readings (e.g. dialysis unit BP readings of <90 or >200 mmHg) and dangerous consequences of excessively low (e.g. syncope, falls) or high (e.g. flash pulmonary edema) BP. Tolerability was defined in the study protocol as the frequency of intra-dialytic hypotension, defined as SBP<90 mmHg during dialysis.36 Additionally, tolerability was gauged by querying participants about symptoms of hypotension such as cramping (yes/no), dizziness/lightheadedness (yes/no) and fatigue (yes/no). We also asked participants “how long did it take you to recover from your last dialysis treatment?” a validated question that is an important patient-centered outcome.37
Secondary outcomes included assessment of preferred modality of home BP measurement transmission among the home BP participants. We also evaluated differences in BP between treatment groups as assessed by: (1) pre-dialysis SBP and DBP; (2) post-dialysis SBP and DBP and (3) 44-hour intra-dialytic ABPM from beginning to the end of the study.
We also evaluated differences in dry weight target, actual observed pre-dialysis weight and actual observed post-dialysis weight in each treatment group from the beginning to the end of the study.
Statistical analyses
Analysis followed the intent to treat principle. Continuous variables are summarized with means with the corresponding standard deviation or standard error as appropriate. Categorical variables are given as proportion per participant or per visit as appropriate. Continuous variables are compared using the t-test, categorical variables by the Fisher exact test and rates were compared using Poisson regression with a robust variance estimator.
RESULTS
Primary outcome: feasibility
After pre-screening, a total of 70 patients were approached. Of those, 50 (71.4%) agreed to participate, 25 in Seattle and 25 in San Francisco (Figure 1). The average recruitment rate was 10 participants per month. 25 participants were randomized to home BP treatment and 25 were randomized to pre-dialysis BP treatment (Figure 1). These 50 participants were cared for by ten different primary nephrologists at eight different dialysis units (7 nephrologists at UW [range of 1-5 patients per nephrologist] and 3 at UCSF [range of 3-17 patients per nephrologist]) operated by three dialysis organizations.
Figure 1.
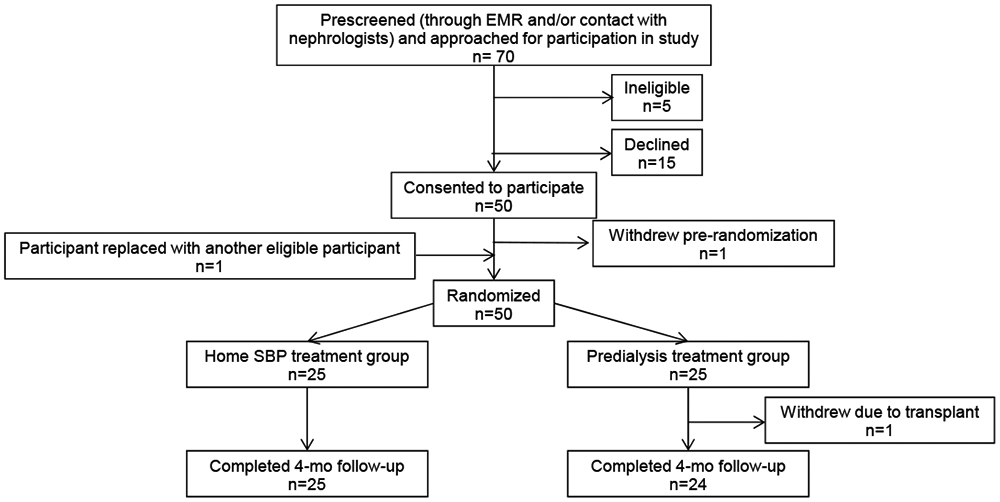
Flow diagram of trial participants. Abbreviations: EMR, electronic medical record; SBP, systolic blood pressure.
Among recruited participants, the mean (±standard deviation [SD]) age was 56 (±14) years, 40% were women; 74% of participants identified as non-white and 8% as Hispanic (Table 1). Among participants randomized to the home SBP treatment group, home SBP was 4.6 mmHg lower on average than pre-dialysis SBP at baseline. Correlation between home SBP and pre-dialysis SBP was modest (r=0.61, p=0.001). Of the 19 participants with a baseline pre-dialysis SBP >140 mmHg; only 10 (52.6%) had a baseline home SBP >140 mmHg.
Table 1.
