Abstract
The authors present a case of a 33-year-old African American male with respiratory diphtheria. The patient was initially assumed to have a peritonsillar abscess before various laboratory tests. He complained of dysphagia, throat pain, and shortness of breath. The patient’s physical examination, supported by video laryngoscopy imaging and a CT scan, showed swelling of his pharynx. The patient reports that he was recently incarcerated for one year and did not receive immunizations as a child. Following his diagnosis, the patient was treated and subsequently recovered.
Keywords: Diphtheria, Pharyngitis, Cervical lymphadenopathy
Introduction
Diphtheria is an acute, infectious disease caused by Corynebacterium diphtheriae, a strain of bacteria that is known to cause respiratory and cutaneous infection. The disease may also manifest as an asymptomatic carrier state. Symptoms include cervical lymphadenopathy, sore throat, fever, and malaise (Fig. 1) [1]. Cyanosis, dyspnea, and bull neck are also signs of diphtheria and could indicate a more severe case. Corynebacterium diphtheriae can release toxins that may cause complications due to toxin-mediated tissue destruction, including myocarditis, heart block, and respiratory failure. For this reason, the disease’s mortality rate is r5−10 % in the general population and even higher in children [2].
Fig. 1.
This infographic discusses the symptoms, complications, treatments, and ways to prevent a diphtheria infection.
Cases of diphtheria can be treated with diphtheria antitoxin. If this is deemed to be a valid treatment option for a case, it is advantageous to administer the antitoxin as early as possible. Additionally, diphtheria can be prevented with the Diphtheria Tetanus Pertussis (DTaP) vaccine, which has proved to be highly effective. Since the vaccine’s introduction in the 1940s, cases of diphtheria have notably declined in the United States [3]. The DT, Tdap, and Td vaccines are other commonly used immunizations against diphtheria. While these vaccines have virtually eradicated the disease in the United States, there are still cases reported in less developed countries, such as Yemen, Bangladesh, and Venezuela from 2016 to 2019, particularly in regions of conflict and social turmoil [4].
Case report
A 33-year-old African American male presented to the emergency department (ED) with a one-week history of sore throat. The patient had been seen five days prior for a presumed peritonsillar abscess but left against medical advice. He began taking prescribed clindamycin without receiving any surgical intervention or additional treatment. The patient later decided to go to a different hospital after brief onset dyspnea when he felt like he was choking. He also reported worsening dysphagia and throat pain. He then claimed that his fever had resolved and did not return to medical attention for two days. The patient received a call from the first ED he visited saying that he had a bacterial infection but did not receive the name of the pathogen. He denied any allergies to medications and reported that he was taking trazadone, quetiapine, methylphenidate, and clindamycin. He also smokes one pack of cigarettes per day.
Upon returning to our ED, the patient was not in visible respiratory distress, had equal chest wall rise and fall, and exhibited no stridor or wheezing. His limbs were warm with bilateral pulses and a capillary refill of two seconds. Despite complaining of shortness of breath, the patient was resting in his bed and his main complaint was throat pain. He was speaking in full sentences and his O2 saturation did not drop below 97 % on the monitor during the primary survey. Though, during the encounter it became clear that he was speaking with a muffled voice and had to frequently and forcibly swallow secretions that would otherwise pool in his oropharynx. At this time, the causative agent had not been confirmed or properly identified by the patient and it was still suspected to be a peritonsillar abscess. Laboratory tests including a complete blood count (CBC), complete metabolic profile (CMP), infectious mononucleosis test, streptococcus screen, and troponin test were performed since one of the presenting complaints was shortness of breath. The patient was given dexamethasone for inflammation, morphine for pain, ondansetron for nausea associated with morphine use, and began penicillin after blood culture acquisition. A computed tomography (CT) scan of the neck soft tissue was ordered (Fig. 2).
Fig. 2.
CT scans of the patient’s neck soft tissue.
After the orders had been placed and fluids and medication were begun, additional information was gathered. The patient reported that he grew up in Chicago and, based on his recollection, did not receive childhood vaccinations due to his parents not believing in the immunization of their children. He reported to have recently been released from prison in the past few weeks after completing a one-year sentence. He recalls having a mild sore throat upon release which has worsened since that time but did not think it may be related until questioned about it now. The patient denied rash, cough, chest pain, abdominal pain, headache, eye pain, and ear pain. He did not report additional symptoms beyond the ones previously stated.
