Abstract
Androgen deficiency (hypogonadism) affects males of all ages. Testosterone replacement therapy (TRT) is effective in restoring serum testosterone and relieving symptoms. TRT, however, is reported to have possible adverse effects in part because administered testosterone is not produced in response to the hypothalamic–pituitary–gonadal (HPG) axis. Progress in stem cell biology offers potential alternatives for treating hypogonadism. Adult Leydig cells (ALCs) are generated by stem Leydig cells (SLCs) during puberty. SLCs persist in the adult testis. Considerable progress has been made in the identification, isolation, expansion and differentiation of SLCs in vitro. In addition to forming ALCs, SLCs are multipotent, with the ability to give rise to all 3 major cell lineages of typical mesenchymal stem cells, including osteoblasts, adipocytes, and chondrocytes. Several regulatory factors, including Desert hedgehog and platelet-derived growth factor, have been reported to play key roles in the proliferation and differentiation of SLCs into the Leydig lineage. In addition, stem cells from several nonsteroidogenic sources, including embryonic stem cells, induced pluripotent stem cells, mature fibroblasts, and mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord have been transdifferentiated into Leydig-like cells under a variety of induction protocols. ALCs generated from SLCs in vitro, as well as Leydig-like cells, have been successfully transplanted into ALC-depleted animals, restoring serum testosterone levels under HPG control. However, important questions remain, including: How long will the transplanted cells continue to function? Which induction protocol is safest and most effective? For translational purposes, more work is needed with primate cells, especially human.
Keywords: stem Leydig cells, steroidogenic stem cells, transdifferentiation, testosterone, hypogonadism, transplantation
Graphical Abstract
Graphical Abstract.
ESSENTIAL POINTS
Adult Leydig cells differentiate from both stem cells of the fetal testis and regressed fetal Leydig cells during puberty
Depending on the species, stem Leydig cells of the adult testis express one or more of the following specific markers: nestin, CD51, P75NTR, ARX, COUP-TF2, CD90, and PDGFRA
The proliferation and differentiation of stem Leydig cells is under the control of multiple paracrine factors, including DHH, PDGF, FGF2, activin, and TGFB
Cells from multiple nonsteroidogenic tissues, including various stem cells and mature fibroblasts, have been transdifferentiated into Leydig-like cells in vitro
The Leydig-like cells generated from testicular stem cells and from nonsteroidogenic organs have been used to increase serum testosterone levels in rodent models upon their transplantation into the testis or to locations outside the testis, with testosterone production under hypothalamic-pituitary-gonad axis control
Outline of Proposed Review
Introduction
- Stem Leydig Cells (SLCs) in Model Animals and Humans
- Leydig cell development in rodents and humans
- Characterization, culture and transplantation of rodent SLCs
- Characterization, culture and in vivo transplantation of human SLCs to rodents
- Generation and In Vivo Transplantation of Leydig-like Cells from Non-Testicular Cells in Model Animals
- Generation of Leydig-like cells from stem cells of non-testicular origin
- Generation of Leydig-like cells by reprogramming fibroblasts
- Transplantation of SLCs to locations outside the testis
- Generation of Steroidogenic Cells from Cells of Nonsteroidogenic Human Tissues
- Differentiation of Leydig-like cells from human stem cells transplanted into rat testes
- Differentiation of steroidogenic cells from human stem cells by virus infection
- Differentiation of steroidogenic cells from human stem cells by defined factors
- Differentiation of steroidogenic cells by reprogramming human fibroblasts
Stem Cells and Hypogonadism: Gaps and Challenges.
Introduction
Androgens, particularly testosterone, are essential for the development of the male reproductive system and for the maintenance of male reproductive functions. Androgen deficiency (hypogonadism) in men results from reduced production of testosterone due to defects at one or more levels of the hypothalamic-pituitary-gonadal (HPG) axis. Hypogonadism is generally seen in 4 major patient groups: fetal-onset hypogonadism, which may result in a disorder of sexual development, including female or ambiguous genitalia (1); childhood-onset hypogonadism, which may delay puberty (2); young adult hypogonadism, which may affect fertility, physical well-being, and quality of life; and late-onset hypogonadism, which may contribute to multiple physical and mental changes in the aged (3–5). Hypogonadism also can be classified into primary and secondary, depending upon the etiology. Primary hypogonadism is due to testicular defects resulting in reduced testosterone despite elevated luteinizing hormone (LH), whereas secondary hypogonadism involves pathology of the pituitary or hypothalamus leading to a disturbance in the HPG axis and subsequently in reduced testosterone (6).
Testosterone replacement therapy (TRT) is the most common way to treat symptoms of hypogonadism (7–9). TRT is effective in providing eugonadal levels of serum testosterone and thus relieving most symptoms associated with hypogonadism. However, there are reports of TRT being associated with cardiovascular and prostate issues, and with reduced spermatogenesis (9–11). Recent progress in stem cell biology has suggested the possibility of restoring testosterone levels by providing newly differentiated testosterone-producing cells. This approach has the potential to provide physiological levels of testosterone with normal circadian rhythm and pulsatility, and under the regulation of the HPG axis. Both daily circadian rhythm and hourly pulsatility could be important factors for elevating testosterone levels safely (10–13).
In this article, we will briefly summarize recent developments in stem cell research in relationship to steroidogenesis, and the potential use of stem cells for the treatment of hypogonadism. Since most such studies were done with model animals, we also will discuss the gaps and challenges in the field before translation to patients might be considered.
Stem Leydig Cells (SLCs) in Model Animals and Humans
Adult Leydig cells (ALCs) develop from SLCs during puberty. Four distinct stages of ALC development have been identified: SLCs, progenitor Leydig cells, immature Leydig cells, and ALCs (14). In this section, we will briefly discuss the origin, identification, differentiation, and in vivo transplantation of testicular SLCs in both rodents and humans.
Leydig cell development in rodents and humans
Distinct populations of Leydig cells appear in the mammalian testis during an individual’s lifetime (15). In rodents, Leydig cells have been identified in the fetal testis (fetal Leydig cells or FLCs) and adult testis (ALCs). In primates, including humans, there is an additional generation that appears briefly during the neonatal stage (16, 17). The androgens produced by FLCs in the embryonic period are crucial for the masculinization of the male fetal genital tract and brain (18). The second generation of Leydig cells in primates occurs before the development of ALCs at puberty (17). The function of the brief neonatal rise in androgen by these cells (also referred to as “mini-puberty”) is still largely unknown, but may be important in imprinting various androgen-targeted tissues such as the prostate, kidney, and brain (19). ALCs develop from stem cells during puberty under the regulation of the HPG axis, and are responsible for testicular androgen production in the adult.