Characteristics of study population enrolled in BOLD trial, stratified by randomized arm
| Overall (N=50) | Home BP (N=25) | Dialysis unit BP (N=25) | |
|---|---|---|---|
| Mean (±SD) age, years | 56.6 (±13.6) | 56.4 (±13.1) | 56.9 (±14.4) |
| N, % women | 20 (40%) | 12 (48%) | 8 (32%) |
| Hispanic ethnicity, N (%) | 4 (8%) | 3 (12%) | 1 (4%) |
| Race, N (%) | |||
| White | 14 (28%) | 6 (24%) | 8 (32%) |
| Black | 20 (40%) | 11 (44%) | 9 (36%) |
| Asian | 11 (22%) | 6 (24%) | 5 (20%) |
| Pacific Islander | 3 (6%) | 1 (4%) | 2 (8%) |
| Other | 3 (6%) | 1 (4%) | 2 (8%) |
| Primary kidney diagnosis, N (%) | |||
| Diabetes Mellitus | 22 (44%) | 10 (40%) | 12 (48%) |
| Hypertension | 9 (18%) | 3 (12%) | 6 (24%) |
| Polycystic Kidney Disease | 1 (2%) | 1 (4%) | 0 (0%) |
| Other/Unknown | 18 (36%) | 11 (42%) | 7 (28%) |
| Vascular access at enrollment, N (%) | |||
| Catheter | 10 (20%) | 5 (20%) | 5 (20%) |
| Fistula | 34 (68%) | 15 (60%) | 19 (76%) |
| Graft | 6 (12%) | 5 (20%) | 1 (4%) |
| Mean (±SD) years on HD at enrollment | 3.0 (2.3) | 3.0 (2.2) | 3.0 (2.4) |
| Dialysis prescription at enrollment | |||
| Mean (±SD) time, minutes | 220 (±26) | 218 (±25) | 221 (±28) |
| Mean (±SD) blood flow, ml/min | 384. (±42) | 380 (±38) | 388 (±46) |
| Mean (±SD) dialysate flow, ml/min | 736 (±85) | 720 (±96) | 752 (±71) |
| Mean (±SD) dry weight, kg | 81 (±21) | 81 (±26) | 80 (±16) |
| Pre-dialysis clinical labs at baseline | |||
| Mean (±SD) sodium, mmol/L | 136 (±3) | 136 (±3) | 135 (±4) |
| Mean (±SD) glucose, mg/dL | 155 (±70) | 145 (±67) | 168 (±74) |
| Mean (±SD) creatinine, mg/dL | 9.6 (±2.8) | 9.7 (±3.1) | 9.5 (±2.5) |
| Mean (±SD) hemoglobin, g/dL | 11.2 (±1.4) | 11.1 (±1.7) | 11.4 (±1.1) |
| Mean (±SD) albumin, g/dL | 3.9 (±0.3) | 4.0 (±0.3) | 3.9 (±0.3) |
| Mean (±SD) calcium, mg/dL | 9.2 (±0.7) | 9.0 (±0.7) | 9.4 (±0.7) |
| Mean (±SD) phosphorus, mg/dL | 6.8 (±2.7) | 7.3 (±3.3) | 6.4 (±1.8) |
| Mean (±SD) potassium, mmol/L | 4.9 (±0.6) | 4.8 (±0.6) | 5.0 (±0.7) |
| Mean (±SD) spKt/V | 1.5 (±0.3) | 1.5 (±0.3) | 1.5 (±0.2) |
| Blood pressure at enrollment | |||
| Mean (±SD) pre-dialysis systolic BP, mmHg | 146 (±25) | 145 (±26) | 147 (±23) |
| Mean (±SD) pre-dialysis diastolic BP, mmHg | 79 (±19) | 76 (±18) | 83 (±20) |
| Mean (±SD) number of blood pressure medications at enrollment | 2.1 (±1.6) | 2.4 (±1.7) | 1.8 (±1.5) |
Primary outcome: adherence
Forty-nine of the 50 enrolled participants (98%) completed the study successfully (Figure 1). The sole participant who withdrew (from the pre-dialysis SBP treatment group) did so when she unexpectedly received a deceased donor kidney transplant. Among participants randomized to the home SBP treatment group, over the 4-month intervention period, 97.4% of study visits in this treatment group had at least one home BP measurement completed and transmitted to the research team (Table 2). Adherence to home BP measurements remained high and consistent throughout the 16 week study (Table 2). Over weeks 1-4, 100% of participants in the home BP treatment arm were able to complete at least 1 home BP measurement; in weeks 5-16, 96% of participants were able to complete 1 home BP measurement.
Table 2.
Adherence to home blood pressure measurements among participants randomized to the home blood pressure treatment group
| Number of study visits with 0, 1, or 2 BP readings transmitted to study team |
|||
|---|---|---|---|
| 0 home blood pressure readings |
1 home blood pressure reading |
2 home blood pressure readings |
|
| Overall, across 16 weeks | 3% | 4% | 94% |
| Week 2 | 0% | 4% | 96% |
| Week 4 | 4% | 4% | 92% |
| Week 6 | 4% | 13% | 83% |
| Week 8 | 0% | 4% | 96% |
| Week 10 | 8% | 0% | 92% |
| Week 12 | 0% | 0% | 100% |
| Week 14 | 0% | 4% | 96% |
| Week 16 | 4% | 0% | 96% |
A total of 21 out of 25 participants in the home BP arm (84%) completed a survey on the home BP experience. Of those, 20/21 (95%) strongly agreed or agreed that "it was easy using the home blood pressure devise to measure blood pressure;” 18/21 (86%) strongly agreed or agreed that "remembering to measure blood pressure using the home BP device twice over two weeks was easy"; and 21/21 (100%) reported they "would recommend patients with ESRD use this home BP device to measure their blood pressure at home."
Primary outcome: safety
The proportion of dialysis treatments with either excessively low or high pre or post dialysis SBP was small and similar in the two treatment groups. The rates of syncope, falls and flash pulmonary edema were also comparable between treatment groups (Table 3).
Table 3.
