Abstract
Background
The super-relaxed state of myosin (SRX) plays a fundamental role in maintaining the low resting metabolic rate of skeletal muscle. Our previous work on this state has been in animal models. Piperine is a small molecule that has been shown to destabilize the SRX in rabbit fast twitch fibers.
Methods
Here we extend this work to human muscle obtained from biopsies of the vastus lateralis of both lean and obese subjects. The slow release of nucleotides by myosin in the SRX was measured by incubating permeable fibers in a fluorescent analog of ATP and chasing with ATP.
Results
The fraction of myosin heads in the SRX was 0.48 ± 0.04 with a lifetime of 148 ± 5 s in lean subjects and a fraction of 0.41 ± 0.05 and a lifetime of 176 ± 7 s in obese subjects. Addition of 100 μM piperine decreased the SRX population by 43 ± 7% in lean subjects and 36 ± 7% in obese subjects, with little change in lifetimes. Addition of piperine to human cardiac cells had no effect on the SRX, a requirement for a drug to treat metabolic diseases.
Conclusions
In human muscle the SRX and its responses to piperine are similar to those seen previously, with no significant differences between muscles from lean and obese subjects. Thus analogs of piperine that have greater specificity could provide effective treatment for metabolic diseases. The SRX provides a potential mechanism contributing to the large dynamic range of metabolic rate.
Keywords: Obesity, Type 2 diabetes, Myosin phosphorylation, Sedentary behavior, Thermogenesis, Piperine
1. Introduction
We previously identified a state of myosin in relaxed muscle with a very slow ATP turnover rate, terming it the “super-relaxed state” (SRX) [1].1 We observed the SRX slow nucleotide turnover in the presence of faster ATPase activities using single nucleotide turnovers, incubating single fibers in a fluorescent analog of ATP (mantATP) and chasing with ATP (see Fig. 1). The SRX has been associated with a structure called the interacting-heads motif (IHM) in which myosin interaction with actin and its basal ATPase are both inhibited by myosin heads interacting with each other and folding back onto the myosin tails making up their filament backbone [2]. The IHM has been observed in a variety of muscles and predates the origin of animals [3]. The SRX and the IHM respond similarly to a number of interventions, including temperature and small molecule inhibitors. For example, we previously showed by EPR spectroscopy that spin-labeled nucleotides bound to myosin in relaxed rabbit skeletal muscle had an oriented spectra in the presence of blebbistatin, which is known to stabilize the IHM [4]. The EPR spectrum was similar to that of tarantula fibers without blebbistatin, known to have a particularly stable IHM and robust SRX, supporting the hypothesis that the IHM structurally underlies the SRX’s low ATP turnover rate [5].
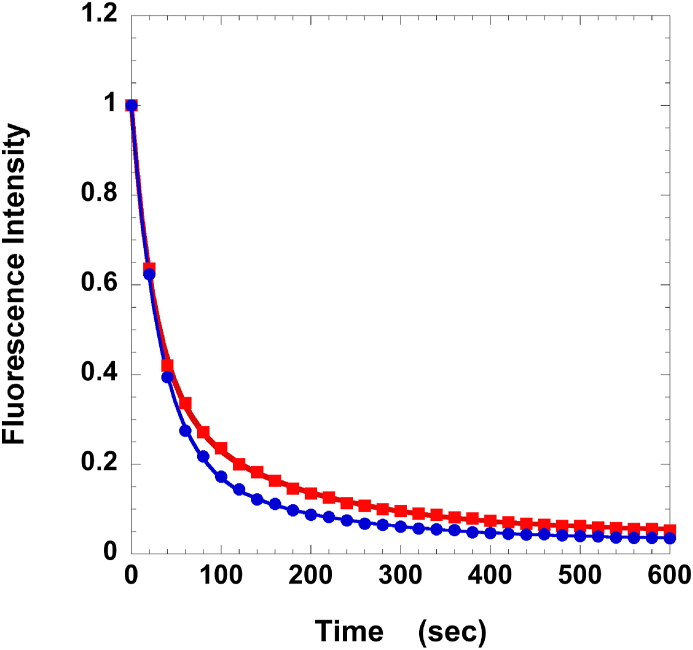
Fig. 1.