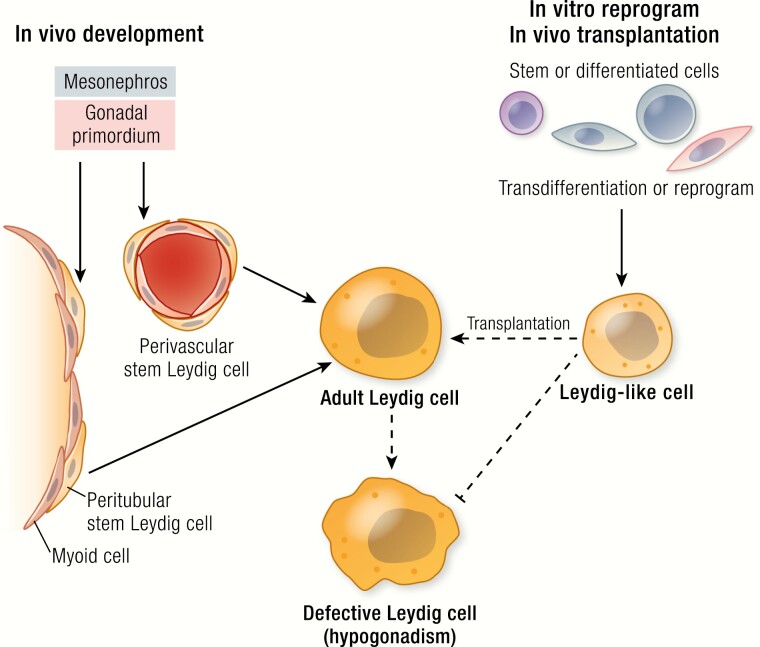
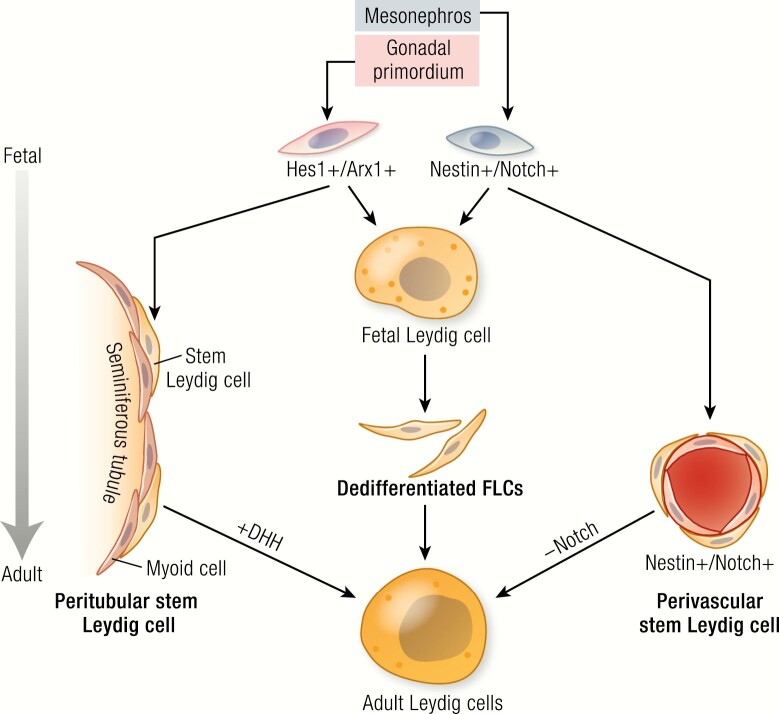
ALCs in rodents arise from SLCs localized to both peritubular and perivascular compartments of the fetal testis (20). The precursors for ALCs in mice derive from 3 fetal sources, identified by lineage-tracing and specific markers: peritubular progenitors (Hes1+/Arx+) of the coelomic epithelium (21), perivascular progenitors (nestin+/Notch+) of the gonadal-mesonephros border (22), and FLCs that are dedifferentiated at the end of the fetal stage (23). The 2 progenitor lineages differ in their dependence on Desert hedgehog (Dhh) and Notch signaling (21, 22); the differentiation of the peritubular lineage depends more on the activation of Dhh signaling (21), while the differentiation of the perivascular lineage depends more on the deactivation of Notch signaling (22). The 3 sources of precursors and their relationships are summarized in Fig. 1. The perinatal generation of Leydig cells in primates is thought to originate from both stem cells and from redifferentiation of the regressed FLCs (16). Similarly, ALCs in primates are also thought to originate from both stem cells and regressed FLCs and/or perinatal Leydig cells, which may undergo repeated dedifferentiation/redifferentiation cycles (15, 16).
Figure 1.
Three possible sources of ALCs in fetal and neonatal mouse testes: peritubular progenitors (Hes1+/Arx+) that come from coelomic epithelium (21); perivascular progenitors (nestin+/Notch+) that come from the gonadal-mesonephros border (22); and dedifferentiated FLCs that occur by the end of the fetal stage (23). Depending on the source, SLCS may differ in their dependence on Dhh and Notch signaling. Thus, the differentiation of the peritubular progenitors depends more on the activation of Dhh signaling (21), while the differentiation of perivascular lineage depends more on the deactivation of Notch signaling (22).
Although ALCs arise from SLCs during puberty, SLCs persist in the adult testis. ALCs are postmitotic and normally do not divide (24). SLCs might therefore play a critical role in maintaining ALC homeostasis in the adult (25–27). While their ability to form ALCs is well established in the adult testis, additional studies utilizing such tools as lineage-tracing and single cell–sequencing are needed to determine whether the SLCs represent a unique stem cell population or, alternatively, a mixed population of specialized mesenchymal progenitors and/or pericytes (27). Detailed information about adult SLCs and their potential for treating primary hypogonadism can be found in several recent reviews (19, 25, 26, 28–31).
Characterization, culture, and transplantation of rodent SLCs
In the adult testis, SLCs are located around both seminiferous tubules and blood vessels of the interstitial compartment. SLCs from both sources have been shown to be capable of generating new Leydig cells upon loss of existing ALCs (25–27, 32). SLCs from the testes of adult rodents and humans have been successfully isolated, expanded, and differentiated in vitro. Several markers have been identified for SLCs across different ages and species. These include CD90 (Thy1) (32), nestin (27, 33), PDGFRA (platelet-derived growth factor receptor alpha) (34, 35), ARX (aristaless-related homeobox) (36), COUP-TF2 (chicken ovalbumin upstream promoter-transcriptional factor 2 (37), CD51 (integrin alpha V) (33, 38), and P75NTR (p75 neurotrophin receptor) (39). There is apparent species specificity for markers. For example, nestin, CD51, and P75NTR overlap for mouse SLCs; PDGFRA, COUP-TF2, and CD90 are most useful for rat; and PDGFRA, nestin, and P75NTR are found in human SLCs.