Safety and tolerability of treatment of home and dialysis blood pressure
| Home BP (N=25 participants) |
Dialysis BP (N=25 participants) |
p-value | |
|---|---|---|---|
| Pre-dialysis SBP<90mmHg | |||
| N, % per # of dialysis sessions | 3/1140 (0.3%) | 2/1086 (0.2%) | 0.8 |
| N, % # of participants | 1/25 (4%) | 2/25 (8%) | 1.0 |
| Post-dialysis SBP<90 mmHg | |||
| N, % per # of dialysis sessions | 2/1137 (0.2%) | 0/1085 (0.0%) | 0.4 |
| N, % # of participants | 2/25 (8%) | 0/25 (0%) | 0.5 |
| Pre-dialysis SBP > 200mmHg | |||
| N, % per # of dialysis sessions | 21/1140 (1.8%) | 13/1086 (1.2%) | 0.7 |
| N, % # of participants | 4/25 (16%) | 4/25 (16%) | 1.0 |
| Post-dialysis SBP>200 mmHg | |||
| N, % per # of dialysis sessions | 12/1137 (1.1%) | 3/1085 (0.3%) | 0.2 |
| N, % # of participants | 3/25 (12%) | 2/25 (8%) | 1.0 |
| Syncope N events | 2 | 1 | 0.8 |
| N, % # of participants | 1/25 (4%) | 1/25 (4%) | 1.0 |
| Fall N events | 3 | 6 | 0.3 |
| N, % # of participants | 3/25 (12%) | 6/25 (20%) | 0.5 |
| Flash pulmonary edema | 0/25 (0%) | 0/25 (0%) | 1.0 |
| Cramping | |||
| N, % of study visits every 2 weeks | 36/186 (19%) | 47/191 (25%) | 0.5 |
| N, % # of participants | 13/25 (52%) | 18/25 (72%) | 0.2 |
| Symptoms of dizziness | |||
| N, % of study visits every 2 weeks | 24/186 (13%) | 22/191 (12%) | 0.8 |
| N, % # of participants | 10/25 (40%) | 14/25 (56%) | 0.4 |
| Symptoms of lightheadedness | |||
| N, % of study visits every 2 weeks | 33/186 (18%) | 31/188 (16%) | 0.8 |
| N, % # of participants | 14/25 (56%) | 12/25 (48%) | 0.8 |
| Symptoms of fatigue | |||
| N, % of study visits every 2 weeks | 59/186 (32%) | 30/191 (16%) | 0.02 |
| N, % # of participants | 15/25 (60%) | 16/25 (64%) | 1.0 |
| Intradialytic hypotension | |||
| N, % of dialysis sessions | 95/1141 (8.3%) | 146/1087 (13.4%) | 0.3 |
| N, % # of participants | 21/25 (84%) | 18/25 (72%) | 0.5 |
| Shortened treatments due to cramping, low BP or symptoms of low BP | |||
| N, % of dialysis sessions | 22/1141 (1.9%) | 21/1087 (1.9%) | 1.0 |
| N, % # of participants | 14/25 (56%) | 10/25 (40%) | 0.4 |
| Mean (±SD) duration of recovery from dialysis, in minutes from study visits every 2 weeks | 327 (±42) | 268 (±37) | 0.6 |
denominators for number of dialysis sessions vary due to missed dialysis visits or missing data
Primary outcome: tolerability
The frequency of intra-dialytic hypotension was lower among participants randomized to the home BP treatment group (8.3% vs 13.4%, p=0.25), although it did not reach statistical significance (Table 3).
The frequency of reported cramping, dizziness, lightheadedness over 4 months among participants randomized to the home BP vs. pre-dialysis BP treatment groups was similar (Table 3). Symptoms of fatigue were reported at more study visits in the home BP treatment group (32% vs. 16%), however the number of participants reporting fatigue did not differ between treatment groups (15/25 vs 16/25).
There was no difference in proportion of dialysis treatments shortened for reasons of cramping, low BP or symptoms of low BP between the treatment groups. The self-reported time to recovery from dialysis was also similar across groups (Table 3). Secondary outcome: transmission of home BP measurements to study team Among participants randomized to the home SBP treatment group, over the 4-month study, telephone (47%) was the most frequent modality to transmit home BPs to the study team, followed by text messaging (33%), paper log (10%), email (7%) and other (e.g. in person, 3%).
Secondary outcome: differences in BP over 4 months across treatment groups
The pre-dialysis SBP was similar in the two arms at baseline, and started to separate by week 6 with SBP becoming lower among those randomized to pre-dialysis BP treatment arm, which was sustained through the last several weeks of the intervention (Figure 2). Over the 4-month intervention period, dry weight adjustment was the most frequent intervention used to reach the target SBP in each treatment group (Table S1). Home SBP was less than pre-dialysis SBP throughout the trial (Figure 3).
Figure 2.
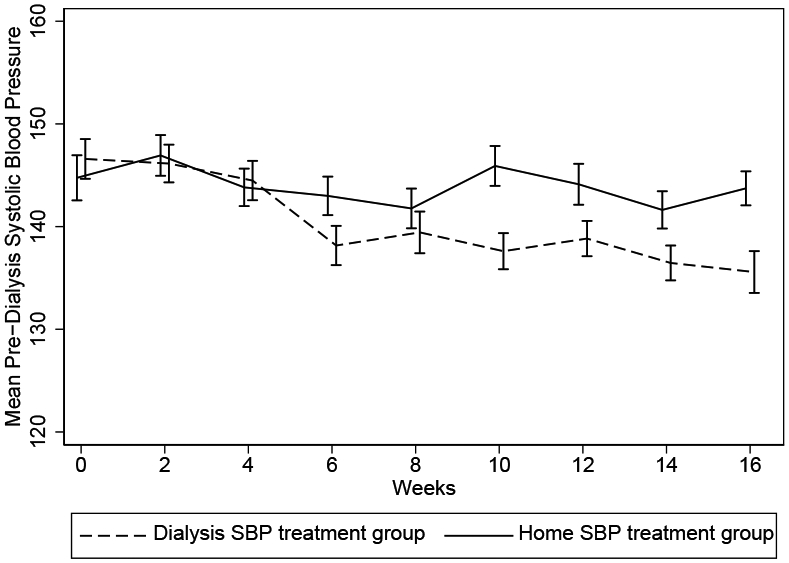
Mean predialysis systolic blood pressures (SBPs) over 4 months among participants randomly assigned to the home SBP versus dialysis SBP treatment groups (N = 50). Error bars represent standard deviation.
Figure 3.