Measuring the SRX in human fibers. The intensity of the fiber fluorescence, relative to the pre-chase value, is plotted as a function of time during the chase phase of the single nucleotide turnover measurements in the presence (blue circles) or absence (red squares) of 100 μM piperine. Human fibers were obtained from lean subjects. The fibers were first incubated in the fluorescent ATP analog, mantATP, and then chased with ATP. Fiber fluorescence decreases in two phases, a fast phase that is largely completed in roughly 20–30 s, followed by a slow phase with a lifetime of minutes. The slow phase arises from the slow release of nucleotides by myosin in the SRX. The slower phase has a decreased population in the presence of piperine, showing that the SRX has been partially destabilized. Fits to the data for control defined P2 = 0.31 ± 0.01, T2 = 157 ± 9 s; and with piperine P2 = 0.21 ± 0.01, T2 = 157 ± 12 s. Data averaged over a number of fibers is summarized in Table 1. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Myosin heads in the SRX are in a dynamic equilibrium with a disordered relaxed state (DRX), in which they have an ATPase activity similar to that observed for purified myosin, approximately tenfold faster than in the SRX. To conserve energy, most myosins at in vivo temperatures are in the SRX. If all the myosins were in the DRX, they would metabolize an additional ∼1000 Cal per day, increasing whole body metabolic rate ∼50% [6]. Thus, pharmaceuticals that destabilize the SRX would provide an effective strategy for treating obesity and type 2 diabetes [7]. This would be particularly useful in cases where efforts to reverse the disease process, such as dieting and/or exercise, may stimulate a loss in resting metabolic rate [8], measured to be as large as 800 Cal per day occurring years after a weight-loss intervention [9]. A number of laboratories and biotechnology companies are actively pursuing the quest to discover such pharmaceuticals.
We recently carried out a screen of 2100 compounds, discovering an effect by the small molecule piperine, the active compound of black pepper, which had many of the qualities desired for treating obesity and type 2 diabetes [7]. This molecule destabilizes the SRX, is specific for fast twitch skeletal muscle having no effect on cardiac muscle, and has no effect on the mechanics of activated muscle. Studies by several other laboratories have shown that piperine was effective in reducing weight gain in rodents and improving blood glucose levels [[10], [11], [12]]. However, the affinity for its target, Kd = 3 μM, is too low to be a pharmaceutical in humans. Thus, further work is needed to find compounds with greater specificity.
All of the above work on the SRX has been carried out in animal models, mostly rabbits [1] and rodents [13]. A study of the parameters of the SRX in vastus lateralis muscle of three human subjects reported on the effects of fiber type [14]. They find that the population of the SRX is smaller in type IIA fibers than in type I or type IIAX. The value of the lifetime is shorter in type IIAX fibers. The data reported here are in broad agreement with theirs with the exception that the lifetime of the SRX in their report is shorter, 80–100 s, than the value reported here of approximately 160 s. This longer value is closer to that observed in rabbit muscle of 250 s [1].
The Holy Grail in the field of the SRX in skeletal muscle is the possibility of developing pharmaceuticals for treating metabolic diseases and the elucidation of the role of the SRX in whole body metabolism [6]. We have oriented our studies on the further development of a possible pharmaceutical to treat metabolic diseases, piperine [7]. We show that piperine-like molecules with greater affinity for myosin would provide effective treatments for the metabolic diseases, obesity and type 2 diabetes. We conclude with a discussion of the role of the SRX in human metabolism in the presence and absence of piperine-like compounds. We focus on the role of muscle activity and its disordering of the SRX via myosin regulatory light chain phosphorylation leading to thermogenesis by myosin heads in the DRX [1,15].
2. Materials and methods
2.1. Fibers and solutions
Biopsy samples taken from the quadriceps (vastus lateralis) were obtained from the Center for Muscle Biology at the University of Kentucky. Samples were acquired from both lean and obese subjects, flash frozen in liquid nitrogen and shipped on dry ice. The only information provided on the donors was their BMI. Strips of human cardiac tissue were obtained from the left ventricle, and were a gift from MyoKardia.
Fibers were stored at −20 °C in a solution of rigor buffer and glycerol mixed 50/50. The Rigor buffer contained: 50 mM 3-(N-morpholino)propanesulfonic acid, 120 mM potassium acetate, 5 mM magnesium chloride, 5 mM EGTA, 4 mM DTT, and 5 mM potassium phosphate, pH = 6.8. Relaxing buffers were obtained by the addition of 4 mM ATP or 250 μM mantATP to the Rigor buffer.