Our laboratory found it possible to expand and differentiate the SLCs associated with the surface of rat seminiferous tubules by culturing isolated tubules after depleting the existing ALCs in vivo with injected ethane dimethanesulfonate (EDS) (32). The SLCs on the tubule surfaces first proliferated during week 1, and then differentiated into testosterone-producing cells during weeks 2 to 3. Interestingly, SLCs associated with stages IX to XI of the cycle of the seminiferous epithelium were more active in proliferation and differentiation than SLCs associated with stages VII to VIII. However, when the SLCs were isolated from each of the 2 groups of tubules and cultured in vitro, no differences were seen in their ability to proliferate or differentiate. These results suggest that stage-dependent local factors affect the SLCs associated with the tubules, and therefore that the paracrine factors derived from the tubular cells (Sertoli, germ, or myoid) must vary depending on the stage of the cycle (40). We were able to screen about 30 different hormones and growth factors for effects on SLC proliferation and/or differentiation (32). Platelet-derived growth factor (PDGF), fibroblast growth factor 2 (FGF2), Dhh, and activin were found to stimulate SLC proliferation, while Dhh, lithium-related signaling, PDGFAA (PDGF alpha dimer), and activin promoted SLC differentiation. TGFB (transforming growth factor beta), FGF2, and PDGFBB (PDGF beta dimer) inhibited proliferation and/or differentiation (32, 41). These factors also may be relevant to humans since human induced pluripotent stem cells (iPSCs) and adipose tissue-derived mesenchymal stem cells (AMSCs) were recently successfully differentiated into Leydig-like cells in vitro in response to some of these same factors (42, 43).
To identify tubule-associated SLCs in the rat, we studied a group of cell surface proteins whose genes were differentially regulated during the commitment of SLCs to the Leydig lineage. We identified CD90 as a marker for SLCs (32). The ability of CD90+ and CD90− cells to differentiate into steroidogenic cells was assessed by culturing the cells with LH for 3 weeks with or without the Dhh agonist SAG (smoothened agonist). CD90+ but not CD90− cells were able to form testosterone-producing cells in the presence of LH plus SAG. These results indicated that CD90 is a suitable marker to identify tubule-associated SLCs. Furthermore, Dhh appeared to be critical for inducing differentiation, since CD90+ cells could not differentiate in the absence of the Dhh agonist (32).
Based on the observation that nestin is expressed in putative SLCs in both fetal and adult testes (27), Xiang and colleagues were able to identify nestin and CD51 as specific markers for SLCs in mice (33, 37). SLCs that were isolated based on these markers were able to expand in vitro for up to 50 passages without significant loss of their differentiation potential. Interestingly, in addition to forming Leydig cells, the cells appeared multipotent, able to give rise to all lineages of typical mesenchymal stem cells (MSCs) including osteogenic, adipogenic, and chondrogenic cells (33, 35, 39), or to transdifferentiate into uterine and prostatic epithelium in vivo, indicating their multipotent potential to form mesodermal and endodermal cells (44). This multipotency is consistent with the results of an early microarray study which showed that rat SLCs had very similar transcriptome profiles to those of bone marrow-derived mesenchymal stem cells (BMSCs) (45). These studies suggest that SLCs may represent one of the specialized MSC populations.
To further test the functions of nestin+ and CD51+ cells in vivo, purified cells based on these 2 markers were transplanted back into testes of rats from which ALCs had been depleted by EDS treatment, the latter resulting in complete loss of ALCs in 3 days and an undetectable level of serum testosterone within 2 weeks (33, 38). Spermatogenesis also was affected, as were androgen-sensitive reproductive organ weights. Normally, a new generation of Leydig cells was gradually restored to the testis by in situ SLCs in 3 to 8 weeks. Transplantation of either nestin+ or CD51+ cells into ALC-depleted rats resulted in the colonization of cells in the interstitium of recipient testes and their further differentiation into Leydig cells. This significantly enhanced the recovery of serum testosterone levels and had positive effects on spermatogenesis and androgen-sensitive reproductive organ weights. Analysis of serum testosterone levels in a 24-hour window revealed a clear circadian rhythm in rats receiving CD51+ cells, similar to the pattern of non–EDS-treated controls (38). These results indicated that the transplanted cells were under the regulation of the HPG axis. The effect of the transplanted cells on aging-related hypogonadism also was tested. When the cells were transplanted into the testes of 22-month-old mice, the age-related reductions in serum testosterone seen in controls were reversed (33). These results demonstrated that SLC transplantation was capable not only of enhancing testosterone levels in ALC-depleted animals, but also of restoring Leydig cell defects that occur with aging.
Characterization, culture, and in vivo transplantation of human SLCs to rodents
As in rodents, the human testis contains SLCs that are associated with the seminiferous tubules (19, 46). Early work indicated that these cells express the SLC markers PDGFRA and LIFR (leukemia inhibitory factor receptor) (46). Their ability to produce testosterone can be induced in vitro by cyclic adenosine monophosphate (cAMP) agonists and PDGF treatments. Xiang and colleagues identified nestin and P75NTR as markers for SLCs in human testes (39). The P75NTR+ cells showed clone-forming properties in vitro and the ability to form Leydig cells. Additionally, the P75NTR+ cells were found to be multipotent, with the ability to form all 3 lineages of typical MSCs, including osteoblasts, adipocytes, and chondrocytes. Transplantation of the cells into ALC-depleted rat testes resulted in their successful differentiation into Leydig cells, and this enhanced the recovery of serum testosterone, spermatogenesis, and reproductive organ weights (39). Recently, a similar cell population was isolated from human testis based on multiple cell surface markers (HLA-A,B,C+/CD34−/PDGFRα+) (35). Interestingly, these cells also expressed common MSC markers, such as CD90, CD73, and CD105, and were able to form all 3 lineages of typical MSCs, including osteoblasts, adipocytes, and chondrocytes. However, unlike the cells derived from P75NTR+ cells (39), the steroidogenic cells differentiated from PDGFRA+ cells produced androstenedione and progesterone, precusors of testosterone, but not testosterone itself. The relationship between these 2 populations deserves further study. Overall, the finding provides the groundwork for further characterization of the cells, study of their regulatory mechanisms, and perhaps future clinical application.
Generation and In Vivo Transplantation of Leydig-like Cells from Non-Testicular Cells in Model Animals
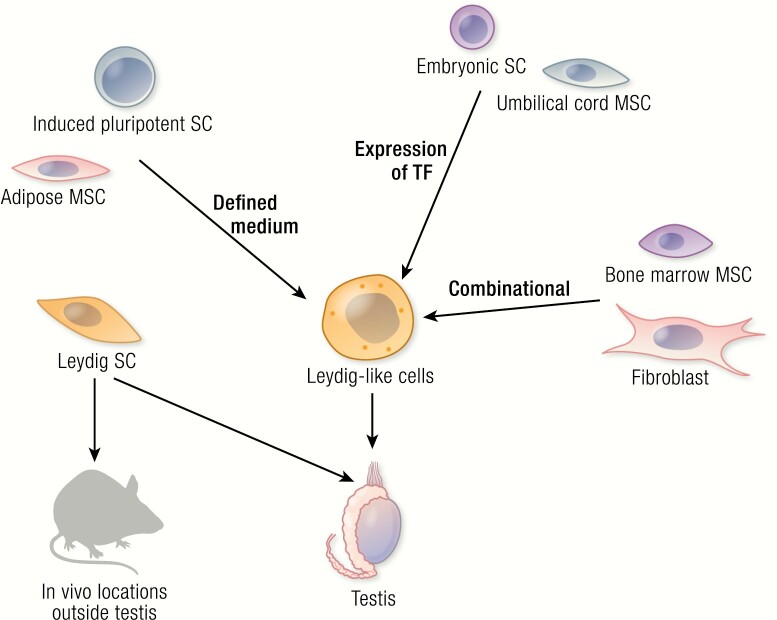
During the past decade, as progress has been made to isolate and culture SLCs from the testis, even more intriguing progress has been made in the generation and in vivo transplantation of steroidogenic cells from cells of nonsteroidogenic tissues (Fig. 2).
