Abstract
In South and Central America, lymphatic filariasis (LF) is caused by Wuchereria bancrofti, which is transmitted by Culex quinquefasciatus, the only vector species in this region. Of the seven countries considered endemic for LF in the Americas in the last decade, Costa Rica, Suriname and Trinidad and Tobago were removed from the World Health Organization list in 2011. The remaining countries, Brazil, Dominican Republic, Guyana and Haiti, have achieved important progress in recent years. Brazil was the first country in the Americas to stop mass drug administration (MDA) and to establish post-MDA surveillance. Dominican Republic stopped MDA in all LF-endemic foci: La Ciénaga and Southwest passed the third Transmission Assessment Survey (TAS) and the Eastern focus passed TAS-1 in 2018. Haiti passed the TAS and interrupted transmission in >80% of endemic communes, achieving effective drug coverage. Guyana implemented effective coverage in MDAs in 2017 and 2018 and in 2019 scaled up the treatment for 100% of the geographical region, introducing ivermectin in the MDA in order to achieve LF elimination by the year 2026. The Americas region is on its way to eliminating LF transmission. However, efforts should be made to improve morbidity management to prevent disability of the already affected populations.
Keywords: Americas region, elimination program, lymphatic filariasis, Wuchereria bancrofti
Introduction
Lymphatic filariasis (LF) is a vector-borne neglected tropical disease that is highly debilitating with economic and social impacts. In the Americas, the disease is caused exclusively by the parasitic worm Wuchereria bancrofti, which was probably introduced to the continent by the migration of African slaves.1 The mosquito Culex quinquefasciatus is the only transmitting species on the American continent2 and the microfilariae of the parasite show nocturnal periodicity in the blood.3
The disease impairs the lymphatic system, leading to chronic disabling consequences such as lymphoedema, hydrocele and elephantiasis. Several measures to interrupt LF transmission are advised by the World Health Organization (WHO), mainly mass drug administration (MDA) followed by Transmission Assessment Surveys (TASs) and monitoring.4
Since 1997, when Resolution WHA 50.29 of the World Health Assembly set the year 2020 as the target for LF elimination as a global public health problem,5 the WHO has been engaged to meet this objective.6
The Global Programme to Eliminate Lymphatic Filariasis (GPELF) was launched in 2000 by the WHO with the objective of eliminating the disease as a public health problem worldwide by 2020.7 This programme has adopted and included two main components: mass drug administration (MDA) to interrupt transmission and morbidity management and disability prevention (MMDP) to support those already affected by the chronic manifestations of LF.7
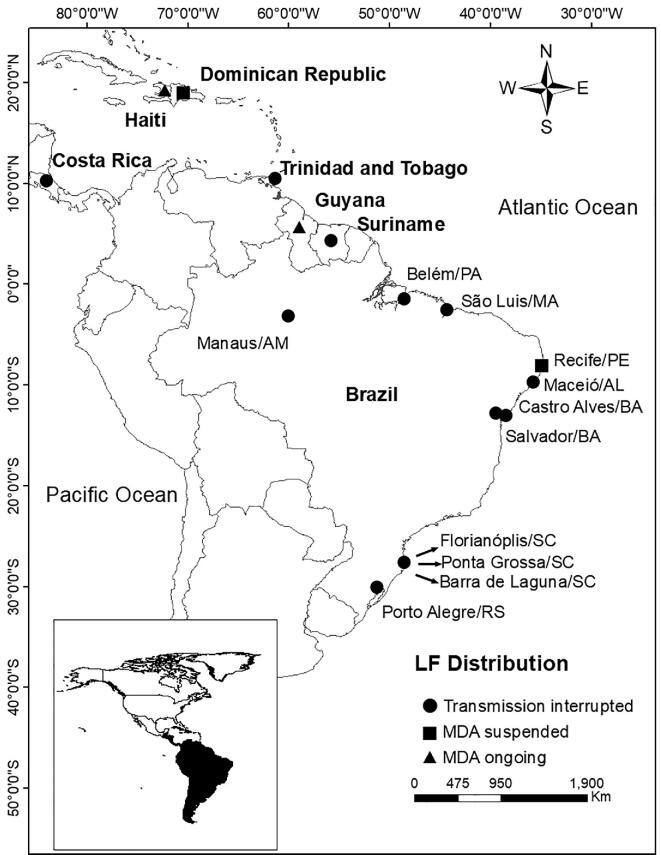
In the Americas region, LF was historically endemic in 24 countries.8 Moreover, in another 10 countries there was some evidence of W. bancrofti transmission, but this was not well documented.8 When the GPELF was initiated, LF transmission was occurring in only seven countries9 (Figure 1). However, Costa Rica, Suriname and Trinidad and Tobago were removed from the WHO list of LF-endemic countries in 2011.7 Brazil, Dominican Republic, Guyana and Haiti, the four remaining endemic countries, have achieved important progress in recent years.
