Abstract
STUDY QUESTION
Does the ovarian sensitivity index (OSI) predict embryo quality, pregnancy and live birth in patients undergoing FSH/hMG stimulation for IVF?
SUMMARY ANSWER
The OSI is predictive of pregnancy and live birth in older women with a more unfavorable prognosis undergoing FSH/hMG stimulation for IVF.
WHAT IS KNOWN ALREADY
The OSI was previously reported to reflect gonadotrophin requirements among high, normal and poor responders and to predict pregnancy potential in younger patients undergoing ovarian stimulation with FSH.
STUDY DESIGN, SIZE, DURATION
A retrospective cohort study that included 1282 women undergoing IVF with FSH/hMG stimulation was carried out between January 2010 and December 2016.
PARTICIPANTS/MATERIALS, SETTING, METHODS
We evaluated 1282 women who underwent fertility treatment with FSH/hMG stimulation and oocyte retrieval at an academically affiliated private fertility center. OSI was calculated as (oocytes ×1000)/total gonadotrophin dose and grouped into two classes based on a receiver operating characteristic (ROC) curve analysis of a randomly selected development sample comprising one-third of the cycles. The remaining cycles comprised the validation group. ROC curves were also used to compare the predictive value of OSI to that of baseline FSH and anti-Müllerian hormone (AMH). Logistic regression models evaluated the effect of high (OSI >0.83) and low (OSI ≤0.83) on clinical pregnancy and live birth in the validation group. Models were adjusted for female age, baseline FSH, AMH and oocyte yield and gonadotrophin dose.
MAIN RESULTS AND THE ROLE OF CHANCE
Women presented with a mean ±SD age of 38.6 ± 5.4 years and showed median AMH levels of 0.65 (95% CI 0.61–0.74) ng/ml. They received 5145 ± 2477 IU of gonadotrophins and produced a median 5.2 (95% CI 5.0–5.5) oocytes. Pregnancy and live birth rates per oocyte retrieval for all women were 20.6% and 15.8%, respectively. Patients with higher OSI (less gonadotrophin required per oocyte retrieved) produced significantly more high-quality embryos than patients with low OSI (3.5 (95% CI 3.2–3.8) versus 0.6 (95% CI 0.5–0.7) (P = 0.0001)) and demonstrated higher pregnancy (23.2% versus 9.7%) and live birth rates (8.8% versus 5.3%) than their counterparts (P = 0.0001 and P = 0.0001, respectively). After adjustments for age, baseline AMH and FSH, total gonadotrophin dosage and oocyte yield, an OSI >0.83 was associated with greater odds of pregnancy (odds ratio 2.12, 95% CI 1.30–3.45, P < 0.003) and live birth (odds ratio 1.91, 95% CI 1.07–3.41, P < 0.028).
LIMITATIONS, REASONS FOR CAUTION
The results may not be applicable to women with excellent pregnancy potential or FSH-only stimulation.
WIDER IMPLICATIONS OF THE FINDINGS
The predictive capacity of OSI for embryo quality, pregnancy and live birth, which is independent of AMH or FSH, may help in counseling patients about their pregnancy potential and live birth chances.
STUDY FUNDING/COMPETING INTEREST(S)
Intramural funding from the Center for Human Reproduction and the Foundation for Reproductive Medicine. A.W., V.A.K., D.F.A., D.H.B. and N.G. have received research grant support, travel funds and speaker honoraria from various pharmaceutical and medical device companies: none, however, related to the topic presented here. D.H.B. and N.G. are listed as inventors on already awarded and still pending US patents, claiming beneficial effects on diminished ovarian reserve and embryo ploidy from dehydroepiandrosterone supplementation.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: ovarian sensitivity index, IVF, live birth, ovarian response, pregnancy, ovarian stimulation, diminished ovarian reserve, oocyte number
WHAT DOES THIS MEAN FOR PATIENTS?
In the course of IVF, a woman’s ovaries are stimulated with hormones to produce more than one egg at a time. This study evaluated whether the amount of hormone medication needed for each oocyte produced was helpful to predict a patient’s chance to get pregnant and deliver a baby.
The analysis of 1282 IVF cycles demonstrated that women who needed more hormones per egg they produced had a lower chance of getting pregnant or delivering a baby. This information may be useful to advise patients about their pregnancy chances during their IVF treatment.
Introduction
A sufficient ovarian response to exogenous gonadotrophin stimulation is considered essential for treatment success of IVF (Sunkara et al., 2011). To correctly assess ovarian function, several ovarian reserve markers have been introduced (Zebitay et al., 2017). While anti-Müllerian hormone (AMH) and antral follicle count (AFC) are appropriate to estimate oocyte yields, their predictive significance in terms of pregnancy rates and live births is limited (Li et al., 2013). The Bologna criteria, a diagnostic algorithm to identify poor responders, features similar shortcomings: women who qualify as poor responder patients generally produce a limited number of oocytes, yet experience a considerable range of pregnancy potential (Bozdag et al., 2017).
These observations raised the assumption of a ‘missing link’ incorrectly predicting pregnancy potential after IVF: diagnostic markers of ovarian reserve as well as the Bologna criteria do not account for the total units of gonadotrophins needed to produce oocytes. However, gonadotrophin consumption is reflective of ovarian function and, thus, widely acknowledged as a prognostic factor for treatment success after IVF (Baker et al., 2015). To better assess the oocyte’s reproductive competence, mathematical interaction models that take the ovary’s responsiveness to stimulation into account were developed. Initially, the follicle output rate (FORT), defined as the proportion of antral follicles that effectively respond to exogenous gonadotrophins and translate into preovulatory stage, was introduced. To quantify the ovary’s follicular competence, FORT groups were categorized as low, medium and high. Pregnancy rates were significantly higher in women with a high FORT when compared to their counterparts with a low output rate (Gallot et al., 2012).