2.2. Characterization of fiber properties
Single nucleotide turnovers were measured in flow cells as described previously at a temperature of 25 °C [1]. Data were fit with a 2 exponential function as described previously [1].
3. Results
3.1. Measuring the SRX
The SRX was measured using permeable muscle fiber preparations. These are fibers from which the cell membrane has been rendered permeable using chemical methods, in this case high concentrations of glycerol. This is a preparation widely used in the field, allowing the proteins of the myofibril to be bathed in a solution of choice. In order to measure the slow release of nucleotides from myosin in the SRX in the presence of other faster enzymes, single muscle fibers were mounted in a flow cell, incubated in the fluorescent ATP analog 2’/3′-O-(N-methylanthraniloyl)-adenosine-5′-triphosphate i.e. mantATP, and then chased with ATP [1]. The decay in fluorescence intensity during the chase phase is shown as a function of time in Fig. 1. Myosin heads in the DRX hydrolyze and release nucleotides quickly, resulting in a rapid phase of fluorescence decay, within roughly 20–30 s. This is followed by a slow release of nucleotides by myosin heads in the SRX. Fig. 1 is a typical decay of fluorescence during the chase phase. The decay in fluorescence was fit with a bi-exponential function having the fast and slow decay fractions P1 and P2, respectively, with lifetimes of T1 and T2 [1]. The averaged data from a number of fibers are shown in Table 1. These data show that human fibers display an SRX with a population and lifetime similar to that seen in rabbit muscle [1]. This result is not surprising given the high degree of conservation of the SRX [3]
Table 1.
Populations and lifetimes (T2) for the SRX
| Control |
Piperine |
|||
|---|---|---|---|---|
| SRX population | T2 | SRX population | T2 | |
| Lean | 0.48 ± 0.04 | 148 ± 5 | 0.36 ± 0.04 | 157 ± 6 |
| Obese | 0.41 ± 0.05 | 176 ± 7 | 0.33 ± 0.04 | 170 ± 7 |
Errors shown are SEM for n =170 for lean subjects and n = 110 for obese subjects. The SRX population is the fraction of myosin heads that are in the SRX state, derived by correcting P2 for non-specifically bound nucleotides as described in section 3.2.
3.2. Correcting raw data for nonspecific binding
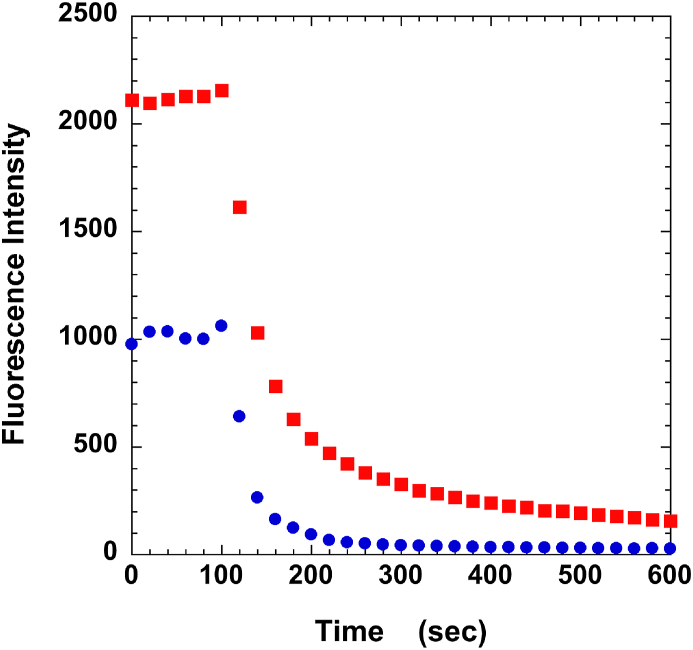
Determining mantATP binding specificity is important for analyzing the data [1]. To address this, fibers were first incubated in mantATP along with an additional 4 mM ATP. The ATP will compete with the mantATP for specific binding sites on the fiber. This is then chased with ATP. Fig. 2 shows that approximately half of the mant nucleotides remain bound in the presence of competing ATP i.e. are nucleotides bound non-specifically to the fiber. Results from multiple fibers showed that 42 ± 2% bound non-specifically. Thus the population of myosin heads in the SRX = P2/(1–0.42). The data of Fig. 2 show that almost all of the nucleotides released slowly arose from nucleotides bound to specific sites in the fiber. These results are very similar to those obtained in rabbit fibers [1]. In the following discussion we will refer to the populations of the SRX adjusted for specificity by the above equation.
