Abstract
Background
The South-East Asia regional programme to eliminate lymphatic filariasis (LF) was launched in 2000. This study presents the progress and impact of the programme in the region.
Methods
Mass drug administration (MDA) and morbidity management data were accessed from the WHO preventive chemotherapy databank. The status of the programme in the nine South-East Asia countries was reviewed and progress was assessed. The impact of the programme on LF disease burden was estimated on the basis of the effectiveness of the MDA drugs against microfilaraemia and chronic disease.
Results
Under the MDA programme, 8.1 billion treatments were delivered in nine countries and 5.7 billion treatments were consumed by the target population during 2001–2018. Three of nine countries eliminated LF. Bangladesh is poised to reach its elimination goal by 2021. In the other five countries, 38–76% of intervention units completed intervention and surveillance is in progress. The MDA programme prevented or cured 74.9 million infections, equivalent to an 84.2% reduction. Close to 1 million lymphoedema patients and 0.5 million hydrocele patients were reported and are being provided with the minimum package of care.
Conclusions
The South-East Asia region's LF elimination programme reduced the burden of LF appreciably and is moving towards achieving the elimination goal in the next 8–10 y.
Keywords: elimination, impact, lymphatic filariasis, mass drug administration, morbidity management
Introduction
Lymphatic filariasis (LF) is a neglected tropical disease (NTD) widely prevalent in the South-East Asia region (SEAR). Chronic disease manifestations such as lymphoedema and hydroceles are disfiguring and disabling and inflict social and economic loss and stigma and poverty. The region consists of nine endemic countries with a population of 1.98 billion, of which 853 million (43%) live in known LF endemic areas. The nine endemic countries are India, Indonesia, Myanmar, Bangladesh, Nepal, Sri Lanka, Timor-Leste, Thailand and Maldives. The LF burden was very high in SEAR, accounting for, as of 2000, 70.0 million of the 129.8 million (54%) globally infected population.1 The economic impact of LF is significant and India alone suffers an annual loss of nearly US$ 1 billion.2
Amidst the escalating LF burden, the Global Programme to Eliminate LF (GPELF), launched in 2000,3 provide the tools and strategies to counter the disease in the region. The scalability of its twin strategies, (1) mass drug administration (MDA) to interrupt transmission and (2) morbidity management and disability prevention (MMDP) measures to alleviate suffering4 have attracted the attention of governments. Following the guidance of GPELF, the SEAR countries initiated the National Programmes to Eliminate LF (NPELF), with 2020 as the target date. The NPELF consists of (1) formation of a national taskforce, (2) mapping of endemic areas, (3) implementation of MDA and MMDP in endemic areas, (4) monitoring and evaluation and post-MDA transmission assessment surveys, (5) preparation of an LF elimination validation dossier and its submission by countries to WHO, (6) review of the dossier by a WHO-constituted expert group and an acknowledgement of countries claiming to have eliminated LF and (7) post-validation surveillance. The national programmes were backed by extensive preparatory work and research studies on mapping, MDA, surveillance and MMDP.5-9 Elimination of LF, along with other NTDs, has been a regional flagship programme of WHO since 2015. This paper presents the progress of the programmes to eliminate LF in SEAR as they complete 20 y (2000–2020) of operations.