Figure 2.
Summary of the cell sources in rodents and humans that have been successfully differentiated into Leydig-like cells in vitro. Differentiation was done by forced expression of transcription factors (Expression of TF), induction by defined chemical medium (Defined medium), or a combination of the two (Combinational). Some of the resulting cells were tested in vivo by their transplantation either into the testes of model animals, or in locations outside the testis. SC, stem cell; MSC, mesenchymal stem cell.
Generation of Leydig-like cells by stem cells of non-testicular origin
Differentiation of steroidogenic cells from stem cells of nonsteroidogenic tissues has been attempted in vitro by many laboratories. Most of the work was done by transfecting the cells with Sf1 (steroidogenic factor 1; also known as Nr5a1), a key steroidogenesis-related transcription factor that is an important inducing factor in the development of steroidogenic cells in both adrenal and testis. Without it, male mice developed female internal genitalia and died during the early neonatal period due to lack of both testosterone- and adrenocortical-producing cells (47). Although the cells transfected with Sf1 eventually became steroidogenic, they did not necessarily produce testosterone, but instead a mixture of steroids (31). For example, BMSCs that were transdifferentiated into steroidogenic cells by Sf1expression produced a mixture of adrenal and gonadal steroids (48), suggesting that BMSCs have the potential to form both adrenal and gonadal lineages. In the presence of cAMP agonist, the cells could be reprogrammed to produce primarily testosterone (49). The flexibility to generate either gonad steroid- or adrenal steroid–producing cells may well be due to the fact that the steroidogenic cells of both organs come from a common Sf1+ progenitor during embryonic development (50).
In a study that compared BMSCs and AMSCs from the same mice for their steroidogenic properties after forced expression of Sf1 plus retinoic acid treatment (51), the resulting cell lines were different in their steroidogenic properties. AMSCs were more likely to produce corticosterone than testosterone, whereas the opposite was true for BMSCs. These novel results suggest the possibility of using adult tissue stem cells for autologous cell regeneration therapy for patients with steroid insufficiency, and point to the need for specific tissue selection and appropriate induction procedures depending on the desired outcome.
In addition to differentiation of stem cells from adult tissues, attempts were also made with embryonic stem cells (ESCs) (52–55). These studies showed that in contrast to tissue-specific stem cells, Sf1 by itself either was not able to differentiate ESCs or did so at extremely low efficiency. However, co-treatment of Sf1-transfected cells with retinoic acid and cAMP agonist greatly enhanced differentiation efficiency (52, 55). Depending on the procedure, the resulting cells produced various steroids, including estradiol (54), corticosterone (53), or testosterone (55). Overall, the results suggest that ESCs have the potential to form multiple steroidogenic lineages, but that the induction protocol is again critical in determining the steroid output.
ESC-derived Leydig-like cells were also tested for their ability to modify testosterone levels in vivo (55). When the cells were transplanted into ALC-depleted rat testes, they survived in vivo and produced testosterone, which enhanced the recovery of serum testosterone levels. Unfortunately, the study did not carefully examine whether the transplanted cells, which did not express luteinizing hormone/choriogonadotropin receptor (LHCGR) before their transplantation, eventually became LH-responsive in vivo (55). This is important since it should be determined whether or not non-LH responsive cells may eventually gain responsiveness to LH after exposure to the testicular environment. Such responsiveness is critical to normal testicular function.
Generation of Leydig-like cells by reprogramming fibroblasts
As indicated above, a number of studies used stem or progenitor cells in attempts to create testosterone-producing cells. A recent study has successfully reprogrammed fibroblasts into such cells (56). Sf1, Gata4, and Dmrt1 were found to be critically required for the reprogramming process. Further studies indicated that Sf1 by itself was sufficient to finish the reprogramming process, confirming early observations that Sf1 is a particularly critical factor in reprogramming Leydig cells (31). Gata4 and Dmrt1 contributed significantly to the expression of LHCGR and to the final testosterone output. Importantly, transcriptome analysis indicated that the reprogramming process converted fibroblasts into cells that not only expressed the steroidogenic pathway genes, but also reset their transcriptome, including global DNA-methylations, to resemble more closely ALCs than fibroblasts (56).
The reprogrammed cells were also tested in vivo by their transplantation into ALC-depleted rat and mouse testes (56). EDS treatment of rats resulted in a complete loss of the ALC population and decline in serum testosterone in the first 2 weeks, with recovery of approximately 25% to 50% by 4 weeks and 100% by 8 weeks. Implantation of reprogrammed cells into the testes of EDS-treated rats dramatically improved the recovery process, so that by 4 weeks the serum testosterone concentrations had already reached control levels. These results indicated that the reprogrammed cells functioned similarly to Leydig cells in vivo, with the ability to enhance the restoration of serum testosterone levels in rats that had chemically-induced hypogonadism.
Transplantation of SLCs to locations outside the testis
Transplantation of stem cells or induced Leydig cells into testes represent approaches to increase serum testosterone. Another is to provide hormone-producing cells implanted at locations outside the testis. This approach would be of particular benefit to castrated individuals or to men whose testes have become incapable of supporting the function of the transplanted cells. Indeed, transplantation and/or generating testosterone-producing cells at locations outside the testis may be preferred, even when not the only choice.
Ramasamy and colleagues recently tested the idea of generating Leydig cells outside the testis (57). Mouse SLCs were encapsulated into matrigel with other testicular cells, including Sertoli and myoid cells, and then subcutaneously autografted into castrated mice. The SLCs underwent self-renewal, as well as differentiation, to give rise to steroidogenic cells within 1 month. As a result, the recipient mice showed significantly increased serum testosterone levels associated with reduced LH, suggesting that the transplanted cells were active in producing testosterone and modifying the HPG axis. Interestingly, if SLCs were implanted in the absence of other testicular cells, such as Sertoli cells, the implanted cells were not capable of self-renewing and differentiating, suggesting that factors generated by testicular cells are critical for the survival and differentiation of transplanted SLCs. However, if the SLCs were incubated with the Sertoli cell product Dhh before implantation, the cells were able to differentiate without other testicular cells, confirming that Dhh is a critically required factor (32). The study established the concept that it is possible to differentiate testosterone-producing cells from SLCs in vivo at locations outside the testis if appropriate supporting factors also are provided.
