Abstract
Emerging evidence suggests that atrial fibrillation is associated with cognitive dysfunction independently of stroke, but the underlying mechanisms remain unclear. In this cross-sectional analysis from the Swiss-atrial fibrillation Study (NCT02105844), we investigated the association of serum neurofilament light protein, a neuronal injury biomarker, with (i) the CHA2DS2-VASc score (congestive heart failure, hypertension, age 65–74 or >75 years, diabetes mellitus, stroke or transient ischaemic attack, vascular disease, sex), clinical and neuroimaging parameters and (ii) cognitive measures in atrial fibrillation patients. We measured neurofilament light in serum using an ultrasensitive single-molecule array assay in a sample of 1379 atrial fibrillation patients (mean age, 72 years; female, 27%). Ischaemic infarcts, small vessel disease markers and normalized brain volume were assessed on brain MRI. Cognitive testing included the Montreal cognitive assessment, trail-making test, semantic verbal fluency and digit symbol substitution test, which were summarized using principal component analysis. Results were analysed using univariable and multivariable linear regression. Neurofilament light was associated with the CHA2DS2-VASc score, with an average 19.2% [95% confidence interval (17.2%, 21.3%)] higher neurofilament per unit CHA2DS2-VASc increase. This association persisted after adjustment for age and MRI characteristics. In multivariable analyses, clinical parameters associated with neurofilament light were higher age [32.5% (27.2%, 38%) neurofilament increase per 10 years], diabetes mellitus, heart failure and peripheral artery disease [26.8% (16.8%, 37.6%), 15.7% (8.1%, 23.9%) and 19.5% (6.8%, 33.7%) higher neurofilament, respectively]. Mean arterial pressure showed a curvilinear association with neurofilament, with evidence for both an inverse linear and a U-shaped association. MRI characteristics associated with neurofilament were white matter lesion volume and volume of large non-cortical or cortical infarcts [4.3% (1.8%, 6.8%) and 5.5% (2.5%, 8.7%) neurofilament increase per unit increase in log-volume of the respective lesion], as well as normalized brain volume [4.9% (1.7%, 8.1%) higher neurofilament per 100 cm3 smaller brain volume]. Neurofilament light was inversely associated with all cognitive measures in univariable analyses. The effect sizes diminished after adjusting for clinical and MRI variables, but the association with the first principal component was still evident. Our results suggest that in atrial fibrillation patients, neuronal loss measured by serum neurofilament light is associated with age, diabetes mellitus, heart failure, blood pressure and vascular brain lesions, and inversely correlates with normalized brain volume and cognitive function.
Keywords: neurofilament light, atrial fibrillation, vascular brain lesions, cognition
In patients with atrial fibrillation, we show that age, diabetes, heart failure, peripheral artery disease and subclinical ischaemic brain lesions are associated with serum levels of neurofilament light chain, a biomarker of neuronal loss. Higher neurofilament levels were associated with smaller brain volume and worse cognitive performance.
Graphical Abstract
Graphical Abstract.
Introduction
Atrial fibrillation (AF) and dementia are highly prevalent in the elderly. Atrial fibrillation is linked to dementia through ischaemic stroke, but evidence has emerged that even in the absence of clinically manifest stroke, the risk of cognitive impairment and dementia is increased in patients with AF (Chen et al., 2018; Madhavan et al., 2018; Kim et al., 2019). Several potential mechanisms have been postulated to explain this association, including silent cerebral infarcts, cerebral small vessel disease (through shared risk factors such as diabetes and hypertension) and cerebral hypoperfusion (Madhavan et al., 2018; Diener et al., 2019), but tangible evidence is lacking. With the progressive ageing of the population, AF and dementia are a continuously growing public health concern, and a deeper understanding of the pathophysiological pathways underlying their association will be crucial in developing strategies to preserve cognitive function in the elderly (Kuhne et al., 2019).
In a cross-sectional analysis from the Swiss Atrial Fibrillation (Swiss-AF) cohort study, we previously showed that cortical and large non-cortical infarcts were common in AF patients and were independently associated with a lower score on the Montreal Cognitive Assessment (MoCA), lending support to the hypothesis that cognitive dysfunction in AF might—at least in part—be mediated through covert cerebral embolic infarcts (Conen et al., 2019).
Here, we used serum neurofilament light protein (sNfL) to further explore the mechanisms that underly neuronal damage and cognitive dysfunction in AF. Neurofilaments are neuron-exclusive cytoskeletal proteins that are released in the extracellular space, cerebrospinal fluid and eventually peripheral blood after neuroaxonal damage. sNfL has emerged as a biomarker for neuronal injury in inflammatory, degenerative, traumatic and vascular neurological disorders (Khalil et al., 2018), but has not yet been investigated as a marker of neurological disease in AF. In this analysis from the Swiss-AF cohort study, we investigated the association of sNfL with (i) clinical parameters and neuroimaging characteristics and (ii) measures of cognitive function.
Materials and methods
Study design, patient population and data collection
This was a cross-sectional analysis using baseline data from the ongoing prospective observational Swiss-AF cohort study (NCT02105844) that enrolled 2415 patients with AF between 2014 and 2017 across 14 centres in Switzerland. The detailed methodology of Swiss-AF has been described previously (Conen et al., 2017, 2019). In short, Swiss-AF included patients with documented AF aged 65 years or older, with an additional 15% of patients aged between 45 and 65 years. Patients with secondary forms of AF, those with a recent ischaemic stroke, transient ischaemic attack (TIA) or other acute illness (<4 weeks) and those unable to provide informed consent (e.g. patients with dementia, psychosis or delirium) were excluded. Baseline information on sociodemographic parameters and comorbidities was collected based on patients’ history and/or medical chart review as applicable, using standardized case report forms. Upon inclusion, weight, height and the mean of three consecutive blood pressure measurements were obtained, and patients underwent blood sampling, brain MRI and cognitive testing.
Baseline blood samples were collected following standard operating procedures (Conen et al., 2017). After centrifugation, serum samples were aliquoted into cryotubes and stored at −80°C in a centralized biobank. The concentrations of sNfL were measured in duplicate using a previously described ultrasensitive single‐molecule array assay (Disanto et al., 2017). Inter-assay coefficients of variation were 10% for low (mean, 6.9 pg/mL), 12% for medium (mean, 19.6 pg/mL) and 5% for high (mean, 84.5 pg/mL) concentration quality control serum samples measured in duplicate in every run. The mean intra-assay coefficient of variation of duplicate determinations for concentration was 5%. Individuals performing sNfL measurements were blinded to clinical, MRI and cognitive patient data.
Baseline brain MRI was acquired on a 1.5 or 3.0 Tesla scanner using a standardized protocol including a 3D T1-weighted magnetization-prepared rapid gradient echo (MPRAGE), a 2D axial fluid-attenuated inversion recovery (FLAIR), a 2D axial diffusion-weighted imaging (DWI) and a 2D axial susceptibility-weighted imaging (SWI) or T2*-weighted sequence (Conen et al., 2017, 2019). All scans were analysed centrally in a core lab (Medical Image Analysis Center AG, Basel, Switzerland) by expert raters blinded to clinical and cognitive patients’ data and measurements of sNfL. We evaluated the following vascular brain lesions, which we defined adapting the Standards for reporting vascular changes on neuroimaging classification of small vessel disease (Wardlaw et al., 2013), as in previous research (Conen et al., 2019): (i) small non-cortical infarcts (SNCIs), defined as hyperintense lesions on FLAIR, ≤20 mm in diameter on axial sections and not involving the cortex, consistent with ischaemic infarction in the territory of a perforating arteriole and located in the white matter, internal or external capsule, deep brain nuclei, thalamus or brainstem. (ii) Large non-cortical infarcts were non-cortical infarcts with a diameter of >20 mm. Cortical infarcts were defined as FLAIR hyperintense lesions involving the cortex irrespective of their size and whether they also involved subcortical areas. Large non-cortical and cortical infarcts (LNCCIs) were grouped together in the analyses. All infarcts were characterized as recent (hyperintense) or chronic according to their appearance on DWI. (iii) FLAIR hyperintensities not meeting the aforementioned criteria for infarcts were identified as white matter lesions (WMLs). (iv) Microbleeds (MBs) were identified and counted as nodular, strongly hypointense lesions on either SWI or T2*-weighted sequences. T2-weighted volumes of SNCIs, LNCCIs and WMLs were segmented and quantified semi-automatically in mm3 using Amira (Mercury Computer Systems Inc., Chelmsford, MA, USA). Lesions with a central FLAIR hypointense core were segmented in total without differentiating between hyperintense and hypointense lesion areas. The normalized brain volume (nBV) was estimated in cm3 on MPRAGE using SIENAX (Smith et al., 2002).