More recently, the ovarian sensitivity index (OSI), which is a function of the total number of oocytes retrieved and the total amount of gonadotrophins utilized in a treatment cycle, was introduced to make allowances for gonadotrophin requirements in the course of ovarian hyperstimulation. Young study subjects with normal ovarian function who were undergoing long agonist stimulation with recombinant FSH were categorized as low, medium and high OSI groups. Among those young women with excellent pregnancy potential, the OSI was shown to be superior in differentiating high, normal and poor responders and superior to age and oocyte yield in predicting pregnancy (Huber et al., 2013).
As women are increasingly deferring family building into more advanced reproductive ages, with the associated compromised ovarian reserve, correct predictions of pregnancy chances become even more important in counseling patients with a more unfavorable prognosis (Lehert et al., 2018). Our academically affiliated fertility center serves a population of infertile women with a very high prevalence of diminished ovarian reserve (DOR), mostly related to advanced female age or premature ovarian aging (POA) (Gleicher et al., 2015). The present study was initiated to investigate whether the OSI is also predictive of pregnancy potential and live birth rate in such an unfavorable patient population.
Materials and methods
This study retrospectively investigated 1282 fresh consecutive IVF cycles in 1282 infertility patients who underwent autologous IVF at the Center for Human Reproduction (CHR) in New York City, NY, USA, between January 2010 and December 2016. Women were eligible for enrollment if they received ovarian hyperstimulation with FSH/hMG, which is routinely given in a 3:1 ratio, and had at least one oocyte retrieved. Patients who underwent IVF for oocyte or embryo banking or served as oocyte donors were excluded.
At initial presentation to our center, all women underwent ovarian reserve testing with FSH and AMH on cycle Days 2/3. FSH and AMH values were also collected at the start of each IVF cycle. Laboratory tests were performed via commercial testing (Laboratory Corporation of America). AMH was measured by ELISA, using the Gen II assay (Beckman Coulter, Inc., Webster, TX, USA). FSH was measured by electrochemiluminescence immunoassay. Additionally, the uterine cavity was routinely evaluated by hysterosonography to exclude uterine pathologies that might compromise pregnancy potential. Indications for fertility treatment were defined and recorded according to the definitions of the Society for Assisted Reproductive Technology for nationwide ART data registry (www.sart.org).
Gonadotrophin dose was chosen according to the clinical estimate of ovarian reserve. In rare cases, dose was adjusted based on ovarian response. Thirty-six hours after ovulation induction with hCG (various manufacturers), oocyte retrieval was performed. Embryo transfer was routinely scheduled on Day 3 after fertilization at cleavage stage. Canceled cycles were excluded from the study. Clinical pregnancies were diagnosed ca. 5.5 weeks after the first positive serum hCG measurements in the presence of a gestational sac on vaginal ultrasound. After the expected date of delivery, patients were contacted for reports of live births.
Statistical analysis
Baseline characteristics of patients were compared using Kruskal–Wallis or chi-square test. The OSI was calculated as the number of oocytes retrieved ×1000 divided by the total gonadotrophin dosage used and categorized as low, medium and high, as previously reported (Huber et al., 2013). We then investigated the relation between female age, baseline FSH and AMH, total gonadotrophin dosage, number of oocytes retrieved, OSI, embryo development, pregnancy and live birth rates.
A random selection of one-third (n = 414) of patients was used to create a predictive model, which could then be tested for validation in the 868 patients representing the other two-thirds of the population. As expected, there were no observed differences in any baseline parameters between these two populations. Receiver operating characteristic (ROC) curves were constructed testing OSI as a predictor of pregnancy and live birth in this development sample. Cutoffs were selected from the ROC curves at a fixed sensitivity of 80% corresponding to an OSI of ≥0.83 for both pregnancy and live birth. The chosen criterion offered a specificity of 46.81 (95% CI 33.95–56.69) for pregnancy and a specificity of 45.54 (95% CI 33.05–55.06) for live birth. A binomial variable was created for these OSI criteria for both pregnancy and live birth.
Logistic regression models were run testing the validity of these selected criteria in prediction of pregnancy and live birth in the 868-validation sample along with covariates for FSH, AMH, oocytes retrieved and total gonadotrophin dose. Oocytes retrieved and gonadotrophin dose were retained in the model since the OSI is a function of the interaction of these two factors. For these models, goodness of fit was tested using the Hosmer and Lemeshow test and was not significant for either pregnancy or live birth, demonstrating the models are well fit. ROC curves comparing the AUC for OSI, AMH and Day-2 FSH were constructed for both pregnancy and live birth (DeLong et al., 1988).
ROC curves were analyzed using MedCalc (MedCalc Statistical Software version 18.11.3, MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2019). SPSS (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY, USA: IBM Corp.) was used for all other analyses. Continuous values are presented as mean ± SD or as geometric mean and 95% CI for non-normal continuous variables. Outcome parameters are presented as proportions. P-value <0.05 was considered statistically significant.
Patients at our center sign at initial consultation an informed consent, which allows for use of their anonymized electronic medical records and, where needed, of their paper records, if the patient’s identity remains protected and the medical record remains confidential. Since the data presented here only involved a retrospective review of the center’s anonymized electronic research database, also used to report the center’s annual IVF outcomes to national registries, these conditions were met. Our Institutional Review Board approved such medical record studies by expedited review.