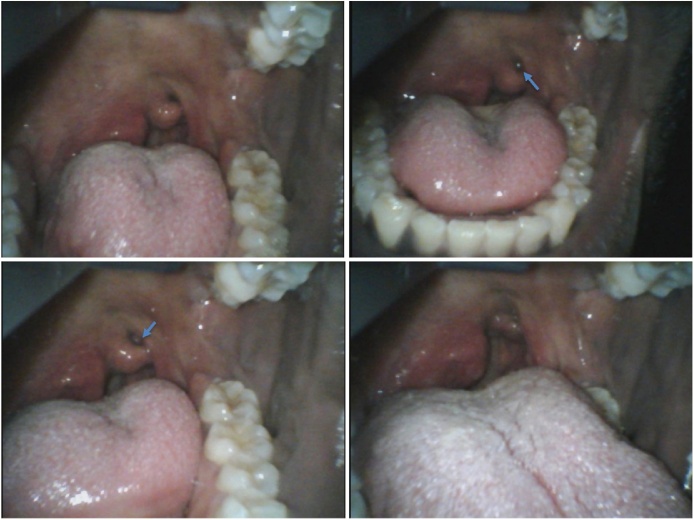
The patient’s physical exam showed that he was positive for neck tenderness on the right side by palpation. Direct visualization of the oropharynx was difficult due to the patient’s discomfort and swelling of his pharynx. His uvula was deviated to the left and a mass was seen on the right side pressing the walls of the pharynx toward the midline. Careful video laryngoscopy performed to bypass the tongue and directly visualize the patient’s pharynx. The airway was determined to be secure and the extent of the swelling was better evaluated. Images were captured at that time (Fig. 3). The remainder of the physical exam was unremarkable. While the patient was having a CT scan of his neck soft tissue, the team attempted to better determine the causative agent cultured in this patient. The laboratory later reported that the cultured agent was Corynebacterium diphtheriae. Report was made to the local health department and the prison from where he came was also notified.
Fig. 3.
Video laryngoscopy images of the patient’s pharynx demonstrating a grey discoloration (arrow) of the left tonsil.
After a verbal confirmation of the culture results had been received over the phone, arrangements were made with the admitting hospitalist team for admission. We continued the previous plan of patient symptomatic management and infection control. Contact precautions were observed and antibiotic treatment was confirmed with the admitting team and infectious disease department. At hospital discharge, the patient was referred to the health department to complete his immunizations.
Discussion
Although diphtheria is rare in the United States, physicians must keep in mind the early symptoms of the disease. At the onset of diphtheria, patients often experience mild erythema, followed by spots of gray and white exudate [1]. In about one-third of reported cases, local toxins induce the formation of the classic pseudomembrane which was visible in this patient’s physical exam. Careful examination of the airway to ensure integrity is critical in the primary assessment of the patient with a suspected diphtheria infection since the pseudomembrane can expand to occlude the airway as it enlarges. Contact and droplet precautions should be observed to prevent additional exposure to vulnerable populations and persons with incomplete immunity to the disease. If left untreated, patients are at risk of respiratory complications due to airway obstruction as the tonsils, uvula, lymph nodes and pseudomembrane expand, which is especially dangerous in small children. Patients may also experience cardiological, renal, and neurological symptoms as a complication when systemic toxins from the bacteria are present [5]. If diphtheria is readily diagnosed and treated, the recovery period from the disease significantly decreases. The median incubation period is 1.4 days. With proper antibiotic use, the median time to no longer being infectious is 5.9 days, compared to 18.5 days without antibiotics [6].