Cognitive testing was performed by trained study personnel in a standardized manner and included:
The MoCA, which assesses visuospatial and executive functions, confrontation naming, memory, attention, language and abstraction. Patients could obtain a maximum of 30 points, with higher scores indicating better cognitive function. One point was added to the test score if the patient had ≤12 years of formal education (Nasreddine et al., 2005).
The Trail Making test (TMT), which assesses visual attention, processing speed and executive functioning. It consists of two parts (A and B), in which the patient was instructed to connect a set of 25 points, either circled numbers in ascending order (TMT-A) or circled numbers and letters in alternating numeric and alphabetic ascending order (TMT-B), as quickly as possible while maintaining accuracy. The number of correct connections and the time to test completion in seconds were measured, with a maximum allowed time of 180 and 300 s for TMT-A and TMT-B, respectively. The test metric was the number of correct answers per second, with higher scores indicating better cognitive function (Tombaugh, 2004).
Semantic Verbal Fluency (SVF), which assesses semantic memory and language production. Patients were asked to name as many words as possible from the semantic category ‘animals’ within 60 s. The test metric was the number or correct responses, with higher scores indicating better cognitive function (Morris et al., 1989).
The Digit Symbol Substitution Test (DSST) of the Wechsler Adult Intelligence Scale, which assesses processing speed, visuomotor coordination and attention. Patients received a key grid of numbers and matching symbols and a test section with numbers and empty boxes. The test consisted of filling as many empty boxes as possible with the matching symbol from the key grid. The score was the number of correct number–symbol matches achieved within 120 s, with higher scores indicating better cognitive function (Petermann, 2011).
The detailed patients’ flowchart for the analyses of this study is shown in Supplementary Fig. 1. We included all Swiss-AF patients with quantifiable sNfL measurement and complete MRI data and excluded those with recent subclinical ischaemic infarcts on DWI (which would expectedly raise the concentrations of sNfL disproportionately (Gattringer et al., 2017; De Marchis et al., 2018; Tiedt et al., 2018)). The Ethics Committee of Northwest and Central Switzerland approved Swiss-AF including this study (PB_2016-00793). Written informed consent was obtained from all study participants according to the Declaration of Helsinki. This study was conducted in accordance with the STROBE Statement for cross-sectional studies (von Elm et al., 2007).
Statistical analyses
Analysis A: association of the CHA2DS2-VASc score, clinical and MRI characteristics with sNfL
To investigate the association of patients’ characteristics with sNfL, we fitted uni- and multivariable linear regression models with various sets of clinical and MRI variables as independent variables and the log-transformed sNfL concentration as dependent variable. Continuous independent variables were centred on their mean (or, in case of skewed data, median) values. We report the back-transformed model-based estimates, which represent multiplicative effects on the geometric mean of sNfL and are denoted by βmult (so that a one-unit increase in the independent variable is associated with an average βmult-fold change in sNfL), along with 95% confidence intervals (CI) and two-sided P-values. We interpret P-values as a continuous measure, with smaller values indicating stronger evidence for an association, but without specifying a threshold value. To compare between models, we used the Akaike’s information criterion (AIC), which estimates the relative quality of different models fitted to a given dataset, while penalizing models for larger number of independent variables. Lower AIC values indicate a better fit. Additionally, we provide the coefficient of determination (R2) of each model as a measure of the proportion of the observed sNfL variance explained by the model. Since R2 tends to increase with the number of independent variables, we also provide the adjusted R2 (), which penalizes R2 for larger numbers of variables. We fitted the following predefined models with log-sNfL as the dependent variable:
The CHA2DS2-VASc score (congestive heart failure, hypertension, age 65–74 or ≥75 years, diabetes mellitus, stroke or TIA, vascular disease, sex) models: CHA2DS2-VASc is a validated clinical score predicting stroke risk in AF patients (Lip et al., 2010; Friberg et al., 2012). We opted to first investigate the association of sNfL with this risk score as a whole, independent of its individual components. Given the known association of sNfL with age (Khalil et al., 2018), we fitted univariable models for the association of sNfL with age, with the CHA2DS2-VASc score and with the CHA2DS2-VASc score after exclusion of its age component. Additionally, we fitted bivariable models for the age-adjusted association of sNfL with the CHA2DS2-VASc score and with the CHA2DS2-VASc score after exclusion of its age component. We selected the best fitting CHA2DS2-VASc score model based on AIC values, and proceeded to further adjust it for MRI markers of small vessel disease (SNCIs, MBs and WMLs) (Wardlaw et al., 2013) as well as for all MRI variables, as detailed below, in two additional multivariable models.
The clinical model: We fitted a multivariable model for the association of sNfL with the following predefined clinical variables: age, sex, history of hypertension, diabetes mellitus, stroke or TIA, coronary heart disease, peripheral artery disease (PAD), heart failure, obstructive sleep apnoea, AF type (paroxysmal, persistent and permanent), body mass index (BMI, calculated as weight in kg/height in m2), smoking status (active, past, non-smoker), alcohol consumption (number of standard drinks daily) and mean arterial pressure [MAP, calculated as 1/3 × systolic blood pressure + 2/3 × diastolic blood pressure (DeMers and Wachs, 2019)]. We opted to use the MAP instead of including both systolic and diastolic blood pressure in the models due to collinearity between those variables. There was no evidence of collinearity upon visual inspection of scatter plots for any of the other continuous clinical variables, but there was evidence for a curvilinear association between sNfL and MAP, which we modelled by introducing an additional quadratic term (MAP2). We reduced the clinical model to a smaller set of variables via stepwise backward elimination based on AIC values. We imputed the few missing values in the clinical variables with simple single imputation, using the mode (i.e., the most common category) for categorical variables and the mean (or, in case of skewed data, the median) for continuous variables (Table 1; one missing value in smoking status and alcohol consumption, imputed with ‘past’ and 0.6 standard drinks daily, respectively; six missing values in systolic and diastolic blood pressure, imputed with 134.7 and 78.4 mmHg, respectively).
The MRI model: We fitted a multivariable model for the age-adjusted association of sNfL with the following predefined MRI variables: nBV, SNCIs’ presence and log-transformed volume, LNCCIs’ presence and log-volume, MBs’ presence and count (truncated at 20 to reduce the influence of outliers) and WMLs’ log-volume.
The combined clinical and MRI model: We fitted a final combined model for the association of sNfL with the chosen clinical and all MRI variables from the models ii and iii.
Table 1.