Fig. 2.
Measuring the fraction of mant nucleotides bound specifically to the fiber. The fiber was first incubated in mantATP and at 100 s chased with ATP similar to the control fiber in Fig. 1, red squares. The fit to the decay curve during the chase defined P2 = 0.29 ± 0.01; T2 = 170 ± 7 s. The fiber was then washed with rigor buffer and incubated again, in mantATP with an additional 4 mM ATP. The presence of the ATP competes off mantATP from sites that bind ATP specifically, blue circles. As can be seen, approximately half of the nucleotides are no longer bound to the fiber. An average of ten experiments showed 42 ± 3% of the nucleotides bound nonspecifically. The fiber was then chased with ATP starting at 100 s. The slow release of nucleotides seen in the absence of competing ATP is much reduced, showing that the slow release of nucleotides comes from ATP specific sites. A fit to the data during the chase defined P2 = 0.05 ± 0.02, T2 = 160 ± 15 s. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. No dependence of the SRX on body mass index (BMI)
Samples were obtained from 3 lean subjects, BMI 24, 24 and 25, and from 3 obese subjects, BMI 33, 38 and 45. Although there were significant differences in SRX populations between individuals, there was little dependence on BMI; a straight line fit to a plot of SRX population as a function of BMI has a slope that is not significantly different from zero. For the lean subjects the SRX populations were 0.41, 0.47 and 0.57, respectively, averaging 0.48. For the obese subjects they were 0.34, 0.50, and 0.36, respectively, averaging 0.41. The populations seen here are close to that found in rabbit, 0.57 ± 0.07 [1] and in a recent study of human muscle [14]. These populations are for our in vitro assays at 25 °C. The SRX is more stable at higher temperatures, and at 37 °C e.g. in vivo the SRX populations would be approximately 75%. The SRX populations may reflect real biological differences between individuals. However, it may also be due to other factors such as sample handling, etc. There was much less variation in T2, which has an average value for all samples of 162 ± 12 s. We conclude that, as measured in our assay system, the parameters of the SRX do not depend significantly on BMI. Measurements within this in vitro system do not preclude the possibility that in vivo post translational modifications could alter the SRX differently as a function of factors related to BMI. Table 1 shows the associated SRX populations and their lifetimes averaged for lean and obese individuals.
3.4. Piperine destabilizes the SRX
Piperine is a small molecule shown previously to destabilize the SRX in rabbit muscle [7]. To determine whether it has a similar effect in humans, muscle fibers were incubated and chased as described above, washed in rigor buffer, and run again using solutions containing 100 μM piperine. As shown in Fig. 1, piperine destabilizes the SRX. Table 1 shows the results for lean and obese subjects, obtained by averaging for each subject then averaging the subjects. If the data are first averaged for each category, piperine is found to destabilize the SRX in lean and obese by 43 ± 7% and 36 ± 7%, respectively. Piperine had no effect on the lifetime of the SRX for samples from either lean or obese humans, in contrast to results found in rabbit where it inhibited lifetime by 29% [7].