Figure 1.
Distribution of LF in countries and cities in the Americas, showing the current situation of LF elimination in each location and the status of MDA.
We present here a summary of the progress towards LF elimination in the Americas region since the inception of the GPELF.
Former LF-endemic countries
Costa Rica
Puerto Limón, a city located on the eastern coast of Costa Rica, was described as the only LF-endemic area in this country in 1946.8 Clinical and epidemiological studies carried out between 1974 and 1983 confirmed that LF was focally distributed in the country, with low infection prevalence and reduced microfilaremia rates.10,11 The elimination strategy was based on selective treatment of the microfilariae carriers with diethylcarbamazine (DEC), followed by a 7 year follow-up of all people detected in 1980–2000.11 Antigenemia mapping performed in 2003 showed interruption of LF transmission in this single focus in Costa Rica11 and in 2011 this country was removed from the WHO list of LF-endemic countries.7
Suriname
The earliest reference of W. bancrofti in Suriname was obtained from reports from Paul C. Flu from 1909.8 Surveys conducted in the 1940s registered a prevalence of microfilariae carriers of up to 30%, leading to a systematic LF control program after 1949. Thus filarial prevalence decreased to 0.1% in 1981.12 The success of the elimination program in Suriname was based on mass detection of microfilaremics and treatment campaigns with DEC, along with the improvement of sanitary conditions. In 2002, a nationwide survey was performed using the rapid immunochromatographic test (ICT) for the detection of circulating filarial antigen among 3000 school-age children, according to the WHO guidelines.13 All blood samples were negative, indicating that LF transmission had been interrupted in the country. In 2011, Suriname was also removed from the WHO list of LF-endemics countries.7
Trinidad and Tobago
The first references to LF in Trinidad and Tobago appear in the annual reports of the surgeon general between 1893 and 1937.8,14 Surveys conducted in 1976, 1979 and 1982 on the north coast of Trinidad indicated that LF was a public health problem in the country, presenting microfilarial prevalences of up to 15%.14,15 In 1982, a community-wide control program was implemented and DEC was given in a single dose of 6 mg/kg at monthly intervals over 12 months. A follow-up survey conducted 12 years later suggested there was no further transmission of W. bancrofti.15 In 1999 a survey conducted in Trinidad among adults and school-age children to detect circulating W. bancrofti antigen showed no positive results.16 In a large-scale nationwide survey conducted in 2002–2003 using ICT cards in school-age children, blood samples were negative for antigen, indicating that LF transmission had been interrupted in the study area.17 In 2011, Trinidad and Tobago was no longer considered by WHO as an endemic country.7
Currently endemic countries
Brazil
From 1951 to 1958, a national epidemiological survey conducted by the public health authorities in Brazil confirmed autochthonous transmission of LF in the country. The distribution of infection was urban and focal, comprising 11 foci in eight states, mainly along the Brazilian coast18,19 (Figure 1).
Control measures were based mainly on selective DEC therapy of the patients, who were followed up after treatment.19 This led to a declining trend in parasite rates.
In the 1980s, only the cities of Belém (North Region) and Recife (Northeast Region) were considered to be endemic.20 But in the 1990s, active transmission of LF was also described in the cities of Maceió, Olinda, Jaboatão dos Guararapes and Paulista on the northeastern coast of Brazil.19,21,22 Evidence for the absence of transmission was obtained in Belém, Salvador and Maceió in the early 2000s, attesting to the effectiveness of the control measures adopted in these cities.19,23 Since then, LF has remained a public health problem in only four cities in the metropolitan region of Recife in Pernambuco State (northeastern coast).19,24 All efforts were concentrated in these areas, using MDA to eliminate LF transmission in the country. Different from other countries that have used double- or triple-drug MDA (DEC+albendazole or ivermectin+DEC+albendazole), Brazil, due to a decision by its Ministry of Health, used only DEC in MDA.