In addition to transplanting cells to organs outside the testis, one study tested the effects of transplanting undifferentiated stem cells into the circulation (58). Glycation product accumulation is one of the phenotypes of aging, especially in slowly renewing tissues. Treatment of young rats with D-galactose (D-gal) can induce aging phenotypes, resulting in significant reductions in both 3β-hydroxysteroid dehydrogenase (HSD3B)+ cells and serum testosterone levels. When AMSCs were directly introduced into the circulation of D-gal treated rats, the aging phenotypes, including changes in the reproductive system, were partially reversed (58). Importantly, some AMSCs were retained in the testis and differentiated into steroidogenic cells. The study suggested that it is possible to supply stem cells to the testis by introducing the cells into the general circulation.
Generation of Steroidogenic Cells from Cells of Nonsteroidogenic Human Tissues
In the last decade, progress has been made in generating steroidogenic cells from cells of nonsteroidogenic tissues in model animals. Additionally, there have been some studies in which steroidogenic cells have been generated using cells from nonsteroidogenic human tissues.
Differentiation of Leydig-like cells from human stem cells transplanted into rat testes
The ability of human stem cells to give rise to testosterone producing cells when transplanted to rat testes was tested (59). MSCs from human umbilical cord (UMSCs) were transplanted into the testes of ALC-depleted rats. The transplanted cells gained steroidogenic capacity after 3 weeks, and this resulted in significantly increased serum testosterone levels compared to the controls. This study with human cells confirmed earlier findings with rodents and pointed to the critical role played by the testicular interstitial compartment in defining the fates of transplanted stem cells (49, 60). It also raises the possibility of treating hypogonadism by directly transplanting undifferentiated stem cells into the testis.
Differentiation of steroidogenic cells from human stem cells by virus infections
As discussed above, forced expression of Sf1 can turn mouse BMSCs into steroidogenic cells (48, 49). Similarly, Sf1 expression in human BMSCs resulted in cells that produced either glucocorticoid (49) or a mixture of steroids, including progesterone, corticosterone, cortisol, dehydroepiandrosterone, testosterone, and estradiol (61). The induced cells also expressed receptors for both adrenocorticotropic hormone (ACTH) and LH, suggesting that the cells are akin to FLCs and are able to respond to both hormones. Thus, as is the case with rodent cells, human BMSCs may have the potential to form different steroidogenic lineages. However, in order to induce cells for a specific steroid, the induction protocol must be fine-tuned.
In addition to BMSCs, human AMSCs (51) and UMSCs (62) were tested in vitro for their potential to form steroidogenic cells. Although all these cells eventually became steroidogenically active by forced expression of Sf1 plus stimulation by small signaling molecules, they were very different in their final steroid outputs. For example, while BMSCs became testosterone-producing (49), AMSCs were much more prone to produce the adrenal steroid corticosterone (51), and UMSCs exhibited characteristics of ovarian steroidogenic cells by producing progesterone and estrogen (62). These results confirmed the observations from studies of mice that had shown that although the MSCs from different sources share the ability to form steroidogenic cells, they have intrinsic differences. Tissue selection could be a key factor when inducing cells for particular steroid outcomes (31).
This lineage preference for forming steroidogenic cells was also found with human ESCs and iPSCs. Unlike tissue stem cells, ESCs and iPSCs were not able to be programmed by expression of Sf1 alone. When the cells were first differentiated into a mesodermal lineage by glycogen synthase kinase 3 beta (GSK-3β) inhibitor, Sf1, and cAMP agonist treatments, the result was their differentiation into steroidogenic lineages (63). However, the cells expressed cytochrome P450 family members of adrenal cells, Cyp21a2and Cyp11b1, and secreted cortisol rather than testosterone. Again, these observations confirmed the findings that although forced expression of Sf1 plus cAMP treatment could make pluripotent stem cells steroidogenic, the cells do not necessarily produce testosterone. A more specific induction procedure will be needed in order to induce cells to produce the desired outcome.
Differentiation of steroidogenic cells from human stem cells by defined factors
Differentiation of steroidogenic cells from stem cells or fibroblasts required transfecting the cells with vectors carrying critical transcriptional factors (48–51, 61–63). Although of considerable basic interest, transfection of the cells in this manner would make the resulting cells unsuitable for therapeutic use because of safety considerations. For the latter purpose, the best solution would be to reprogram cells by exogenous induction factors, such as hormones, growth factors, and small molecules. Recently such attempts have been made with human stem cells (42, 43, 64, 65). Guo and colleagues (42, 43) successfully differentiated human iPSCs and AMSCs into testosterone-producing cells with a chemically defined medium. The studies adapted a procedure that included “commitment, expansion, differentiation, maturation and enrichment” to convert both iPSCs and AMSCs into Leydig-like cells. The commitment and differentiation medium, formulated based on factors previously identified for SLC differentiation (32), consisted of 10 different components, including hormones, growth factors, and small signal-modifying molecules. The entire induction process took 35 days. With this complex procedure, iPSCs and AMSCs were successfully differentiated into Leydig-like cells that were able to produce testosterone. The cells also showed similar gene expression profiles to that of human Leydig cells, as well as loss of iPSC-related pluripotency markers including NANOG, OCT4, and SOX2, suggesting a complete conversion. The study also tested the function of the cells by transplanting them into ALC-depleted rat testes (42, 43). The transplanted cells survived in vivo and produced testosterone, resulting in recovery of serum testosterone levels and testis weights. These results demonstrated that it is possible to differentiate both pluripotent (iPSCs) and multipotent (AMSCs) cells to testosterone-producing cells by chemically defined medium, without introducing exogenous genes. Unfortunately, the studies did not characterize the cells for their potential to produce steroids other than testosterone, and it is also not known whether the cells will respond to pituitary hormones other than LH.
Differentiation of steroidogenic cells by reprogramming human fibroblasts
In addition to differentiation of steroidogenic cells from stem cells, reprogramming human fibroblasts to form steroidogenic cells has been achieved (66–68). When human foreskin fibroblasts were transfected with vectors carrying 4 transcription factors (Sf1, Gata4, Coup-TF2 and Nr4a1) the cells became steroidogenic and produced testosterone (66). Sf1 and Gata4 were particularly important for the expression of the major steroidogenic genes, while Sf1, Gata4, and Nr4a1 were necessary to generate testosterone-producing cells. Unlike the results with mouse cells (56), Sf1 by itself was not able to fully reprogram human cells. Instead, the transcription factors Sf1, Gata4, and Nr4a1 were all required to make human cells steroidogenic. This was confirmed by a recent study from the same group showing that targeted activation of Sf1, Gata4, and Dmrt1 via the CRISPR/dCas9 system can reprogram human fibroblasts into testosterone-producing cells (67). This latter approach does not involve the introduction of exogenous genes, but rather relies on the forced expression of endogenous genes. However, it still requires viral infections, which would make the cells problematic for clinical usage.