Results
Patient characteristics and cycle parameters for the whole cohort as well as subgroups are displayed in Tables I and II. Patients had a mean age of 38.6 ± 5.4 years and presented with mean AMH levels of 0.65 (95% CI 0.61–0.74) ng/ml. The reasons for their fertility treatment included DOR in 74.4%, male factor in 25.1%, tubal factor in 13.3% and ovulation disorders, including polycystic ovary syndrome, in 27.0% (many patients presented with more than one infertility diagnosis). They received a total gonadotrophin dosage of 5145 ± 2477 IU, and produced 5.2 (95% CI 5.0–5.5) oocytes and 2.9 ± 3.5 high-quality embryos. Pregnancy and live birth rates per retrieval were 20.6% and 15.8%, respectively.
Table I.
Patient characteristics and cycle parameters for the whole group and for development and validation samples.
| All | Development sample a | Validation sample | |
|---|---|---|---|
| N | 1282 | 414 | 868 |
| Female age (years) | 38.6 ± 5.4 | 38.4 ± 5.4 | 38.6 ± 5.4 |
| BMI (kg/m2)b | 23.8 (95% CI 23.6–24.1) | 23.8 (95% CI 23.4–24.3) | 23.8 (95% CI 23.5–24.1) |
| AMH (ng/ml)b | 0.65 (95% CI 0.61–0.74) | 0.75 (95% CI 0.65–0.88) | 0.63 (95% CI 0.56–0.71) |
| Max FSH (mIU/ml)b | 10.7 (95% CI 10.4–11.1) | 10.6 (95% CI 9.9–11.1) | 10.8 (95% CI 10.4–11.3) |
| Total gonadotrophins used (IU) | 5145 ± 2477 | 5419 ± 2510 | 5216 ± 2458 |
| Oocytes retrieved (n)b | 5.2 (95% CI 5.0–5.5) | 5.5 (95% CI 5.0–6.1) | 5.1 (95% CI 4.9–5.5) |
| High-quality embryos | 2.9 ± 3.5 | 2.9 ± 3.4 | 2.9 ± 3.6 |
| Pregnancy rate (%) | 20.6 (95% CI 18.4–22.8) | 21.0 (95% CI 17.1–25.0) | 20.4 (95% CI 17.7–23.1) |
| Live birth rate (%) | 15.8 (95% CI 13.8–17.8) | 16.4 (95% CI 12.8–20.0) | 15.6 (95% CI 13.1–18.0) |
Data are mean ± SD.
No characteristics were significantly different between the Development sample and the Validation sample.
Backtransformed after logarithmic transformation.
AMH, anti-Müllerian hormone.
Table II.
Patient characteristics and cycle parameters according to ovarian sensitivity to gonadotrophins.
| High OSI (≥0.83) | Low OSI (<0.83) | P -value | |
|---|---|---|---|
| N | 699 | 169 | |
| Female age (years) | 38.1 ± 5.4 | 40.4 ± 4.8 | <0.0001 |
| BMI (kg/m2)a | 23.9(95% CI 23.5–24.2) | 23.5 (95% CI 22.9–24.2) | 0.98 |
| AMH (ng/ml)a | 0.81 (95% CI 0.73–0.88) | 0.25 (95% CI 0.21–0.30) | <0.0001 |
| Maximum FSH (mIU/ml)a | 9.8 (95% CI 9.4–10.3) | 16.2 (95% CI 14.7–17.8) | <0.0001 |
| Total gonadotrophins used (IU) | 4757 (95% CI 4587–4927) | 6769 (95% CI 6444–7109) | <0.0001 |
| Oocytes retrieved (n)a | 7.0 (95% CI 6.6–7.5) | 1.5 (95% CI 1.4–1.6) | <0.0001 |
| High-quality embryos | 3.5 (95% CI 3.2–3.8) | 0.6 (95% CI 0.5–0.7) | <0.0001 |
| Pregnancy rate (%) | 23.2 (95% CI 20–26.3) | 8.8 (95% CI 4.5–13.2) | <0.0001 |
| Live birth rate (%) | 18.0 (95% CI 15.2–20.9) | 5.3 (95% CI 1.9–8.8) | <0.0001 |
Backtransformed after logarithmic transformation.
OSI, ovarian sensitivity index.
Women with high or low OSI classifications were 38.1 ± 5.4 and 40.4 ± 4.8 years old (P < 0.0001); they demonstrated median AMH levels of 0.81 (95% CI 0.73–0.88) and 0.25 (95% CI 0.21– 0.30) ng/ml (P < 0.0001) and received gonadotrophin dosages of 4757 (95% CI 4587–4927) and 6769 (95% CI 6444–7109) IU, respectively (P < 0.0001). Controlled ovarian hyperstimulation resulted in 7.0 (95% CI 6.6–7.5) and 1.5 (95% CI 1.4–1.6) oocytes. Pregnancy occurred in 23.2% and 8.8% of cycles and resulted in live birth rates of 18.0% and 5.3% in women with high and low OSI, respectively (P < 0.0001 for both) (Table II).
OSI is a function of the number of oocytes produced in a cycle of ovulation induction per unit of gonadotrophin used. Figure 1 plots the oocytes retrieved against the total dose of FSH. Loess regression is a non-parametric technique that uses local weighted regression to fit a smooth curve through points in a scatter plot. The plots were made with an Epanechnikov kernel fitting 50% of data around each point (data near the current point receive higher weights than extreme data receive). Loess curves are useful for data exploration and hypothesis development.
Figure 1.