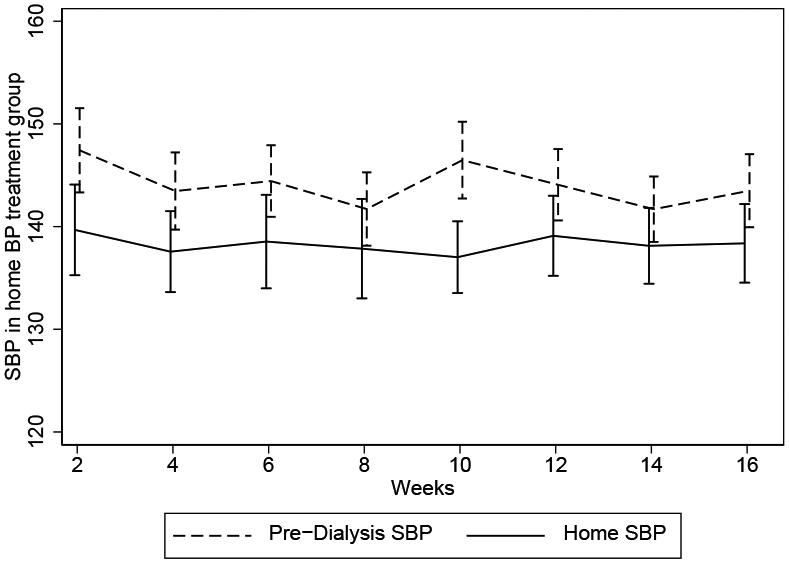
Mean home and predialysis systolic blood pressures (SBPs) over 4 months among participants randomly assigned to the home BP treatment group (n = 25). Error bars represent standard deviation.
Trends in the secondary outcomes of pre-dialysis DBP, post-dialysis SBP and post-dialysis DBP are shown in_Figures S1-3. Trends indry weight targets, actual observed pre-dialysis weight andactual observed post-dialysis weight are shown in Figures S4-6.
31 out of 50 participants agreed to have a baseline ABPM assessment and 21 of the 31 agreed to a repeat ABPM at the end of the study. Overall, there was no significant discordance between home BP and 44-hour ABPM at baseline in terms of classifying blood pressure as being above or below 140 mmHg (Table S2). The results of longitudinal changes in 44-hour ABPM measures are shown in Table S3.
DISCUSSION
Numerous observational studies spanning several decades have reported a paradoxical, U-shaped association between BP measure in the dialysis unit and adverse outcomes.8-14 Although fewer in number, observational studies of home BP, in contrast, have shown that high BP measured outside of the dialysis is associated with higher risk of CVD and all-cause mortality among in-center hemodialysis patients.10,19 Despite this data, there has not been widespread adoption of measuring and targeting out-of-dialysis unit BP in clinical practice. A persistent concern has been feasibility. For example, in the recently published BP in Dialysis (BID) trial, the rationale for targeting pre-dialysis SBP was explained as: “Predialysis SBP may be inferior to home BP measurements (HBPM) and ambulatory BP monitoring (ABPM) in predicting clinical outcomes. However, the long-term adherence of patients on HD with requirements for repeated HBPM and ABPM is unknown.”22 In this context, we believe that our pilot trial demonstrating that measurement and treatment of home BP is feasible, well tolerated and safe, is important as possible future adoption of home BP is considered in the future and for the design of future BP trials in hemodialysis patients.
Compared with other approaches to obtaining out-of-dialysis unit BP measurements, home BP a pragmatic approach. Our survey data suggests that 44-hour ABPM30 may be too burdensome for many hemodialysis patients and may not feasible to be repeated at a frequency sufficient for titration of dry weight and BP medications in all patients.38 In the DRIP trial, which randomized patients in Indiana to an ultrafiltration intervention, 85-90% of participants agreed to repeat ABPM studies 4 or 8 weeks apart.39 However, this success rate of repeat ABPMs has not been tested for longer periods of time or in other centers. Also, it may also not be practical to ask hemodialysis patients to attend another clinic routinely to have BP measured.14 Home BP also has the advantage of eliminating white coat effect, identifying masked hypertension, and capturing multiple measurements over several days.40-42 There are data validating home BP measurements in hemodialysis patients with 44-hour inter-dialytic ABPM readings.10, 17, 19, 20, 43-45 Observational studies in patients on hemodialysis17, 19, 20, 40-42, 46 confirm the prognostic value of home BP measurement. For these reasons, home BP appears to be a pragmatic and important target for therapies in hemodialysis patients.
In our pilot trial, our recruitment rate and adherence to home BP measurement was high (and remained high over the 16 week trial), demonstrating that hemodialysis patients are interested and able to successfully measure and transmit their home BP recordings to the health care team. Our qualitative survey results also demonstrated enthusiasm from study participants to measure home BP. This is consistent with prior work in other populations that has shown that providing care outside the traditional health care setting has been linked to improved patient satisfaction and engagement, 47, 48 particularly with increased adoption of mobile health technologies. Furthermore, text messaging was one of the preferred modality to transmit home SBP in our study; and participants were enthusiastic about an app-based home BP intervention in future studies. These data support future studies to use repeat home BP measures to guide interventions in hemodialysis patients.