Patient demographic, clinical and MRI characteristics
| All patients (N = 1379) |
Patients without stroke/TIA (N = 1125) | ||
|---|---|---|---|
| Demographic and clinical data | Missing values rate (%) | ||
| Age, years, mean (SD) | 72.3 (8.6) | 0 | 71.7 (8.8) |
| Sex, female, N (%) | 374 (27.1) | 0 | 297 (26.4) |
| AF type, N (%) | 0 | ||
| Paroxysmal | 636 (46.1) | 510 (45.3) | |
| Persistent | 408 (29.6) | 349 (31.0) | |
| Permanent | 335 (24.3) | 266 (23.6) | |
| History of | |||
| Hypertension, N (%) | 930 (67.4) | 0 | 741 (65.9) |
| Diabetes mellitus, N (%) | 190 (13.8) | 0 | 146 (13.0) |
| Stroke or transient ischaemic attack, N (%) | 254 (18.4) | 0 | 0 (0) |
| Coronary heart disease, N (%) | 364 (26.4) | 0 | 292 (26.0) |
| Peripheral artery disease, N (%) | 87 (6.3) | 0 | 64 (5.7) |
| Heart failure, N (%) | 297 (21.5) | 0 | 238 (21.2) |
| Obstructive sleep apnoea, N (%) | 171 (12.4) | 0 | 128 (11.4) |
| CHA2DS2-VASc score, median (IQR) | 3 (2–4) | 0 | 3 (2–4) |
| Smoking status, N (%) | 0.1 | ||
| Non-smoker | 603 (43.8) | 488 (43.4) | |
| Past smoker | 671 (48.7) | 553 (49.2) | |
| Active smoker | 104 (7.5) | 84 (7.5) | |
| Alcohol consumption, standard drinks/day, median (IQR) | 0.6 (0.1–1.3) | 0.1 | 0.6 (0.1–1.3) |
| Education level, N (%) | 0.1 | ||
| Basic | 157 (11.4) | 128 (11.4) | |
| Middle | 679 (49.3) | 555 (49.3) | |
| Advanced | 541 (39.3) | 442 (39.3) | |
| Body mass index (kg/m2), mean (SD) | 27.5 (4.6) | 0 | 27.6 (4.7) |
| Systolic blood pressure (mmHg) mean (SD) | 134.7 (18.7) | 0.4 | 134.7 (18.6) |
| Diastolic blood pressure (mmHg ) mean (SD) | 78.4 (11.9) | 0.4 | 78.7 (11.9) |
| Mean arterial pressure (mmHg) mean (SD) | 97.2 (12.6) | 0.4 | 97.3 (12.7) |
| Oral anticoagulation, N (%) | 1240 (89.9) | 0 | 1,004 (89.2) |
| MRI data | |||
| Small non-cortical infarcts, N (%) | 293 (21.2) | 0 | 200 (17.8) |
| Volume (if present) (mm3), median (IQR) | 60 (30–150) | 56 (30–123) | |
| Large non-cortical and cortical infarcts, N (%) | 288 (20.9) | 0 | 153 (13.6) |
| Volume (if present) (mm3), median (IQR) | 1374 (252–7454) | 585 (162–4002) | |
| White matter lesions, N (%) | 1368 (99.2) | 0 | 1116 (99.2) |
| Volume (if present) (mm3) median (IQR) | 3662 (1350–9197) | 3335 (1224–8252) | |
|
Microbleeds, N (%) Count (if present), median (IQR) |
291 (21.1) | 0 | 220 (19.6) |
| 1 (1–2) | 1 (1–2) | ||
| Normalized brain volume (cm3), median (IQR) | 1411 (1354–1478) | 0 | 1417 (1358–1487) |
SD, standard deviation; IQR, interquartile range.
Analysis B: association of sNfL with measures of cognitive function
To investigate the association of sNfL with cognitive function, we fitted linear regression models with the score of each of the cognitive tests (MoCA, TMT-A, TMT-B, SVF and DSST) as the dependent variable and the log-transformed sNfL concentration as independent variable. We report the model-based estimates, which represent additive effects on the mean of the test score and are denoted by β, along with the 95% CI and two-sided P-values. A one-unit increase in log-sNfL is associated with an average change in the test score of β units (or a 10% increase in sNfL is associated with a change of 0.095 × β units in the test score). For each cognitive test, we fitted the following predefined models with test score as the dependent variable:
univariable model (including only log-sNfL as independent variable);
age-adjusted model (including log-sNfL and age as independent variables);
clinical multivariable model, including log-sNfL, age, sex, education level (basic, middle and advanced), history of hypertension, diabetes mellitus, stroke or TIA, coronary heart disease, PAD, heart failure, obstructive sleep apnoea, BMI, smoking status (active, past, non-smoker) and alcohol consumption (number of standard drinks daily) as independent variables. We imputed the few missing values in the clinical variables with simple single imputation, as described above.
MRI multivariable model, including log-sNfL, age, nBV, SNCIs’ presence and log-volume, LNCCIs’ presence and log-volume, burden of MBs (categorized as 0, 1, 2 and ≥3) and WMLs’ log-volume as independent variables;
combined clinical and MRI multivariable model, including all aforementioned variables.
To summarize performance over all cognitive tests, we used principal component analysis. The first principal component (PC1) explained 61.3% of the observed variance and the loading of each test on PC1 (representing the covariance between each test and PC1) was positive (MoCA: +0.40, TMT-A: +0.45, TMT-B: +0.50, SVF: +0.39, DSST: +0.49), thereby allowing for the use of PC1 as a single, summary measure of cognitive function, with higher values indicating better cognitive performance. We additionally fitted all the above-described models i–v with PC1 as the dependent variable.
As a sensitivity analysis, we repeated all models described under analyses A and B in the subset of patients without history of stroke or TIA.
All analyses were performed with R version 3.5.2 (2018-12-20).
Data availability
The Swiss-AF consent forms, as approved by the ethics committee, do not allow for the data to be made publicly available. Researchers may contact the authors for the potential submission of research proposals for future analyses or independent verification of our results.
Results
A total of 1379 patients [mean (SD) age, 72.3 (8.6) years, 27.1% female] with quantifiable sNfL measurement and complete MRI data were available for analysis A (Supplementary Fig. 1). The median (IQR) sNfL concentration was 38.2 (26.6–56.4) pg/ml. The detailed demographic, clinical and MRI characteristics of all patients are summarized in Table 1.
Association of the CHA2DS2-VASc score with sNfL
The CHA2DS2-VASc score was associated with sNfL in univariable analysis, with an average 19.2% increase in sNfL concentration per point increase in the CHA2DS2-VASc score [βmult = 1.192, 95% CI (1.172, 1.213), P < 0.001; Fig. 1]. Age was also strongly associated with sNfL in univariable analysis [βmult = 1.489 per 10 years, 95% CI (1.440, 1.539), P < 0.001]. The association between the CHA2DS2-VASc score and sNfL persisted after excluding the age component of the score, after adjusting for age and after both excluding the age component and adjusting for age. The model with the best fit was the one including the unmodified CHA2DS2-VASc score and adjusting for age, which was used in the rest of the analyses. The CHA2DS2-VASc score remained associated with sNfL after adjusting for MRI markers of small vessel disease and for all MRI variables combined (Supplementary Table 1).
Figure 1.
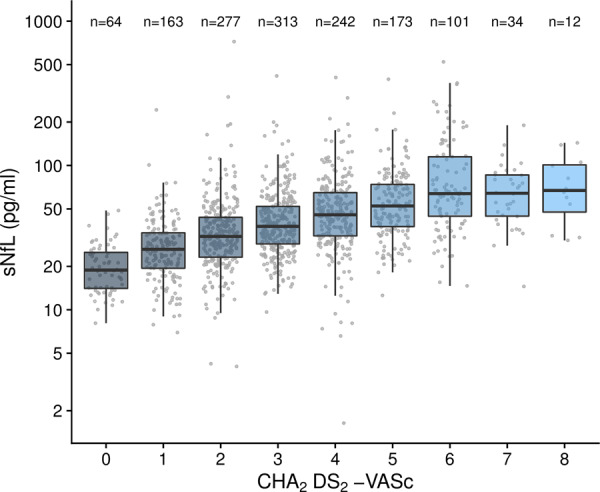
Boxplots of sNfL distribution stratified to CHA2DS2-VASc score.
Association of clinical and MRI characteristics with sNfL
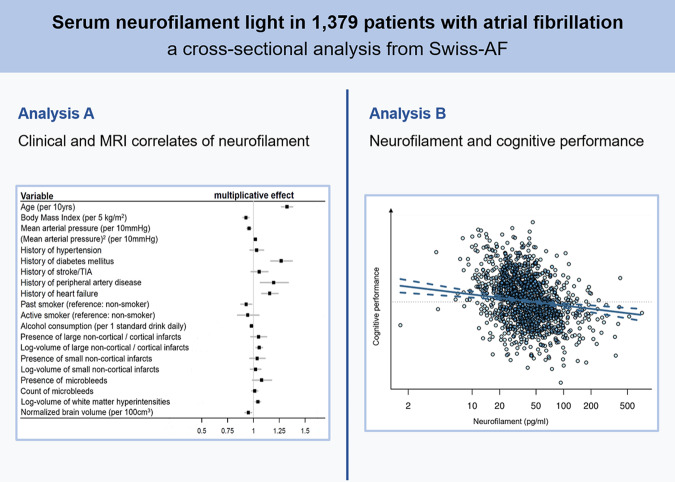
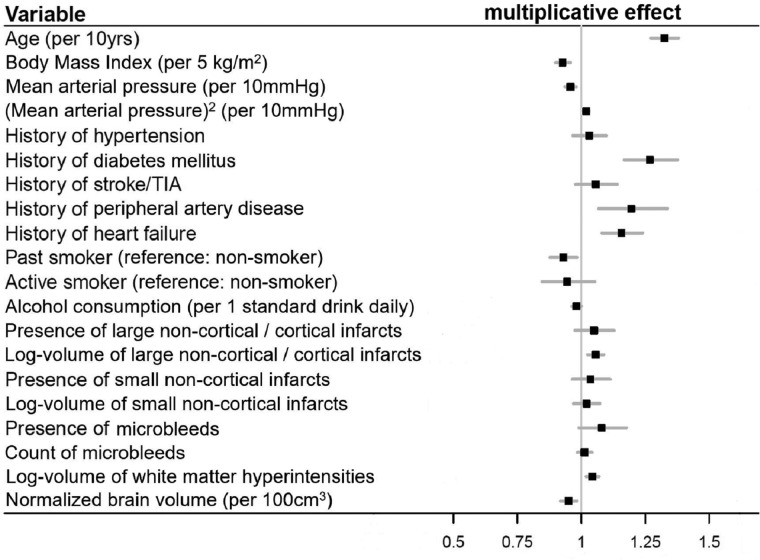
The detailed results of the clinical, MRI and combined models are summarized in Table 2. The clinical model fitted the data better and explained a larger proportion of the observed sNfL variance compared to the MRI model. The combined clinical and MRI model fitted the data best. Figure 2 shows the effect size estimates for the association of all clinical and MRI variables with sNfL from the combined model: Parameters positively associated with sNfL were age (on average 32.5% higher sNfL per 10 years), history of diabetes mellitus (26.8% higher sNfL), PAD (19.5% higher sNfL) and heart failure (15.7% higher sNfL), as well as volume of LNCCIs and WMLs (5.5% and 4.3% higher sNfL per unit increase in log-volume of the respective lesion). Mean arterial pressure showed a curvilinear association with sNfL, with an inverse linear and U-shaped component (Fig. 3A). Parameters inversely associated with sNfL were BMI (7.3% lower sNfL per 5 kg/m2 higher BMI), past smoker status (7% lower sNfL compared to non-smoker), alcohol consumption (1.9% lower sNfL per 1 standard drink daily) and nBV (4.9% lower sNfL per 100 cm3 larger nBV; Fig. 3B).