MDA programme
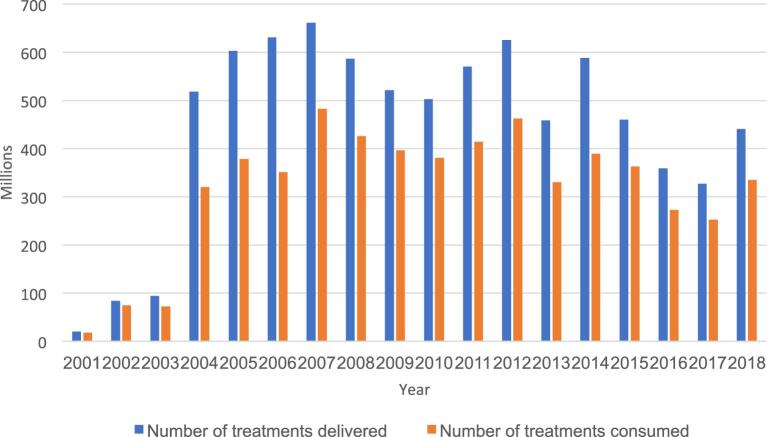
The population requiring MDA (using diethylcarbamazine [DEC] plus albendazole) in the first year of intervention (2001) was 700.8 million and it had increased to 853.4 million by 2018. While countries with a smaller endemic population such as Maldives, Thailand and Sri Lanka achieved 100% MDA geographic coverage by 2004, Indonesia reached the milestone in 2017, as far flung provinces and districts required additional logistic support and resources. Mobilising the resources, government machinery and health system, countries with large endemic populations expanded the MDA programme gradually. With a modest start covering 20.0 million living in 16 intervention units (IUs) in 2001, the drugs were delivered to a staggering 660.9 million living in 442 IUs in 2007. In 2018, the drugs were delivered to 440.3 million living in 333 IUs10 (Figure 1).
Figure 1.
The number of annual treatments delivered and consumed in SEAR during 2001–2018.
During 2001–2018, the national programmes delivered a total of 8.1 billion treatments, of which 5.7 billion were consumed by the target population of 853.4 million11 (Table 1). The gap between the number of treatments delivered and consumed was due to some people refusing to participate in treatment on account of a fear of adverse events, poor perceived benefits of treatment, the personal health status of individuals and poor drug delivery in some places.12 The regional reported treatment coverage varied from year to year ranging from 55.7% in 2006 to 88.7% in 2002. The average number of treatments delivered per person was 9.43 and treatments consumed was 6.7. In 66% of IUs in the region, MDA was completed and stopped after meeting the Transmission Assessment Survey (TAS) criteria. In countries where MDA is in progress, 38% to 76% of IUs (Table 1) and 403.4 million of the target population no longer require MDA.
Table 1.
MDA details and current status of the programme to eliminate LF in SEAR countries as of 2018–2019
| Country | Number of IUs | Population of IUs (millions) | Number of treatments delivered (millions) 2001–2019 | Number of treatments consumed (millions) 2001–2019 | % IUs MDA no longer required as of 2019 | Current programme status |
|---|---|---|---|---|---|---|
| India | 256 | 630 | 6727.3 | 47 103.4 | 38 | MDA and surveillance |
| Indonesia | 236 | 102 | 494.9 | 312.6 | 42 | MDA and surveillance |
| Myanmar | 45 | 40 | 327.4 | 292.8 | 71 | MDA and surveillance |
| Nepal | 63 | 25 | 175.9 | 130.8 | 76 | MDA and surveillance |
| Timor-Leste | 13 | 12.8 | 5.9 | 4.5 | 100* | MDA likely to be stopped |
| Bangladesh | 19 | 33 | 265.3 | 221 | 100 | Surveillance |
| Thailand | 350 | 0.17 | 1.2 | 1.1 | 100 | LF elimination acknowledged |
| Sri Lanka | 8 | 10.46 | 52.8 | 44.8 | 100 | LF elimination acknowledged |
| Maldives | 1 | <0.01 | 0.007 | 0.006 | 100 | LF elimination acknowledged |
Abbreviations: IU, intervention unit; LF, lymphatic filariasis; MDA, mass drug administration; TAS, Transmission Assessment Survey.
*Subject to TAS outcome.
MMDP
The underlying analyses presented in Table 2 show that there were an estimated 8.1 million lymphoedema cases and 5.6 million hydrocele cases in SEAR as of 2018, spread over hundreds of thousands of communities. The MMDP strategy envisages providing the minimum package of care to all chronic disease patients, which should be evident from surveys on the quality of lymphoedema services. To implement such a huge MMDP programme is challenging, particularly in countries with a high disease burden and vast endemic areas.13,14 As of 2018, 944 957 lymphoedema patients and 452 891 hydrocele patients were reported by the countries’ programmes.10 Enlisting the patients in all IUs using robust methodologies15,16 and expansion of the MMDP measures remains a priority for larger countries in the region. This has been well accomplished in Bangladesh, where more than 30 616 lymphoedema patients and 12 824 hydrocele patients were identified and are being provided with the recommended package of care.16 Countries with a relatively low disease burden such as Maldives, Thailand and Sri Lanka met the MMDP criteria required for validation of the elimination of LF.