In a recent study, it was found possible to reprogram human fibroblasts only with Sf1 by providing a defined small molecule cocktail (68). The cocktail formula consisted of forskolin (cAMP/PKA signaling activator), DAPT (Notch signaling inhibitor) and purmorphamine (Hedgehog signaling activator). With these small molecules, the human foreskin fibroblasts were successfully converted into testosterone-producing cells by the introduction of only 1 gene, Sf1. The study moves closer to a virus-free reprogramming procedure based entirely on defined medium. However, the reprogrammed cells either were not carefully checked for their responsiveness to LH (66, 68) or were found to be very weak in responsiveness (67). Also, the studies did not determine whether the reprogrammed cells also produced steroids other than testosterone. These are important characteristics to define before the resulting cells can be considered for clinical usage.
Stem Cells and Hypogonadism: Gaps and Challenges
In the last decade, great progress has been made in basic understanding of how testosterone-producing Leydig cells form from stem cells. With this basic understanding, progress also has been made in the generation of steroidogenic cells from cells of nonsteroidogenic tissues. Gaps and challenges remain if such cells are to be used successfully and safely in a clinical setting. First, most of the studies with steroidogenesis-related stem cells were done with rodents. More work is needed with cells from primates, especially humans. On the basic side, researchers must identify more specific markers and critical regulatory factors that control the self-renewal and differentiation of SLCs.
On the reprogramming front, most transdifferentiation experiments have been done by introducing exogenous genes or by forced expression of endogenous genes (Fig. 2). Advances in chemical medium-based transdifferentiation of cells from nonsteroidogenic tissues would be of great potential clinical value if the need for exogenous genes was eliminated. That is, for cells to be usable in the clinic, there should be ways to elicit their reprogramming without the introduction of genes with viruses. To date, reprogrammed cells from tissues other than steroidogenic organs typically have produced unexpected or mixed steroids. Tissue selection and suitable induction protocols are necessary to generate cells for specific steroid output. In addition, to make sure the cells are under the proper control of the HPG axis, they must express gonadotropin receptors.
For in vivo transplantations, most studies have focused only on the initial establishment of the transplanted cells, not the long-term survival and function of the cells. Future studies will need to focus on long-term effects. In addition, more work is needed to test the possibility of transplanting cells to locations outside the testis. For such implantations to be successful, better carrier-substrates, including nanomaterials, need to be developed so that the cells can be better packed and protected in vivo. Since transplantation experiments, either within or outside the testis, have been done mostly in animals in which the in situ ALC populations were completely eliminated (by EDS treatment or castration), questions remain as whether similar benefits of transplanted cells would be obtained if the transplantations were done in intact animals that contain at least some working ALCs. Lastly, for the transplantation experiments, short- and long-term safety issues will need to be addressed, including the risks of rejection and tumorigenesis.
Acknowledgments
Financial Support: This work was supported by a Major Research Plan Grant 91949123 (HC) from National Natural Science Foundation of China, a Wenzhou City Public Welfare Science and Technology Project grant Y20150012 (HC) and NIH grant R01 AG21092 (BZ).
Disclosure Summary: The authors have nothing to disclose. The author has submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Glossary
Abbreviations:
- ACTH
adrenocorticotropic hormone
- Hes1
hairy and enhancer of split-1
- ALC
adult Leydig cell
- AMSC
adipose tissue-derived mesenchymal stem cell
- ARX
Aristaless related homeobox
- BMSC
bone marrow–derived mesenchymal stem cell
- cAMP
cyclic adenosine monophosphate
- CD90
cluster of differentiation 90, also known as Thy-1
- CD51
cluster of differentiation 51, also known as integrin alpha V
- CD73
cluster of differentiation 73, also known as ecto-5′-nucleotidase (NT5E)
- CD105
cluster of differentiation 105, also known as endoglin (ENG)
- COUP-TF2
chicken ovalbumin upstream promoter-transcriptional factor 2, also known as NR2F2
- Cyp21a2
cytochrome P450 family 21 subfamily a member 2
- Cyp11b1
cytochrome P450 family 11 subfamily b member 1
- DAPT
N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester; a γ-secretase inhibitor
- D-gal
D-galactose
- Dmrt1
doublesex and mab-3 related transcription factor 1
- Dhh
Desert hedgehog
- EDS
ethane dimethanesulphonate
- ESC
embryonic stem cell
- FGF2
fibroblast growth factor 2
- FLC
fetal Leydig cell
- Gata4
GATA-binding factor 4
- GSK-3β
glycogen synthase kinase 3 beta
- HPG axis
hypothalamic-pituitary-gonad axis
- HSD3B
3β-hydroxysteroid dehydrogenase
- iPSC
induced pluripotent stem cells
- LH
luteinizing hormone
- LHCGR
luteinizing hormone/choriogonadotropin receptor
- MSC
mesenchymal stem cell
- NANOG
Nanog homeobox transcription factor
- NR4A1
nuclear receptor subfamily 4 group A member 1
- NR5A1
nuclear receptor subfamily 5 group A member 1, also known as Sf1
- Oct4
octamer-binding transcription factor 4
- P75NTR
p75 neurotrophin receptor
- PKA
protein kinase A
- PDGF
platelet-derived growth factor
- PDGFAA
platelet derived growth factor alpha dimer
- PDGFBB
platelet derived growth factor beta dimer
- PDGFRA
platelet derived growth factor receptor alpha
- SAG
smoothened agonist
- Sf1
steroidogenic factor 1, also known as NR5A1
- SLC
stem Leydig cell
- SOX2
sex-determining region Y-box 2
- TGFB
transforming growth factor beta
- TRT
testosterone replacement therapy
- UMSC
umbilical cord mesenchymal stem cells
References
- 1. Grinspon RP, Loreti N, Braslavsky D, et al. Spreading the clinical window for diagnosing fetal-onset hypogonadism in boys. Front Endocrinol (Lausanne). 2014;5:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Howard SR, Dunkel L. Management of hypogonadism from birth to adolescence. Best Pract Res Clin Endocrinol Metab. 2018;32(4):355–372. [DOI] [PubMed] [Google Scholar]
- 3. Hackett G. Type 2 Diabetes and Testosterone Therapy. World J Mens Health. 2019;37(1):31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nam YS, Lee G, Yun JM, Cho B. Testosterone Replacement, Muscle Strength, and Physical Function. World J Mens Health. 2018;36(2):110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yialamas MA, Hayes FJ. Androgens and the ageing male and female. Best Pract Res Clin Endocrinol Metab. 2003;17(2):223–236. [DOI] [PubMed] [Google Scholar]
