Abstract
Rationale & Objective:
Identification of novel risk factors for chronic kidney disease (CKD) progression may inform mechanistic investigations and improve identification of high-risk subgroups. The current study aimed to characterize CKD progression across levels of numerous risk factors and to identify independent risk factors for CKD progression among those with and without diabetes.
Study Design:
The Chronic Renal Insufficiency Cohort (CRIC) Study is a prospective cohort study of adults with CKD conducted at seven US clinical centers.
Setting & Participants:
Participants (N=3379) had up to 12.3 years of follow-up; 47% had diabetes.
Predictors:
Thirty risk factors for CKD progression across sociodemographic, behavioral, clinical and biochemical domains at baseline.
Outcomes:
Study outcomes were estimated glomerular filtration rate (eGFR) slope and the composite of halving of eGFR or initiation of kidney replacement therapy (KRT).
Analytical Approach:
Stepwise selection of independent risk factors was performed stratified by diabetes status using linear mixed effects and Cox proportional hazards models.
Results:
Among those without and with diabetes, respectively, the mean (SD) eGFR slope was −1.4 (3.3) and −2.7 (4.7) mL/min/1.73m2/year. Among participants with diabetes, multivariable-adjusted hazard of the composite outcome was approximately twofold or greater with higher levels of the inflammatory chemokine CXCL12, the cardiac marker N-terminal pro-B-type natriuretic peptide (NTproBNP) and the kidney injury marker urine neutrophil gelatinase-associated lipocalin (NGAL). Among those without diabetes, low serum bicarbonate as well as higher high-sensitivity troponin T, NTproBNP and urine NGAL were all significantly associated with a 1.5-fold or greater rate of the composite outcome.
Limitations:
The observational study design precludes causal inference.
Conclusions:
Strong associations for cardiac markers, plasma CXCL12 and urine NGAL exceeded that of systolic blood pressure ≥140 mmHg, a well-established risk factor for CKD progression. This warrants further investigation into the potential mechanisms these markers indicate and opportunities to use them to improve risk stratification.
ClinicalTrials.gov identifier:
Keywords: Chronic kidney disease (CKD), diabetes, kidney replacement therapy (KRT), end-stage renal disease (ESRD), halving of estimated glomerular filtration rate (eGFR), eGFR slope, neutrophil gelatinase-associated lipocalin (NGAL), N-terminal pro-B-type natriuretic peptide (NTproBNP), inflammatory chemokines, CXCL12
Graphical Abstract
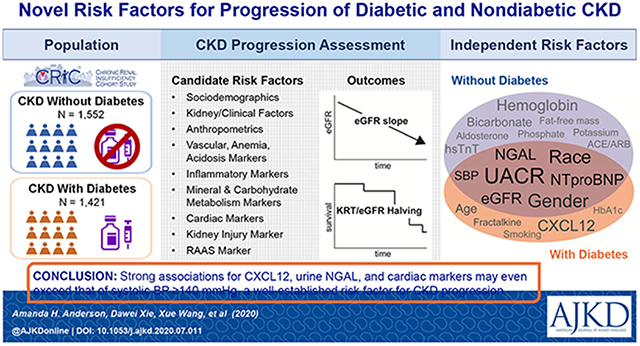
Plain language summary
Several novel biomarkers associated with increased risk of CKD progression The primary goal of this study was to identify independent risk factors of CKD progression among participants with and without diabetes in a prospective CKD cohort study (N=3379). Among those with diabetes, CKD progression rates approximately doubled with higher levels of the inflammatory chemokine CXCL12, the cardiac marker NTproBNP and the kidney injury marker urine NGAL. Among those without diabetes, rates increased over 1.5-fold with higher levels of high-sensitivity troponin T, NTproBNP and urine NGAL. The strength of these associations exceeded that of systolic blood pressure ≥140 mmHg, a well-established risk factor for kidney disease progression. These findings provide insights into potential mechanisms of CKD progression and will guide future research in defining subgroups at highest risk for CKD progression.
INTRODUCTION
Approximately 15% of adult Americans have chronic kidney disease (CKD).1 CKD is a condition characterized by elevated levels of morbidity and mortality and an elevated risk of cardiovascular disease (CVD). As kidney function declines, metabolic and hemodynamic disturbances emerge, and rates of hospitalization, CVD, and death increase. The set of known risk factors for progression of CKD is relatively small, and effective therapies and strategies to slow CKD progression are limited.
As a result, identification of novel risk factors is the focus of a large body of ongoing research. In recent years, factors found to be associated with incidence or progression of CKD include the APOL1 high-risk genotype,2 circulating levels of soluble urokinase-type plasminogen activator receptor (suPAR) in adults and in children,3, 4 urinary epithelial growth factor (EGF),5 and both urinary and circulating levels of uromodulin.6, 7 Efforts to identify and confirm these and other risk factor-outcome relationships are complicated by the multiple etiologies of CKD and frequently occurring comorbidities. Additionally, the strength or significance of findings in a risk factor analysis can differ depending on the CKD progression metric utilized (e.g., time to initiation of kidney replacement therapy [KRT] or estimated glomerular filtration rate [eGFR] decline) and adjustment for other risk factors. Given these complexities, a large, deeply phenotyped CKD cohort with a long duration of follow-up is needed to identify factors that may elucidate important disease progression mechanisms, lead to future interventions targeting these pathways, and identify high-risk groups of CKD patients who may benefit from more aggressive strategies using available therapies.
The Chronic Renal Insufficiency Cohort (CRIC) Study, established by the National Institute of Diabetes and Digestive and Kidney Diseases, is the largest prospective cohort study of CKD in the United States (US) and was specifically designed to identify risk factors for progression of diabetic and nondiabetic CKD. The goals of the current study were 1) to characterize rates of CKD progression across levels of numerous risk factors and 2) to identify independent risk factors that most significantly relate to CKD progression from across and within a broad set of domains among those with and without diabetes.
METHODS
Study Design and Population
Seven clinical centers from across the US enrolled participants into the CRIC Study between June 2003 and August 2008. The cohort of 3939 adult men and women with moderate and advanced CKD has been previously described.8–10 Inclusion in the CRIC Study was partially based on age-specific eGFR criteria as follows: 20-70 mL/min/1.73m2 for those aged 21-44 years, 20-60 mL/min/1.73m2 for those aged 45-64 years, and 20-50 mL/min/1.73m2 for those aged 65-74 years. Major exclusion criteria included prior dialysis longer than one month, HIV infection, polycystic kidney disease, or other primary renal diseases requiring active immunosuppression. Participants completed annual in-person clinic visits during which data were obtained across multiple domains and blood and urine specimens were collected. After excluding participants with only one eGFR measure during follow-up, 3379 participants were eligible for this analysis. Written informed consent was obtained from all study participants, and the study protocol was approved by institutional review boards at each of the CRIC Study clinical centers.
Study Data
We considered a set of thirty risk factors for CKD progression representing the major potential mechanistic pathways examined in the CRIC Study, and when possible, multiple markers within pathways to identify the most significant markers. Available risk factors were ascertained at study baseline. Demographic risk factors were age, gender, and race/ethnicity. Kidney function measures included eGFR and urine albumin:creatinine ratio (UACR or albuminuria). Blood pressure factors were systolic blood pressure (SBP) and self-reported use of an angiotensin converting enzyme inhibitor or angiotensin receptor blocker (ACE/ARB). Clinical risk factors included history of CVD and serum uric acid. The socioeconomic factor was level of education, and behavioral factor was current smoking. Body composition measures were body mass index and fat-free mass. Ankle-brachial index was the peripheral vascular measure and hemoglobin was the measure of anemia. High-sensitivity C-reactive protein (hsCRP) was the general inflammatory marker, and serum fractalkine (CX3CL1) and plasma CXCL12 were the available inflammatory chemokines. Mineral metabolism markers included fibroblast growth factor-23 (FGF-23), serum phosphate, and intact parathyroid hormone (iPTH), and carbohydrate metabolism markers were hemoglobin A1c (HbA1c) and insulin resistance estimated by the Homeostatic Model Assessment (HOMA-IR). High-sensitivity Troponin T (hsTnT) and N-terminal pro-B-type natriuretic peptide (NTproBNP) were the considered cardiac markers. Serum bicarbonate served as the acidosis measure, urine sodium and urine potassium were the included urinary electrolytes, and urine neutrophil gelatinase-associated lipocalin (NGAL) was used as a marker of kidney injury. Serum aldosterone was included as a marker of the renin-angiotensin-aldosterone system. Additional details regarding risk factors and CRIC data collection are provided in Item S1.
Stratifying Factor
Diabetes mellitus, selected a priori as the primary stratifying factor, was defined as a fasting glucose >126 mg/dL, a non-fasting glucose >200 mg/dL, or use of insulin or other medications for glycemic control at baseline.
Outcomes and Censoring Events
The two CKD progression outcomes were: 1) annual change of eGFR (i.e., eGFR slope), and 2) time to KRT or eGFR halving from baseline (i.e., the composite renal outcome). Participant follow-up was censored at time of death, loss to follow-up, or the end of the follow-up period, whichever occurred first. Outcomes were ascertained from study entry through late-2015. Outcomes and censoring events are described in more detail in Item S1.
Statistical Analysis
Summary statistics for baseline characteristics were calculated overall and by diabetes status using frequencies and percentages for categorical variables and mean (standard deviation [SD]) and median (interquartile range [IQR]) for continuous variables, as appropriate. Differences were assessed using the Chi-Square test, analysis of variance (ANOVA) and the Kruskal Wallis test, respectively. Using ordinary least squares regression, the mean eGFR change over follow-up for each participant was calculated, and the distribution of eGFR slope was graphically depicted by diabetes status and summarized into categories. The mean event rate per 1000 person-years for the composite renal endpoint was calculated across baseline eGFR categories (<30, 30-44.9, 45-59.9, 60-75, ≥75 mL/min/1.73m2) by diabetes status. Each CKD progression outcome was summarized by baseline characteristics and diabetes status.
Multivariable-adjusted modeling of CKD progression was performed stratified by diabetes status. Each CKD progression outcome was modeled separately following the same three steps. In Step 1, all demographic, kidney function, and blood pressure factors were fit in a model that also included CRIC clinical center. In Step 2, each remaining factor was individually added to the model from Step 1. Demographic, kidney function, and blood pressure factors were forced into the model, but additional factors were retained in the next step if it had a P value <.2. In Step 3, all demographic, kidney function, and blood pressure factors and any identified additional factors were entered into a model, and, in a backward selection process, risk factors with a P value ≥.1 were removed to create a parsimonious model. Linear mixed effects models with random intercepts and slopes and variance components covariance structure were employed to model eGFR slope. To account for the competing risk of death, cause-specific Cox proportional hazards models were used for time to the development of the composite renal outcome. Violations of the proportionality assumption for Cox proportional hazards analysis were assessed using standard techniques and statistical tests of the interaction coefficients for all covariates with time in the multivariable-adjusted models. Factors that violated the proportionality assumption are included in the final model with time-stratified (years 0-6 and ≥6) hazard ratios. Due to missing data, a total of N=1552 without diabetes and N=1421 with diabetes were included in linear mixed effects models and N=1534 without diabetes and N=1347 with diabetes in Cox proportional hazards models. Differences across participants included in the complete case analyses and those eligible but excluded due to missing data were assessed and deemed small with all standardized differences <0.2.11
Secondary and Sensitivity Analyses
We repeated all model-building after removing albuminuria from the set of factors as a secondary analysis. Additionally, we chose a priori to explore for effect modification of the final models by race/ethnicity and albuminuria. As a sensitivity analysis, we refit all final models among the subset of participants free of self-reported heart failure at baseline and further adjusted for left ventricular ejection fraction and left ventricular hypertrophy from echocardiograms performed at the Year 1 study visit.