Table 2.
Impact of the mass drug administration (MDA) programme (2001–2018) on reducing the lymphatic filariasis burden in the South-East Asia region
| Indicator | W. bancrofti | B. malayi | Total |
|---|---|---|---|
| Number of infections in 2000 | 63 | 7 | 70 |
| Estimated number of infections in 2018 with no MDA in place | 80.4 | 8.6 | 89 |
| Estimated number of total infections in 2018 after MDA impact | 12.4 | 1.5 | 13.9 |
| Estimated number of microfilaraemia cases in 2018 after MDA impact | 1.0 | 0.25 | 1.25 |
| Estimated number of lymphedema cases in 2018 after MDA impact | 6.7 | 1.4 | 8.1 |
| Estimated number of hydrocele cases in 2018 after MDA impact | 5.6 | 0 | 5.6 |
| Estimated number of cases prevented | 68.2 | 6.7 | 74.9 |
| % reduction in cases due to MDA | 84.8 | 77.9 | 84.2 |
Impact on LF burden
Three countries in the region, namely, Maldives, Sri Lanka17 and Thailand,18 met the LF elimination criteria and were validated by the WHO. All three countries eliminated LF by 2017, with five to six rounds of effective MDA (only the Narathiwat province in Thailand required nine rounds). Commitment, good health systems, effective implementation of intervention and surveillance measures enabled these countries to accomplish elimination relatively early. Bangladesh has completed the intervention measures and most of the surveillance and MMDP activities16,19 and is to submit the LF elimination dossier to WHO shortly. Various provinces of the other five countries, namely, India,20-22 Indonesia,11 Myanmar,23,24 Nepal25 and Timor-Leste,11 implemented multiple rounds of MDA. They are at different levels of progress and are making sustained efforts to reach the LF elimination goal in forthcoming years (Table 1).
The impact of the MDA programme on LF infection is assessed using a model described earlier.1 The 18 y (2001–2018) of the MDA programme in the region, under which 5.7 billion treatments were consumed, prevented or cured 74.9 million infections, which include microfilaria (Mf) carriers and chronic disease patients. This is equivalent to an 84.2% reduction in infections (Table 2) compared with the actual number of infections (89.0 million) that would have been prevalent had there been no MDA intervention.
The Bangladesh programme's best practice
Bangladesh, a lower to middle income country, stands out in the region by virtue of some best practice followed by the NPELF. Proactive programme leadership, good teamwork and consistent support by the Ministry of Health enabled the programme to move closer to elimination of LF.16,19 Some of the best practice of the programme includes (1) quality MDA and monitoring and evaluation practices, (2) robust delineation of endemicity and assessment of chronic disease burden in 19 endemic districts16 and 15 uncertain districts26 and implementation of MMDP measures and (3) active partnership with stakeholders and undertaking programme-specific research studies.27-30 The NPELF is likely to submit the dossier by 2021 for validation of elimination of LF, globally the first large high burden country to do so.
Acceleration of elimination of LF
The triple drug therapy of ivermctin plus DEC plus albendazole (IDA), shown to be more efficacious than the currently used two-drug therapy (DEC plus albendazole),31-33 was introduced into the region to accelerate the elimination of LF. Studies showed that all individuals treated with triple drug therapy remained Mf negative after 1 y compared with 1 of 12 individuals on the two-drug regimen31 and that this impressive Mf clearance was sustained for as long as 3 y.33 This instilled confidence that triple drug therapy will be able to eliminate LF with fewer rounds of MDA compared with two-drug therapy and that it will accelerate elimination of LF.32,34 Timor-Leste deployed the triple drug therapy in the entire nationwide MDA of 2019 and is the first country to do so globally. The programme covered 1.3 million and reported 76% treatment coverage. In 2018, India piloted IDA therapy in four districts with a population of >10 million and expanded this to 19 districts in 2019. The country has ambitious plans to expand further to all eligible districts. Indonesia, Myanmar and Nepal also have plans to introduce IDA therapy, particularly in those districts that fail in TAS. Implementation of triple drug therapy requires recalibration of some programme activities such as administering the drugs according to height as well as extensive social mobilisation and microplanning. Timor-Leste and India were able to make such changes, paving the way for other countries in the region. The community-level impact of the triple drug therapy is being evaluated.