Only a few prior clinical trials have studied BP in hemodialysis patients and most have evaluated BP medications, dialysis prescription, ultrafiltration and more recently BP targets. 22, 39, 49-54 Only a few studies have included home BP as a target for interventions in hemodialysis patients. In a trial of 64 hemodialysis patients in Brazil, treatment of home BP vs. pre-dialysis BP over 6 months led to better BP control during the interdialytic period (measured by ABPM) but no difference in left ventricular mass index.55 The HDPAL trial randomized 200 hemodialysis patients in Indiana to treatment with atenolol vs. lisinopril;54 and participants were asked to measure mid-week home BP. However, data on adherence to the home BP measures was not reported and home BP measurement was not the primary focus of the study. The BID trial randomized 126 hypertensive hemodialysis patients to target pre-dialysis SBP of 110-140 mmHg (intensive arm) vs. 155-165 mmHg (standard arm).22 The BID study protocol did also include morning and afternoon home BP measurement the day after each midweek dialysis session. Consistent with our study, home SBP was lower on average than pre-dialysis SBP (by 6.1 [±0.7] mmHg). However, compared with our study, adherence to home BP measures was lower; by month 4, BID investigators obtained at least 1 home BP measure per month in only 73% of participants compared to 96% in our study. The better adherence to home BP measures in our study may be explained by the focus on home BP as the primary intervention and a simpler, more pragmatic study protocol. Thus, our approach could facilitate more widespread implementation of home BP into clinical practice or use in future clinical trials of BP interventions.
We found a similar frequency of intradialytic symptoms and adverse events (e.g. falls and syncope) among participants randomized to the home vs. dialysis BP treatment arms. However, we noted lower frequency of intra-dialytic hypotension in those randomized to the home BP treatment group compared with the dialysis BP treatment group (although this difference did not reach statistical significance). Intradialytic hypotension is an important outcome that is associated with numerous downstream clinical consequences36, 56-66 On the other hand, we did find a higher frequency of fatigue in patients randomized to the home BP treatment arm. Larger studies are thus needed to better define the impact of treatment of home BP on patient symptoms and intra-dialytic events.
There was separation in measured BPs between the two treatment groups with standard therapies for BP; and dry weight adjustment was the most frequently used intervention to reach SBP targets, an approach that has been used in other clinical trials as well.22, 51 All adjustments in dry weight or medications were performed in collaboration with the primary nephrologist; an approach that is pragmatic and can be adopted to future home BP intervention studies.
The study had several strengths. The study population was diverse and included patients in 2 cities cared for by 10 nephrologists practicing out of 8 different dialysis units (operated by 3 providers). A pragmatic approach to BP management utilizing dry weight adjustment and BP medications was used in both treatment groups. We recognize a few weaknesses as well. The sample size was relatively small and the intervention was short in duration and not designed to study "hard" clinical outcomes such as mortality. Home BP was only measured in one randomization group; so the effect of treating pre-dialysis SBP on home SBP was not able to be determined from the present study. We chose to include normotensive patients by pre-dialysis SBP (the only readings we had at study screening) since these patients could have hypertension according to their home BP and/or become hypertensive over time. We also did not screen patients based on ambulatory blood pressure for eligibility as this did not align with the primary objective to assess feasibility of home BP measurement and treatment. Some of the secondary outcomes of interest may be affected by many other factors besides BP and interventions targeting BP. We recognize that clinical pre-dialysis BP readings may have considerable variability;67 however this approach is pragmatic and reflects "real-world" practice of BP management. Finally, the study was conducted among patients cared for by two academic faculty practices in San Francisco and Seattle; the findings may differ in other hemodialysis patients.
To summarize, in this pilot trial, we found that measuring and treating home BP was feasible and well-tolerated. Further we did not observe any strong signals to suggest any safety concerns. Our data support the notion that repeat measurements of home BP is a pragmatic way to obtain out-of-dialysis unit BP in many dialysis patients in the “real world.” A larger trial with more participants and longer duration of follow-up will be needed to understand the value of home BP measurements as a guide to antihypertensive therapies among hemodialysis patients.
Supplementary Material
Figure S1. Mean pre-dialysis diastolic blood pressure (DBP) over 4 months among participants randomized to the home SBP vs dialysis SBP treatment groups (N=50)
Figure S2. Mean post-dialysis SBP over 4 months among participants randomized to the home SBP vs dialysis SBP treatment groups (N=50)
Figure S3. Mean post-dialysis diastolic blood pressure (DBP) over 4 months among participants randomized to the home SBP vs dialysis SBP treatment groups (N=50)
Figure S4. Mean dry weight target over 4 months among participants randomized to the home SBP vs dialysis SBP treatment groups (N=50)
Figure S5. Mean actual observed pre-dialysis weight over 4 months among participants randomized to the home SBP vs dialysis SBP treatment groups (N=50)
Figure S6. Mean actual observed post-dialysis weight over 4 months among participants randomized to the home SBP vs dialysis SBP treatment groups (N=50)
Table S1. Dry weight and BP medication adjustments over 4 months in both treatment groups
Table S2. Concordance of home BP and 44-hour ABPM measures at baseline
Table S3. Blood pressure measures by 44-hour ambulatory blood pressure monitoring
Acknowledgements:
We appreciate the support from the following nephrologists who collaborated with us: Drs. Scott Beiber, Fionnuala Cormack, Stephen Gluck, Yoshio Hall, Leah Haseley, Raymond Hsu, Lowell Lo, Kimberley Muczynski. Matthew Rivara, Brendan Shannon. We would like to thank our Data and Safety Monitoring Board: Dr. Joaquim Ix, Dr. Glenn Chertow and Dr. Stephen Shiboski. We also thank the nurses and staff at the following maintenance hemodialysis units UCSF Mt Zion (medical director Dr. Lowell Lo), Satellite San Francisco (medical directors Drs. Hanlon Fong and K.T. Linga) and Northwest Kidney Centers in the greater Seattle area (Chief Medical Officer Dr. Suzanne Watnick).