Table 2.
Association of patients’ clinical and MRI characteristics with sNfL
| Variables (N = 1379) | Clinical modela AIC = 2079.05 R2 = 0.36, = 0.36 |
MRI model AIC = 2148.29 R2 = 0.33, = 0.32 |
Combined model AIC = 2040.56 R2 = 0.39, = 0.38 |
|||
|---|---|---|---|---|---|---|
| β mult b (95% CI) | P-value | β mult b (95% CI) | P-value | β mult b (95% CI) | P-value | |
| Age (per 10 years) |
1.411 (1.365, 1.460) |
<0.001 |
1.367 (1.312, 1.424) |
<0.001 |
1.325 (1.272, 1.380) |
<0.001 |
| BMI (per 5 kg/m2) |
0.925 (0.897, 0.955) |
<0.001 |
0.927 (0.899, 0.957) |
<0.001 | ||
| MAP (per 10 mmHg) |
0.961 (0.939, 0.983) |
<0.001 |
0.958 (0.937, 0.980) |
<0.001 | ||
| MAP2 (per 10 mmHg) |
1.019 (1.008, 1.031) |
0.001 |
1.019 (1.007, 1.030) |
0.002 | ||
| History of hypertension |
1.068 (1.003, 1.138) |
0.042 |
1.030 (0.967, 1.098) |
0.351 | ||
| History of diabetes mellitus |
1.283 (1.181, 1.394) |
<.001 |
1.268 (1.168, 1.376) |
<0.001 | ||
| History of stroke or TIA |
1.137 (1.059, 1.220) |
<0.001 |
1.056 (0.978, 1.141) |
0.166 | ||
| History of peripheral artery disease |
1.231 (1.098, 1.380) |
<0.001 |
1.195 (1.068, 1.337) |
0.002 | ||
| History of heart failure |
1.181 (1.102, 1.266) |
<0.001 |
1.157 (1.081, 1.239) |
<0.001 | ||
| Past smoker (ref: non-smoker) |
0.925 (0.873, 0.980) |
0.008 |
0.930 (0.878, 0.984) |
0.012 | ||
| Active smoker (ref: non-smoker) |
0.950 (0.850, 1.060) |
0.358 |
0.944 (0.847, 1.053) |
0.301 | ||
| Alcohol consumption (per 1 standard drink daily) |
0.984 (0.965, 1.002) |
0.086 |
0.981 (0.963, 1.000) |
0.045 | ||
| Presence of LNCCIs |
1.100 (1.025, 1.180) |
0.008 |
1.049 (0.975, 1.128) |
0.199 | ||
| Log-volume of LNCCIs |
1.066 (1.035, 1.099) |
<0.001 |
1.055 (1.025, 1.087) |
<0.001 | ||
| Presence of SNCIs |
1.055 (0.980, 1.136) |
0.156 |
1.036 (0.965, 1.113) |
0.330 | ||
| Log-volume of SNCIs |
1.030 (0.978, 1.085) |
0.262 |
1.020 (0.970, 1.072) |
0.442 | ||
| Presence of MBs |
1.104 (1.011, 1.207) |
0.028 |
1.079 (0.991, 1.176) |
0.079 | ||
| Count of MBs |
1.017 (0.987, 1.047) |
0.266 |
1.013 (0.985, 1.042) |
0.372 | ||
| Log-volume of WMLs |
1.045 (1.019, 1.071) |
<0.001 |
1.043 (1.018, 1.068) |
<0.001 | ||
| nBV (per 100 cm3) |
0.945 (0.914, 0.978) |
0.001 |
0.951 (0.919, 0.983) |
0.003 | ||
AIC, Akaike’s information criterion; BMI, body mass index; MAP, mean arterial pressure; TIA, transient ischaemic attack; LNCCIs, large non-cortical or cortical infarcts; SNCIs, small non-cortical infarcts; MBs, micro-bleeds; WMLs, white-matter lesions; nBV, normalized brain volume.
Sex, atrial fibrillation type, history of coronary heart disease and obstructive sleep apnoea were eliminated from the final, reduced clinical model.
The back-transformed model-based estimates βmult represent multiplicative effects on sNfL (e.g. βmult = 1.325 for age denotes an average 1.325-fold increase in sNfL concentration, that is an average 32.5% sNfL increase, per 10 years older age).
Figure 2.
Multiplicative effect sizes of the association of clinical and MRI variables with sNfL from the combined model.
Figure 3.
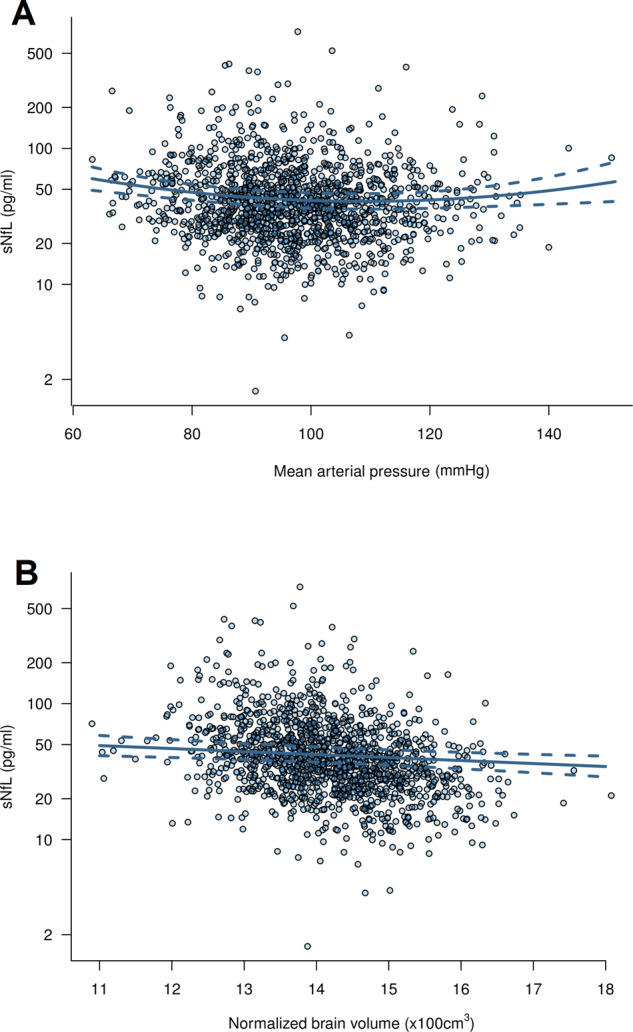
Scatter plot of the association of (A) mean arterial pressure and (B) normalized brain volume with sNfL. The solid line represents the predicted values from the combined clinical and MRI model and the dashed lines represent the 95% pointwise confidence intervals.