Analyses were performed using SAS v9.4 (SAS Institute Inc).
RESULTS
Participants had a mean age of 57.9 years, 55.1% were men and 7.5% were non-Hispanic black with high-risk APOL1 genotypes (Table 1). The mean (SD) eGFR at baseline was 49.3 (17.6) mL/min/1.73m2 for those without diabetes and 42.2 (14.5) for those with diabetes. During a median (range) of 7 (1-12) years of follow-up, the mean (SD) eGFR slope was −1.4 (3.3) mL/min/1.73m2/year and −2.7 (4.7) among those without and with diabetes, respectively (Figure 1, Panel A). Nearly 10% and 20% of study participants without and with diabetes, respectively, had annual declines in eGFR greater than 5 mL/min/1.73m2/year. The unadjusted rate (95% CI) of the composite renal endpoint was 33.5 (29.6-36.5) per 1000 person-years among those without diabetes, and 81.6 (75.9-88.6) among those with diabetes (Figure 1, Panel B).
Table 1.
Baseline characteristics of Chronic Renal Insufficiency Cohort (CRIC) Study participants overall and by diabetes status
| All Participants (N=3379) | Diabetes Status | P valuea | |||
|---|---|---|---|---|---|
| Without Diabetes (N=1797) | With Diabetes (N=1582) | ||||
| Mean (SD), Median (IQR) or N (%) | |||||
| Demographic Factors | |||||
| Age, years | Mean (SD) | 57.9 (10.8) | 56.7 (11.6) | 59.2 (9.7) | <.001 |
| Gender | Male | 1863 (55.1%) | 969 (53.9%) | 894 (56.5%) | .1 |
| Female | 1516 (44.9%) | 828 (46.1%) | 688 (43.5%) | ||
| Race/ethnicity | NH-White | 1530 (45.3%) | 932 (51.9%) | 598 (37.8%) | <.001 |
| NH-Black, APOL1 low-risk | 1058 (31.3%) | 494 (27.5%) | 564 (35.7%) | ||
| NH-Black, APOL1 high-risk | 255 (7.5%) | 154 (8.6%) | 101 (6.4%) | ||
| Hispanic | 398 (11.8%) | 145 (8.1%) | 253 (16.0%) | ||
| Other | 138 (4.1%) | 72 (4.0%) | 66 (4.2%) | ||
| Kidney Function Measures | |||||
| Baseline eGFR, mL/min/1.73m2 | <30 | 591 (17.5%) | 250 (13.9%) | 341 (21.6%) | <.001 |
| 30-44.9 | 1157 (34.2%) | 536 (29.8%) | 621 (39.3%) | ||
| 45-59.9 | 990 (29.3%) | 546 (30.4%) | 444 (28.1%) | ||
| ≥60 | 641 (19.0%) | 465 (25.9%) | 176 (11.1%) | ||
| UACR, mg/g | Median (IQR) | 43.4 (7.9-392.5) | 19.4 (5.7-163.2) | 118.1 (16.8-787.7) | <.001 |
| Blood Pressure Factors | |||||
| Systolic BP, mmHg | Median (IQR) | 124.7 (112.7-140.0) | 121.3 (109.3-134.0) | 130.0 (116.7-144.7) | <.001 |
| ACE/ARB | No | 1037 (30.9%) | 735 (41.2%) | 302 (19.2%) | <.001 |
| Yes | 2317 (69.1%) | 1048 (58.8%) | 1269 (80.8%) | ||
| Clinical Factors | |||||
| History of CVD | No | 2290 (67.8%) | 1376 (76.6%) | 914 (57.8%) | <.001 |
| Yes | 1089 (32.2%) | 421 (23.4%) | 668 (42.2%) | ||
| Uric acid, mg/dL | Median (IQR) | 7.3 (6.0-8.6) | 7.1 (5.9-8.4) | 7.4 (6.3-8.8) | <.001 |
| Socioeconomic Status Factor | |||||
| Education | Less than high school | 648 (19.2%) | 245 (13.6%) | 403 (25.5%) | <.001 |
| High school/some college | 1609 (47.6%) | 837 (46.6%) | 772 (48.8%) | ||
| Graduated college | 1121 (33.2%) | 714 (39.8%) | 407 (25.7%) | ||
| Behavioral Factor | |||||
| Current smoker | No | 2959 (87.6%) | 1562 (86.9%) | 1397 (88.3%) | .2 |
| Yes | 420 (12.4%) | 235 (13.1%) | 185 (11.7%) | ||
| Body Composition Measures | |||||
| BMI, kg/m2 | Median (IQR) | 30.9 (26.8-36.1) | 29.5 (25.7-33.7) | 32.8 (28.4-38.3) | <.001 |
| Fat-free mass, kg | Median (IQR) | 59.1 (49.0-69.8) | 55.9 (46.2-66.4) | 62.5 (52.7-73.3) | <.001 |
| Peripheral Vascular Measure | |||||
| Ankle-brachial index | Median (IQR) | 1.1 (1.0-1.2) | 1.1 (1.0-1.2) | 1.0 (0.9-1.1) | <.001 |
| Measure of Anemia | |||||
| Hemoglobin, g/dL | Median (IQR) | 12.7 (11.5-13.9) | 13.2 (12.1-14.4) | 12.0 (11.0-13.2) | <.001 |
| Inflammatory Marker and Chemokines | |||||
| hsCRP, mg/L | Median (IQR) | 2.5 (1.0-6.2) | 2.4 (1.0-5.8) | 2.6 (1.1-6.6) | .2 |
| Serum fractalkine (CX3CL1), ng/mL | Median (IQR) | 0.8 (0.6-1.1) | 0.8 (0.6-1.0) | 0.9 (0.7-1.1) | <.001 |
| Plasma CXCL12, pg/mL | Median (IQR) | 2413.9 (2066.7-2797.7) | 2341.4 (2021.3-2717.1) | 2493.8 (2143.6-2880.0) | <.001 |
| Mineral Metabolism Markers | |||||
| FGF23, RU/mL | Median (IQR) | 138.6 (93.9-222.6) | 119.1 (83.2-187.6) | 166.0 (110.4-262.4) | <.001 |
| Serum phosphate, mg/dL | Median (IQR) | 3.7 (3.2-4.1) | 3.5 (3.1-3.9) | 3.8 (3.4-4.3) | <.001 |
| Intact PTH, pg/mL | Median (IQR) | 52.0 (34.0-83.9) | 48.0 (32.3-75.7) | 57.0 (36.8-94.0) | <.001 |
| Carbohydrate Metabolism Markers | |||||
| HbA1c, % | Median (IQR) | 6.1 (5.6-7.3) | 5.7 (5.3-6.0) | 7.3 (6.5-8.4) | <.001 |
| HOMA-IR | Median (IQR) | 4.1 (2.5-7.2) | 3.0 (2.0-4.5) | 6.3 (3.9-11.2) | <.001 |
| Cardiac Markers | |||||
| hsTnT, pg/mL | Median (IQR) | 11.3 (5.4-21.9) | 7.7 (3.4-14.4) | 17.1 (9.5-31.9) | <.001 |
| NTproBNP, pg/mL | Median (IQR) | 140.9 (60.8-368.6) | 106.5 (47.5-262.4) | 194.7 (80.9-484.3) | <.001 |
| Acidosis Marker | |||||
| Serum bicarbonate, mEq/L | Median (IQR) | 25.0 (22.0-27.0) | 25.0 (23.0-27.0) | 24.0 (22.0-26.0) | .02 |
| Urinary Electrolytes | |||||
| Urine sodium, mEq/24h | Median (IQR) | 151.5 (108.8-203.5) | 145.2 (103.6-197.0) | 159.4 (114.0-209.3) | <.001 |
| Urine potassium, mmol/24h | Median (IQR) | 52.6 (38.0-70.0) | 52.5 (36.7-69.9) | 52.7 (39.4-70.6) | .08 |
| Kidney Injury Marker | |||||
| Urine NGAL, ng/mL | Median (IQR) | 14.0 (6.2-32.3) | 12.6 (5.9-27.7) | 16.1 (6.5-37.0) | <.001 |
| RAAS Marker | |||||
| Serum aldosterone, pg/mL | Median (IQR) | 101.3 (71.3-152.4) | 103.4 (72.3-156.6) | 98.8 (70.1-147.6) | .06 |
Abbreviations: ACE: angiotensin converting enzyme; ARB: angiotensin receptor blocker; BMI: body mass index; BP: blood pressure; CKD: chronic kidney disease; CVD: cardiovascular disease; eGFR: estimated glomerular filtration rate; FGF23: fibroblast growth factor-23; HbA1c: glycosylated hemoglobin; HOMA-IR: Homeostasis Model Assessment-insulin resistance; hsCRP: high-sensitivity C-reactive protein; hsTnT: high-sensitivity troponin T; IQR: inter-quartile range; NGAL: neutrophil gelatinase-associated lipocalin; NH: non-Hispanic; NTproBNP: N-terminal pro-B-type natriuretic peptide; PTH: parathyroid hormone; RAAS: renin-angiotensin-aldosterone system; SD: standard deviation; UACR: urine albumin:creatinine ratio.
SI conversion: To convert Uric acid to μmol/L, multiply by 59.485. To convert hsCRP to nmol/L, multiply by 9.524. To convert serum phosphate to mmol/L, multiply by 0.323. For serum bicarbonate, 1 mEq/L is equivalent to 1 mmol/L. For urine sodium and urine potassium, 1 mEq/24h is equivalent to 1 mmol/d. To convert serum aldosterone to pmol/L, multiply by 2.774.
P-value for comparison across those with and without diabetes.
Figure 1.