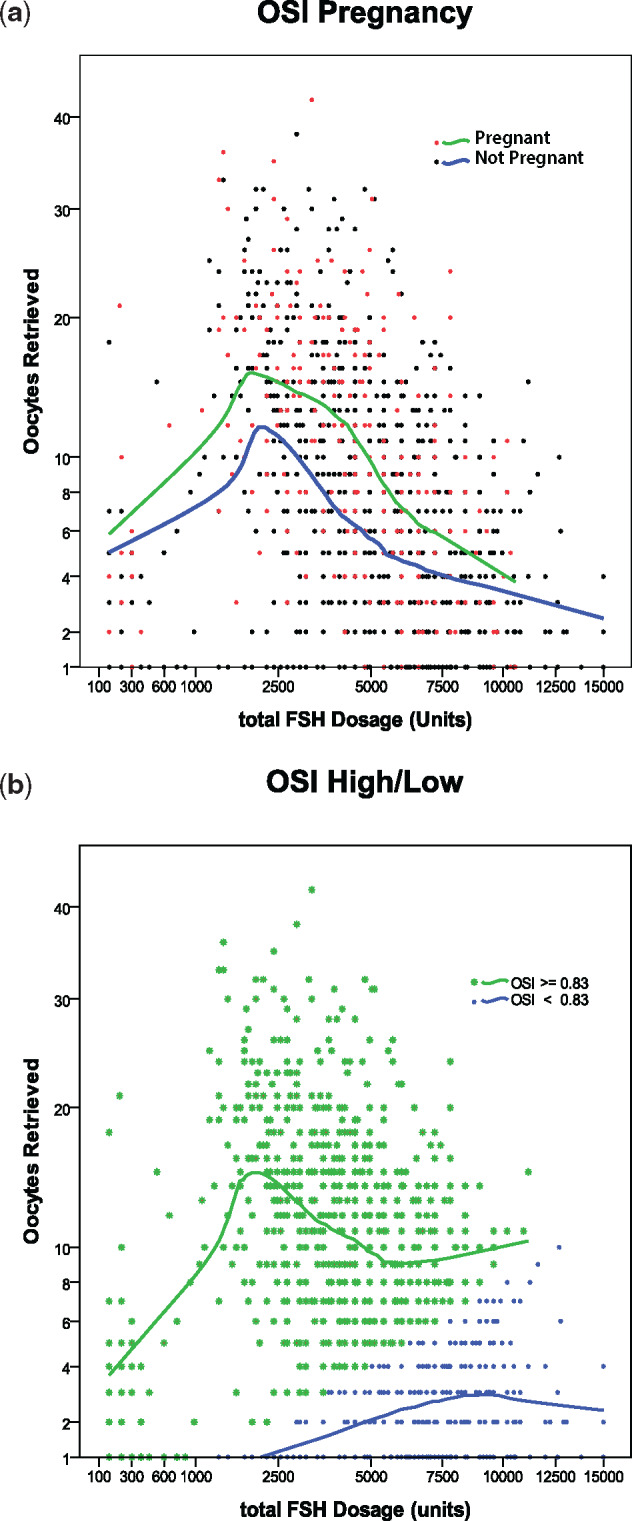
Number of oocytes retrieved with varying total dose of gonadotrophin. (a) Ovarian sensitivity index (OSI*) in pregnant and non-pregnant women. (b) Oocytes retrieved per total gonadotrophin dose for patients with high or low OSI. *OSI is a function of the number of oocytes produced in a cycle of ovulation induction per unit of gonadotrophin used.
In Fig. 1a, the Loess curve is a function of the OSI (oocyte per gonadotrophin) for cycles that resulted in pregnancy versus those that did not. Not surprisingly, the curves suggest that patients who achieve pregnancy produce more oocytes per dose of gonadotrophin than those who did not become pregnant.
Similarly, in Fig. 1b, the fitted curves represent the oocytes retrieved per total gonadotrophin dose for patients with high or low OSI. Although it is clear from these curves that women with higher OSI show increasing oocyte recovery with increased gonadotrophin dosage, with diminishing results above about 2400 units of gonadotrophin, it is also clear that for women with lower OSI higher doses of gonadotrophin continue to be associated with increased oocyte recovery.
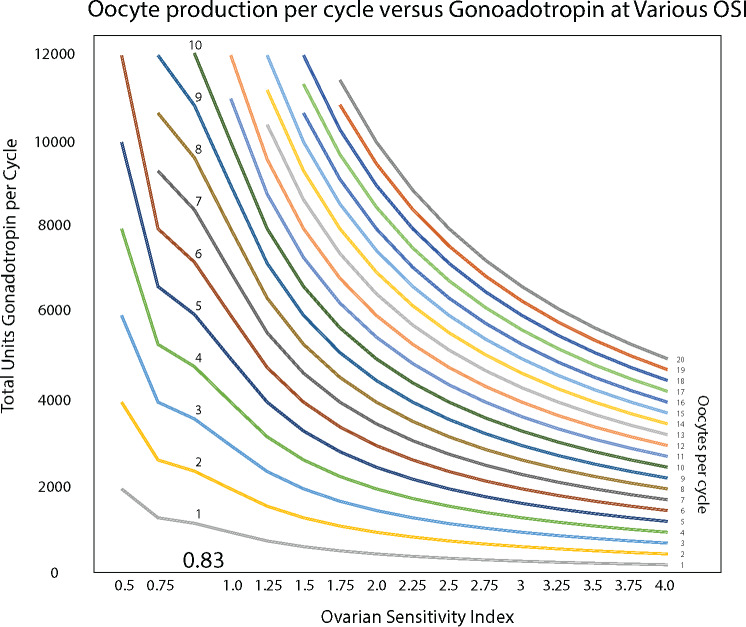
A comparison of ROC curves for OSI, AMH and Days 2–3 FSH among the 868 women in the validation sample (Fig. 2a and b) revealed that OSI had a significantly greater AUC for both pregnancy (Fig. 2a) and live birth (Fig. 2b) compared to both AMH (P = 0.014; P = 0.05, respectively) and Day 2–3 FSH (P = 0.0001; P = 0.0001, respectively). Figure 3 describes this relationship at various OSIs.