Association of sNfL with measures of cognitive function
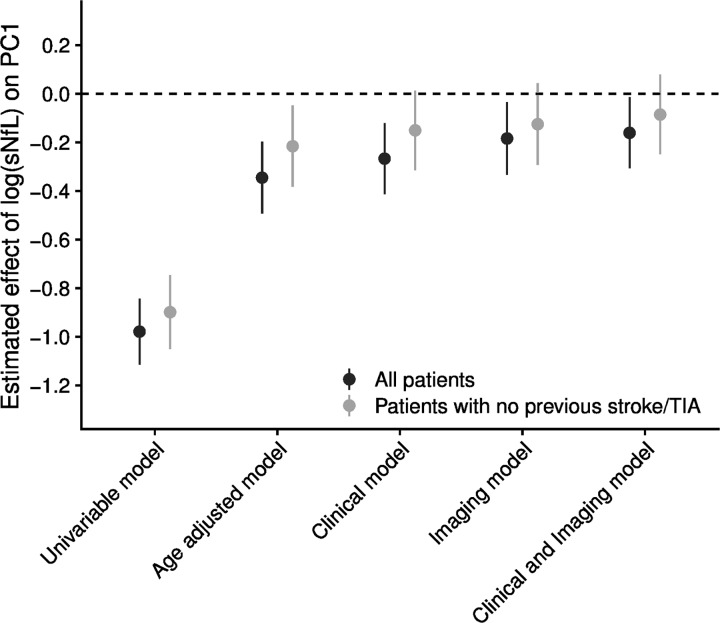
Of the 1379 patients with sNfL and MRI data, cognitive testing was incomplete in 16, leaving 1363 patients available for analysis B. The median (IQR) MoCA score was 26 (24–28) points, TMT-A and TMT-B scores were 0.53 (0.39–0.68) and 0.21 (0.14–0.28) correct connections per second, respectively, SVF score was 19 (15–23) correct responses and DSST score was 45 (36–54) correct matches. The detailed results of all models for the association of sNfL with all cognitive measures are summarized in Table 3. In univariable analyses, log-sNfL was strongly associated with all cognitive tests and the PC1, with higher sNfL concentrations indicating worse cognitive performance. The effect sizes generally diminished for all cognitive measures after adjusting for age, clinical variables and MRI characteristics. In the combined models adjusting for all aforementioned parameters, the association of log-sNfL with TMT-A, TMT-B and PC1 persisted. Figure 4 shows the model-based estimates for the association of sNfL with PC1. The scatter plot of the association of sNfL with PC1 is shown in Fig. 5.
Table 3.
Association of log-sNfL with measures of cognitive function
| Cognitive measures (N = 1363) | Univariable |
Age-adjusted |
Multivariable clinical model
a
|
Multivariable MRI model
b
|
Multivariable combined model
c
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| β d (95% CI) | P-value | β d (95% CI) | P-value | β d (95% CI) | P-value | β d (95% CI) | P-value | β d (95% CI) | P-value | |
| MoCA |
−0.93 (−1.17, −0.69) |
<0.001 |
−0.37 (−0.65, −0.10) |
0.008 |
−0.23 (−0.51, 0.05) |
0.114 |
−0.22 (−0.51, 0.06) |
0.123 |
−0.15 (−0.44, 0.14) |
0.307 |
| TMT-A |
−0.11 (−0.13, −0.10) |
<0.001 |
−0.04 (−0.06, −0.02) |
<0.001 |
−0.04 (−0.06, −0.02) |
<0.001 |
−0.03 (−0.04, −0.01) |
0.009 |
−0.03 (−0.04, −0.01) |
0.012 |
| TMT-B |
−0.06 (−0.07, −0.05) |
<0.001 |
−0.02 (−0.03, −0.01) |
<0.001 |
−0.02 (−0.03, −0.01) |
<0.001 |
−0.01 (−0.02, −0.00) |
0.015 |
−0.01 (−0.02, −0.00) |
0.023 |
| SVF |
−1.56 (−2.00, −1.12) |
<0.001 |
−0.46 (−0.97, 0.05) |
0.076 |
−0.29 (−0.81, 0.24) |
0.283 |
−0.14 (−0.67, 0.38) |
0.587 |
−0.09 (−0.62, 0.45) |
0.752 |
| DSST |
−6.95 (−8.07, −5.83) |
<0.001 |
−2.40 (−3.64, −1.16) |
<0.001 |
−1.75 (−2.96, −0.54) |
0.005 |
−0.98 (−2.23, 0.27) |
0.124 |
−0.84 (−2.05, 0.37) |
0.175 |
| PC1 |
−0.98 (−1.11, −0.84) |
<0.001 |
−0.34 (−0.49, −0.20) |
<0.001 |
−0.27 (−0.41, −0.12) |
<0.001 |
−0.18 (−0.33, −0.03) |
0.016 |
−0.16 (−0.31, −0.01) |
0.032 |
MoCA, Montreal Cognitive Assessment; TMT, Trail Making Test; SVF, Semantic Verbal Fluency; DSST, Digit Symbol Substitution Test; PC1, first principal component.
Adjusted for age, sex, education level, history of hypertension, diabetes mellitus, stroke or TIA, coronary heart disease, peripheral artery disease, heart failure, obstructive sleep apnoea, BMI, smoking status and alcohol consumption.
Adjusted for age, normalized brain volume, presence and volume of small non-cortical infarcts, presence and volume of large non-cortical or cortical infarcts, burden of MBs and volume of white matter lesions.
Adjusted for age, sex, education level, history of hypertension, diabetes mellitus, stroke or TIA, coronary heart disease, peripheral artery disease, heart failure, obstructive sleep apnoea, BMI, smoking status, alcohol consumption, normalized brain volume, presence and volume of small non-cortical infarcts, presence and volume of large non-cortical or cortical infarcts, burden of MBs and volume of white matter lesions.
The model-based estimates β represent additive effects on test score (e.g. β = −0.93 for the association of log-sNfL with MoCA denotes an average decrease of 0.93 points in the MoCA score per unit higher log-sNfL, or an average decrease of approx. 0.09 points in the MoCA score per 10% higher sNfL concentration).
Figure 4.
Model-based estimates for the association of log-sNfL with PC1.
Figure 5.
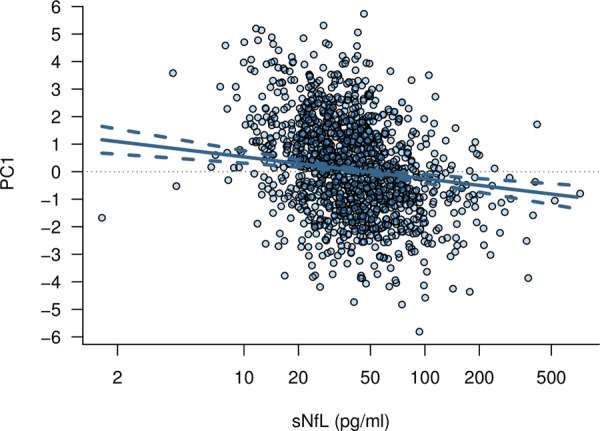
Scatter plot of the age-adjusted association of sNfL with PC1. The solid line represents the model-based predicted values and the dashed lines represent the pointwise 95% confidence intervals.
Sensitivity analyses in patients without history of stroke or transient ischaemic attack
After excluding those with history of stroke or TIA, 1125 patients were available for sensitivity analysis A. Their median (IQR) sNfL concentration was 36.7 (25.5–53.2) pg/ml and their detailed demographic, clinical and MRI characteristics are summarized in Table 1. Of those, 12 patients had incomplete cognitive testing, leaving 1113 patients available for the sensitivity analysis B. Both sensitivity analyses in patients without history of stroke or TIA yielded consistent results with the main analysis (Supplementary Tables 2–4).
Discussion
This cross-sectional study on the clinical, neuroimaging and cognitive correlates of sNfL in a large sample of AF patients showed the following key findings: (i) Higher CHA2DS2-VASc scores indicated increasing neuronal injury, independent of age and vascular brain lesions visible on MRI. (ii) Besides age, clinical factors associated with increased neuronal loss were diabetes mellitus, PAD, heart failure and lower MAP. (iii) MRI characteristics associated with higher sNfL were higher volume of WMLs and LNCCIs, as well as lower nBV. (iv) sNfL was associated with worse cognitive performance, an association which was largely but not exclusively explained by age, comorbidities and vascular brain lesions.