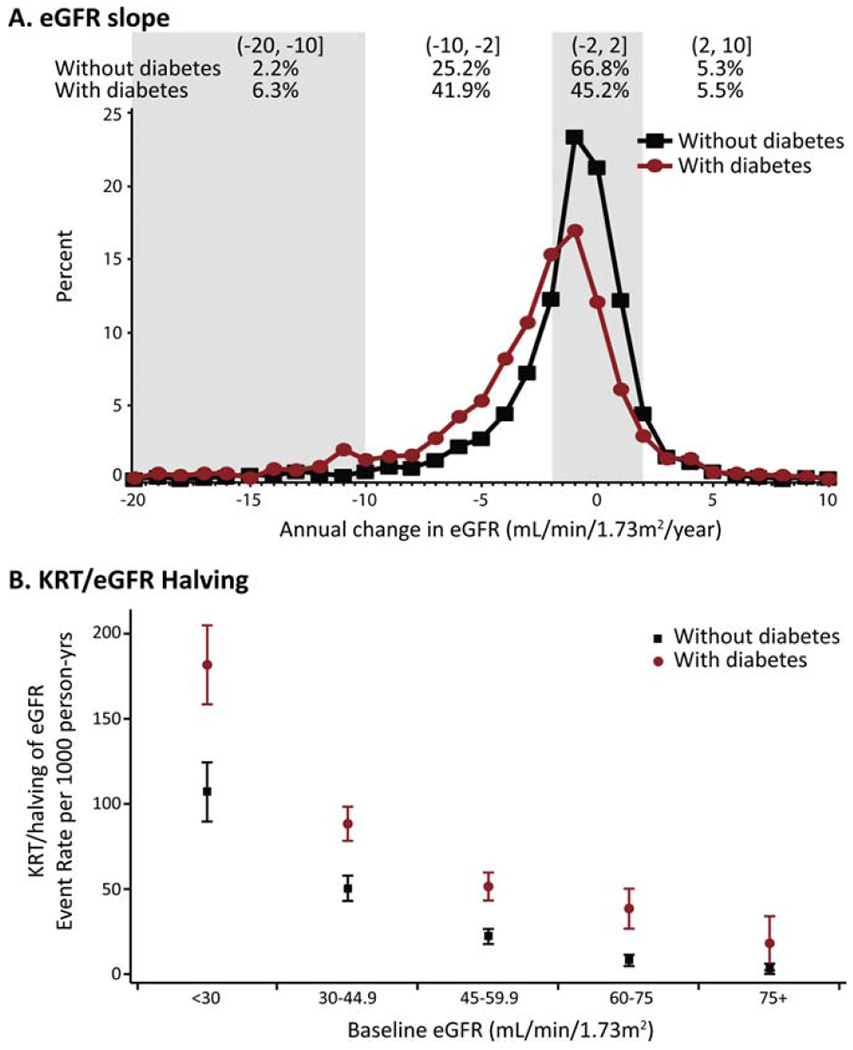
Rates and patterns of chronic kidney disease progression for Chronic Renal Insufficiency Cohort participants with and without diabetes.
Panel A: Annual change in estimated glomerular filtration rate (eGFR) in mL/min/1.73m2/year and percentage within categories of eGFR change by diabetes status. Percentages do not add up to 100%, as the tails of the distributions were truncated.
Panel B: Unadjusted event rates of kidney replacement therapy (KRT) or eGFR halving per 1,000 person-years by diabetes status and level of baseline eGFR.
Statistically significant differences (P<.05) in mean eGFR slope and rates of the composite renal endpoint were observed across categories of most of the considered risk factors (Table 2). The subgroups with the fastest eGFR decline among those without diabetes were participants with macroalbuminuria (UACR ≥300 mg/g; mean (SD) eGFR slope: −4.1 (4.1) mL/min/1.73m2/year) and non-Hispanic blacks with APOL1 high risk genotype (−3.2 (4.2)). Among those with diabetes, eGFR declined the fastest in participants with macroalbuminuria (mean (SD) eGFR slope: −5.2 (5.1) mL/min/1.73m2/year) and youngest age (<44 years; −4.9 (7.5)). Highest rates of the composite renal endpoint were observed among those with macroalbuminuria and with baseline eGFR <30 mL/min/1.73m2 regardless of diabetes status.
Table 2.
Measures of chronic kidney disease progression by baseline characteristics among CRIC participants without or with diabetes
| Without Diabetes | With Diabetes | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| eGFR Slope, mL/min/1.73m2/year | KRT/eGFR Halving | eGFR Slope, mL/min/1.73m2/year | KRT/eGFR Halving | ||||||
| Mean (SD) | P value | Events per 1,000 person-yrs (95% CI) | P value | Mean (SD) | P value | Events per 1,000 person-yrs (95% CI) | P value | ||
| Demographic Factors | |||||||||
| Age, years | <44 | −2.1 (3.9) | <.001 | 50.0 (40.7, 59.3) | <.001 | −4.9 (7.5) | <.001 | 112.7 (88.2,137.3) | <.001 |
| 45-64 | −1.2 (3.2) | 28.4 (24.6, 32.1) | −2.9 (4.4) | 88.0 (80.1, 95.9) | |||||
| 65-75 | −1.2 (2.7) | 34.5 (28.2, 40.9) | −1.9 (4.0) | 62.1 (53.0, 71.1) | |||||
| Gender | Male | −1.6 (3.4) | .004 | 36.8 (32.3, 41.3) | .01 | −2.8 (4.7) | .3 | 87.8 (79.5, 96.0) | .001 |
| Female | −1.1 (3.1) | 29.9 (25.6, 34.2) | −2.6 (4.6) | 74.1 (65.7, 82.5) | |||||
| Race/ethnicity | NH-White | −1.0 (2.8) | <.001 | 22.3 (18.9, 25.7) | <.001 | −1.5 (4.1) | <.001 | 56.2 (48.7, 63.7) | <.001 |
| NH-Black, APOL1 low-risk | −1.4 (3.6) | 40.4 (33.8, 47.1) | −3.0 (4.4) | 88.1 (77.7, 98.5) | |||||
| NH-Black, APOL1 high-risk | −3.2 (4.2) | 65.9 (50.1, 81.7) | −4.4 (5.4) | 123.8 (93.7,153.9) | |||||
| Hispanic | −1.7 (3.1) | 54.3 (39.1, 69.4) | −4.3 (5.6) | 127.4 (106.9,147.9) | |||||
| Other | −1.9 (2.9) | 44.6 (26.4, 62.9) | −3.1 (4.7) | 82.9 (53.7,112.1) | |||||
| Kidney Function Measures | |||||||||
| Baseline eGFR, mL/min/1.73m2 | <30 | −2.1 (3.2) | <.001 | 107.0 (89.6, 124.4) | <.001 | −2.9 (3.9) | .4 | 181.7 (158.5, 204.9) | <.001 |
| 30-44.9 | −1.8 (3.5) | 50.4 (43.0, 57.8) | −2.9 (4.8) | 88.3 (78.3, 98.3) | |||||
| 45-59.9 | −1.2 (3.1) | 22.1 (17.7, 26.5) | −2.6 (4.7) | 51.5 (43.3, 59.7) | |||||
| ≥60 | −0.7 (3.0) | 6.4 (4.0, 8.9) | −2.3 (5.5) | 34.3 (24.4, 44.2) | |||||
| UACR, mg/g | <30 | −0.3 (2.4) | <.001 | 8.3 (6.3, 10.3) | <.001 | −0.4 (3.4) | <.001 | 20.7 (16.1, 25.2) | <.001 |
| 30-299 | −1.7 (2.8) | 46.0 (38.6, 53.5) | −1.8 (3.3) | 69.4 (59.1, 79.6) | |||||
| ≥300 | −4.1 (4.1) | 133.8 (116.1, 151.6) | −5.2 (5.1) | 192.6 (174.9, 210.4) | |||||
| Blood Pressure Factors | |||||||||
| Systolic BP, mmHg | <120 | −0.8 (2.6) | <.001 | 24.9 (21.1, 28.7) | <.001 | −1.1 (4.2) | <.001 | 46.5 (38.9, 54.0) | <.001 |
| 120-139 | −1.5 (3.4) | 33.6 (28.3, 38.9) | −2.3 (4.2) | 72.0 (63.1, 80.8) | |||||
| ≥140 | −2.6 (4.0) | 61.6 (50.8, 72.4) | −4.7 (4.9) | 143.9 (128.5, 159.3) | |||||
| ACE/ARB | No | −1.3 (3.4) | .6 | 27.8 (23.4, 32.2) | <.001 | −3.0 (4.2) | .2 | 93.3 (78.4, 108.3) | .009 |
| Yes | −1.4 (3.1) | 37.6 (33.2, 41.9) | −2.6 (4.8) | 78.9 (72.4, 85.3) | |||||
| Clinical Factors | |||||||||
| History of CVD | No | −1.3 (3.2) | .2 | 32.6 (29.2, 36.1) | .2 | −2.7 (4.9) | .9 | 73.1 (65.9, 80.2) | <.001 |
| Yes | −1.6 (3.4) | 36.8 (29.7, 43.9) | −2.7 (4.3) | 94.8 (84.6, 104.9) | |||||
| Uric acid, mg/dL | <6.5 | −0.9 (3.1) | <.001 | 17.7 (14.1, 21.3) | <.001 | −2.8 (5.0) | .1 | 54.1 (45.5, 62.7) | <.001 |
| 6.5-8.9 | −1.7 (3.4) | 37.3 (31.6, 43.0) | −3.0 (4.4) | 82.2 (72.1, 92.3) | |||||
| ≥8.2 | −1.6 (3.2) | 51.8 (44.1, 59.4) | −2.4 (4.7) | 106.9 (95.2, 118.5) | |||||
| Socioeconomic Status Factor | |||||||||
| Education | Less than High School | −1.8 (3.8) | <.001 | 54.5 (42.8, 66.1) | <.001 | −3.4 (4.7) | <.001 | 107.9 (93.4, 122.4) | <.001 |
| High School/Some College | −1.6 (3.5) | 36.5 (31.7, 41.4) | −2.8 (5.0) | 81.5 (73.1, 89.9) | |||||
| Graduated College | −1.0 (2.7) | 24.9 (20.8, 28.9) | −2.0 (4.0) | 61.9 (52.3, 71.4) | |||||
| Behavioral Factor | |||||||||
| Current Smoker | No | −1.3 (3.1) | <.001 | 31.4 (28.2, 34.6) | <.001 | −2.6 (4.7) | .007 | 78.2 (72.1, 84.3) | <.001 |
| Yes | −2.1 (4.0) | 51.3 (39.5, 63.2) | −3.6 (4.4) | 111.4 (89.9, 132.9) | |||||
| Body Composition Measures | |||||||||
| BMI, kg/m2 | <28 | −1.4 (3.0) | .9 | 34.2 (29.3, 39.2) | .9 | −3.5 (5.1) | .001 | 90.9 (77.4, 104.4) | .02 |
| 28-34.9 | −1.4 (3.5) | 32.8 (27.9, 37.8) | −2.6 (4.7) | 75.3 (66.3, 84.2) | |||||
| ≥35 | −1.4 (3.3) | 33.5 (26.7, 40.3) | −2.4 (4.3) | 83.4 (73.8, 93.0) | |||||
| Fat-free mass, kg | Q1: [25.8, 49.1) | −1.3 (3.1) | <.001 | 34.5 (28.8, 40.1) | .006 | −2.6 (4.5) | .9 | 71.0 (57.9, 84.1) | <.001 |
| Q2: [49.1, 59.1) | −1.2 (3.1) | 31.8 (25.6, 38.0) | −2.7 (4.5) | 68.7 (58.1, 79.4) | |||||
| Q3: [59.1, 69.9) | −1.2 (2.9) | 28.0 (22.4, 33.6) | −2.6 (4.5) | 79.6 (67.9, 91.3) | |||||
| Q4: [69.9, 167.1) | −2.0 (4.0) | 41.1 (32.8, 49.3) | −2.8 (4.8) | 96.3 (84.6, 108.0) | |||||
| Peripheral Vascular Measure | |||||||||
| Ankle-brachial index | <0.9 | −1.6 (3.6) | .2 | 47.7 (35.1, 60.3) | .004 | −2.7 (4.5) | .6 | 91.5 (76.9,106.2) | <.001 |
| 0.9-1.09 | −1.4 (3.1) | 34.3 (29.8, 38.8) | −2.8 (4.7) | 76.4 (67.9, 84.8) | |||||
| 1.1-1.39 | −1.3 (3.3) | 29.4 (24.8, 34.0) | −2.7 (4.7) | 79.0 (68.7, 89.4) | |||||
| ≥1.4 | −2.6 (4.9) | 38.1 (7.6, 68.6) | −3.5 (4.1) | 118.3 (79.1, 157.5) | |||||
| Measure of Anemia | |||||||||
| Hemoglobin, mg/dL | M: <12, F: <11 | −2.1 (4.0) | <.001 | 76.0 (61.5, 90.4) | <.001 | −3.6 (4.6) | <.001 | 117.1 (104.3, 129.8) | <.001 |
| M: 12-13.9, F: 11-12.9 | −1.5 (3.3) | 38.2 (33.2, 43.3) | −2.3 (4.6) | 68.7 (60.9, 76.4) | |||||
| M: ≥14, F: ≥13 | −1.1 (2.9) | 19.3 (15.8, 22.7) | −2.2 (4.8) | 60.0 (48.8, 71.3) | |||||
| Inflammatory Marker and Chemokines | |||||||||
| hsCRP, mg/L | Q1: [0.1, 1.0) | −1.3 (3.2) | .1 | 29.6 (23.9, 35.2) | .01 | −2.8 (4.4) | .009 | 82.4 (70.3, 94.6) | .7 |
| Q2: [1.0, 2.5) | −1.1 (2.8) | 29.5 (23.8, 35.2) | −3.3 (4.9) | 80.7 (69.1, 92.3) | |||||
| Q3: [2.5, 6.3) | −1.4 (3.5) | 35.1 (28.7, 41.5) | −2.1 (4.8) | 78.4 (66.9, 89.9) | |||||
| Q4: [6.3, 187) | −1.6 (3.5) | 40.9 (33.6, 48.3) | −2.7 (4.6) | 85.2 (73.3, 97.2) | |||||