Figure 2.
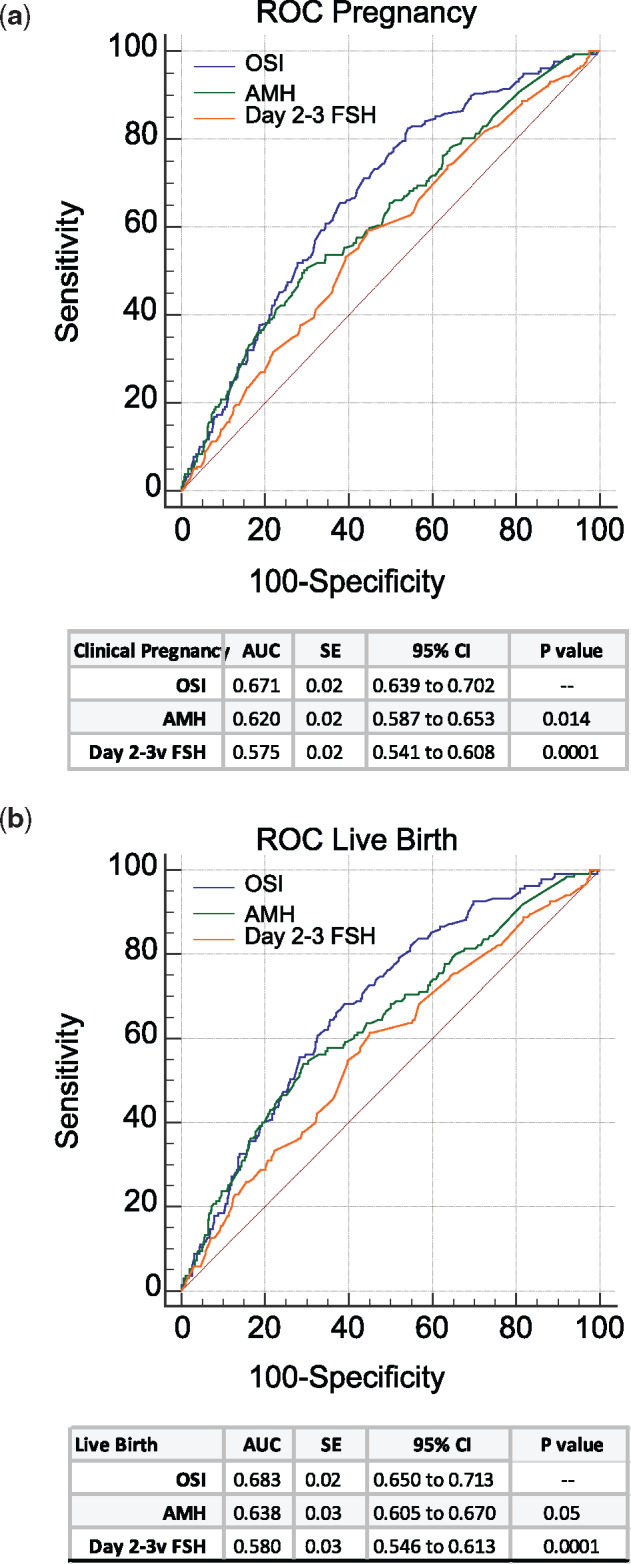
Comparison of receiver operating characteristic (ROC) curves for the prediction of pregnancy and live birth in the 868 women in the validation group. (a) ROC curve for prediction of pregnancy. (b) ROC curve for prediction of live birth. AMH, anti-Müllerian hormone.
Figure 3.
Oocyte production per cycle versus gonadotrophin at various OSIs.
Logistic regression models were run to examine the association of OSI ≥0.83 with the outcomes of clinical pregnancy and live birth (Tables III and IV). The effects of each of the covariates were tested as well as the cumulative model.
Table III.
Logistic regression model on the predictive capacity of the OSI on pregnancy chances after assisted reproduction.
| Parameters | Unadjusted OR (95%CI) |
P-value | Adjusted OR (95% CI)a |
P-value |
|---|---|---|---|---|
| OSI > 0.83 | 2.98 (2.13–4.16)b | <0.001 | – | – |
| Patient age | 2.20 (1.55–3.11)c | <0.001 | 2.20 (1.55–3.11) | <0.001 |
| Total gonadotrophins | 3.10 (2.14–4.49)c | <0.001 | 2.81 (1.89–4.17) | <0.001 |
| Oocytes retrieved | 1.92 (1.29–2.86)c | 0.001 | 2.26 (1.44–3.54) | <0.001 |
| FSH | 2.50 (1.72–3.65)c | <0.001 | 2.04 (1.27–3.29) | 0.003 |
| AMH | 2.18 (1.45–3.28)c | <0.001 | 2.12 (1.30–3.45) | 0.003 |
Model includes OSI >0.83 and all previous parameters.
Logistic model of OSI >0.83 as a predictor of clinical pregnancy.
Model includes only OSI > 0.83 plus parameter in the first column.
OR, odds ratio.
After adjustments for age, baseline AMH and FSH, total gonadotrophin dosage and oocyte yield, OSI ≥0.83 was associated with greater odds of pregnancy (odds ratio (OR) 2.12, 95% CI 1.30–3.45, P < 0.003) (Table III). OSI also demonstrated increased odds of live birth: OSI: OR 1.91, 95% CI 1.07–3.41, P < 0.028 (Table IV).
Table IV.