The CHA2DS2-VASc score, a validated clinical score predicting ischaemic stroke risk in AF patients (Lip et al., 2010; Friberg et al., 2012), was associated with sNfL. This was independent of age, history of stroke and ischaemic infarcts visible on MRI, as well as MRI markers of small vessel disease, and might therefore reflect ongoing ischaemic brain injury in AF that evades detection on conventional MRI (Brundel et al., 2012). Higher CHA2DS2-VASc scores have also been previously associated with an increasing risk for dementia in stroke-free AF patients (Kim et al., 2019).
Our study in AF patients confirms the strong independent association of sNfL with age, which has been demonstrated across a wide variety of patient populations and healthy controls, probably reflecting neurodegenerative processes associated with normal ageing (Khalil et al., 2018, 2020). Furthermore, we found that diabetes mellitus was associated with a sNfL increase by a similar magnitude as 10 years of age. In line with this, poor glycemic control was independently associated with sNfL in a previous study on sNfL among diabetics (Korley et al., 2019). The association of diabetes mellitus with sNfL was independent of small vessel disease markers and potentially embolic infarcts on MRI. It remains, therefore, unknown whether this association reflects increasing ischaemic neuronal injury in the presence of diabetes mellitus due to micro-infarcts that go undetected on conventional MRI (Brundel et al., 2012), some other non-ischaemic, diabetes-induced mechanism of neuronal damage in the central nervous system (Malone, 2016) or the potential contribution of diabetic neuropathy in the peripheral nervous system (Mariotto et al., 2018). Peripheral artery disease was another independent determinant of sNfL, even after adjustment for vascular MRI brain lesions, which might again reflect increasing ischaemic brain injury that evades detection on conventional MRI (Brundel et al., 2012) in the presence of manifest atherosclerotic disease and is in line with cumulating evidence for the association of PAD with cognitive dysfunction independently of manifest cerebrovascular disease (Rafnsson et al., 2009).
Interestingly, we found a curvilinear association of MAP with sNfL, with evidence for both an inverse linear and a U-shaped relationship, indicating increasing neuronal loss with lower MAP. This novel finding is in contrast to a previous smaller study in diabetics, which found a positive linear association of systolic blood pressure and no association of diastolic blood pressure with sNfL (Korley et al., 2019). As MAP is a measure of the organ perfusion pressure (DeMers and Wachs, 2019), our finding suggests that neuronal damage in AF may be partly attributable to cerebral hypoperfusion. This was independent of history of heart failure, which was another independent determinant of sNfL, suggesting that hemodynamic changes in AF might adversely affect brain health above and beyond clinically manifest heart failure. Taken together, these findings refine and further support the hypoperfusion hypothesis for the association of AF with cognitive dysfunction (Madhavan et al., 2018; Diener et al., 2019), which has been proposed based on the known associations of cerebral hypoperfusion with dementia (Austin et al., 2011; Wolters et al., 2017; Iadecola et al., 2019), AF with cerebral hypoperfusion (Lavy et al., 1980; Gardarsdottir et al., 2018), and heart failure with cerebral hypoperfusion (Roy et al., 2017) and cognitive dysfunction (Vogels et al., 2007). Of note, recent studies in healthy individuals provide evidence for a U-shaped association of blood pressure with cognitive dysfunction (Lv et al., 2017) and for an inverse association of diastolic blood pressure with white matter disease (Fuhrmann et al., 2019) and cognitive decline (Levine et al., 2019). Putting our findings in this context, the curvilinear association of MAP with sNfL that we observed might not be specific to AF, but rather reflect a universal effect of blood pressure on brain health.
Of all vascular MRI brain lesions, the volume of WMLs and chronic LNCCIs were the strongest independent determinants of sNfL, also after excluding patients with stroke history. This indicates that both small vessel disease and infarcts of potentially embolic origin, even clinically silent ones, contribute to neuronal injury in AF. Our finding is in line with previous research on the association of sNfL with the burden of small vessel disease (Gattringer et al., 2017; Duering et al., 2018; Uphaus et al., 2019) and the size of acute ischaemic infarcts (Gattringer et al., 2017; Tiedt et al., 2018), and might suggest a greater severity of ongoing neurodegenerative processes secondary to ischaemia (Tiedt et al., 2018) or persistent, active microischaemic phenomena in the brain of AF patients with a higher burden of established, embolic or microangiopathic, ischaemic MRI lesions. These findings demonstrate the potential of sNfL as a blood biomarker to select AF patients who would benefit from further MRI investigations to uncover potential subclinical vascular brain disease, considering that mass screening with MRI is not feasible and that sNfL in our cohort appeared to be sensitive to potential mechanisms of brain injury independent of structural changes visualized on MRI. Of note, the presence of MBs was only marginally associated with sNfL after adjustment for other brain lesions, an association that was further weakened in the combined model. Thus, MBs might represent a proxy marker of vascular brain disease and contribute little if any to neuronal injury per se, in line with the previous observations (Akoudad et al., 2016; Conen et al., 2019).
Another major finding was the inverse association of nBV with sNfL, which was independent of age, history of stroke and vascular MRI brain lesions. This association, which has been described in neurological diseases including multiple sclerosis and dementias (Khalil et al., 2018), might reflect an underlying ongoing neurodegenerative process in AF. Indeed, AF has been previously associated with reduced brain volume independent of ischaemic infarcts, with putative explanations being cerebral microinfarcts or hypoperfusion leading to brain atrophy (Stefansdottir et al., 2013; Piers et al., 2016).
Despite the in-depth neuroimaging patients’ characterization, the clinical model still explained a larger proportion of the sNfL variance than the MRI model. We can only speculate on the reasons for this: It is possible that microischaemic, hemodynamic, degenerative or other, yet unknown processes lead to neuronal damage in AF while remaining undetected on conventional MRI.
Finally, sNfL was inversely associated with cognitive performance in patients with AF. This is in line with previous research on the association of sNfL with cognitive measures in patients with small vessel (Duering et al., 2018) and neurodegenerative diseases (Byrne et al., 2017; Mattsson et al., 2017; Lin et al., 2018; van der Ende et al., 2019), and suggests that sNfL is a non-disease-specific marker of neuronal damage resulting in cognitive dysfunction. The association of sNfL with cognitive measures grew markedly weaker after adjusting for age and was further attenuated in the multivariable clinical and MRI models, indicating that a multitude of factors including ageing, comorbidities and vascular brain lesions contribute to or mediate the association of neuronal damage with cognitive dysfunction in AF. Importantly, the association of sNfL with PC1, the summary cognitive measure, persisted after adjustment for all clinical and neuroimaging parameters, suggesting that additional unknown factors might contribute to this association. Among the separate cognitive measures, the strongest associations of sNfL were with TMT-A, TMT-B and—to a lesser extent—DSST, all measures of executive function, processing speed and attention. Preferential changes in these cognitive domains are known to reflect a vascular profile of cognitive dysfunction (O’Brien et al., 2003) and have been previously associated with AF (Nishtala et al., 2018).
The strengths of our study include (i) its large sample size of AF patients with detailed clinical, neuroimaging and cognitive characterization, allowing for adjustment for several confounding factors and thus reducing the risk of spurious findings, (ii) the standardized manner of data acquisition, high rate of data completeness and blinded MRI assessment and sNfL measurement, reducing the risk of bias and (iii) its multicentre design, indicating a certain generalizability of our results, at least within the Caucasian population of central Europe. However, the following limitations must be acknowledged: (i) The study’s cross-sectional design, which allows only for the assessment of association but not causality thereof. (ii) As Swiss-AF included exclusively patients with AF, we did not have a comparison group of patients with other heart diseases or healthy controls. It is, therefore, unknown whether our results are specific to AF. (iii) A large number of patients with Swiss-AF did not undergo brain MRI due to contraindications or claustrophobia and were thus ineligible for this study. It is, therefore, unknown whether our results are generalizable to patients with AF unsuited for brain MRI. (iv) We were not able to adjust our analyses for diseases of the peripheral nervous system, which were not systematically collected in Swiss-AF but might contribute to sNfL (Khalil et al., 2018). (v) Neuroimaging was performed on 1.5 or 3.0 Tesla scanners, which might miss a relevant proportion of microinfarcts compared to higher resolution MRI (van Veluw et al., 2013).