Challenges
To achieve elimination of LF in the region, the following challenges need to be addressed:
Treatment coverage has been suboptimal in some districts across the large countries,35,36 particularly where health systems are weaker. So steps should be taken to attain effective treatment coverage.
Hotspots and residual infection37-39 in bancroftian and brugian filariasis endemic areas, which is seen in up to 34% of the communities in some situations,38 remain a threat. To overcome this, post-TAS surveillance methods40,41 should be standardised and introduced into the programme to eliminate residual microfilaraemia.
Comprehensive enlisting of chronic disease cases across the districts of large countries through robust and feasible methods15,16 and providing them with the minimum package of care by strengthening the necessary components of primary healthcare systems is required to meet the MMDP criteria to validate elimination of LF.
A considerable proportion of the at-risk population lives in urban areas where MDA implementation and treatment coverage have been suboptimal.35 A strategy to assess LF distribution and implementation of intervention measures in needy localities is required.
The LF status of some districts is uncertain42 and needs to be reassessed using robust sampling strategies.43 And it should be examined if some of these districts require intervention measures.
Continuous advocacy remains a high priority for the region to sustain the governmental and donor support to the programme for a sufficiently long time.
The ongoing pandemic of COVID-19 has disrupted community-based interventions, including MDA and pre-TAS/TAS assessment, in many IUs across countries. The effect of COVID-19 on global economies will also change the NTD funding landscape, both locally and globally, which may impact the LF elimination programmes in the region. So stakeholders need to work together to sustain the priority activities and gains of the NPELF.
Conclusions
The SEAR has made tremendous progress towards elimination of LF and has already reduced the disease burden significantly. The larger countries in the region are likely to complete intervention in 3–5 y and surveillance in another 4 y. The region is well placed to achieve the goal of LF elimination by 2028–2030.
Contributor Information
D Ramaiah Kapa, Consultant LF Epidemiologist, Puducherry 605008, India.
Ahmed Jamsheed Mohamed, Depa rtment of Communicable Diseases, WHO Regional Office for South-East Asia, New Delhi 110002, India.
Authors’ contributions
KDR prepared the first draft of the manuscript and analysed the data. AMJ identified and separated data and created a database. Both authors read, reviewed and approved the final manuscript. KDR is the guarantor of the paper.
Funding
The publication of the papers within this supplement were supported by MSD, GSK and Eisai through the Mectizan Donation Program (MDP) and the Global Alliance for LF Elimination (GAELF).
Competing interests
None declared.
Ethical approval
The work was approved by the South-East Asia Regional Office, WHO, New Delhi, India.
References
- 1. Ramaiah KD, Ottesen EA.. Progress and impact of 13 years of the global programme to eliminate lymphatic filariasis on reducing the burden of filarial disease. PLoS Negl Trop Dis. 2014;8(11):e3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ramaiah KD, Das PK, Michael E et al. The economic burden of lymphatic filariasis in India. Parasitol Today. 2000;16(6):251–3. [DOI] [PubMed] [Google Scholar]
- 3. Ottesen EA. The global programme to eliminate lymphatic filariasis. Trop Med Int Hlth. 2000;5(9):591–4. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization Lymphatic Filariasis. Global Programme to Eliminate Lymphatic filariasis; https://www.who.int/lymphatic_filariasis/elimination-programme/en/ [accessed 16 April 2020]. [Google Scholar]
- 5. Sabesan S, Palaniyandi M, Das PK et al. Mapping of lymphatic filariasis in India. Ann Trop Med Parasitol. 2000;94(6):591–606. [DOI] [PubMed] [Google Scholar]
- 6. Sherchand JB, Obsomer V, Thakur GD et al. Mapping of lymphatic filariasis in Nepal. Filaria J. 2003;2(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ramaiah KD, Das PK.. Mass drug administration to eliminate lymphatic filariasis in India. Trends Parasitol. 2004;20(11):499–502. [DOI] [PubMed] [Google Scholar]