| Serum fractalkine (CX3CL1), pg/mL | Q1: [0.1, 0.6) | −1.0 (2.9) | <.001 | 17.6 (13.7, 21.6) | <.001 | −1.6 (3.6) | <.001 | 37.7 (29.1, 46.3) | <.001 |
| Q2: [0.6, 0.8) | −1.4 (3.4) | 32.1 (26.2, 37.9) | −1.9 (3.7) | 62.4 (52.1, 72.8) | |||||
| Q3: [0.8, 1.1) | −1.3 (2.9) | 40.3 (33.0, 47.7) | −3.1 (5.5) | 94.9 (82.4, 107.5) | |||||
| Q4: [1.1, 3.6] | −2.1 (3.8) | 60.5 (50.1, 70.9) | −3.7 (4.9) | 123.0 (108.9, 137.1) | |||||
| Plasma CXCL12, pg/mL | Q1: [832.1, 2066.4) | −0.9 (2.7) | <.001 | 20.4 (16.1, 24.8) | <.001 | −2.1 (4.6) | .02 | 50.8 (41.3, 60.4) | <.001 |
| Q2: [2066.4, 2410.5) | −1.4 (3.5) | 27.9 (22.3, 33.4) | −2.5 (3.9) | 65.8 (55.3, 76.2) | |||||
| Q3: [2410.5, 2797.8) | −1.5 (3.2) | 40.4 (33.2, 47.6) | −3.1 (4.9) | 94.0 (81.2, 106.8) | |||||
| Q4: [2797.8, 6173.3] | −1.9 (3.6) | 55.3 (45.9, 64.7) | −3.0 (5.2) | 115.6 (101.5, 129.7) | |||||
| Mineral Metabolism Markers | |||||||||
| FGF23, RU/mL | Q1: [1.4, 94.1) | −0.9 (2.8) | <.001 | 16.0 (12.5, 19.5) | <.001 | −2.1 (3.8) | .04 | 42.3 (32.5, 52.1) | <.001 |
| Q2: [94.1, 139.1) | −1.3 (3.0) | 28.5 (23.0, 34.0) | −2.5 (4.6) | 58.7 (49.0, 68.4) | |||||
| Q3: [139.1, 225.5) | −1.8 (3.5) | 49.3 (40.7, 57.9) | −3.0 (5.0) | 88.8 (77.4, 100.3) | |||||
| Q4: [225.5, 14 318.9] | −1.9 (3.8) | 67.8 (56.3, 79.4) | −3.0 (4.9) | 129.3 (114.3, 144.3) | |||||
| Serum phosphate, mg/dL | Q1: [1.7, 3.3) | −1.4 (3.1) | .006 | 26.1 (21.3, 30.9) | <.001 | −2.2 (3.9) | .006 | 52.7 (42.3, 63.2) | <.001 |
| Q2: [3.3, 3.7) | −1.1 (2.9) | 27.6 (22.3, 32.9) | −2.2 (3.7) | 68.3 (56.9, 79.8) | |||||
| Q3: [3.7, 4.1) | −1.3 (3.7) | 36.9 (29.9, 43.9) | −2.8 (4.7) | 81.8 (69.8, 93.7) | |||||
| Q4: [4.1, 9.3] | −1.9 (3.5) | 56.9 (46.2, 67.5) | −3.2 (5.5) | 111.0 (98.6, 123.3) | |||||
| Intact PTH pg/mL | Q1: [1.9, 34.1) | −0.8 (2.5) | <.001 | 15.9 (12.1, 19.7) | <.001 | −1.8 (4.7) | <.001 | 48.9 (39.9, 58.0) | <.001 |
| Q2: [34.1, 52.4) | −1.1 (3.0) | 21.3 (16.6, 26.0) | −2.6 (4.2) | 55.9 (46.5, 65.4) | |||||
| Q3: [52.4, 84.7) | −1.6 (3.6) | 40.2 (33.1, 47.4) | −3.0 (4.6) | 85.5 (73.0, 98.1) | |||||
| Q4: [84.5, 1483] | −2.5 (3.8) | 84.9 (72.0, 97.8) | −3.3 (5.0) | 145.1 (128.7, 161.5) | |||||
| Carbohydrate Metabolism Markers | |||||||||
| HbA1c, % | Q1: [3.5, 5.6) | −1.4 (3.2) | .9 | 34.4 (29.4, 39.5) | .9 | −2.5 (3.6) | <.001 | 62.5 (38.5, 86.5) | <.001 |
| Q2: [5.6, 6.2) | −1.3 (3.2) | 33.0 (28.3, 37.8) | −1.7 (4.2) | 68.8 (53.4, 84.2) | |||||
| Q3: [6.2, 7.3) | −1.4 (3.5) | 31.9 (24.0, 39.7) | −2.4 (4.3) | 70.0 (60.4, 79.5) | |||||
| Q4: [7.3, 15.2] | −1.7 (4.0) | 41.2 (5.1, 77.2) | −3.2 (5.0) | 94.8 (85.8,103.9) | |||||
| HOMA-IR | Q1: [0, 2.5) | −1.4 (3.2) | .3 | 33.0 (27.9, 38.1) | .6 | −2.9 (4.9) | .7 | 83.2 (63.3,103.2) | .008 |
| Q2: [2.5, 4.1) | −1.2 (3.0) | 32.6 (26.9, 38.2) | −2.5 (3.7) | 66.6 (53.9, 79.4) | |||||
| Q3: [4.1, 7.2) | −1.6 (3.6) | 37.6 (30.3, 44.8) | −2.8 (4.5) | 84.8 (73.3, 96.3) | |||||
| Q4: [7.2, 224.8) | −1.4 (3.5) | 33.5 (22.6, 44.5) | −2.7 (5.1) | 86.0 (76.4, 95.7) | |||||
| Cardiac Markers | |||||||||
| hsTnT, pg/mL | Q1: [1.5, 5.5) | −1.1 (3.2) | .002 | 23.6 (19.5, 27.8) | <.001 | −1.3 (4.8) | <.001 | 34.6 (24.6, 44.6) | <.001 |
| Q2: [5.5, 11.4) | −1.3 (3.1) | 32.5 (26.8, 38.2) | −2.0 (3.4) | 47.8 (38.9, 56.7) | |||||
| Q3: (11.4, 22.2) | −1.5 (3.1) | 38.6 (31.3, 46.0) | −2.5 (4.7) | 73.5 (63.1, 83.8) | |||||
| Q4: (22.2, 738.7) | −2.1 (3.8) | 70.2 (54.6, 85.7) | −3.6 (5.1) | 141.3 (127.1, 155.6) | |||||
| NTproBNP, pg/mL | Q1: [2.5, 60.2) | −1.0 (2.9) | <.001 | 18.7 (14.8, 22.7) | <.001 | −1.6 (4.9) | <.001 | 37.2 (28.9, 45.6) | <.001 |
| Q2: [60.2, 139.4) | −1.2 (3.2) | 29.1 (23.6, 34.7) | −2.5 (4.2) | 63.0 (52.5, 73.4) | |||||
| Q3: [139.4, 366.6) | −1.6 (3.3) | 42.9 (35.4, 50.5) | −2.4 (4.2) | 84.6 (73.0, 96.2) | |||||
| Q4: [366.6, 33 742] | −2.0 (3.6) | 63.2 (51.8, 74.7) | −3.8 (5.0) | 137.0 (121.6, 152.4) | |||||
| Acidosis Marker | |||||||||
| Serum bicarbonate, mmol/L | ≤22 | −1.9 (3.4) | .002 | 64.4 (54.6, 74.2) | <.001 | −3.2 (4.7) | .002 | 100.1 (87.2, 113.1) | <.001 |
| (22, 24] | −1.4 (3.3) | 30.5 (24.4, 36.6) | −3.1 (5.0) | 90.4 (77.4, 103.4) | |||||
| (24, 26] | −1.2 (3.2) | 26.6 (21.3, 32.0) | −2.4 (4.8) | 76.1 (64.5, 87.7) | |||||
| >26 | −1.1 (3.0) | 21.1 (16.5, 25.7) | −2.2 (4.1) | 62.6 (52.7, 72.5) | |||||
| Urinary Electrolytes | |||||||||
| Urine sodium, mEq/24h | Q1: [4.3, 108.6) | −1.4 (3.4) | .05 | 32.2 (26.2, 38.2) | .4 | −2.7 (4.1) | .6 | 90.9 (77.0, 104.8) | .002 |
| Q2: [108.6, 151.3) | −1.1 (2.9) | 31.2 (25.3, 37.1) | −2.9 (4.9) | 71.0 (59.4, 82.6) | |||||
| Q3: [151.3, 203.1) | −1.3 (3.0) | 34.3 (27.6, 41.0) | −2.5 (4.3) | 86.4 (74.6, 98.3) | |||||
| Q4: [203.1, 699.8] | −1.7 (3.7) | 37.3 (30.3, 44.3) | −2.6 (4.9) | 74.1 (63.4, 84.9) | |||||
| Urine potassium, mmol/24h | Q1: [3.0, 37.5) | −1.4 (2.8) | .002 | 43.3 (36.1, 50.6) | <.001 | −2.9 (4.2) | .002 | 89.9 (75.8, 104.0) | <.001 |
| Q2: [37.5, 51.8) | −1.6 (3.7) | 37.8 (30.5, 45.0) | −2.9 (4.8) | 84.2 (72.4, 95.9) | |||||
| Q3: [51.8, 69.4) | −1.6 (3.6) | 33.3 (27.1, 39.5) | −3.0 (5.1) | 84.8 (72.4, 97.2) | |||||
| Q4: [69.4, 417.7] | −0.9 (2.9) | 21.6 (16.7, 26.5) | −1.9 (4.1) | 66.0 (55.9, 76.1) | |||||
| Kidney Injury Marker | |||||||||
| Urine NGAL, ng/ml | Q1: [0.4, 6.3) | −0.7 (2.3) | <.001 | 14.0 (10.2, 17.9) | <.001 | −1.1 (3.8) | <.001 | 48.7 (40.0, 57.5) | <.001 |
| Q2: [6.3, 14.2) | −1.3 (3.5) | 28.9 (23.4, 34.5) | −2.1 (4.2) | 57.4 (47.2, 67.6) | |||||
| Q3: [14.2, 32.9) | −1.5 (3.2) | 39.9 (32.7, 47.1) | −2.7 (4.0) | 78.6 (66.9, 90.4) | |||||
| Q4: [32.9, 2743.8] | −2.2 (3.8) | 63.5 (53.3, 73.7) | −4.6 (5.4) | 157.6 (139.3, 175.8) | |||||
| RAAS Marker | |||||||||
| Serum aldosterone, pg/mL | Q1: [0.8, 71.2) | −1.2 (3.5) | .03 | 21.0 (16.1, 26.0) | <.001 | −3.1 (4.3) | .2 | 74.0 (63.0, 84.9) | .07 |
| Q2: [71.2, 101.4) | −1.5 (3.3) | 33.9 (27.5, 40.3) | −2.9 (5.1) | 85.6 (73.7, 97.5) | |||||
| Q3: [101.4, 152.8) | −1.7 (3.3) | 45.1 (37.7, 52.5) | −2.6 (4.5) | 79.9 (68.2, 91.6) | |||||
| Q4: [152.8, 15 630.9] | −1.2 (3.0) | 35.6 (29.2, 42.0) | −2.4 (4.9) | 89.0 (75.8, 102.3) | |||||
Abbreviations: ACE: angiotensin converting enzyme; ARB: angiotensin receptor blocker; BP: blood pressure; CI: confidence interval; CKD: chronic kidney disease; CVD: cardiovascular disease; eGFR: estimated glomerular filtration rate; KRT: kidney replacement therapy; FGF23: fibroblast growth factor-23; hsCRP: high-sensitivity C-reactive protein; hsTnT: high-sensitivity troponin T; NGAL: neutrophil gelatinase-associated lipocalin; NH: non-Hispanic; NTproBNP: N-terminal pro-B-type natriuretic peptide; PTH: parathyroid hormone; RAAS: renin-angiotensin-aldosterone system; SD: standard deviation; UACR: urine albumin:creatinine ratio
SI conversion: To convert Uric acid to μmol/L, multiply by 59.485. To convert Hemoglobin to g/L, multiply by 0.01. To convert hsCRP to nmol/L, multiply by 9.524. To convert serum phosphate to mmol/L, multiply by 0.323. For serum bicarbonate, 1 mEq/L is equivalent to 1 mmol/L. For urine potassium, 1 mEq/24h is equivalent to 1 mmol/d. To convert serum aldosterone to pmol/L, multiply by 2.774.