Conclusion
In conclusion, our study demonstrates the potential of sNfL as a tool to explore the mechanisms that underly cognitive dysfunction in AF. It seems likely that neuronal damage in AF results from a complex interplay between subclinical brain ischaemia, altered hemodynamics and neurodegeneration. Serum neurofilament light holds promise not only as an instrument to investigate the intricate mechanisms underlying the heart–brain interactions, but also as a surrogate outcome parameter for brain health and cognitive function in cardiovascular research. In future Swiss-AF analyses, we plan to investigate the prognostic significance of sNfL and other blood-based biomarkers of cardiovascular disease longitudinally with regard to the development of vascular brain lesions, brain atrophy and cognitive dysfunction over time.
Supplementary material
Supplementary material is available at Brain Communications online.
Supplementary Material
Acknowledgements
The authors thank all Swiss-AF investigators, listed in Appendix I, for their contributions in data collection.
Funding
Swiss-AF was supported by grants from the Swiss National Science Foundation (33CS30_148474, 33CS30_177520), the Foundation for Cardiovascular Research Basel and the University of Basel. This study was supported by the Science Funds (Wissenschaftspool), Medical Division of the University Hospital Basel, Switzerland. David Conen holds a McMaster University Department of Medicine Mid-Career Research Award. His work is supported by the Hamilton Health Sciences RFA Strategic Initiative Program.
Competing interests
U.F.: Research grants for the Swiss National Science Foundation, Swiss Heart Foundation, Medtronic; Consultant for Stryker, Medtronic and CSL Behring; G.M.D.M.: support from the Swiss National Science Foundation; Spezialprogramm Nachwuchsförderung Klinische Forschung, University of Basel; Science Funds (Wissenschaftspool) of the University Hospital Basel; Swiss Heart Foundation; Bangerter-Rhyner-Stiftung; Swisslife Jubiläumsstiftung for Medical Research; Swiss Neurological Society; consulting honoraria by Bayer and BMS/Pfizer; speaker honoraria by Bayer and Medtronic; L.H.B.: grants from the Swiss National Science Foundation, University of Basel, Swiss Heart Foundation, Stroke Association, AstraZeneca; consulting and advisory board fees from Amgen, Bayer, Bristol-Myers Squibb, Claret Medical; The other authors have no competing interests to declare.
Glossary
- AF =
atrial fibrillation
- AIC =
Akaike’s information criterion
- BMI =
body mass index
- CI =
confidence interval
- DSST =
Digit Symbol Substitution Test
- FLAIR =
fluid-attenuated inversion recovery
- IQR =
interquartile range
- LNCCIs =
large non-cortical or cortical infarcts
- MAP =
mean arterial pressure
- MBs =
microbleeds
- MoCA =
Montreal Cognitive Assessment
- nBV =
normalized brain volume
- PC1 =
first principal component
- PAD =
peripheral artery disease
- SD =
standard deviation
- SNCIs =
small non-cortical infarcts
- sNfL =
serum neurofilament light
- SVF =
Semantic Verbal Fluency
- TIA =
transient ischaemic attack
- TMT =
Trail Making Test
Appendix I
List of all Swiss-AF investigators according to participating center:
University Hospital Basel and University of Basel: Stefan Osswald, Michael Kühne, Stefanie Aeschbacher, Chloe Auberson, Steffen Blum, Leo Bonati, Selinda Ceylan, David Conen, Simone Doerpfeld, Ceylan Eken, Marc Girod, Peter Hämmerle, Philipp Krisai, Michael Kühne, Christine Meyer-Zürn, Pascal Meyre, Andreas U. Monsch, Christian Müller, Stefan Osswald, Philipp Reddiess, Anne Springer, Fabienne Steiner, Christian Sticherling, Thomas Szucs, Gian Voellmin, Leon Zwimpfer. University Hospital Bern: Nicolas Rodondi, Drahomir Aujesky, Urs Fischer, Juerg Fuhrer, Laurent Roten, Simon Jung, Heinrich Mattle; Luise Adam, Carole Elodie Aubert, Martin Feller, Claudio Schneider, Axel Loewe, Elisavet Moutzouri, Tanja Flückiger, Cindy Groen, Damiana Rakovic, Rylana Wenger, Lukas Ehrsam, Alexandra Nuoffer, Nathalie Schwab. Triemli Hospital Zurich: Andreas Müller, Christopher Beynon, Roger Dillier, Michèle Deubelbeiss, Franz Eberli, Christine Franzini, Isabel Juchli, Claudia Liedtke, Jacqueline Nadler, Thayze Obst, Noreen Tynan, Xiaoye Schneider, Katrin Studerus, Dominik Weishaupt. Cantonal Hospital Baden: Jürg-Hans Beer, Simone Fontana, Silke Kuest, Karin Scheuch, Denise Hischier, Nicole Bonetti, Alexandra Grau, Jonas Villinger, Eva Laube, Philipp Baumgartner, Mark Filipovic, Marcel Frick, Giulia Montrasio, Stefanie Leuenberger, Franziska Rutz. Cardiocentro Lugano: Tiziano Moccetti, Angelo Auricchio, Adriana Anesini, Cristina Camporini, Giulio Conte, Maria Luce Caputo, Francois Regoli. Cantonal Hospital St.Gallen: Peter Ammann, Roman Brenner, David Altmann, Michaela Gemperle. Cantonal Hospital Fribourg: Daniel Hayoz, Mathieu Firmann, Sandrine Foucras. Cantonal Hospital Lucerne: Richard Kobza, Benjamin Berte, Virgina Justi, Frauke Kellner-Weldon, Brigitta Mehmann, Myriam Roth, Andrea Ruckli-Kaeppeli, Ian Russi, Kai Schmidt, Mabelle Young, Melanie Zbinden. EOC Ospedale Regionale di Lugano: Giorgio Moschovitis, Jane Frangi-Kultalahti, Anica Pin, Luisa Vicari. University Hospital Geneva: Dipen Shah, Georg Ehret, Hervé Gallet, Elise Guillermet, Francois Lazeyras, Karl-Olof Lovblad, Patrick Perret, Philippe Tavel, Cheryl Teres. University Hospital Lausanne: Jürg Schläpfer, Nathalie Lauriers, Marie Méan, Sandrine Salzmann. Public Hospital Solothurn: Frank-Peter Stephan, Andrea Grêt, Jan Novak, Sandra Vitelli. EOC Ospedale San Giovanni di Bellinzona: Marcello Di Valentino, Jane Frangi-Kultalahti, Augusto Gallino. University Hospital Zurich and University of Zurich: Fabienne Witassek, Matthias Schwenkglenks. Medical Image Analysis Center AG: Jens Würfel, Anna Altermatt, Michael Amann, Petra Huber, Esther Ruberte, Tim Sinnecker, Vanessa Zuber. Clinical Trial Unit Basel: Michael Coslovsky, Pascal Benkert, Gilles Dutilh, Milica Markovic, Patrick Simon. Schiller AG: Ramun Schmid.