Since 2003, annual rounds of MDA with DEC have been implemented in the LF foci in Recife, Olinda and Jaboatão, which are currently under surveillance to confirm the goal of eliminating this disease (MDA was not introduced in Paulista because the prevalence was <0.3%).19
Brazil was the first country in the Americas to stop MDA and establish post-MDA surveillance. The third and last TAS will be conducted in 2020.
An important morbidity management initiative in the Recife LF-endemic area, lead by Dr Gerusa Dreyer, was a pioneering proposal called Hope Clubs.25,26 Support groups of chronic LF patients and their families are trained to practice self-care lymphoedema management to prevent acute dermatolymphangioadenitis (ADLA) through hygiene measures. It also offers peer support among its members, reducing social isolation and providing emotional assistance.25,26
The country is now preparing the dossier to be submitted and validated by the Regional Program Review Group (RPRG), thus reaching the goal of eliminating LF as a public health problem by 2020.
Dominican Republic
The initial record of LF in the Dominican Republic dates back to 1947 in Santo Domingo.8,27 Over the years, many epidemiological studies have shown LF prevalence of up to 7.8% of microfilariae carriers in the national capital.27,28 The documented foci of the disease in the 1980s and 1990s were located in the cities of Santo Domingo and San Cristobal and in provinces of the Southwest and East regions of the country.27–29
The LF Elimination Program was launched in 199824 and baseline mapping identified the following three focal areas of active transmission: La Ciénaga (a slum area of the capital Santo Domingo) and the Southwest (Barahona) and East regions (eastern bateyes, which are rural settlements around sugar cane mills).29 The first MDA and morbidity management were implemented in 2002 in the Southwest focus,24 where the population received five rounds of annual DEC–albendazole between 2002 and 2007.29 The foci of La Ciénaga and the East received three MDA rounds from 2004 to 2006 and from 2014 to 2017, respectively.30,31 In 2018, La Ciénaga and the Southwest had already completed and passed TAS-3 and are in post-treatment surveillance.30
In La Ciénega and the Southwest, the TAS indicated that LF transmission was interrupted.29–31 The East focus passed TAS-1 in 2018 and will carry out TAS-2 and TAS-3 in 2020 and 2022, respectively, to move from MDA to post-treatment surveillance.31 In this last focus, LF transmission appears to be absent or at least substantially reduced.31
In the Dominican Republic, one important achievement was the integration of the LF elimination program with other disease control activities of the Ministry of Health.32 This collaborative integration scaled up MDA coverage, improved information systems and strengthened relationships between the health services and the community.32
The main activities and challenges of the programme to eliminate LF in the Dominican Republic after 2018 are to consolidate morbidity management, finish the TAS in the East focus and prepare the report in order to request from the Pan American Health Organization (PAHO)/WHO validation of the elimination of LF as a public health problem.31
Guyana
In Guyana, and other English-speaking parts of the Caribbean, it is believed that LF could have come via indentured labour imported from India and China, as well as from the African slaves brought to work on the plantations.8
The first evidence of LF affecting people in Guyana was reported in 1877.8 Epidemiological studies carried out since the 1940s showed prevalences of up to 15.9%.33 The situation of LF in the country remained almost unchanged until the creation, in 1999, of a national programme to interrupt LF transmission and to control morbidity.24 At that time, in a survey covering the capital, Georgetown, and adjoining areas, microfilariae prevalence ranged from 1.7 to 31.4%.15 In another study, screening of W. bancrofti circulating antigen resulted in positivity rates of 21.9% and 29.3% in children and adults, respectively.16 Subsequently, in 2001, a nationwide mapping of school-age children showed 9.3% positive exams for LF, particularly in urban areas.34 In 2003, the highest LF prevalence was found in the northern coastal belt, where 90% of the country's population lives.24
Progress toward elimination of the disease was made from 2003 to 2007, based on the introduction of DEC-fortified salt.34 Because of technical problems with the production and distribution of the DEC salt, the elimination strategy changed to MDA with DEC and albendazole from 2014 onwards.31
In Guyana, disease management combining patient education and access to appropriate treatments had a significant health benefit, improving the quality of life of patients with lymphoedema.35
During the years 2017 and 2018 Guyana achieved high MDA coverage (>65%).31 In 2019 the country changed its strategy by adding ivermectin to treatment with DEC and albendazole (IDA) and expanded the MDA from four to eight regions covering 100% of the geographical endemic area.31 If all goes as planned, the expected year for the validation of elimination of LF as a public health problem by the PAHO/WHO will be 2026.31
Haiti
Elephantiasis, one of the most characteristic clinical forms of LF, has been reported in Haiti since the mid-1700s, and studies during the 20th century indicated that W. bancrofti was widely distributed throughout the country.8 However, only in the 2000s was a national filariasis survey conducted, after the National Program to Eliminate LF (NPELF) was announced in 2001. A baseline survey was carried out to detect circulating W. bancrofti antigen in school-age children from all 133 communes (districts) in the country, in which 117 (87.9%) positive cases were found.36 Thus almost the entire population in Haiti was considered at risk of infection.