- 8. Fischer P, Supali T, Maizels RM. Lymphatic filariasis and Brugia timori: prospects for elimination. Trends Parasitol. 2004;20(8):351–5. [DOI] [PubMed] [Google Scholar]
- 9. Shenoy RK, Suma TK, Kumaraswami V. A qualitative study on the feasibility and benefits of food hygiene measures practiced by patients with brugian filariasis. J Commun Dis. 2003;35(1):9–16. [PubMed] [Google Scholar]
- 10. World Health Organization Weekly Epidemiological Record. 2019;94:457–72. [Google Scholar]
- 11. World Health Organization Neglected Tropical Diseases. PCT Databank; https://www.who.int/neglected_diseases/preventive_chemotherapy/databank/en [accessed 16 April 2020]. [Google Scholar]
- 12. Krentel A, Fischer PU, Weil GJ. A review of factors that influence individual compliance with mass drug administration for elimination of lymphatic filariasis. PLoS Negl Trop Dis. 2013;7(11):e2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chandrasena N, Premaratna R, Gunaratna IE et al. Morbidity management and disability prevention for lymphatic filariasis in Sri Lanka: Current status and future prospects. PLoS Negl Trop. 2018;12(5):e0006472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cassidy T, Worrell CM, Little K et al. Experience of a community-based lymphoedema management programme for lymphatic filariasis in Odisha state, India: An analysis of focus group discussions with patients, families, community members and programme volunteers. PLoS Negl Trop Dis. 2016;10(2):e0004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Walsh V, Little K, Wiegand R et al. Evaluating the burden of lymphedema due to lymphatic filariasis in 2005 in Khurda district, Odisha state, India. PLoS Negl Trop Dis. 2016;10(8):e0004917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karim MJ, Haq R, Mableson HE et al. Developing the first national database and map of lymphatic filariasis clinical cases in Bangladesh: Another step closer to the elimination goals. PLoS Negl Trop Dis. 2019;13(7):e0007542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization, South-East Asia News Release SEAR/PR/1626.
- 18. Rojanapanus S, Toothong T, Boondej P, et al. How Thailand eliminated lymphatic filariasis as a public health problem. Infect Dis Poverty. 2019;8(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shamsuzzaman AK, Haq R, Karim MJ et al. The significant scale up of Transmission Assessment Surveys ‘TAS’ for endgame surveillance of lymphatic filariasis in Bangladesh: One step closer to the elimination goal of 2020. PLoS Negl Trop Dis. 2017;11(1):e0005340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. National Vector Borne Disease Control Programme Director General of Health Services. Ministry of Health & Family Welfare; https://nvbdcp.gov.in/index1 [accessed 16 April 2020]. [Google Scholar]
- 21. Shriram AN, Premkumar A, Krishnamoorthy K et al. Elimination of diurnally sub-periodic Wuchereria bancrofti in Andaman and Nicobar Islands, India, using mass DEC-fortified salt as a supplementary intervention to MDA. Parasitol Res. 2020; DOI:10.1007/s00436-020-06659-7. [DOI] [PubMed] [Google Scholar]
- 22. Srivastava PK, Dhillon GP. Elimination of lymphatic filariasis in India–a successful endeavour. J Indian Med Assoc. 2008;106(10):673–7. [PubMed] [Google Scholar]
- 23. Aye NN, Lin Z, Lon KN et al. Mapping and modelling the impact of mass drug administration on filariasis prevalence in Myanmar. Infect Dis Poverty. 2018;7(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Win KM, Tripathy JP, Maung TM et al. Rapid progress towards elimination of lymphatic filariasis in endemic regions of Myanmar as a result of 16 years of anti-filarial activities (2001–2016). Trop Med Health. 2018;46:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ojha CR, Joshi B, Kc KP et al. Impact of mass drug administration for elimination of lymphatic filariasis in Nepal. PLoS Negl Trop Dis. 2017;11(7):e0005788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sultan Mahmood ASM, Haq R, Rahman M et al. Methods for assessing lymphatic filariasis transmission in low endemic areas of Bangladesh: one step closer to the elimination goal. American Society of Tropical Medicine and Hygiene, 63rd Annual Meeting, New Orleans, 2014.