In intermediate models stratified by diabetes status (Step 2), only race/ethnicity, baseline eGFR, albuminuria, SBP, and the cardiac marker NTproBNP were statistically significant across all outcomes and diabetes groups (Table S1a and Table S1b).
Final models among participants without diabetes
Among those without diabetes, multivariable-adjusted models of both the composite renal outcome and eGFR slope identified faster progression among men, non-Hispanic blacks with and without the APOL1 high-risk genotype and “other” races (all non-Hispanic, non-white, non-black race groups combined), and those with higher albuminuria, SBP, NTproBNP, and urine NGAL (Table 3). Multivariable-adjusted rates were significantly lower with greater levels of hemoglobin (≥14 g/dL for men and ≥13 for women; hazard ratio (HR;95% confidence interval): 0.7 (0.5-0.9)). Serum bicarbonate ≤22 mmol/L as well as higher high-sensitivity troponin T, NTproBNP and urine NGAL were all significantly associated with a 1.5-fold or greater rate of the composite outcome. These associations were similar or stronger than that of SBP ≥140 mmHg (HR (95% CI): 1.5 (1.1-2.0)). Those with lowest baseline eGFR (<30 mL/min/1.73m2) were 1.8 times as likely to initiate KRT or experience eGFR halving as those with eGFR of 30-44.9 mL/min/1.73m2, but those with eGFR of 30-59.9 mL/min/1.73m2 had the fastest eGFR decline over time. Faster eGFR decline was observed with the lowest and highest serum phosphate quartiles and urine potassium levels of 51.8-69.4 mmol/day.
Table 3.
Final multivariable-adjusted models of chronic kidney disease progression among CRIC participants without or with diabetes
| Without Diabetes | With Diabetes | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| eGFR Slope, mL/min/1.73m2/year | KRT/eGFR Halving | eGFR Slope, mL/min/1.73m2/year | KRT/eGFR Halving | ||||||
| Beta Coefficienta (SE) | P value | HR (95% CI)b | P valueb | Beta Coefficienta (SE) | P value | HR (95% CI)b | P valueb | ||
| Demographic Factors | |||||||||
| Age, years | <45 | - | - | - | - | −0.6 (0.2) | .02 | Y0-6: 1.2 (0.9, 1.7) |
Y0-6: .007 Y6+: .05 |
| Y6+: 1.7 (1.1, 2.8) | |||||||||
| 45-64 | - | - | Ref | Ref | |||||
| 65-75 | - | - | 0.1 (0.1) | Y0-6: 0.7 (0.6, 0.9) | |||||
| Y6+: 0.9 (0.6, 1.3) | |||||||||
| Gender | Male | Ref | <.001 | Ref | <.001 | - | - | Ref | <.001 |
| Female | 0.4 (0.1) | 0.5 (0.4, 0.7) | - | 0.7 (0.5, 0.8) | |||||
| Race/ethnicity | NH-White | Ref | <.001 | Ref |
Y0-6: <.001 Y6+: .1 |
Ref | <.001 | Ref | <.001 |
| NH-Black, APOL1 low-risk | −0.1 (0.1) | Y0-6: 1.9 (1.4, 2.6) | −0.5 (0.1) | 1.5 (1.2, 1.8) | |||||
| Y6+: 1.6 (1.0, 2.4) | |||||||||
| NH-Black, APOL1 high-risk | −0.7 (0.2) | Y0-6: 3.7 (2.5, 5.4) | −1.1 (0.3) | 1.9 (1.4, 2.6) | |||||
| Y6+: 1.4 (0.7, 2.8) | |||||||||
| Hispanic | 0.2 (0.2) | Y0-6: 1.2 (0.8, 1.9) | −0.6 (0.2) | 1.4 (1.0, 1.9) | |||||
| Y6+: 1.7 (0.9, 3.2) | |||||||||
| Other | −0.5 (0.2) | Y0-6: 2.7 (1.5, 4.8) | −0.3 (0.3) | 1.4 (0.9, 2.2) | |||||
| Y6+: 2.3 (0.9, 6.0) | |||||||||
| Kidney Measures | |||||||||
| Baseline eGFR, mL/min/ 1.73m2 | <30 | 0.2 (0.2) | .04 | 1.8 (1.4, 2.4) | <.001 | 0.6 (0.2) | <.001 | Y0-6: 1.6 (1.3, 1.9) |
Y0-6: <.001 Y6+: .01 |
| Y6+: 2.0 (1.2, 3.3) | |||||||||
| 30-44.9 | Ref | Ref | Ref | Ref | |||||
| 45-59.9 | 0.0 (0.1) | 0.6 (0.4, 0.8) | −0.5 (0.1) | Y0-6: 0.7 (0.6, 1.0) | |||||
| Y6+: 0.9 (0.6, 1.3) | |||||||||
| ≥60 | 0.2 (0.1) | 0.2 (0.1, 0.4) | −0.8 (0.2) | Y0-6: 0.5 (0.3, 0.9) | |||||
| Y6+: 1.1 (0.6, 1.9) | |||||||||
| UACR, mg/g | <30 | Ref | <.001 | Ref | <.001 | Ref | <.001 | Ref |
Y0-6: <.001 Y6+: <.001 |
| 30-299 | −0.9 (0.1) | 3.5 (2.5, 4.8) | −1.1 (0.1) | Y0-6: 2.5 (1.7, 3.7) | |||||
| Y6+: 3.0 (1.9, 4.7) | |||||||||
| ≥300 | −2.2 (0.1) | 10.6 (7.7, 14.8) | −2.5 (0.2) | Y0-6: 6.3 (4.4, 9.1) | |||||
| Y6+: 5.4 (3.3, 8.7) | |||||||||
| Blood Pressure Factors | |||||||||
| Systolic BP, mmHg | <120 | Ref | .002 | Ref | .03 | Ref | <.001 | Ref | <.001 |
| 120-139 | −0.2 (0.1) | 1.2 (1.0, 1.6) | −0.2 (0.1) | 1.2 (0.9, 1.5) | |||||
| ≥140 | −0.4 (0.1) | 1.5 (1.1, 2.0) | −0.6 (0.2) | 1.6 (1.2, 2.0) | |||||
| ACE/ARB | No | Ref | .07 | - | - | - | - | - | - |
| Yes | 0.2 (0.1) | - | - | - | |||||
| Behavioral Factor | |||||||||
| Current smoker | No | - | - | - | - | Ref | .08 | - | - |
| Yes | - | - | −0.3 (0.2) | - | |||||
| Body Composition Measures | |||||||||
| Fat-free mass, kg | Q1: [25.8, 49.1) | - | - | Ref | .02 | - | - | - | - |
| Q2: [49.1, 59.1) | - | 0.7 (0.5, 1.0) | - | - | |||||
| Q3: [59.1, 69.9) | - | 0.8 (0.5, 1.1) | - | - | |||||
| Q4: [69.9, 167.1) | - | 1.1 (0.8, 1.6) | - | - | |||||
| Measure of Anemia | |||||||||
| Hemoglobin, g/dL | M: <12, F: <11 | - | - | 1.1 (0.8, 1.4) | .004 | - | - | - | - |
| M: 12-13.9, F: 11-12.9 | - | Ref | - | - | |||||
| M: ≥14, F: ≥13 | - | 0.7 (0.5, 0.9) | - | - | |||||
| Inflammatory Marker and Chemokines | |||||||||
| Serum fractalkine (CX3CL1), pg/mL | Q1: [0.1, 0.6) | - | - | - | - | Ref | .02 | Ref | .04 |
| Q2: [0.6, 0.8) | - | - | 0.1 (0.2) | 1.0 (0.7, 1.3) | |||||
| Q3: [0.8, 1.1) | - | - | −0.4 (0.2) | 1.3 (1.0, 1.8) | |||||
| Q4: [1.1, 3.6] | - | - | −0.3 (0.2) | 1.3 (0.9, 1.7) | |||||
| Plasma CXCL12, pg/mL | Q1: [832.1, 2066.4) | - | - | - | - | - | - | Ref |
Y0-6: .005 Y6+: .03 |
| Q2: [2066.4, 2410.5) | - | - | - | Y0-6: 1.2 (0.9, 1.6) | |||||
| Y6+: 1.3 (0.8, 2.2) | |||||||||
| Q3: [2410.5, 2797.8) | - | - | - | Y0-6: 1.7 (1.2, 2.3) | |||||
| Y6+: 1.1 (0.6, 1.8) | |||||||||
| Q4: [2797.8, 6173.3] | - | - | - | Y0-6: 1.5 (1.1, 2.0) | |||||
| Y6+: 1.9 (1.2, 3.1) | |||||||||
| Mineral Metabolism Markers | |||||||||
| Serum phosphate, mg/dL | Q1: [1.7, 3.3) | −0.3 (0.1) | .03 | - | - | - | - | - | - |
| Q2: [3.3, 3.7) | Ref | - | - | - | |||||
| Q3: [3.7, 4.1) | 0.0 (0.1) | - | - | - | |||||
| Q4: [4.1, 9.3] | −0.2 (0.1) | - | - | - | |||||
| Carbohydrate Metabolism Markers | |||||||||
| HbA1c, % | Q1: [3.5, 5.6) | - | - | - | - | - | - | Ref | .05 |
| Q2: [5.6, 6.2) | - | - | - | 1.5 (0.9, 2.5) | |||||
| Q3: [6.2, 7.3) | - | - | - | 1.0 (0.6, 1.6) | |||||
| Q4: [7.3, 15.2] | - | - | - | 1.2 (0.7, 1.8) | |||||