Logistic regression model on the predictive capacity of the OSI on live birth chances after assisted reproduction.
| Parameters | Unadjusted OR (95%CI) |
P-value | Adjusted OR (95% CI)a |
P-value |
|---|---|---|---|---|
| OSI > 0.83 | 3.05 (2.07–4.50)b | <0.001 | – | – |
| Patient age | 2.09 (1.39–3.14)c | <0.001 | 2.09 (1.39–3.14) | <0.001 |
| Total gonadotrophins | 2.98 (1.95–4.57)c | <0.001 | 2.68 (1.69–4.25) | <0.001 |
| Oocytes retrieved | 1.80 (1.14–2.86)c | 0.012 | 2.06 (1.22–3.47) | 0.007 |
| FSH | 2.68 (1.72–4.16)c | <0.001 | 1.89 (1.07–3.31) | 0.028 |
| AMH | 1.92 (1.20–3.09)c | 0.007 | 1.91 (1.07–3.41) | 0.028 |
Model includes OSI >0.83 and all previous parameters.
Logistic model of OSI >0.83 as a predictor of live birth.
Model includes only OSI >0.83 plus parameter in the first column.
Discussion
Our findings demonstrate that, even after adjustment for female age, baseline AMH and FSH and oocyte numbers, ovarian responsiveness to exogenous gonadotrophin stimulation (i.e. higher OSI) is associated with increased odds of live birth. Adjustment for each of these factors did little to change the OR of pregnancy and live birth modeled on OSI alone. ROC curves for pregnancy and live birth, both showed that OSI had a significantly greater sensitivity and specificity than either AMH or Day 2–3 FSH (Fig. 2a and b). As true pregnancy potential is even more precious in women with limited embryo numbers, this suggests that the OSI may be particularly helpful in counseling IVF patients with a more unfavorable prognosis owing to advanced reproductive age or POA.
One may, of course, argue that the information OSI offers only becomes available during an IVF cycle, when the decision to undergo treatment has already been made. Ovarian reserve markers, in contrast, can be utilized to counsel patients before they initiate treatment. Although this point appears valid, two aspects deserve consideration. First, OSI was demonstrated to be superior to baseline FSH or AMH in predicting pregnancy potential (Fig. 2). The predictive capacity of currently available tools to assess pregnancy potential in women undergoing ART is still limited. Improvement is therefore welcome. Second, addressing a woman’s pregnancy chances prior to treatment start is crucial for all patients, irrespective of age and ovarian function. Once patients are confronted with a limited number of oocytes at retrieval or a failed IVF attempt, counseling women of advanced reproductive age with diminished ovarian function about their reproductive potential and future treatment options becomes of importance. The OSI may, at least in part, bridge this gap between the purely quantitative aspect of ovarian reserve and the more qualitative approach of ovarian competence and, thus, aid those patients to make appropriate decisions for subsequent cycles. OSI may also be useful when comparing different protocols for ovulation induction to see if the sensitivity of ovarian response can be improved.
As this study clearly demonstrates, a greater number of oocytes produced per unit of gonadotrophin are associated with increased pregnancy and live birth with IVF, which then after fertilization, downstream, is most likely to be reflected in embryo numbers. Using a cutoff of ≥0.83 suggests that patients need to produce at least one oocyte per 1205 units of gonadotrophin stimulation to achieve the best chances of pregnancy and live birth, and that an OSI of <0.83 greatly reduces the chance of a positive outcome. By being more upstream, OSI may, therefore, be superior in predicting pregnancies and live births in comparison to embryo grading since it precisely represents the interaction between cause and effect, i.e. gonadotrophin stimulus and egg numbers (Vaegter et al., 2017). By contrast, commonly used predictive parameters, such as female age, ovarian reserve markers and oocyte yields, either reflect a starting position or an endpoint, but fail to picture the stimulus required to maximize oocyte yields (Iliodromiti et al., 2015). This study, therefore, offers further evidence for the potential importance of retrieved oocyte quality in predicting IVF cycle outcomes and, therefore, also supports previously voiced opinions that a more detailed oocyte assessment during IVF cycles than currently is practiced may be of clinical value (Lazzaroni-Tealdi et al., 2015).
Higher pregnancy rates in women with larger oocyte yields after mild stimulation in comparison to patients after prolonged and/or accelerated controlled ovarian hyperstimulation also support our findings (Baker et al., 2015), as does an earlier report from our own center that demonstrated that the number of good quality embryos available for transfer is at all ages directly associated with pregnancy and live birth chances from individual embryos. The more good quality embryos a woman produced, the higher were the pregnancy and live birth rates, even if number of embryos transferred remained the same (Gleicher et al., 2016). These findings also potentially clarify an earlier observation we reported in 2016, when we demonstrated that in poor prognosis patients increasing numbers of retrieved oocytes, independently, were associated with improved pregnancy and live birth rates in IVF, even if the number of embryos was the same (Gleicher et al., 2016). Increasing egg numbers, thus, is an obvious indicator of egg quality.
What all of this means is that this ‘embryo quality factor’ associated with increasing embryo numbers and better pregnancy and live birth chances is, likely, a programmed maternal oocyte factor that is transmitted to the embryo during fertilization. These findings also confirm previous publications on maximum pregnancy potential in the presence of a large oocyte yield (Sunkara et al., 2011; Drakopoulos et al., 2016). Differences in follicular responsiveness to gonadotrophins were also demonstrated in women with unexplained infertility: patients with a large proportion of responding follicles experienced higher pregnancy rates than their counterparts with comparable AFC, but smaller preovulatory follicle numbers (Hassan et al., 2017). Furthermore, Alvaro Mercadal et al. (2018) reported significantly lower cumulative pregnancy rates in women with 4–9 oocytes when compared to patients with 10–15 oocytes.