3.5. Piperine does not affect human cardiac muscle
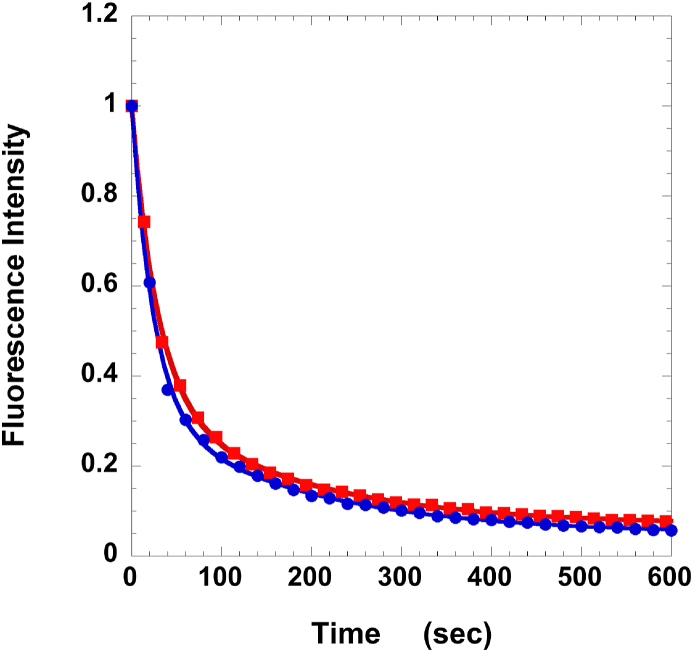
Of particular importance in the development of pharmaceuticals to treat metabolic disease in humans is the requirement that the pharmaceutical be specific for skeletal muscle and have no effect on cardiac muscle. In our previous work on piperine in rabbit muscles this was in fact the case [7]. To check whether this was the case in human cardiac tissue, we repeated the experiment using human cardiac ventricular strips. As shown in Fig. 3, piperine had no effect on the population or lifetime of the SRX. The value of P2 seen here, 0.27 ± 0.02, is similar to the P2 observed previously in human cardiac cells of 0.25 ± 0.02 [16].
Fig. 3.
Effect of piperine on human cardiac cells in the absence, red squares, and presence of 100 μM piperine, blue circles. The experiment was carried out following the same protocols as described in the legend to Fig. 1, with the exception that the muscle sample was a strip of cardiac cells taken from the left ventricle of a human subject. As can be seen, piperine has little effect on the SRX. Fits to the data showed that the fractions of fluorescence that decayed slowly, P2, were 0.27 ± 0.02 for control and 0.29 ± 0.02 with piperine, with respective lifetimes of 180 ± 18 and 171 ± 14 s. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Here we show that the population of the SRX in human skeletal muscle has the same magnitude as other organisms previously studied (rabbit and rodent), as well as in a recent study of human muscle [14]. It is remarkably resilient (similar magnitude) with different body mass indices, and is reduced by piperine [1,13]. The lifetime averaged over all conditions, 162 ± 13 s, is a little shorter than for rabbit, ∼250 s [1], but longer than previously seen for human, ∼90 s. The specificity of fluorescent nucleotide binding is very similar in rabbit and human fibers. This parameter is necessary to interpret the data in terms of populations of the SRX (discussed in section 3.2). Critical to the future consideration of piperine-like pharmacological agents, there is no effect of piperine on human cardiac muscle cells. For a pharmaceutical used to treat obesity and type 2 diabetes, any effect on cardiac cells would cause an unacceptable side effect. This is similar to previous observations of no effect on rabbit cardiac cells [7]. We conclude that previous observations of the effects of piperine on the SRX in other model systems are applicable to human muscles. Previous studies of the effect of piperine in rodents had shown that it is effective at preventing diet-induced obesity and type 2 diabetes [[10], [11], [12]]. No mechanism for this effect was identified, and we have suggested that it was due to the effect of piperine on the SRX [7]. Assuming that a piperine-like compound disordered 40% of the myosin heads from the SRX to the DRX, one can calculate using known human myosin kinetics that whole-body metabolic rate would increase ∼400 Calories per day, providing an effective treatment for obesity and type 2 diabetes [7].