- 27. Hafiz I, Graves P, Haq R et al. Clinical case estimates of lymphatic filariasis in an endemic district of Bangladesh after a decade of mass drug administration. Trans R Soc Trop Med Hyg. 2015;109(11):700–9. [DOI] [PubMed] [Google Scholar]
- 28. Mableson H, Martindale S, Stanton MC et al. Community-based field implementation scenarios of an SMS reporting tool for lymphatic filariasis case estimates in Africa and Asia. mHealth. 2016;3:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Irish SR, Al-Amin HM, Paulin HN et al. Molecular xenomonitoring for Wuchereria bancrofti in Culex quinquefasciatus in two districts in Bangladesh supports transmission assessment survey findings. PLoS Negl Trop Dis. 2018;12:e0006574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Douglass J, Mableson HE, Martindale S et al. An enhanced self-care protocol for people affected by moderate to severe lymphedema. Methods Protoc. 2019;2(3):E77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thomsen EK, Sanuku N, Baea M, Satofan S, Maki E et al. Efficacy, safety, and pharmacokinetics of coadministered diethylcarbamazine, albendazole, and ivermectin for treatment of bancroftian filariasis. Clin Infect Dis. 2016;62:334–41. [DOI] [PubMed] [Google Scholar]
- 32. Irvine MA, Stolk WA, Smith ME et al. Effectiveness of a triple-drug regimen for global elimination of lymphatic filariasis: a modelling study. Lancet Infect Dis. 2017;17(4):451–8. [DOI] [PubMed] [Google Scholar]
- 33. King CL, Suamani J, Sanuku N et al. A trial of triple-drug treatment for lymphatic filariasis. N Eng J Med. 2018;379(19):1801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. WHO Guideline Alternative mass drug administration regimens to eliminate lymphatic filariasis. World Health Organization 2017. [PubMed] [Google Scholar]
- 35. Babu BV, Babu GR.. Coverage of and compliance with mass drug administration under the programme to eliminate lymphatic filariasis in India: a systematic review. Trans R Soc Trop Med Hyg. 2014;108(9):538–49. [DOI] [PubMed] [Google Scholar]
- 36. Krentel A, Damayanti R, Titaley CR et al. Improving coverage and compliance in mass drug administration for the elimination of LF in two ‘endgame’ districts in Indonesia using micronarrative surveys. PLoS Negl Trop Dis. 2016;10(11):e0005027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dickson BFR, Graves PM, Aye NN et al. The prevalence of lymphatic filariasis infection and disease following six rounds of mass drug administration in Mandalay Region, Myanmar. PLoS Negl Trop Dis. 2018;12(11):e0006944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Subramanian S, Vanamail P, Srividya A et al. Epidemiological assessment of eight rounds of mass drug administration for lymphatic filariasis in India: implications for monitoring and evaluation. PLoS Negl Trop Dis. 2012;6(11):e1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rao RU, Nagodavithana KC, Samarasekera SD et al. A comprehensive assessment of lymphatic filariasis in Sri Lanka six years after cessation of mass drug administration. PLoS Negl Trop Dis. 2014;8(11):e3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rao RU, Samarasekera SD, Nagodavithana KC et al. Systematic sampling of adults as a sensitive means of detecting persistence of lymphatic filariasis following mass drug administration in Sri Lanka. PLoS Negl Trop Dis. 2019;13(4):e0007365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Subramanian S, Jambulingam P, Krishnamoorthy K et al. Molecular xenomonitoring as a post-MDA surveillance tool for global programme to eliminate lymphatic filariasis: Field validation in an evaluation unit in India. PLoS Negl Trop Dis. 2020;14(1):e0007862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chand G, Kaushal LS, Choudari NK et al. Mapping is a prerequisite for elimination of filariasis and effective targeting of filarial ‘hot spots’. Pathog Glob Health. 2016;110(4-5):157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sime H, Gass KM, Mekasha S et al. Results of a confirmatory mapping tool for lymphatic filariasis endemicity classification in areas where transmission was uncertain in Ethiopia. PLoS Negl Trop Dis. 2018;12(3):e0006325. [DOI] [PMC free article] [PubMed] [Google Scholar]