What in general underlies impaired follicular competence and consequently suboptimal or even poor responses to gonadotrophin stimulation remains to be elucidated. One can, however, assume that at least some of these patients suffer from so-called POA, sometimes also called occult primary ovarian insufficiency (oPOI) and, therefore, in view of their DOR may benefit from medication adjustments. Klinkert et al. (2004) reported a higher proportion of normal response to treatment and improved pregnancy potential in unexpected poor responders after higher gonadotrophin dosage in subsequent cycles. In our center’s experience, higher gonadotrophin dosages are especially effective in younger women with POA/oPOI but less so in older women.
A subset of women with compromised follicular competence apparently also benefit from combined FSH/hMG stimulation for sustained follicular development and maturation. In a recent meta-analysis, Alviggi et al. (2018) describe improved cycle outcomes after controlled ovarian hyperstimulation with FSH and LH in women with unexpectedly poor response and in patients above age 35 years.
The observation of Alviggi et al. (2018) likely reflects different, yet pivotal, roles of both gonadotrophins during folliculogenesis, as described in the ‘two cell-two gonadotropin’ hypothesis (Ryan et al., 1968), which assumes a synergistic interaction of FSH and LH for follicular development and maturation. LH stimulates theca cells and thereby promotes androgen production, while FSH primarily enhances granulosa cell proliferation and, thus, estrogen synthesis. Expanding on this concept, granulosa cells were recently reported to also express LH receptors, with their numbers increasing during the more advanced stages of follicular development (Jeppesen et al., 2012). FSH and LH also promote growth factor secretion, such as insulin-like growth factor-1 and -2 in granulosa as well as theca cells. Both cell types in turn, of course, reflect follicular development (Huang et al., 1994).
These observations raise the possibility that follicular growth may be interrupted, or at least impaired, in absence of adequate LH levels. Recently, the Cochrane Collaboration group reported moderate evidence of increased ongoing pregnancy rates after LH-containing gonadotrophin stimulation when compared to FSH-only cycles (Mochtar et al., 2017). Mitochondrial gene expression data in granulosa cells support these findings: Saito et al. (2013) demonstrated higher gene expression ratios and improved rates of high-quality embryos in women undergoing LH-containing stimulation with hMG when compared to a recombinant FSH regimen. While younger women may still be able to cope with such unfavorable conditions, older women, with an already somewhat compromised follicular competence, will not. This is one reason why older patients at our center are stimulated with LH-containing gonadotrophins. The data presented here, however, suggest that even at a relatively advanced age, and with significant DOR, OSI is still highly predictive of pregnancy and live birth chances after FSH/hMG stimulation, thus confirming initial reports in better prognosis patients who received ovarian stimulation with FSH-only protocols (Huber et al., 2013; Hassan et al., 2017). The large study cohort used here and the reassuring results from the validation sample strengthen our findings, though they may not be entirely applicable to young women with excellent pregnancy potential in whom a direct continuous relationship of OSI with pregnancy outcomes might well be observed.
In conclusion, the ovary’s response to stimulation with exogenous gonadotrophins reflected by an OSI ≥0.83 is predictive of embryonic development, pregnancy potential and live birth. These findings suggest that the results of ovarian stimulation efforts should be included in counseling IVF patients about their chances of pregnancy.
Authors’ roles
A.W.: study design, execution, manuscript drafting and critical discussion; D.H.B.: study design, execution, manuscript drafting, analysis and critical discussion; S.K.D.: execution, analysis and critical discussion; V.A.K.: execution and critical discussion; D.F.A.: execution and critical discussion; and N.G.: study design, manuscript drafting and critical discussion.
Funding
Intramural funds from the Center for Human Reproduction and from the Foundation for Reproductive Medicine.
Conflict of interest
A.W., V.A.K., D.F.A., D.H.B. and N.G. have received research grant support, travel funds and speaker honoraria from various pharmaceutical and medical device companies, none, however, related to the topic presented here. D.H.B. and N.G. are listed as inventors on already awarded and still pending US patents, claiming beneficial effects on diminished ovarian reserve and embryo ploidy from dehydroepiandrosterone supplementation.
References
- Alvaro Mercadal B, Rodriguez I, Arroyo G, Martinez F, Barri PN, Coroleu B. Characterization of a suboptimal IVF population and clinical outcome after two IVF cycles. Gynecol Endocrinol 2018;34:125–128. [DOI] [PubMed] [Google Scholar]
- Alviggi C, Conforti A, Esteves SC, Andersen CY, Bosch E, Buhler K, Ferraretti AP, De Placido G, Mollo A, Fischer R et al. Recombinant luteinizing hormone supplementation in assisted reproductive technology: a systematic review. Fertil Steril 2018;109:644–664. [DOI] [PubMed] [Google Scholar]
- Baker VL, Brown MB, Luke B, Smith GW, Ireland JJ. Gonadotropin dose is negatively correlated with live birth rate: analysis of more than 650,000 assisted reproductive technology cycles. Fertil Steril 2015;104:1145–1152.e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdag G, Polat M, Yarali I, Yarali H. Live birth rates in various subgroups of poor ovarian responders fulfilling the Bologna criteria. Reprod Biomed Online 2017;34:639–644. [DOI] [PubMed] [Google Scholar]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–845. [PubMed] [Google Scholar]