In 2002, MDA was started with DEC+albendazole and by 2005 1.6 million people in 24 communes were targeted at least once for MDA.37 Despite a slow start during the first 8 years of the NPELF, treatment numbers rapidly increased after 2008.
By 2012, Haiti's NPELF had reached full geographic coverage and in 2014 about 20 communes had satisfied the criteria to stop MDA.38 In 2015, following TAS, 45 communes had stopped MDA,38 resulting in a scaling down of geographical coverage.
Despite many challenges (political crisis, interruption in financial support, hurricanes, earthquake), the NPELF stands as an exceptional public health success in Haiti.37,38
Currently 117 of the 140 communes have passed the TAS and no longer need treatment, meaning that >80% of the country is in the post-treatment surveillance period. Only 22 communes remain endemic, most of them in urban areas, highlighting the importance of achieving effective coverage to progress towards the LF elimination goal.31
Although there is no definitive cure for lymphoedema, a study carried out in Haiti confirmed the effectiveness of proper hygiene and skin care in the reduction acute episodes of ADLA under field conditions in a resource-limited area.39
The experience of MDA 2018 and 2019 using microplanning, reinforcing social communication and supervision, improved the outcome of the campaign and opens up new opportunities to move forward to the LF elimination goal.31
Conclusions
Given the progress in Latin America and the Caribbean (LAC), the population requiring MDA in the region has decreased by 12.9 million (67% reduction). In order to speed up the process towards regional elimination of LF in the Americas, efforts have been intensified and some strategies were implemented to optimize the MDA coverage, such as microplanning using a bottom-up approach, improving communication and social mobilization to engage the targeted population, combining drug distribution strategies (fixed posts, door to door and schools), rapid coverage monitoring and mop-up almost immediately after the MDA is implemented.
Recent evidence indicates that MDA using the IDA combination rather than the routine two-medicine combination (DEC+albendazole) clears microfilaria more efficiently from the blood.40 A high-coverage MDA using IDA could accelerate the elimination of LF as a public health problem in the Americas. However, one of the pillars of the national programme, morbidity management and disability prevention, is still a great challenge in the Americas. While all four countries working towards LF elimination have submitted some information to their ministries of public health, the burden of the disease is still underestimated and the coverage of the basic care package for management of chronic morbidity at the primary healthcare level needs to be expanded.
Acknowledgements
None.
Contributor Information
Gilberto Fontes, Laboratório de Parasitologia, Campus Centro Oeste, Universidade Federal de São João del Rei, Minas Gerais, Brazil.
Eliana Maria Mauricio da Rocha, Laboratório de Parasitologia, Campus Centro Oeste, Universidade Federal de São João del Rei, Minas Gerais, Brazil.
Ronaldo Guilherme Carvalho Scholte, Neglected Infectious Diseases Program, Neglected, Tropical and Vector-Borne Diseases Unit, Department of Communicable Diseases and Environmental Determinants of Health, Pan American Health Organization (PAHO), Washington DC, USA.
Rubén Santiago Nicholls, Neglected Infectious Diseases Program, Neglected, Tropical and Vector-Borne Diseases Unit, Department of Communicable Diseases and Environmental Determinants of Health, Pan American Health Organization (PAHO), Washington DC, USA.
Authors’ contributions
All authors were responsible for the conceptualization, literature search and drafting, review and editing of the manuscript for publication.
Funding
The publication of the papers within this supplement were supported by MSD, GSK and Eisai through the Mectizan Donation Program (MDP) and the Global Alliance for LF Elimination (GAELF).