- 6. Traish AM. Negative Impact of Testosterone Deficiency and 5α-Reductase Inhibitors Therapy on Metabolic and Sexual Function in Men. Adv Exp Med Biol. 2017;1043:473–526. [DOI] [PubMed] [Google Scholar]
- 7. Seftel AD, Kathrins M, Niederberger C. Critical Update of the 2010 Endocrine Society Clinical Practice Guidelines for Male Hypogonadism: A Systematic Analysis. Mayo Clin Proc. 2015;90(8):1104–1115. [DOI] [PubMed] [Google Scholar]
- 8. Snyder PJ, Bhasin S, Cunningham GR, et al. Lessons From the Testosterone Trials. Endocr Rev. 2018;39(3):369–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yeap BB, Page ST, Grossmann M. Testosterone treatment in older men: clinical implications and unresolved questions from the Testosterone Trials. Lancet Diabetes Endocrinol. 2018;6(8):659–672. [DOI] [PubMed] [Google Scholar]
- 10. Baburski AZ, Andric SA, Kostic TS. Luteinizing hormone signaling is involved in synchronization of Leydig cell’s clock and is crucial for rhythm robustness of testosterone production†. Biol Reprod. 2019;100(5):1406–1415. [DOI] [PubMed] [Google Scholar]
- 11. Patel AS, Leong JY, Ramos L, Ramasamy R. Testosterone Is a Contraceptive and Should Not Be Used in Men Who Desire Fertility. World J Mens Health. 2019;37(1):45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klotz L. Testosterone therapy and prostate cancer–safety concerns are well founded. Nat Rev Urol. 2015;12(1):48–54. [DOI] [PubMed] [Google Scholar]
- 13. Yeap BB. Testosterone and cardiovascular disease risk. Curr Opin Endocrinol Diabetes Obes. 2015;22(3):193–202. [DOI] [PubMed] [Google Scholar]
- 14. Chen H, Stanley E, Jin S, Zirkin BR. Stem Leydig cells: from fetal to aged animals. Birth Defects Res C Embryo Today. 2010;90(4):272–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Teerds KJ, Huhtaniemi IT. Morphological and functional maturation of Leydig cells: from rodent models to primates. Hum Reprod Update. 2015;21(3):310–328. [DOI] [PubMed] [Google Scholar]
- 16. Svechnikov K, Izzo G, Landreh L,Weisser J, Söder O. Endocrine disruptors and Leydig cell function. J Biomed Biotechnol. 2010. pii: 684504. doi: 10.1155/2010/684504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prince FP. Ultrastructural evidence of mature Leydig cells and Leydig cell regression in the neonatal human testis. Anat Rec. 1990;228(4):405–417. [DOI] [PubMed] [Google Scholar]
- 18. Griswold SL, Behringer RR. Fetal Leydig cell origin and development. Sex Dev. 2009;3(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Svechnikov K, Landreh L, Weisser J, et al. Origin, development and regulation of human Leydig cells. Horm Res Paediatr. 2010;73(2):93–101. [DOI] [PubMed] [Google Scholar]
- 20. DeFalco T, Takahashi S, Capel B. Two distinct origins for Leydig cell progenitors in the fetal testis. Dev Biol. 2011;352(1):14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rotgers E, Jørgensen A, Yao HH. At the Crossroads of Fate-Somatic Cell Lineage Specification in the Fetal Gonad. Endocr Rev. 2018;39(5):739–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kumar DL, DeFalco T. A perivascular niche for multipotent progenitors in the fetal testis. Nat Commun. 2018;9(1):4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shima Y, Miyabayashi K, Sato T, et al. Fetal Leydig cells dedifferentiate and serve as adult Leydig stem cells. Development. 2018;145(23). pii: dev169136. [DOI] [PubMed] [Google Scholar]
- 24. Teerds KJ, De Rooij DG, Rommerts FF, van der Tweel I, Wensing CJ. Turnover time of Leydig cells and other interstitial cells in testes of adult rats. Arch Androl. 1989;23(2):105–111. [DOI] [PubMed] [Google Scholar]
- 25. Chen H, Stanley E, Jin S, Zirkin BR. Stem Leydig cells: from fetal to aged animals. Birth Defects Res C Embryo Today. 2010;90(4):272–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen H, Wang Y, Ge R, Zirkin BR. Leydig cell stem cells: Identification, proliferation and differentiation. Mol Cell Endocrinol. 2017;445:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Davidoff MS, Middendorff R, Enikolopov G, Riethmacher D, Holstein AF, Müller D. Progenitor cells of the testosterone-producing Leydig cells revealed. J Cell Biol. 2004;167(5):935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Inoue M, Baba T, Morohashi KI. Recent progress in understanding the mechanisms of Leydig cell differentiation. Mol Cell Endocrinol. 2018;468:39–46. [DOI] [PubMed] [Google Scholar]
- 29. Peak TC, Haney NM, Wang W, DeLay KJ, Hellstrom WJ. Stem cell therapy for the treatment of Leydig cell dysfunction in primary hypogonadism. World J Stem Cells. 2016;8(10):306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Davidoff MS. The Pluripotent Microvascular Pericytes Are the Adult Stem Cells Even in the Testis. Adv Exp Med Biol. 2019;1122:235–267. [DOI] [PubMed] [Google Scholar]
- 31. Yazawa T, Imamichi Y, Miyamoto K, Khan MR, Uwada J, Umezawa A, Taniguchi T. Induction of steroidogenic cells from adult stem cells and pluripotent stem cells. Endocr J. 2016;63(11):943–951. [DOI] [PubMed] [Google Scholar]
- 32. Li X, Wang Z, Jiang Z, et al. Regulation of seminiferous tubule-associated stem Leydig cells in adult rat testes. Proc Natl Acad Sci U S A. 2016;113(10):2666–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jiang MH, Cai B, Tuo Y, et al. Characterization of Nestin-positive stem Leydig cells as a potential source for the treatment of testicular Leydig cell dysfunction. Cell Res. 2014;24(12):1466–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ge RS, Dong Q, Sottas CM, Papadopoulos V, Zirkin BR, Hardy MP. In search of rat stem Leydig cells: identification, isolation, and lineage-specific development. Proc Natl Acad Sci U S A. 2006;103(8):2719–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eliveld J, van den Berg EA, Chikhovskaya JV, et al. Primary human testicular PDGFRα+ cells are multipotent and can be differentiated into cells with Leydig cell characteristics in vitro. Hum Reprod. 2019;34(9):1621–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miyabayashi K, Katoh-Fukui Y, Ogawa H, et al. Aristaless related homeobox gene, Arx, is implicated in mouse fetal Leydig cell differentiation possibly through expressing in the progenitor cells. Plos One. 2013;8(6):e68050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kilcoyne KR, Smith LB, Atanassova N, et al. Fetal programming of adult Leydig cell function by androgenic effects on stem/progenitor cells. Proc Natl Acad Sci U S A. 2014;111(18):E1924–E1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zang ZJ, Wang J, Chen Z, et al. Transplantation of CD51+ Stem Leydig Cells: A New Strategy for the Treatment of Testosterone Deficiency. Stem Cells. 2017;35(5):1222–1232. [DOI] [PubMed] [Google Scholar]