- Drakopoulos P, Blockeel C, Stoop D, Camus M, de Vos M, Tournaye H, Polyzos NP. Conventional ovarian stimulation and single embryo transfer for IVF/ICSI. How many oocytes do we need to maximize cumulative live birth rates after utilization of all fresh and frozen embryos? Hum Reprod 2016;31:370–376. [DOI] [PubMed] [Google Scholar]
- Gallot V, Berwanger da Silva AL, Genro V, Grynberg M, Frydman N, Fanchin R. Antral follicle responsiveness to follicle-stimulating hormone administration assessed by the Follicular Output RaTe (FORT) may predict in vitro fertilization-embryo transfer outcome. Hum Reprod 2012;27:1066–1072. [DOI] [PubMed] [Google Scholar]
- Gleicher N, Kushnir VA, Sen A, Darmon SK, Weghofer A, Wu YG, Wang Q, Zhang L, Albertini DF, Barad DH. Definition by FSH, AMH and embryo numbers of good-, intermediate- and poor-prognosis patients suggests previously unknown IVF outcome-determining factor associated with AMH. J Transl Med 2016;14:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleicher N, Vega MV, Darmon SK, Weghofer A, Wu YG, Wang Q, Zhang L, Albertini DF, Barad DH, Kushnir VA. Live-birth rates in very poor prognosis patients, who are defined as poor responders under the Bologna criteria, with nonelective single embryo, two-embryo, and three or more embryos transferred. Fertil Steril 2015;104:1435–1441. [DOI] [PubMed] [Google Scholar]
- Hassan A, Kotb M, AwadAllah A, Wahba A, Shehata N. Follicular output rate can predict clinical pregnancy in women with unexplained infertility undergoing IVF/ICSI: a prospective cohort study. Reprod Biomed Online 2017. a;34:598–604. [DOI] [PubMed] [Google Scholar]
- Hassan AMA, Kotb MMM, AwadAllah AMA, Shehata NAA, Wahba A. Follicular sensitivity index (FSI): a novel tool to predict clinical pregnancy rate in IVF/ICSI cycles. J Assist Reprod Genet 2017. b;34:1317–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZH, Clayton PE, Brady G, Morris ID. Insulin-like growth factor-I gene expression in human granulosa-lutein cells. J Mol Endocrinol 1994;12:283–291. [DOI] [PubMed] [Google Scholar]
- Huber M, Hadziosmanovic N, Berglund L, Holte J. Using the ovarian sensitivity index to define poor, normal, and high response after controlled ovarian hyperstimulation in the long gonadotropin-releasing hormone-agonist protocol: suggestions for a new principle to solve an old problem. Fertil Steril 2013;100:1270–1276. [DOI] [PubMed] [Google Scholar]
- Iliodromiti S, Anderson RA, Nelson SM. Technical and performance characteristics of anti-Mullerian hormone and antral follicle count as biomarkers of ovarian response. Hum Reprod Update 2015;21:698–710. [DOI] [PubMed] [Google Scholar]
- Jeppesen JV, Kristensen SG, Nielsen ME, Humaidan P, Dal Canto M, Fadini R, Schmidt KT, Ernst E, Yding Andersen C. LH-receptor gene expression in human granulosa and cumulus cells from antral and preovulatory follicles. J Clin Endocrinol Metab 2012;97:E1524–E1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkert ER, Broekmans FJM, Looman CWN, Te Velde ER. A poor response in the first in vitro fertilization cycle is not necessarily related to a poor prognosis in subsequent cycles. Fertil Steril 2004;81:1247–1253. [DOI] [PubMed] [Google Scholar]
- Lazzaroni-Tealdi E, Barad DH, Albertini DF, Yu Y, Kushnir VA, Russell H, Wu YG, Gleicher N. Oocyte scoring enhances embryo-scoring in predicting pregnancy chances with IVF where it counts most. PLoS One 2015;10:e0143632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehert P, Chin W, Schertz J, D'Hooghe T, Alviggi C, Humaidan P. Predicting live birth for poor ovarian responders: the PROsPeR concept. Reprod Biomed Online 2018;37:43–52. [DOI] [PubMed] [Google Scholar]
- Li HW, Lee VC, Lau EY, Yeung WS, Ho PC, Ng EH. Role of baseline antral follicle count and anti-Mullerian hormone in prediction of cumulative live birth in the first in vitro fertilisation cycle: a retrospective cohort analysis. PLoS One 2013;8:e61095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochtar MH, Danhof NA, Ayeleke RO, Van der Veen F, van Wely M. Recombinant luteinizing hormone (rLH) and recombinant follicle stimulating hormone (rFSH) for ovarian stimulation in IVF/ICSI cycles. Cochrane Database Syst Rev 2017;5:CD005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KJ, Petro Z, Kaiser J. Steroid formation by isolated and recombined ovarian granulosa and tehcal cells. J Clin Endocrinol Metab 1968;28:355–358. [DOI] [PubMed] [Google Scholar]
- Saito N, Yamashita Y, Ono Y, Higuchi Y, Hayashi A, Yoshida Y, Yamamoto H, Kawabe S, Kamada M, Terai Y et al. Difference in mitochondrial gene expression in granulosa cells between recombinant FSH and hMG cycles under in vitro fertilization and transfer. Reprod Med Biol 2013;12:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkara SK, Rittenberg V, Raine-Fenning N, Bhattacharya S, Zamora J, Coomarasamy A. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod 2011;26:1768–1774. [DOI] [PubMed] [Google Scholar]
- Vaegter KK, Lakic TG, Olovsson M, Berglund L, Brodin T, Holte J. Which factors are most predictive for live birth after in vitro fertilization and intracytoplasmic sperm injection (IVF/ICSI) treatments? Analysis of 100 prospectively recorded variables in 8,400 IVF/ICSI single-embryo transfers. Fertil Steril 2017;107:641–648.e2. [DOI] [PubMed] [Google Scholar]
- Zebitay AG, Cetin O, Verit FF, Keskin S, Sakar MN, Karahuseyinoglu S, Ilhan G, Sahmay S. The role of ovarian reserve markers in prediction of clinical pregnancy. J Obstet Gynaecol 2017;37:492–497. [DOI] [PubMed] [Google Scholar]