Competing interests
None declared.
Ethical approval
Not required.
References
- 1. Orihel TC. Filariae. In: Beaver PC, Jung RC (). Animals agents and vectors of human disease, 5th ed Philadelphia: Lea & Febiger; 1985, p. 171–91. [Google Scholar]
- 2. World Health Organization Lymphatic filariasis: a handbook of practical entomology. WHO/HTM/NTD/PCT/2013.10; Geneva: World Health Organization; 2013. [Google Scholar]
- 3. Fontes G, Rocha EMM, Brito AC et al. . The microfilarial periodicity of Wuchereria bancrofti in northeastern Brazil. Ann Trop Med Parasitol. 2000;94(4):373–9. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization Lymphatic filariasis: monitoring and epidemiological assessment of mass drug administration. A manual for national elimination programmes. WHO/HTM/NTD/PCT/2011.4 Geneva: World Health Organization; 2011. [Google Scholar]
- 5. World Health Organization Fiftieth World Health Assembly, Geneva, 5–14 May 1997: resolutions and decisions, annexes. WHA50/1997/REC/1 Geneva: World Health Organization; 1997, p. 5–14. [Google Scholar]
- 6. World Health Organization Preparing and implementing a national plan to eliminate lymphatic filariasis: a guideline for programme managers. WHO/CDS/CPE/CEE/2000.15; Geneva: World Health Organization; 2000, p. 65. [Google Scholar]
- 7. World Health Organization Global programme to eliminate lymphatic filariasis: progress report on mass drug administration, 2010. Wkly Epidemiol Rec. 2011;86(35):377–88. [PubMed] [Google Scholar]
- 8. Addiss DG, Chuke SO.. Lymphatic filariasis in the Americas: an epidemiologic history. Atlanta, GA:Centers for Disease Control and Prevention; 2002. [Google Scholar]
- 9. World Health Organization Global Programme to Eliminate Lymphatic Filariasis: annual report on lymphatic filariasis. WHO/CDS /CPE/CEE/2002.28; Geneva: World Health Organization; 2002. [Google Scholar]
- 10. Paniagua F, Garcés JL, Granados C et al. . Prevalence of bancroftian filariasis in the city of Puerto Limón and the province of Limón, Costa Rica. Am J Trop Med Hyg. 1983;32(6):1294–7. [DOI] [PubMed] [Google Scholar]
- 11. Ministerio de Salud de Costa Rica Interrupción de la transmissión de Filariasis Bancrofti em La ciudad de Porto Limón. Serie documentos técnicos n° 3. Elaborado por Garcés JL and Paniagua F. Costa Rica/Ministerio de Salud. 1ª ed San José, Costa Rica: Ministerio de Salud de Costa Rica;2003. [Google Scholar]
- 12. Oostburg BFJ. Is wuchereriasis a disappearing disease in Suriname? Acta Leiden. 1985;53:37–50. [PubMed] [Google Scholar]
- 13. Pan American Health Organization. Lymphatic filariasis elimination in the Americas: report In: Proceedings of the 5th Regional Program Manager's Meeting, Suriname. Washington, DC: Pan American Health Organization; 2004. [Google Scholar]
- 14. Nathan MB, Beckles G, Tikasingh ES et al. . Parasitological and clinical studies of Wuchereria bancrofti and Mansonella ozzardi in coastal north Trinidad, West Indies. West Indian Med J. 1982;31(4):168–76. [PubMed] [Google Scholar]
- 15. Pan American Health Organization. Lymphatic filariasis elimination in the Americas: report In: Proceedings of the First Regional Program Manager's Meeting, Dominican Republic. Washington, DC: Pan American Health Organization; 2000. [Google Scholar]
- 16. Rawlins SC, Lammie L, Tiwari T et al. . Lymphatic filariasis in the Caribbean region: the opportunity for its elimination and certification. Rev Panam Salud Publica. 2000;7(5):319–24. [DOI] [PubMed] [Google Scholar]
- 17. Rawlins SC, Siung-Chang A, Baboolal S et al. . Evidence for the interruption of transmission of lymphatic filariasis among schoolchildren in Trinidad and Tobago. Trans R Soc Trop Med Hyg. 2004;98(8):473–7. [DOI] [PubMed] [Google Scholar]
- 18. Rachou RG. Conceito e programa de profilaxia da filariose bancroftiana no Brasil. Rev Bras Malariol Doenças Trop. 1960;12:11–40.14038551 [Google Scholar]