| Cardiac Markers | |||||||||
| hsTnT, pg/mL | Q1: [1.5, 5.5) | - | - | Ref |
Y0-6: <.001 Y6+: .5 |
- | - | - | - |
| Q2: [5.5, 11.4) | - | Y0-6: 1.6 (1.1, 2.3) | - | - | |||||
| Y6+: 0.8 (0.5, 1.2) | - | - | |||||||
| Q3: (11.4, 22.2) | - | Y0-6: 0.8 (0.5, 1.2) | - | - | |||||
| Y6+: 0.7 (0.5, 1.2) | - | - | |||||||
| Q4: (22.2, 738.7) | - | Y0-6: 1.0 (0.6, 1.5) | - | - | |||||
| Y6+: 0.7 (0.4, 1.4) | - | - | |||||||
| NTproBNP, pg/mL | Q1: [2.5, 60.2) | Ref | .03 | Ref |
Y0-6: .04 Y6+: .4 |
Ref | <.001 | Ref |
Y0-6: <.001 Y6+: .4 |
| Q2: [60.2, 139.4) | −0.2 (0.1) | Y0-6: 1.6 (1.1, 2.5) | −0.5 (0.2) | Y0-6: 1.8 (1.2, 2.7) | |||||
| Y6+: 1.0 (0.6, 1.7) | Y6+: 1.2 (0.7, 2.1) | ||||||||
| Q3: [139.4, 366.6) | −0.4 (0.1) | Y0-6: 1.8 (1.2, 2.8) | −0.5 (0.2) | Y0-6: 1.8 (1.2, 2.7) | |||||
| Y6+: 1.5 (0.9, 2.4) | Y6+: 1.4 (0.8, 2.3) | ||||||||
| Q4: [366.6, 33 742] | −0.3 (0.2) | Y0-6: 1.8 (1.2, 2.9) | −0.8 (0.2) | Y0-6: 2.3 (1.5, 3.4) | |||||
| Y6+: 1.2 (0.7, 2.2) | Y6+: 1.6 (0.9, 2.7) | ||||||||
| Acidosis Marker | |||||||||
| Serum bicarbonate, mmol/L | ≤22 | - | - | 1.5 (1.1, 2.0) | .007 | - | - | - | - |
| (22, 24] | - | Ref | - | - | |||||
| (24, 26] | - | 1.0 (0.7, 1.3) | - | - | |||||
| >26 | - | 1.1 (0.8, 1.6) | - | - | |||||
| Urinary Electrolytes | |||||||||
| Urine potassium, mmol/24h | Q1: [3.0, 37.5) | Ref | .03 | - | - | - | - | - | - |
| Q2: [37.5, 51.8) | −0.1 (0.1) | - | - | - | |||||
| Q3: [51.8, 69.4) | −0.3 (0.1) | - | - | - | |||||
| Q4: [69.4, 417.7] | 0.1 (0.1) | - | - | - | |||||
| Kidney Injury Marker | |||||||||
| Urine NGAL, ng/ml | Q1: [0.4, 6.3) | Ref | .08 | Ref | .02 | Ref | .01 | Ref |
Y0-6: <.001 Y6+: .1 |
| Q2: [6.3, 14.2) | −0.3 (0.1) | 1.6 (1.1, 2.3) | −0.3 (0.2) | Y0-6: 1.3 (0.9, 1.8) | |||||
| Y6+: 1.0 (0.6, 1.6) | |||||||||
| Q3: [14.2, 32.9) | −0.2 (0.1) | 1.3 (0.9, 1.9) | −0.4 (0.2) | Y0-6: 1.4 (1.0, 1.9) | |||||
| Y6+: 1.2 (0.7, 1.9) | |||||||||
| Q4: [32.9, 2743.8] | −0.3 (0.1) | 1.6 (1.1, 2.3) | −0.6 (0.2) | Y0-6: 2.4 (1.8, 3.3) | |||||
| Y6+: 1.7 (1.1, 2.8) | |||||||||
| RAAS Marker | |||||||||
| Serum aldosterone, pg/mL | Q1: [0.8, 71.2) | Ref | .07 | - | - | - | - | - | - |
| Q2: [71.2, 101.4) | −0.2 (0.1) | - | - | - | |||||
| Q3: [101.4, 152.8) | −0.2 (0.1) | - | - | - | |||||
| Q4: [152.8, 15 630.9] | 0.1 (0.1) | - | - | - | |||||
Abbreviations: ACE: angiotensin converting enzyme; ARB: angiotensin receptor blocker; BP: blood pressure; CI: confidence interval; CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; KRT: kidney replacement therapy; HbA1c: glycosylated hemoglobin; HR: hazard ratio; hsTnT: high-sensitivity troponin T; NGAL: neutrophil gelatinase-associated lipocalin; NH: non-Hispanic; NTproBNP: N-terminal pro-B-type natriuretic peptide; RAAS: renin-angiotensin-aldosterone system; Ref: reference; SE: standard error; UACR: urine albumin:creatinine ratio
SI conversion: To convert serum phosphate to mmol/L, multiply by 0.323. For serum bicarbonate, 1 mEq/L is equivalent to 1 mmol/L. For urine potassium, 1 mEq/24h is equivalent to 1 mmol/d. To convert serum aldosterone to pmol/L, multiply by 2.774.
Factors with >3% missingness included UACR (3.3%), HOMA_IR (6.1%), urine sodium (4.1%), urine potassium (4.1%), and urine NGAL (5.4%). All other risk factors had <2.7% missing data.
Negative coefficients represent a faster decline in eGFR compared to the reference group.
HRs and p-values for factors with significant interactions with time are depicted separately for years 0 through 6 (Y0-6) and 6+ years (Y6+).
Final models among participants with diabetes
In the final multivariable-adjusted models for those with diabetes, both steeper eGFR slopes and higher rates of KRT or eGFR halving were observed among those < 45 years of age, men, non-Hispanic blacks with and without the APOL1 high-risk genotype, and those with higher albuminuria, SBP, serum fractalkine, NTproBNP, and urine NGAL (Table 3). Hazard ratios approximately doubled with higher levels of the inflammatory chemokine CXCL12, the cardiac marker NTproBNP and the kidney injury marker urine NGAL. These strong associations exceeded that of SBP ≥140 mmHg (HR (95% CI): 1.6 (1.2-2.0)). A contrasting pattern of progression emerged for baseline eGFR, with eGFR > 45 mL/min/1.73m2 associated with faster eGFR decline, despite eGFR < 30 mL/min/1.73m2 being associated with increased rates of KRT or eGFR halving.
Figure 2 summarizes the statistical significance of the associations between risk factors included in each of the final CKD progression models for those with and without diabetes. Race/ethnicity, baseline eGFR, albuminuria, SBP, NTproBNP, and urine NGAL were strong and consistent predictors across models including participants with and without diabetes.
Figure 2.
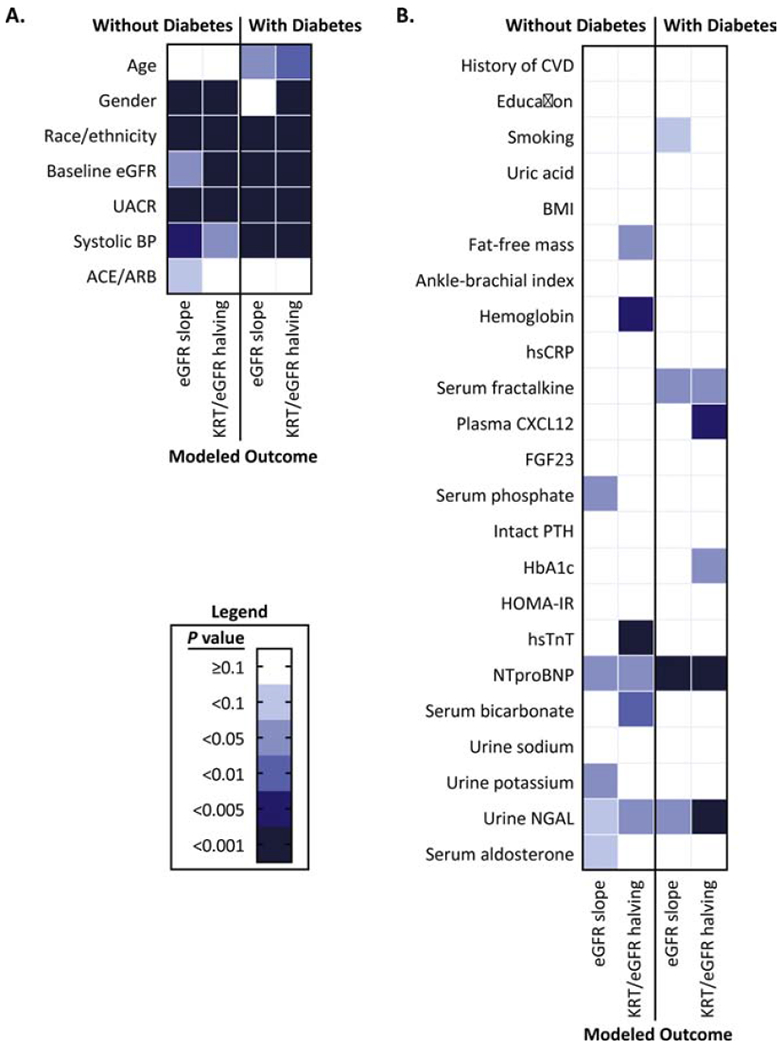
Summary of the statistical significance of chronic kidney disease progression risk factors in the Chronic Renal Insufficiency Cohort Study
Panel A: Demographic, kidney function, and blood pressure risk factors for estimated glomerular filtration rate (eGFR) slope and the composite renal outcome of kidney replacement therapy (KRT) and eGFR halving.
Panel B: Clinical, socioeconomic, behavioral, body composition, vascular, anemia, inflammatory, mineral or carbohydrate metabolism, cardiac, acidosis, urinary electrolyte, kidney injury, and RAAS factors for estimated glomerular filtration rate (eGFR) slope and the composite renal outcome of kidney replacement therapy (KRT) and eGFR halving.
Alternative final models fit without albuminuria are summarized in Figure 3 and Tables S2a and S2b. Several effect estimates became stronger, and while risk factor selection was largely consistent with or without albuminuria in the model, there were a few noteworthy exceptions. Several demographic and blood pressure risk factors including age, gender, and ACE/ARB use were incrementally retained in these models. Other factors that emerged were smoking, fat-free mass, and FGF23 for those without diabetes, and a history of CVD, smoking, intact PTH, HbA1c, hsTnT, and serum bicarbonate for those with diabetes.
Figure 3.