There were fewer than 20,000 cases of diphtheria diagnosed in the United States in 2018, without major outbreaks in the general population since the Seattle outbreak on Skid Road from 1972 to 1974, where 558 cases were reported. This outbreak most significantly affected the Native American population. Diphtheria remains a significant global illness despite near-eradication levels in developed countries. Most cases in the United States are imported with patients being diagnosed but not infected while in the country [7]. Nevertheless, one must consider that the unimmunized population is significantly more vulnerable to the disease. According to a Canadian study, 49 % of prison inmates were incompletely vaccinated for diphtheria, tetanus, and pertussis. As inmates live in close quarters and often make physical contact with one another, there is a significant capacity for disease to spread [8]. As the patient in this case was recently incarcerated, this could be a potential cause of his infection. In addition to incarcerated individuals, there are also groups of people who do not “believe” in immunizations and thus do not vaccinate their children (as was the case with our patient.) These groups pose a threat to the near eradicated levels of many vaccine preventable diseases such as diphtheria.
Conclusion
Although diphtheria is essentially eradicated in the United States, physicians must be aware of the possibility of infection based on the patient’s symptoms, such as cervical lymphadenopathy and dyspnea in the case of respiratory diphtheria, and immunization history. This is important because the time it takes to begin treatment is proportional to the recovery period. Physicians should also be particularly suspicious of bacterial infection in recently incarcerated patients. This case underscores the importance of childhood immunizations to combat vaccine preventable illnesses.
Funding
No funding was received for this work.
Consent
HCA Centralized Algorithms for Research Rules on IRB Exemptions (CARRIE)/IRB manager issued approval [2020-696]. Based on the information provided and attested as true, the research plan described does not require IRB oversight. This is because [the investigators] are either a) not engaging in research with human subjects as defined by federal regulations; b) engaging in research with human subjects deemed excluded from IRB oversight per 45CFR46.102(l) OR c) engaging in research with sufficient human subject protections in the design to meet one or more IRB exemption criteria set forth in 45CFR46.104.
Author contributions
PA was the treating clinician. MS, SR, PRB, LG wrote the discussion, conducted literature review, and prepared the manuscript. PA, LG wrote the case report section of manuscript and reviewed the manuscript. All authors approved the final manuscript.
Declaration of Competing Interest
The authors report no declarations of interest.
References
- 1.Nikiforov V.N., Tur’ianov M.Kh, Beliaeva N.M. Klinicheskie proiavleniia, diagnostika i lechenie difterii u vzroslykh [The clinical manifestations, diagnosis and treatment of diphtheria in adults] Ter Arkh. 1995;67(11):16–18. [PubMed] [Google Scholar]
- 2.Washington C.H., Issaranggoon na ayuthaya S., Makonkawkeyoon K., Oberdorfer P. A 9-year-old boy with severe diphtherial infection and cardiac complications. BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2014-206085. bcr2014206085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogden S.A., Ludlow J.T., Alsayouri K. StatPearls. StatPearls Publishing; Treasure Island, FL: 2020. Diphtheria Tetanus Pertussis (DTaP) vaccine. [PubMed] [Google Scholar]
- 4.Clarke K.E.N., MacNeil A., Hadler S., Scott C., Tiwari T.S.P., Cherian T. Global epidemiology of diphtheria, 2000-20171. Emerg Infect Dis. 2019;25(10):1834–1842. doi: 10.3201/eid2510.190271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Both L., Collins S., de Zoysa A., White J., Mandal S., Efstratiou A. Molecular and epidemiological review of toxigenic diphtheria infections in England between 2007 and 2013. J Clin Microbiol. 2015;53(2):567–572. doi: 10.1128/JCM.03398-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Truelove S.A., Keegan L.T., Moss W.J. Clinical and epidemiological aspects of diphtheria: a systematic review and pooled analysis. Clin Infect Dis. 2020;71(1):89–97. doi: 10.1093/cid/ciz808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pedersen A.H., Spearman J., Tronca E., Bader M., Harnisch J. Diphtheria on Skid Road, Seattle, Wash., 1972-75. Public Health Rep. 1977;92(4):336–342. [PMC free article] [PubMed] [Google Scholar]
- 8.Sequera V.G., Valencia S., García-Basteiro A.L., Marco A., Bayas J.M. Vaccinations in prisons: a shot in the arm for community health. Hum Vaccin Immunother. 2015;11(11):2615–2626. doi: 10.1080/21645515.2015.1051269. [DOI] [PMC free article] [PubMed] [Google Scholar]