Support: This study was supported by the National Institute for Diabetes, Digestive and Kidney Diseases (R21DK114213), Satellite Healthcare and an unrestricted gift from the Northwest Kidney Centers. Dr Hsu is also supported by K24 DK92291. The funders did not have a role in study design, data collection, analysis, reporting, or the decision to submit for publication.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
Data Sharing: De-identified individual participant data that underlie the results reported in this article and the study protocol will be shared for individual participant data meta-analysis. Data will be shared with investigators whose proposed use of the data has been approved by an independent review committee identified for this purpose. Proposals may be submitted to the primary investigators.
Peer Review: Received December 16, 2019. Evaluated by 2 external peer reviewers, with direct editorial input from a Statistics/Methods Editor and an International Editor, who served as Acting Editor-in-Chief. Accepted in revised form June 11, 2020. The involvement of an Acting Editor-in-Chief was to comply with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Collaboration PS. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349): 1903–1913. [DOI] [PubMed] [Google Scholar]
- 2.Flack JM, Neaton J, Grimm R Jr., et al. Blood pressure and mortality among men with prior myocardial infarction. Multiple Risk Factor Intervention Trial Research Group. Circulation. 1995;92(9): 2437–2445. [DOI] [PubMed] [Google Scholar]
- 3.MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335(8692): 765–774. [DOI] [PubMed] [Google Scholar]
- 4.Vamos EP, Harris M, Millett C, et al. Association of systolic and diastolic blood pressure and all cause mortality in people with newly diagnosed type 2 diabetes: retrospective cohort study. BMJ (Clinical research ed.). 2012;345: e5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension (Dallas, Tex. : 1979). 2003;42(5): 1050–1065. [DOI] [PubMed] [Google Scholar]
- 6.Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. Journal of the American Society of Nephrology : JASN. 1998;9(12 Suppl): S16–23. [PubMed] [Google Scholar]
- 7.Locatelli F, Marcelli D, Conte F, et al. Survival and development of cardiovascular disease by modality of treatment in patients with end-stage renal disease. Journal of the American Society of Nephrology : JASN. 2001;12(11): 2411–2417. [DOI] [PubMed] [Google Scholar]
- 8.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney international. 2003;63(3): 793–808. [DOI] [PubMed] [Google Scholar]
- 9.Cheung AK, Sarnak MJ, Yan G, et al. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney international. 2000;58(1): 353–362. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal R Blood pressure and mortality among hemodialysis patients. Hypertension (Dallas, Tex. : 1979). 2010;55(3): 762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zager PG, Nikolic J, Brown RH, et al. "U" curve association of blood pressure and mortality in hemodialysis patients. . Kidney international. 1998;54(2): 561–569. [DOI] [PubMed] [Google Scholar]
- 12.Robinson BM, Tong L, Zhang J, et al. Blood pressure levels and mortality risk among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney international. 2012;82(5): 570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Port FK, Hulbert-Shearon TE, Wolfe RA, et al. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1999;33(3): 507–517. [DOI] [PubMed] [Google Scholar]
- 14.Bansal N, McCulloch CE, Rahman M, et al. Blood pressure and risk of all-cause mortality in advanced chronic kidney disease and hemodialysis: the chronic renal insufficiency cohort study. Hypertension (Dallas, Tex. : 1979). 2015;65(1): 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duranti E, Imperiali P, Sasdelli M. Is hypertension a mortality risk factor in dialysis? Kidney Int Suppl. 1996;55: S173–174. [PubMed] [Google Scholar]
- 16.Bansal N, McCulloch CE, Lin F, et al. Blood Pressure and Risk of Cardiovascular Events in Patients on Chronic Hemodialysis: The CRIC Study (Chronic Renal Insufficiency Cohort). Hypertension (Dallas, Tex. : 1979). 2017;70(2): 435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal R, Andersen MJ, Light RP. Location not quantity of blood pressure measurements predicts mortality in hemodialysis patients. American journal of nephrology. 2008;28(2): 210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levin NW, Kotanko P, Eckardt KU, et al. Blood pressure in chronic kidney disease stage 5D-report from a Kidney Disease: Improving Global Outcomes controversies conference. Kidney international. 2010;77(4): 273–284. [DOI] [PubMed] [Google Scholar]
- 19.Alborzi P, Patel N, Agarwal R. Home blood pressures are of greater prognostic value than hemodialysis unit recordings. Clinical journal of the American Society of Nephrology : CJASN. 2007;2(6): 1228–1234. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal R, Satyan S, Alborzi P, et al. Home blood pressure measurements for managing hypertension in hemodialysis patients. American journal of nephrology. 2009;30(2): 126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muntner P, Einhorn PT, Cushman WC, et al. Blood Pressure Assessment in Adults in Clinical Practice and Clinic-Based Research: JACC Scientific Expert Panel. J Am Coll Cardiol. 2019;73(3): 317–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miskulin DC, Gassman J, Schrader R, et al. BP in Dialysis: Results of a Pilot Study. Journal of the American Society of Nephrology : JASN. 2018;29(1): 307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kidney Disease Outcomes Quality Initiative Work Group. K/DOQI Clinical Practice Guidelines on Cardiovascular Disease in Dialysis Patients. Am J Kidney Dis. 2005:45(6)(suppl 3):1–154 [Google Scholar]
- 24.Bolton K, Beddhu S, Campese VM, et al. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2005;45(4): S7–S153. [PubMed] [Google Scholar]