References
- Akoudad S, Wolters FJ, Viswanathan A, de Bruijn RF, van der Lugt A, Hofman A, et al. Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol 2016; 73: 934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin BP, Nair VA, Meier TB, Xu G, Rowley HA, Carlsson CM, et al. Effects of hypoperfusion in Alzheimer’s disease. J Alzheimer Dis 2011; 26: 123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundel M, de Bresser J, van Dillen JJ, Kappelle LJ, Biessels GJ. Cerebral microinfarcts: a systematic review of neuropathological studies. J Cereb Blood Flow Metab 2012; 32: 425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne LM, Rodrigues FB, Blennow K, Durr A, Leavitt BR, Roos RAC, et al. Neurofilament light protein in blood as a potential biomarker of neurodegeneration in Huntington’s disease: a retrospective cohort analysis. Lancet Neurol 2017; 16: 601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LY, Norby FL, Gottesman RF, Mosley TH, Soliman EZ, Agarwal SK, et al. Association of Atrial fibrillation with cognitive decline and dementia over 20 years: The ARIC‐NCS (Atherosclerosis Risk in Communities Neurocognitive Study.). J Am Heart Assoc 2018; 7: e007301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conen D, Rodondi N, Muller A, Beer JH, Ammann P, Moschovitis G, et al. Relationships of overt and silent brain lesions with cognitive function in patients with atrial fibrillation. J Am Coll Cardiol 2019; 73: 989–99. [DOI] [PubMed] [Google Scholar]
- Conen D, Rodondi N, Mueller A, Beer J, Auricchio A, Ammann P, et al. Design of the Swiss Atrial Fibrillation Cohort Study (Swiss-AF): structural brain damage and cognitive decline among patients with atrial fibrillation. Swiss Med Wkly 2017; 147: w14467. [DOI] [PubMed] [Google Scholar]
- De Marchis GM, Katan M, Barro C, Fladt J, Traenka C, Seiffge DJ, et al. Serum neurofilament light chain in patients with acute cerebrovascular events. Eur J Neurol 2018; 25: 562–8. [DOI] [PubMed] [Google Scholar]
- DeMers D, Wachs D, Physiology, mean arterial pressure. Treasure Island (FL: ): StatPearls Publishing LLC; 2019. [PubMed] [Google Scholar]
- Diener HC, Hart RG, Koudstaal PJ, Lane DA, Lip GYH. Atrial fibrillation and cognitive function: JACC review topic of the week. J Am Coll Cardiol 2019; 73: 612–9. [DOI] [PubMed] [Google Scholar]
- Disanto G, Barro C, Benkert P, Naegelin Y, Schadelin S, Giardiello A, the Swiss Multiple Sclerosis Cohort Study Group, et al. Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol 2017; 81: 857–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duering M, Konieczny MJ, Tiedt S, Baykara E, Tuladhar AM, Leijsen EV, et al. Serum neurofilament light chain levels are related to small vessel disease burden. J Stroke 2018; 20: 228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J 2012; 33: 1500–10. [DOI] [PubMed] [Google Scholar]
- Fuhrmann D, Nesbitt D, Shafto M, Rowe JB, Price D, Gadie A, et al. Strong and specific associations between cardiovascular risk factors and white matter micro- and macrostructure in healthy aging. Neurobiol Aging 2019; 74: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardarsdottir M, Sigurdsson S, Aspelund T, Gudnason V, Arnar DO, Rokita H, et al. Atrial fibrillation is associated with decreased total cerebral blood flow and brain perfusion. EP Europace 2018; 20: 1252–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattringer T, Pinter D, Enzinger C, Seifert-Held T, Kneihsl M, Fandler S, et al. Serum neurofilament light is sensitive to active cerebral small vessel disease. Neurology 2017; 89: 2108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Duering M, Hachinski V, Joutel A, Pendlebury ST, Schneider JA, et al. Vascular cognitive impairment and dementia: JACC scientific expert panel. J Am Coll Cardiol 2019; 73: 3326–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil M, Pirpamer L, Hofer E, Voortman MM, Barro C, Leppert D, et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun 2020; 11: 812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 2018; 14: 577–89. [DOI] [PubMed] [Google Scholar]
- Kim D, Yang PS, Yu HT, Kim TH, Jang E, Sung JH, et al. Risk of dementia in stroke-free patients diagnosed with atrial fibrillation: data from a population-based cohort. Eur Heart J 2019; 40: 2313–23. [DOI] [PubMed] [Google Scholar]
- Korley FK, Goldstick J, Mastali M, Van Eyk JE, Barsan W, Meurer WJ, et al. Serum NfL (neurofilament light chain) levels and incident stroke in adults with diabetes mellitus. Stroke 2019; 50: 1669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhne M, Krisai P, Conen D, Osswald S. The heart-brain connection: further establishing the relationship between atrial fibrillation and dementia? Eur Heart J 2019; 40: 2324–6. [DOI] [PubMed] [Google Scholar]
- Lavy S, Stern S, Melamed E, Cooper G, Keren A, Levy P. Effect of chronic atrial fibrillation on regional cerebral blood flow. Stroke 1980; 11: 35–8. [DOI] [PubMed] [Google Scholar]
- Levine DA, Galecki AT, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, et al. Blood pressure and cognitive decline over 8 years in middle-aged and older black and white Americans. Hypertension 2019; 73: 310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-S, Lee W-J, Wang S-J, Fuh J-L. Levels of plasma neurofilament light chain and cognitive function in patients with Alzheimer or Parkinson disease. Sci Rep 2018; 8: 17368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010; 137: 263–72. [DOI] [PubMed] [Google Scholar]
- Lv Y-B, Zhu P-F, Yin Z-X, Kraus VB, Threapleton D, Chei C-L, et al. A U-shaped association between blood pressure and cognitive impairment in Chinese elderly. J Am Med Dir Assoc 2017; 18: 193.e7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan M, Graff-Radford J, Piccini JP, Gersh BJ. Cognitive dysfunction in atrial fibrillation. Nat Rev Cardiol 2018; 15: 744–56. [DOI] [PubMed] [Google Scholar]
- Malone JI. Diabetic central neuropathy: CNS damage related to hyperglycaemia. Diabetes 2016; 65: 355–7. [DOI] [PubMed] [Google Scholar]
- Mariotto S, Farinazzo A, Magliozzi R, Alberti D, Monaco S, Ferrari S. Serum and cerebrospinal neurofilament light chain levels in patients with acquired peripheral neuropathies. J Peripher Nerv Syst 2018; 23: 174–7. [DOI] [PubMed] [Google Scholar]
- Mattsson N, Andreasson U, Zetterberg H, Blennow K, for the Alzheimer’s Disease Neuroimaging Initiative. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol 2017; 74: 557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989; 39: 1159–65. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bã©Dirian VéR, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–9. [DOI] [PubMed] [Google Scholar]
- Nishtala A, Piers RJ, Himali JJ, Beiser AS, Davis-Plourde KL, Saczynski JS, et al. Atrial fibrillation and cognitive decline in the Framingham Heart Study. Heart Rhythm 2018; 15: 166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JT, Erkinjuntti T, Reisberg B, Roman G, Sawada T, Pantoni L, et al. Vascular cognitive impairment. Lancet Neurol 2003; 2: 89–98. [DOI] [PubMed] [Google Scholar]
- Petermann F, Wechsler Adult Intelligence Scale. 4th edn Frankfurt: Pearson; 2011. [Google Scholar]
- Piers RJ, Nishtala A, Preis SR, DeCarli C, Wolf PA, Benjamin EJ, et al. Association between atrial fibrillation and volumetric magnetic resonance imaging brain measures: Framingham Offspring Study. Heart Rhythm 2016; 13: 2020–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafnsson SB, Deary IJ, Fowkes FG. Peripheral arterial disease and cognitive function. Vasc Med) 2009; 14: 51–61. [DOI] [PubMed] [Google Scholar]
- Roy B, Woo MA, Wang DJJ, Fonarow GC, Harper RM, Kumar R. Reduced regional cerebral blood flow in patients with heart failure. Eur J Heart Fail 2017; 19: 1294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. NeuroImage 2002; 17: 479–89. [DOI] [PubMed] [Google Scholar]
- Stefansdottir H, Arnar DO, Aspelund T, Sigurdsson S, Jonsdottir MK, Hjaltason H, et al. Atrial fibrillation is associated with reduced brain volume and cognitive function independent of cerebral infarcts. Stroke 2013; 44: 1020–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedt S, Duering M, Barro C, Kaya AG, Boeck J, Bode FJ, et al. Serum neurofilament light: a biomarker of neuroaxonal injury after ischaemic stroke. Neurology 2018; 91: e1338–47. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol 2004; 19: 203–14. [DOI] [PubMed] [Google Scholar]
- Uphaus T, Bittner S, Groschel S, Steffen F, Muthuraman M, Wasser K, et al. NfL (neurofilament light chain) levels as a predictive marker for long-term outcome after ischaemic stroke. Stroke 2019; 50: 3077–84. [DOI] [PubMed] [Google Scholar]
- van der Ende EL, Meeter LH, Poos JM, Panman JL, Jiskoot LC, Dopper EGP, et al. Serum neurofilament light chain in genetic frontotemporal dementia: a longitudinal, multicentre cohort study. Lancet Neurol 2019; 18: 1103–11. [DOI] [PubMed] [Google Scholar]
- van Veluw SJ, Zwanenburg JJ, Engelen-Lee J, Spliet WG, Hendrikse J, Luijten PR, et al. In vivo detection of cerebral cortical microinfarcts with high-resolution 7T MRI. J Cereb Blood Flow Metab 2013; 33: 322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels RL, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Cognitive impairment in heart failure: a systematic review of the literature. Eur J Heart Fail 2007; 9: 440–9. [DOI] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453–7. [DOI] [PubMed] [Google Scholar]
- Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters FJ, Zonneveld HI, Hofman A, van der Lugt A, Koudstaal PJ, Vernooij MW, et al. cerebral perfusion and the risk of dementia: a population-based study. Circulation 2017; 136: 719–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Swiss-AF consent forms, as approved by the ethics committee, do not allow for the data to be made publicly available. Researchers may contact the authors for the potential submission of research proposals for future analyses or independent verification of our results.