What role could the SRX play in whole body metabolic rate during everyday life? One pathway known to regulate the SRX is phosphorylation of the myosin regulatory light chain (RLC), which disorders the binding of the myosin heads to the core of the thick filament (for a review see Ref. [15]). The disordered heads are available to bind to actin, potentiating twitch force. However, they would also lead to greater ATPase activity in the DRX and thus greater thermogenesis [1,6], analogous to futile cycling of protons in mitochondria or calcium in the sarcoplasmic reticulum [17]. There have been only a few papers exploring the extent of myosin phosphorylation in humans. Houston and coworkers measured phosphorylation before and after exercise in human subjects in a series of papers, see for example [18,19] reviewed in Refs. [15]. Resting levels of RLC phosphorylation ranged from 0.30 to 0.50, rose to 0.4–0.8 after maximum voluntary contractions (nearly doubling phosphorylation after 10 s contractions), and dephosphorylated back to resting levels after 10–30 min. Results were similar in fast and slow twitch fibers. These results, showing high levels of initial RLC phosphorylation and increasing close to maximum during brief exercise, then decaying relatively slowly, all argue that RLC phosphorylation levels are high in active individuals. Previous work in rabbit muscle showed that >50% RLC phosphorylation disordered 35% of the SRX [1]. Assuming a similar result for human muscle, and further assuming that active individuals have up to 50% phosphorylation during waking hours suggests that metabolism due to myosin phosphorylation would approach 300 Calories/day, or about 15% of total daily expenditure. Another physiological process influencing the SRX is mechano-sensing, whereby a stretch of the muscle disorders the SRX [20]. However, this is probably playing a role mainly in activating the muscle, with tension partitioning the myosin population into the DRX, further increasing the force being generated in proportion to the tension against which the force is applied.
While increased metabolism due to myosin RLC phosphorylation has not been measured directly, in fact greater metabolic activity is observed following active contraction, called excess post-exercise oxygen consumption (EPOC) [21]. The time course of EPOC is complex, but one component, the initial 5–10 min, is similar to twitch potentiation and RLC phosphorylation. The thermogenesis associated with EPOC adds to the energetic costs of the activity, especially for brief and/or light activities [22]. This helps to explain the potentially larger-than-expected benefits of standing, walking, and fidgeting [[23], [24], [25]]. EPOC therefore represents a mechanism whereby regular movement through the day could contribute significantly to metabolic rate, with particularly short bursts of activity (seconds to minutes in duration) potentially harvesting the greatest caloric expenditure after movement relative to the movement itself. Although many factors contribute to EPOC, including obligatory replacement of metabolites, etc., we propose that thermogenesis by myosin in the DRX is one of them, particularly for short bursts of activity that might fall more into the category of daily living rather than exercise per se.
Recent research has shown that long periods of inactivity lead to increased risk factors for cardiovascular disease and type 2 diabetes. Interrupting the inactivity with periodic bouts of moderate exercise improves cardiovascular risk factors and glycemic control [[26], [27], [28]]. Brief bouts of activity split up over time metabolize more energy than the same total activity exerted in a single bout. For example, when relatively sedentary humans walk briskly for 2 min to interrupt sedentary behavior, EPOC occurs for roughly another 5 min, doubling the caloric expenditure relative to that of the movement itself [29]. We propose that myosin phosphorylation leading to thermogenesis by myosin heads in the DRX contributes to the benefits of brief bouts of activity.
5. Summary
Properties of the SRX in human muscles are similar to those seen earlier in animal models. The effect of piperine is also similar, suggesting piperine would be a reasonable lead compound to look for pharmaceuticals to treat metabolic disease. The properties of the SRX are similar in muscles of lean and obese individuals. Thus, BMI for the six individuals studied here was not determined by genetic differences in the SRX, although the SRX could still play a role due to differences in post-translational modifications in vivo. The SRX has the potential to play a role in a number of areas related to human metabolic rate, including advancing models designed around lifestyle interventions to reduce the metabolically-related diseases, obesity and type-2 diabetes.
The major limitation of our study is that, due to the limited resources available to our laboratory, we could only study 6 subjects. A more extensive study could have allowed better statistical analysis and examination of more varied conditions. In particular we think that studies of the effects of myosin phosphorylation on the SRX in vitro, coupled with measurements of phosphorylation levels in vivo, would be most informative.
CRediT authorship contribution statement
Clyde Wilson: Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing, Supervision. Nariman Naber: Methodology, Investigation, Data curation, Writing - review & editing. Roger Cooke: Conceptualization, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing.
Acknowledgements
Data for this study were acquired at the Nikon Imaging Center at UCSF/QB3. The authors would like to thank Dr. Delaine Larson and Dr. Kari Herrington for their generous help in using the microscopes. We also thank Drs. Charlotte Peterson and Grace Walton at the University of Kentucky Center for Muscle Biology for providing human muscle tissue.