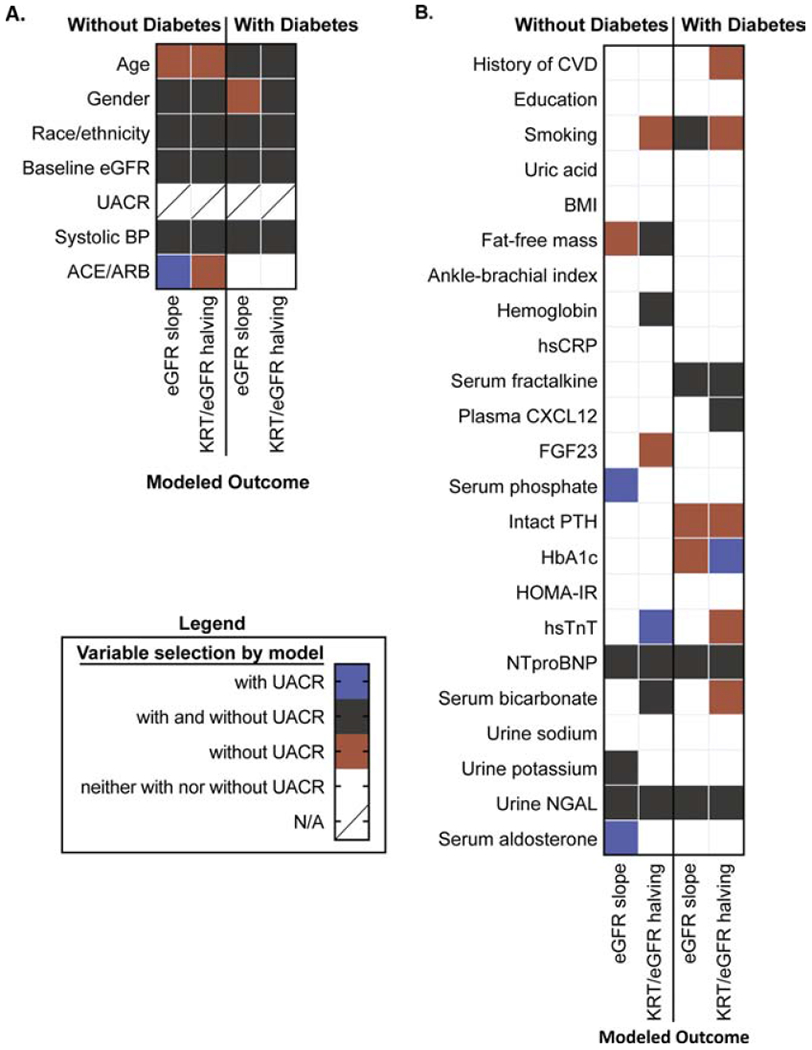
Summary of final variable selection of chronic kidney disease progression risk factors among CRIC participants without or with diabetes before and after exclusion of albuminuria measure UACR. Variables selected in modeling without exclusion of albuminuria indicated in blue, with exclusion of albuminuria indicated in orange, and selected both with and without albuminuria indicated in gray.
Panel A: Demographic, kidney function, and blood pressure risk factors for estimated glomerular filtration rate (eGFR) slope and the composite renal outcome of kidney replacement therapy (KRT) and eGFR halving.
Panel B: Clinical, socioeconomic, behavioral, body composition, vascular, anemia, inflammatory, mineral or carbohydrate metabolism, cardiac, acidosis, urinary electrolyte, kidney injury, and RAAS factors for estimated glomerular filtration rate (eGFR) slope and the composite renal outcome of kidney replacement therapy (KRT) and eGFR halving.
Tables 2 and 3 provide the specific magnitude and direction of the risk factor associations, which include both linear and non-linear associations.
Exploration of potential effect modification by albuminuria (Tables S3a and S3b) and race/ethnicity (Tables S4a and Table S4b) identified a significant interaction of albuminuria with race, SBP, hemoglobin, and NTproBNP among those without diabetes and with race and urine NGAL among participants with diabetes. Significant effect modification by race/ethnicity was observed for albuminuria, SBP, fat-free mass, and NTproBNP among those without diabetes and for gender and albuminuria among those with diabetes.
DISCUSSION
Chronic kidney disease progression is associated with significant morbidity and mortality and varies substantially across certain subgroups (e.g., diabetes versus no diabetes, Blacks compared to Whites) and between individuals within the same subgroup. Despite our knowledge of a small set of well-established risk factors such as albuminuria and diabetes, the pathophysiology of CKD progression is not well understood. In the current study, demographic, kidney function, and blood pressure risk factors, including non-Hispanic black race (especially those with APOL1 high-risk genotypes), baseline eGFR, higher proteinuria, and higher SBP, were consistently associated with higher rates of CKD progression among both those with and without diabetes after adjustment for additional measures. Novel markers including higher levels of the inflammatory chemokine plasma CXCL12, the cardiac marker NTproBNP, and the kidney injury marker urine NGAL were all independently associated with an approximate twofold or greater rate of the composite renal endpoint among participants with diabetes. Low serum bicarbonate as well as higher high-sensitivity troponin T, NTproBNP and urine NGAL were all independently associated with a 1.5-fold or greater rate, and higher hemoglobin was associated with a significantly lower rate of the composite outcome among those without diabetes. Indeed, strong associations for cardiac markers, plasma CXCL12, and urine NGAL exceeded that of systolic blood pressure ≥140 mmHg, a well-established risk factor for CKD progression.
Among the set of potential novel risk factors for CKD progression, the strongest signal was observed with the cardiac marker NTproBNP. NTproBNP levels are elevated following myocardial stretch due to pressure or volume overload in the general population and with lower eGFR levels in the setting of CKD, and are strongly associated with heart failure.12–15 In the current study, NTproBNP is also strongly associated with CKD progression both among those with and without diabetes. In models restricted to those free of self-reported heart failure at baseline and adjusted for left ventricular ejection fraction and left ventricular hypertrophy, the strength of the NTproBNP findings remain similar, suggesting that the observed relationship between NTproBNP and CKD progression may reflect more than heart failure-induced kidney function decline. In other words, NTproBNP may be a marker of cardiorenal syndrome, but this requires confirmation in additional populations. A few previous studies have reported an association of NTproBNP with CKD progression,16–18 although these studies had limitations of smaller sample sizes and less representative study populations. The current study confirms these earlier reports and extends them by demonstrating that NTproBNP is a robust marker of diabetic and non-diabetic CKD progression, using several measures of kidney function and an iterative model selection process to identify factors most strongly associated with renal outcomes.
Urinary NGAL was initially studied in the setting of acute kidney injury, given its release from tubular epithelial cells following damage.19 Following the discovery that NGAL levels are also elevated in the setting of CKD, this marker has been found to be associated with CKD progression, including in a previous CRIC Study analysis.20, 21 In the current analysis, urine NGAL was strongly associated with progression of diabetic and non-diabetic CKD, suggesting that it captures some of the variability in rates of kidney function decline not explained by albuminuria or other risk factors.
Although we identified a number of risk factors for CKD progression shared among patients with and without diabetes, we found several notable differences in risk factor patterns when diabetes status was considered, including a specific relationship between inflammatory chemokines and progression of diabetic CKD. The C-X-C motif, ligand 12 chemokine (CXCL12, also known as stromal cell-derived factor-1) is ubiquitous across numerous tissues and cell types and plays complex and tissue-specific biologic roles.22, 23 CXCL12 was previously reported as being associated with increased risk of incident myocardial infarction and death in the CRIC Study.24 Fractalkine, or C-X3-C motif, ligand 1 (CX3CL1), is also an inflammatory chemokine and possibly promotes atherosclerotic CVD and the pathogenesis of diabetes mellitus.25, 26 The CRIC Study previously reported an independent, increased odds of prevalent diabetes and risk of mortality associated with elevated levels of fractalkine.27 A few studies have examined the potential relationships of these chemokines to kidney disease.28–31 Taken with the current study, this growing evidence supports the need for further investigation into these possible diabetes-specific CKD progression pathways. It should be noted that both inflammatory chemokines were identified among the most significant risk factors for CKD progression in the current study rather than hsCRP, a general marker of inflammation. Given the role of inflammatory chemokine release in triggering destructive invasion into essential organs, exploration of these markers as possible mechanistic factors for CKD progression among those with diabetes is warranted.32
Albuminuria levels above 300 mg/g were associated with the highest risk of CKD progression among all risk factors considered in this study regardless of diabetes. Because albuminuria may be on the causal pathway between many risk factors studied and kidney disease progression, we reimplemented analyses after excluding albuminuria, and observed two principal findings. First, several effect estimates increased for factors included in the models. Second, several factors including smoking, mineral metabolism markers, and HbA1c become newly significant, thereby highlighting pathways that may be mediated through albuminuria. Alternatively, these risk factors may truly be confounded by albuminuria but additional analyses with repeated measures of the risk factors and albuminuria are necessary to distinguish between these two explanations. Among those without diabetes, the association of ACE/ARB use with eGFR slope became non-significant, but with the composite renal endpoint became newly significant after albuminuria was omitted. With the well-documented anti-proteinuric and possible reno-protective effects of ACE/ARBs, this finding is not unexpected.33 While cigarette smoking does not appear in our primary models, it is significantly associated with time to KRT/eGFR halving regardless of diabetes status after removal of albuminuria. Previous reports linking smoking to CKD progression are heterogeneous and to our knowledge did not investigate possible mediation through proteinuria, so this remains an area for future research.34, 35 Of note, both FGF23 (for those without diabetes) and intact PTH (for those with diabetes) come forward in models once albuminuria is no longer accounted for, lending further support to the importance of mineral metabolism pathways in the progression of CKD.36–40 Finally, HbA1c, which has been shown to be associated with proteinuria and CKD progression, demonstrated mixed findings in our diabetic subgroup.41–43 In secondary analyses, albuminuria was found to significantly modify the effect of some of the key novel markers including urine NGAL among those with diabetes and NTproBNP among participants without diabetes. Albuminuria also significantly interacted with race/ethnicity regardless of diabetes status. These differential associations across levels of albuminuria should be explored further in future investigations of these risk factors for CKD progression.
Strengths of this study are the inclusion of a large number of men and women with and without diabetes, follow-up for as long as 12 years, and the simultaneous investigation of 30 risk factors for CKD progression. The extensive characterization of sociodemographic factors, clinical and physical attributes, and biochemical profiles of CRIC Study participants enabled the identification of unique sets of risk factors for CKD progression by diabetes status. Models of both KRT/eGFR halving and eGFR slope permitted investigation of both clinical endpoints and measures of kidney function decline.
Several limitations of the current study should also be noted. First, while the CRIC Study population included CKD patients with and without diabetes, it is not entirely representative of all patients with CKD in the United States. Additionally, the heterogeneity of CKD patients without diabetes who were combined together in these analyses may have obscured some important signals relevant to smaller subgroups embedded within this population. Future efforts to better sub-phenotype these CKD patients will likely improve efforts to identify potential targets for future therapies and refine risk estimates for CKD progression. Second, there may be other important risk factors that were not considered in our study. However, factors in the current study were selected a priori based on existing literature, availability of measures at baseline, and ongoing CRIC Study analyses, with the goal of identifying the most significant risk factors across and within major potential mechanistic pathways. Third, GFR estimates in the CRIC Study are obtained only annually, and the number of eGFR values ranges from two to twelve. Given estimates of GFR slope over time are less precise for those participants with shorter follow-up, we utilized linear mixed effects models to more heavily weight slope estimates from individuals with longer follow-up. Fourth, it remains to be determined if some of the risk factors identified serve as proxies for reduced renal clearance or if they represent potential explanatory pathways. Fifth, measurement error varied across candidate risk factors and may have influenced retention of factors and magnitude of effects in the final models.44 Lastly, participants ineligible for this study due to the availability of only one eGFR measurement for slope models were more likely to have a baseline eGFR <30 mL/min/.73m2 and higher levels of albuminuria. As such, rates of CKD progression in the current study are likely an underestimate of the entire CRIC population at baseline. In addition, use of complete case analyses excluded approximately 400 participants from the final models and may have influenced selection of risk factors. However, differences across those included and excluded from the analyses revealed only small differences across all considered factors, so the impact of this modeling approach should be minimal.