- 25.Harper J, Nicholas J, Webb L, Casula A, Williams AJ. UK Renal Registry 12th Annual Report (December 2009): Chapter 11 Blood Pressure Profile of Prevalent Patients Receiving Dialysis in the UK in 2008: national and centre-specific analyses. Nephron Clin Pract. 2010;115: C239–C260. [DOI] [PubMed] [Google Scholar]
- 26.Jindal K, Chan CT, Deziel C, et al. Hemodialysis clinical practice guidelines for the Canadian Society of Nephrology. Journal of the American Society of Nephrology : JASN. 2006;17(3 Suppl 1): S1–27. [DOI] [PubMed] [Google Scholar]
- 27.Roberts MA, Pilmore HL, Tonkin AM, et al. Challenges in blood pressure measurement in patients treated with maintenance hemodialysis. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012;60(3): 463–472. [DOI] [PubMed] [Google Scholar]
- 28.Hirakata H, Nitta K, Inaba M, et al. Japanese Society for Dialysis Therapy Guidelines for Management of Cardiovascular Diseases in Patients on Chronic Hemodialysis. Ther Apher Dial. 2012;16(5): 387–435. [DOI] [PubMed] [Google Scholar]
- 29.Sarafidis PA, Persu A, Agarwal R, et al. Hypertension in dialysis patients: a consensus document by the European Renal and Cardiovascular Medicine (EURECA-m) working group of the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) and the Hypertension and the Kidney working group of the European Society of Hypertension (ESH). Nephrol Dial Transpl. 2017;32(4): 620–640. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal R, Lewis RR. Prediction of hypertension in chronic hemodialysis patients. Kidney international. 2001;60(5): 1982–1989. [DOI] [PubMed] [Google Scholar]
- 31.Leypoldt JK, Cheung AK, Delmez JA, et al. Relationship between volume status and blood pressure during chronic hemodialysis. Kidney international. 2002;61(1): 266–275. [DOI] [PubMed] [Google Scholar]
- 32.Moriya H, Ohtake T, Kobayashi S. Aortic stiffness, left ventricular hypertrophy and weekly averaged blood pressure (WAB) in patients on haemodialysis. Nephrol Dial Transpl. 2007;22(4): 1198–1204. [DOI] [PubMed] [Google Scholar]
- 33.McManus RJ, Mant J, Bray EP, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomised controlled trial. Lancet. 2010;376(9736): 163–172. [DOI] [PubMed] [Google Scholar]
- 34.McManus RJ, Mant J, Haque MS, et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. Jama. 2014;312(8): 799–808. [DOI] [PubMed] [Google Scholar]
- 35.Thompson AM, Eguchi K, Reznik ME, Shah SS, Pickering TG. Validation of an oscillometric home blood pressure monitor in an end-stage renal disease population and the effect of arterial stiffness on its accuracy. Blood Press Monit. 2007;12(4): 227–232. [DOI] [PubMed] [Google Scholar]
- 36.Flythe JE, Xue H, Lynch KE, Curhan GC, Brunelli SM. Association of mortality risk with various definitions of intradialytic hypotension. Journal of the American Society of Nephrology : JASN. 2015;26(3): 724–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindsay RM, Heidenheim PA, Nesrallah G, Garg AX, Suri R. Minutes to recovery after a hemodialysis session: a simple health-related quality of life question that is reliable, valid, and sensitive to change. Clinical journal of the American Society of Nephrology : CJASN. 2006;1(5): 952–959. [DOI] [PubMed] [Google Scholar]
- 38.Jardine AG. Con: Ambulatory blood pressure measurement in patients receiving haemodialysis: a sore arm and a waste of time? Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2015;30(9): 1438–1441. [DOI] [PubMed] [Google Scholar]
- 39.Agarwal R, Alborzi P, Satyan S, Light RP. Dry-weight reduction in hypertensive hemodialysis patients (DRIP): a randomized, controlled trial. Hypertension (Dallas, Tex. : 1979). 2009;53(3): 500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asayama K, Thijs L, Li Y, et al. Setting thresholds to varying blood pressure monitoring intervals differentially affects risk estimates associated with white-coat and masked hypertension in the population. Hypertension (Dallas, Tex. : 1979). 2014;64(5): 935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banegas JR, Ruilope LM, de la Sierra A, et al. Relationship between Clinic and Ambulatory Blood-Pressure Measurements and Mortality. The New England journal of medicine. 2018;378(16): 1509–1520. [DOI] [PubMed] [Google Scholar]
- 42.Tientcheu D, Ayers C, Das SR, et al. Target Organ Complications and Cardiovascular Events Associated With Masked Hypertension and White-Coat Hypertension: Analysis From the Dallas Heart Study. Journal of the American College of Cardiology. 2015;66(20): 2159–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogura M, Yamada Y, Terawaki H, Hamaguchi A, Kimura Y, Hosoya T. Home systolic blood pressure on the morning of dialysis days has prognostic impact for hypertensive hemodialysis patients. Clinical and experimental nephrology. 2012;16(3): 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheikh S, Sinha AD, Agarwal R. Home blood pressure monitoring: how good a predictor of long-term risk? Current hypertension reports. 2011;13(3): 192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agarwal R How should hypertension be assessed and managed in hemodialysis patients? Home BP, not dialysis unit BP, should be used for managing hypertension. Seminars in dialysis. 2007;20(5): 402–405. [DOI] [PubMed] [Google Scholar]
- 46.Agarwal R, Peixoto AJ, Santos SF, Zoccali C. Pre- and postdialysis blood pressures are imprecise estimates of interdialytic ambulatory blood pressure. Clinical journal of the American Society of Nephrology : CJASN. 2006;1(3): 389–398. [DOI] [PubMed] [Google Scholar]
- 47.Kerr EA, Zikmund-Fisher BJ, Klamerus ML, Subramanian U, Hogan MM, Hofer TP. The role of clinical uncertainty in treatment decisions for diabetic patients with uncontrolled blood pressure. Annals of internal medicine. 2008;148(10): 717–727. [DOI] [PubMed] [Google Scholar]