- 39. Zhang M, Wang J, Deng C, et al. Transplanted human p75-positive stem Leydig cells replace disrupted Leydig cells for testosterone production. Cell Death Dis. 2017;8(10):e3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guan X, Chen F, Chen P, et al. Effects of spermatogenic cycle on Stem Leydig cell proliferation and differentiation. Mol Cell Endocrinol. 2019;481:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen P, Zhao X, Guan X, Chen H. Origin and regulation of stem Leydig cells in the adult testis. Curr Opin in Endo and Metab Res. 2019;6:49–53. [Google Scholar]
- 42. Chen X, Li C, Chen Y, et al. Differentiation of human induced pluripotent stem cells into Leydig-like cells with molecular compounds. Cell Death Dis. 2019;10(3):e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen Y, Li C, Ji W, et al. Differentiation of human adipose derived stem cells into Leydig-like cells with molecular compounds. J Cell Mol Med. 2019. Jul 10. doi: 10.1111/jcmm.14427. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nanjappa MK, Medrano TI, Prins GS, Chen H, Zirkin BR, Cooke PS. Transdifferentiation of adult rat stem Leydig cells into prostatic and uterine epithelium, but not epidermis. Andrology. 2017;5(6):1165–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stanley EL, Johnston DS, Fan J, et al. Stem Leydig cell differentiation: gene expression during development of the adult rat population of Leydig cells. Biol Reprod. 2011;85(6):1161–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Landreh L, Spinnler K, Schubert K, et al. Human testicular peritubular cells host putative stem Leydig cells with steroidogenic capacity. J Clin Endocrinol Metab. 2014;99(7):E1227–E1235. [DOI] [PubMed] [Google Scholar]
- 47. Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77(4):481–490. [DOI] [PubMed] [Google Scholar]
- 48. Gondo S, Yanase T, Okabe T, et al. SF-1/Ad4BP transforms primary long-term cultured bone marrow cells into ACTH-responsive steroidogenic cells. Genes Cells. 2004;9(12):1239–1247. [DOI] [PubMed] [Google Scholar]
- 49. Yazawa T, Mizutani T, Yamada K, et al. Differentiation of adult stem cells derived from bone marrow stroma into Leydig or adrenocortical cells. Endocrinology. 2006;147(9):4104–4111. [DOI] [PubMed] [Google Scholar]
- 50. Meinsohn MC, Smith OE, Bertolin K, Murphy BD. The Orphan Nuclear Receptors Steroidogenic Factor-1 and Liver Receptor Homolog-1: Structure, Regulation, and Essential Roles in Mammalian Reproduction. Physiol Rev. 2019;99(2):1249–1279. [DOI] [PubMed] [Google Scholar]
- 51. Gondo S, Okabe T, Tanaka T, et al. Adipose tissue-derived and bone marrow-derived mesenchymal cells develop into different lineage of steroidogenic cells by forced expression of steroidogenic factor 1. Endocrinology. 2008;149(9):4717–4725. [DOI] [PubMed] [Google Scholar]
- 52. Crawford PA, Sadovsky Y, Milbrandt J. Nuclear receptor steroidogenic factor 1 directs embryonic stem cells toward the steroidogenic lineage. Mol Cell Biol. 1997;17(7):3997–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yazawa T, Kawabe S, Inaoka Y, et al. Differentiation of mesenchymal stem cells and embryonic stem cells into steroidogenic cells using steroidogenic factor-1 and liver receptor homolog-1. Mol Cell Endocrinol. 2011;336(1-2):127–132. [DOI] [PubMed] [Google Scholar]
- 54. Jadhav U, Jameson JL. Steroidogenic factor-1 (SF-1)-driven differentiation of murine embryonic stem (ES) cells into a gonadal lineage. Endocrinology. 2011;152(7):2870–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang Y, Su Z, Xu W, et al. Directed mouse embryonic stem cells into leydig-like cells rescue testosterone-deficient male rats in vivo. Stem Cells Dev. 2015;24(4):459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yang Y, Li Z, Wu X, et al. Direct Reprogramming of Mouse Fibroblasts toward Leydig-like Cells by Defined Factors. Stem Cell Reports. 2017;8(1):39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Arora H, Zuttion MSSR, Nahar B, Lamb D, Hare JM, Ramasamy R. Subcutaneous Leydig Stem Cell Autograft: A Promising Strategy to Increase Serum Testosterone. Stem Cells Transl Med. 2019;8(1):58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yang C, Du YK, Wang J, et al. Transplanted Adipose-Derived Stem Cells Ameliorate Testicular Dysfunction In A D-Galactose-Induced Aging Rat Model. J Cell Physiol. 2015;230(10):2403–2414. [DOI] [PubMed] [Google Scholar]
- 59. Zhang ZY, Xing XY, Ju GQ, Zhong L, Sun J. Mesenchymal stem cells from human umbilical cord ameliorate testicular dysfunction in a male rat hypogonadism model. Asian J Androl. 2017;19(5):543–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lue Y, Erkkila K, Liu PY, et al. Fate of bone marrow stem cells transplanted into the testis: potential implication for men with testicular failure. Am J Pathol. 2007;170(3):899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tanaka T, Gondo S, Okabe T, et al. Steroidogenic factor 1/adrenal 4 binding protein transforms human bone marrow mesenchymal cells into steroidogenic cells. J Mol Endocrinol. 2007;39(5):343–350. [DOI] [PubMed] [Google Scholar]
- 62. Yazawa T, Inaoka Y, Okada R, et al. PPAR-gamma coactivator-1alpha regulates progesterone production in ovarian granulosa cells with SF-1 and LRH-1. Mol Endocrinol. 2010;24(3):485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sonoyama T, Sone M, Honda K, et al. Differentiation of human embryonic stem cells and human induced pluripotent stem cells into steroid-producing cells. Endocrinology. 2012;153(9):4336–4345. [DOI] [PubMed] [Google Scholar]
- 64. Xing X, Zhang Z, Zhong L, et al. Differentiation of human umbilical cord mesenchymal stem cells into steroidogenic cells in vitro. Exp Ther Med. 2016;12(6):3527–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hou L, Dong Q, Wu YJ, Sun YX, Guo YY, Huo YH. Gonadotropins facilitate potential differentiation of human bone marrow mesenchymal stem cells into Leydig cells in vitro. Kaohsiung J Med Sci. 2016;32(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hou YP, Zhang ZY, Xing XY, Zhou J, Sun J. Direct conversion of human fibroblasts into functional Leydig-like cells by SF-1, GATA4 and NGFI-B. Am J Transl Res. 2018;10(1):175–183. [PMC free article] [PubMed] [Google Scholar]
- 67. Huang H, Zou X, Zhong L, et al. CRISPR/dCas9-mediated activation of multiple endogenous target genes directly converts human foreskin fibroblasts into Leydig-like cells. J Cell Mol Med. 2019. Jul 2. doi: 10.1111/jcmm.14470. [Epub ahead of print]. PMID: 31264792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhou J, Hou Y, Zhang Z, et al. Conversion of human fibroblasts into functional Leydig-like cells by small molecules and a single factor. Biochem Biophys Res Commun. 2019;516(1):1–7. [DOI] [PubMed] [Google Scholar]