The current study confirms the strong relationships of demographic, kidney function, and blood pressure risk factors including race/ethnicity, baseline eGFR, albuminuria, and SBP with CKD progression among those with and without diabetes. This study also observed higher levels of the novel markers inflammatory chemokine plasma CXCL12, cardiac marker NTproBNP, and kidney injury marker urine NGAL were each independently associated with an approximate doubling of the rate of the composite renal endpoint among participants with diabetes. Low serum bicarbonate as well as higher high-sensitivity troponin T, NTproBNP and urine NGAL were all independently and significantly associated with a 1.5-fold or greater rate, and higher hemoglobin was associated with a significantly lower rate of the composite outcome among those without diabetes. Increase in hazard with elevated cardiac markers, plasma CXCL12, and urine NGAL exceeded that of systolic blood pressure ≥140 mmHg, a well-established risk factor for CKD progression. Taken together, these strong, novel risk factors for CKD progression provide avenues for future investigation into potential pathways of progression as well as opportunities to better define sub-phenotypes of patients with higher CKD progression risk profiles.
Supplementary Material
Acknowledgments:
The authors would like to thank the CRIC Study participants, study personnel, and investigators for their contributions to this work.
Support:_Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131, and K23DK097201. The NIDDK provided input to the CRIC Steering Committee in the analysis of the study and approved the submission of the manuscript for publication.
Financial Disclosure: AHA declares travel and consulting fees from Kyowa Hakko Kirin. LMD declares membership on a Data Monitoring Committee for Proteon Therapeutics, and serving as a consultant for GlaxoSmithKline. SDN declares serving as a consultant for Tricida and Reata, and being an independent event adjudication committee member for Bayer and Boeringer-Ingelheim, and receipt of investigator-initiated research support from Keryx Pharmaceuticals. MR declares receipt of research support from Bayer and Duke Clinical Research Institute, and receipt of honoraria from Relypsa. JJS declares receipt of research support from Lilly, Sanofi, and GlaxoSmithKline. HIF declares receipt of travel and consulting fees from Kyowa Hakko Kirin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2018 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2019;73(3)(suppl 1):A7–A8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parsa A, Kao WH, Xie D, et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369(23): 2183–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayek SS, Sever S, Ko YA, et al. Soluble Urokinase Receptor and Chronic Kidney Disease. N Engl J Med. 2015;373(20): 1916–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaefer F, Trachtman H, Wuhl E, et al. Association of Serum Soluble Urokinase Receptor Levels With Progression of Kidney Disease in Children. JAMA Pediatr. 2017;171(11): e172914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ju W, Nair V, Smith S, et al. Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med. 2015;7(316): 316ra193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kottgen A, Hwang SJ, Larson MG, et al. Uromodulin levels associate with a common UMOD variant and risk for incident CKD. J Am Soc Nephrol. 2010;21(2): 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leiherer A, Muendlein A, Saely CH, et al. The value of uromodulin as a new serum marker to predict decline in renal function. J Hypertens. 2018;36(1): 110–118. [DOI] [PubMed] [Google Scholar]
- 8.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J.Am.Soc.Nephrol. 2003;14(7 Suppl 2): S148–S153. [DOI] [PubMed] [Google Scholar]
- 9.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin.J Am Soc Nephrol. 2009;4(8): 1302–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer MJ, Go AS, Lora CM, et al. CKD in Hispanics: Baseline characteristics from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic-CRIC Studies. Am.J.Kidney Dis. 2011;58(2): 214–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2): 175–191. [DOI] [PubMed] [Google Scholar]
- 12.Yasue H, Yoshimura M, Sumida H, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90(1): 195–203. [DOI] [PubMed] [Google Scholar]
- 13.Bjurman C, Petzold M, Venge P, Farbemo J, Fu ML, Hammarsten O. High-sensitive cardiac troponin, NT-proBNP, hFABP and copeptin levels in relation to glomerular filtration rates and a medical record of cardiovascular disease. Clin Biochem. 2015;48(4-5): 302–307. [DOI] [PubMed] [Google Scholar]
- 14.Taylor CJ, Roalfe AK, lies R, Hobbs FD. The potential role of NT-proBNP in screening for and predicting prognosis in heart failure: a survival analysis. BMJ Open. 2014;4(4): e004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bansal N, Hyre Anderson A, Yang W, et al. High-sensitivity troponin T and N-terminal pro-B-type natriuretic peptide (NT-proBNP) and risk of incident heart failure in patients with CKD: the Chronic Renal Insufficiency Cohort (CRIC) Study. J Am Soc Nephrol. 2015;26(4): 946–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spanaus KS, Kronenberg F, Ritz E, et al. B-type natriuretic peptide concentrations predict the progression of nondiabetic chronic kidney disease: the Mild-to-Moderate Kidney Disease Study. Clin Chem. 2007;53(7): 1264–1272. [DOI] [PubMed] [Google Scholar]
- 17.Desai AS, Toto R, Jarolim P, et al. Association between cardiac biomarkers and the development of ESRD in patients with type 2 diabetes mellitus, anemia, and CKD. Am J Kidney Dis. 2011;58(5): 717–728. [DOI] [PubMed] [Google Scholar]
- 18.Bansal N, Katz R, Dalrymple L, et al. NT-proBNP and troponin T and risk of rapid kidney function decline and incident CKD in elderly adults. Clin J Am Soc Nephrol. 2015;10(2): 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolignano D, Donato V, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am J Kidney Dis. 2008;52(3): 595–605. [DOI] [PubMed] [Google Scholar]
- 20.Liu KD, Yang W, Anderson AH, et al. Urine neutrophil gelatinase-associated lipocalin levels do not improve risk prediction of progressive chronic kidney disease. Kidney Int. 2013;83(5): 909–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith ER, Lee D, Cai MM, et al. Urinary neutrophil gelatinase-associated lipocalin may aid prediction of renal decline in patients with non-proteinuric Stages 3 and 4 chronic kidney disease (CKD). Nephrol Dial Transplant. 2013;28(6): 1569–1579. [DOI] [PubMed] [Google Scholar]
- 22.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J Exp Med. 1996;184(3): 1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Askari AT, Unzek S, Popovic ZB, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362(9385): 697–703. [DOI] [PubMed] [Google Scholar]
- 24.Mehta NN, Matthews GJ, Krishnamoorthy P, et al. Higher plasma CXCL12 levels predict incident myocardial infarction and death in chronic kidney disease: findings from the Chronic Renal Insufficiency Cohort study. Eur Heart J. 2014;35(31): 2115–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Apostolakis S, Spandidos D. Chemokines and atherosclerosis: focus on the CX3CL1/CX3CR1 pathway. Acta Pharmacol Sin. 2013;34(10): 1251–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah R, Hinkle CC, Ferguson JF, et al. Fractalkine is a novel human adipochemokine associated with type 2 diabetes. Diabetes. 2011;60(5): 1512–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah R, Matthews GJ, Shah RY, et al. Serum Fractalkine (CX3CL1) and Cardiovascular Outcomes and Diabetes: Findings From the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2015;66(2): 266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh V, Jaiswal PK, Tiwari P, Kapoor R, Mittal RD. Association of chemokine gene variants with end stage renal disease in North Indian population. Transpl Immunol. 2013;28(4): 189–192. [DOI] [PubMed] [Google Scholar]
- 29.Cui C, Cui Y, Fu Y, Ma S, Zhang S. Microarray analysis reveals gene and microRNA signatures in diabetic kidney disease. Mol Med Rep. 2018;17(2): 2161–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darisipudi MN, Kulkarni OP, Sayyed SG, et al. Dual blockade of the homeostatic chemokine CXCL12 and the proinflammatory chemokine CCL2 has additive protective effects on diabetic kidney disease. Am J Pathol. 2011;179(1): 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hochheiser K, Heuser C, Krause TA, et al. Exclusive CX3CR1 dependence of kidney DCs impacts glomerulonephritis progression. J Clin Invest. 2013;123(10): 4242–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishikawa F, Miyazaki S. New biodefense strategies by neutrophils. Arch Immunol Ther Exp (Warsz). 2005;53(3): 226–233. [PubMed] [Google Scholar]
- 33.Ruggenenti P, Cravedi P, Remuzzi G. Mechanisms and treatment of CKD. J Am Soc Nephrol. 2012;23(12): 1917–1928. [DOI] [PubMed] [Google Scholar]
- 34.Xia J, Wang L, Ma Z, et al. Cigarette smoking and chronic kidney disease in the general population: a systematic review and meta-analysis of prospective cohort studies. Nephrol Dial Transplant. 2017;32(3): 475–487. [DOI] [PubMed] [Google Scholar]
- 35.Bundy JD, Bazzano LA, Xie D, et al. Self-Reported Tobacco, Alcohol, and Illicit Drug Use and Progression of Chronic Kidney Disease. Clin J Am Soc Nephrol. 2018;13(7): 993–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Seigneux S, Delitsikou V, Martin PY. The KNOW-CKD study: evidence for a link between proteinuria and alterations of mineral metabolism. Nephrol Dial Transplant. 2020;35(3): 382–385. [DOI] [PubMed] [Google Scholar]
- 37.Lundberg S, Qureshi AR, Olivecrona S, Gunnarsson I, Jacobson SH, Larsson TE. FGF23, albuminuria, and disease progression in patients with chronic IgA nephropathy. Clin J Am Soc Nephrol. 2012;7(5): 727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305(23): 2432–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Seigneux S, Courbebaisse M, Rutkowski JM, et al. Proteinuria Increases Plasma Phosphate by Altering Its Tubular Handling. J Am Soc Nephrol. 2015;26(7): 1608–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Seigneux S, Wilhelm-Bals A, Courbebaisse M. On the relationship between proteinuria and plasma phosphate. Swiss Med Wkly. 2017;147: w14509. [DOI] [PubMed] [Google Scholar]
- 41.Kang SH, Jung DJ, Choi EW, Cho KH, Park JW, Do JY. HbA1c Levels Are Associated with Chronic Kidney Disease in a Non-Diabetic Adult Population: A Nationwide Survey (KNHANES 2011-2013). PLoS One. 2015;10(12): e0145827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang SH, Park JW, Do JY, Cho KH. Glycated hemoglobin A1c level is associated with high urinary albumin/creatinine ratio in non-diabetic adult population. Ann Med. 2016;48(6): 477–484. [DOI] [PubMed] [Google Scholar]
- 43.Ritz E, Viberti GC, Ruilope LM, et al. Determinants of urinary albumin excretion within the normal range in patients with type 2 diabetes: the Randomised Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) study. Diabetologia. 2010;53(1): 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brakenhoff TB, van Smeden M, Visseren FLJ, Groenwold RHH. Random measurement error: Why worry? An example of cardiovascular risk factors. PLoS One. 2018;13(2): e0192298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