Footnotes
Abbreviations: SRX, super-relaxed state; IHM, interacting-heads motif; DRX, disordered relaxed state; mantATP, 2’/3′-O-(N-methylanthraniloyl)-adenosine-5′-triphosphate; RLC, myosin regulatory light chain; EPOC, excess post-exercise oxygen consumption; BMI, Body mass index; P2, the fraction of fluorescent nucleotides that are released slowly during the chase phase; T2, the lifetime of P2 i.e. the slow phase.
Contributor Information
Clyde Wilson, Email: Clyde@drclydewilson.com.
Nariman Naber, Email: nariman.naber@ucsf.edu.
Roger Cooke, Email: cooke@cgl.ucsf.edu.
References
- 1.Stewart M.A., Franks-Skiba K., Chen S., Cooke R. Myosin ATP turnover rate is a mechanism involved in thermogenesis in resting skeletal muscle fibers. Proc Natl Acad Sci USA. 2010;107:430–435. doi: 10.1073/pnas.0909468107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irving T.C., Craig R. Getting into the thick (and thin) of it. J Gen Physiol. 2019;151:610–613. doi: 10.1085/jgp.201812307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee K.H., Sulbaran G., Yang S., Mun J.Y., Alamo L., Pinto A. Interacting-heads motif has been conserved as a mechanism of myosin II inhibition since before the origin of animals. Proc Natl Acad Sci USA. 2018;115:E1991–E2000. doi: 10.1073/pnas.1715247115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao F.Q., Padron R., Craig R. Blebbistatin stabilizes the helical order of myosin filaments by promoting the switch 2 closed state. Biophys J. 2008;95:3322–3329. doi: 10.1529/biophysj.108.137067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson C., Naber N., Pate E., Cooke R. The myosin inhibitor blebbistatin stabilizes the super-relaxed state in skeletal muscle. Biophys J. 2014;107:1637–1646. doi: 10.1016/j.bpj.2014.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooke R. The role of the myosin ATPase activity in adaptive thermogenesis by skeletal muscle. Biophysical reviews. 2011;3:33–45. doi: 10.1007/s12551-011-0044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nogara L., Naber N., Pate E., Canton M., Reggiani C., Cooke R. Piperine’s mitigation of obesity and diabetes can be explained by its up-regulation of the metabolic rate of resting muscle. Proc Natl Acad Sci USA. 2016;113:13009–13014. doi: 10.1073/pnas.1607536113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller M.J., Enderle J., Bosy-Westphal A. Changes in energy expenditure with weight gain and weight loss in humans. Curr Obes Rep. 2016;5:413–423. doi: 10.1007/s13679-016-0237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fothergill E., Guo J., Howard L., Kerns J.C., Knuth N.D., Brychta R. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity. 2016;24(8):1612–1619. doi: 10.1002/oby.21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.BrahmaNaidu P., Nemani H., Meriga B., Mehar S.K., Potana S., Ramgopalrao S. Mitigating efficacy of piperine in the physiological derangements of high fat diet induced obesity in Sprague Dawley rats. Chem Biol Interact. 2014;221:42–51. doi: 10.1016/j.cbi.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Choi S., Choi Y., Choi Y., Kim S., Jang J., Park T. Piperine reverses high fat diet-induced hepatic steatosis and insulin resistance in mice. Food Chem. 2013;141:3627–3635. doi: 10.1016/j.foodchem.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 12.Kim K.J., Lee M.S., Jo K., Hwang J.K. Piperidine alkaloids from Piper retrofractum Vahl. protect against high-fat diet-induced obesity by regulating lipid metabolism and activating AMP-activated protein kinase. Biochem Biophys Res Commun. 2011;411:219–225. doi: 10.1016/j.bbrc.2011.06.153. [DOI] [PubMed] [Google Scholar]