- 48.Little P, Barnett J, Barnsley L, Marjoram J, Fitzgerald-Barron A, Mant D. Comparison of acceptability of and preferences for different methods of measuring blood pressure in primary care. BMJ (Clinical research ed.). 2002;325(7358): 258–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Group FHNT, Chertow GM, Levin NW, et al. In-center hemodialysis six times per week versus three times per week. The New England journal of medicine. 2010;363(24): 2287–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agodoa LY, Appel L, Bakris GL, et al. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. Jama. 2001;285(21): 2719–2728. [DOI] [PubMed] [Google Scholar]
- 51.Agarwal R, Alborzi P, Satyan S, Light RP. Dry-weight reduction in hypertensive hemodialysis patients (DRIP): a randomized, controlled trial. Hypertension (Dallas, Tex.: 1979). 2009;53(3): 500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agarwal R, Sinha AD, Pappas MK, Abraham TN, Tegegne GG. Hypertension in hemodialysis patients treated with atenolol or lisinopril: a randomized controlled trial. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2014;29(3): 672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inrig JK, Molina C, D'Silva K, et al. Effect of low versus high dialysate sodium concentration on blood pressure and endothelial-derived vasoregulators during hemodialysis: a randomized crossover study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2015;65(3): 464–473. [DOI] [PubMed] [Google Scholar]
- 54.Agarwal R, Sinha AD, Pappas MK, Abraham TN, Tegegne GG. Hypertension in hemodialysis patients treated with atenolol or lisinopril: a randomized controlled trial. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2014;29(3): 672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.da Silva GV, de Barros S, Abensur H, Ortega KC, Mion D Jr. Home blood pressure monitoring in blood pressure control among haemodialysis patients: an open randomized clinical trial. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2009;24(12): 3805–3811. [DOI] [PubMed] [Google Scholar]
- 56.Breidthardt T, Burton JO, Odudu A, Eldehni MT, Jefferies H, McIntyre CW. N-terminal Pro-B-type natriuretic peptide and its correlation to haemodialysis-induced myocardial stunning. Nephron Clin Pract. 2013;123(1-2): 118–122. [DOI] [PubMed] [Google Scholar]
- 57.Breidthardt T, Burton JO, Odudu A, Eldehni MT, Jefferies HJ, McIntyre CW. Troponin T for the detection of dialysis-induced myocardial stunning in hemodialysis patients. Clinical journal of the American Society of Nephrology : CJASN. 2012;7(8): 1285–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Breidthardt T, McIntyre CW. Dialysis-induced myocardial stunning: the other side of the cardiorenal syndrome. Reviews in cardiovascular medicine. 2011; 12(1): 13–20. [DOI] [PubMed] [Google Scholar]
- 59.Buchanan C, Mohammed A, Cox E, et al. Intradialytic Cardiac Magnetic Resonance Imaging to Assess Cardiovascular Responses in a Short-Term Trial of Hemodiafiltration and Hemodialysis. Journal of the American Society of Nephrology : JASN. 2017;28(4): 1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clinical journal of the American Society of Nephrology : CJASN. 2009;4(12): 1925–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clinical journal of the American Society of Nephrology : CJASN. 2009;4(5): 914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stefansson BV, Brunelli SM, Cabrera C, et al. Intradialytic hypotension and risk of cardiovascular disease. Clinical journal of the American Society of Nephrology : CJASN. 2014;9(12): 2124–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.MacEwen C, Sutherland S, Daly J, Pugh C, Tarassenko L. Relationship between Hypotension and Cerebral Ischemia during Hemodialysis. Journal of the American Society of Nephrology : JASN. 2017;28(8): 2511–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Assimon MM, Flythe JE. Intradialytic Blood Pressure Abnormalities: The Highs, The Lows and All That Lies Between. American journal of nephrology. 2015;42(5): 337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seong EY, Zheng Y, Winkelmayer WC, Montez-Rath ME, Chang TI. The Relationship between Intradialytic Hypotension and Hospitalized Mesenteric Ischemia: A Case-Control Study. Clinical journal of the American Society of Nephrology : CJASN. 2018;13(10): 1517–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flythe JE, Katsanos SL, Hu Y, Kshirsagar AV, Falk RJ, Moore CR. Predictors of 30-Day Hospital Readmission among Maintenance Hemodialysis Patients: A Hospital's Perspective. Clinical journal of the American Society of Nephrology : CJASN. 2016;11(6): 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rahman M, Griffin V, Kumar A, Manzoor F, Wright JT Jr., Smith MC. A comparison of standardized versus "usual" blood pressure measurements in hemodialysis patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2002;39(6): 1226–1230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Mean pre-dialysis diastolic blood pressure (DBP) over 4 months among participants randomized to the home SBP vs dialysis SBP treatment groups (N=50)
Figure S2. Mean post-dialysis SBP over 4 months among participants randomized to the home SBP vs dialysis SBP treatment groups (N=50)
Figure S3. Mean post-dialysis diastolic blood pressure (DBP) over 4 months among participants randomized to the home SBP vs dialysis SBP treatment groups (N=50)
Figure S4. Mean dry weight target over 4 months among participants randomized to the home SBP vs dialysis SBP treatment groups (N=50)
Figure S5. Mean actual observed pre-dialysis weight over 4 months among participants randomized to the home SBP vs dialysis SBP treatment groups (N=50)
Figure S6. Mean actual observed post-dialysis weight over 4 months among participants randomized to the home SBP vs dialysis SBP treatment groups (N=50)
Table S1. Dry weight and BP medication adjustments over 4 months in both treatment groups
Table S2. Concordance of home BP and 44-hour ABPM measures at baseline
Table S3. Blood pressure measures by 44-hour ambulatory blood pressure monitoring