- 13.Colson B.A., Peterson K.J., Collins B.C., Lowe D.A., Thomas D.D. The myosin super-relaxed state is disrupted by estradiol deficiency. Biochem Biophys Res Commun. 2015;456:151–155. doi: 10.1016/j.bbrc.2014.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phung L.A., Foster A.D., Miller M.S., Lowe D.A., Thomas D.D. Super-relaxed state of myosin in human skeletal muscle is fiber-type dependent. Am J Physiol Cell Physiol. 2020 doi: 10.1152/ajpcell.00396. Advanced online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandenboom R. Modulation of skeletal muscle contraction by myosin phosphorylation. Comp Physiol. 2016;7:171–212. doi: 10.1002/cphy.c150044. [DOI] [PubMed] [Google Scholar]
- 16.Anderson R.L., Trivedi D.V., Sarkar S.S., Henze M., Ma W., Gong H. Deciphering the super relaxed state of human beta-cardiac myosin and the mode of action of mavacamten from myosin molecules to muscle fibers. Proc Natl Acad Sci USA. 2018;115:E8143–E8152. doi: 10.1073/pnas.1809540115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuller-Jackson J., Henry B.A. Adipose and skeletal muscle thermogenesis: studies from large animals. J Endocrinol. 2018;237:R99–R115. doi: 10.1530/JOE-18-0090. [DOI] [PubMed] [Google Scholar]
- 18.Houston M.E., Grange R.W. Myosin phosphorylation, twitch potentiation, and fatigue in human skeletal muscle. Can J Physiol Pharmacol. 1990;68:908–913. doi: 10.1139/y90-139. [DOI] [PubMed] [Google Scholar]
- 19.Houston M.E., Grange R.W. Torque potentiation and myosin light-chain phosphorylation in human muscle following a fatiguing contraction. Can J Physiol Pharmacol. 1991;69:269–273. doi: 10.1139/y91-041. [DOI] [PubMed] [Google Scholar]
- 20.Fusi L., Brunello E., Yan Z., Irving M. Thick filament mechano-sensing is a calcium-independent regulatory mechanism in skeletal muscle. Nat Commun. 2016;7:13281. doi: 10.1038/ncomms13281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Speakman J.R., Selman C. Physical activity and resting metabolic rate. Proc Nutr Soc. 2003;62:621–634. doi: 10.1079/PNS2003282. [DOI] [PubMed] [Google Scholar]
- 22.Baker E.J., Gleeson T.T. The effects of intensity on the energetics of brief locomotor activity. J Exp Biol. 1999;202:3081–3087. doi: 10.1242/jeb.202.22.3081. [DOI] [PubMed] [Google Scholar]
- 23.Bouchard C., Blair S.N., Katzmarzyk P.T. Less sitting, more physical activity, or higher fitness? Mayo Clin Proc. 2015;90:1533–1540. doi: 10.1016/j.mayocp.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Levine J.A., Eberhardt N.L., Jensen M.D. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283:212–214. doi: 10.1126/science.283.5399.212. New York, NY. [DOI] [PubMed] [Google Scholar]
- 25.Gunn S.M., van der Ploeg G.E., Withers R.T., Gore C.J., Owen N., Bauman A.E. Measurement and prediction of energy expenditure in males during household and garden tasks. Eur J Appl Physiol. 2004;91:61–70. doi: 10.1007/s00421-003-0932-1. [DOI] [PubMed] [Google Scholar]
- 26.Dempsey P.C., Blankenship J.M., Larsen R.N., Sacre J.W., Sethi P., Straznicky N.E. Interrupting prolonged sitting in type 2 diabetes: nocturnal persistence of improved glycaemic control. Diabetologia. 2017;60:499–507. doi: 10.1007/s00125-016-4169-z. [DOI] [PubMed] [Google Scholar]
- 27.Dempsey P.C., Larsen R.N., Sethi P., Sacre J.W., Straznicky N.E., Cohen N.D. Benefits for type 2 diabetes of interrupting prolonged sitting with brief bouts of light walking or simple resistance activities. Diabetes Care. 2016;39:964–972. doi: 10.2337/dc15-2336. [DOI] [PubMed] [Google Scholar]
- 28.Duvivier B.M.F.M., Schaper N.C., Hesselink M.K.C., van Kan L., Steinen N., Winkens B. Breaking sitting with light activities vs structured exercise: a randomised crossover study demonstrating benefits for glycaemic control and insulin sensitivity in type 2 diabetes. Diabetologia. 2017;60:490–498. doi: 10.1007/s00125-016-4161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fenemor S.P., Homer A.R., Perry T.L., Skeaff C.M., Peddie M.C., Rehrer N.J. Energy utilization associated with regular activity breaks and continuous physical activity: a randomized crossover trial. Nutr Metabol Cardiovasc Dis. 2018;28:557–564. doi: 10.1016/j.numecd.2018.02.003. [DOI] [PubMed] [Google Scholar]