- 19. Fontes G, Leite AB, Lima ARV et al. . Lymphatic filariasis in Brazil: epidemiological situation and outlook for elimination. Parasit Vectors. 2012;5:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ministério da Saúde do Brasil O Controle das Endemias no Brasil (de 1979 a 1984). Brasília: Superintendência de Campanhas de Saúde Pública, SUCAM; 1985. [Google Scholar]
- 21. Maciel MAV, Marzochi KBF, Silva EC et al. . [Comparative studies on endemic areas of bancroftian filariasis in Greater Recife, Brazil]. Cad Saude Publica. 1994;10(Suppl 2):301–9. [PubMed] [Google Scholar]
- 22. Medeiros Z, Bonfim C, Alves A et al. . The epidemiological delimitation of lymphatic filariasis in an endemic area of Brazil, 41 years after the first recorded case. Ann Trop Med Parasitol. 2008;102(6):509–19. [DOI] [PubMed] [Google Scholar]
- 23. Fontes G, Braun RF, Fraiha-Neto H et al. . Filariose linfática em Belém Estado do Pará Norte do Brasil e a perspectiva de eliminação. Rev Soc Bras Med Trop. 2005;38(2):131–6. [DOI] [PubMed] [Google Scholar]
- 24. Pan American Health Organization Lymphatic filariasis elimination in the Americas. In: Proceedings of the 4th Regional Lymphatic Filariasis Elimination Program Manager's Meeting, Maceió. Washington, DC: Pan American Health Organization; 2003. [Google Scholar]
- 25. Dreyer G, Addiss D.. Hope Clubs: new strategy for lymphatic filariasis endemic areas. Newslett R Soc Trop Med Hyg. 2000;8:8. [Google Scholar]
- 26. Dreyer G, Norões J, Mattos D. [Hope Clubs as adjunct therapeutic measure in bancroftian filariasis endemic areas]. Rev Soc Bras Med Trop. 2006;39(4):365–9. [DOI] [PubMed] [Google Scholar]
- 27. Vincent AL, Vargas de Goméz M, Gonzalvo A et al. . Filariasis in the Dominican Republic. Am J Trop Med Hyg. 1981;30(3):739–41. [DOI] [PubMed] [Google Scholar]
- 28. Vincent AL, Gonzalvo A, Cowell BC et al. . A survey of Bancroftian filariasis in the Dominican Republic. J Parasitol. 1987;73(4):839–40. [PubMed] [Google Scholar]
- 29. Noland GS, Blount S, Gonzalez M. Post-mass drug administration transmission assessment survey for elimination of lymphatic filariasis in La Ciénaga, Dominican Republic. Am J Trop Med Hyg. 2015;93(6):1292–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Keys HM, Noland GS, De Rochars MB et al. . Prevalence of malaria and lymphatic filariasis in bateyes of the Dominican Republic. Infect Dis Poverty. 2019;8(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pan American Health Organization Lymphatic filariasis elimination in the Americas. In: Proceedings of the 18th Regional Lymphatic Filariasis Elimination Program Manager's Meeting. Washington, DC: Pan American Health Organization; 2018. [Google Scholar]
- 32. Baker MC, McFarland DA, Gonzales M et al. . The impact of integrating the elimination programme for lymphatic filariasis into primary health care in the Dominican Republic. Int J Health Plann Manage. 2007;22(4):337–52. [DOI] [PubMed] [Google Scholar]
- 33. Nathan MB, Stroom V.. Prevalence of Wuchereria bancrofti in Georgetown, Guyana. Bull Pan Am Health Org. 1990;24(3):301–6. [PubMed] [Google Scholar]
- 34. Pan American Health Organization Lymphatic filariasis elimination in the Americas: report. In: Proceedings of the 11th Regional Program Manager's Meeting. Washington, DC: Pan American Health Organization; 2010. [Google Scholar]
- 35. McPherson T. Impact on the quality of life of lymphoedema patients following introduction of a hygiene and skin care regimen in a Guyanese community endemic for lymphatic filariasis: a preliminary clinical intervention study. Filaria J. 2003;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beau de Rochars MVE, Milord MD, St Jean Y et al. . Geographic distribution of Lymphatic filariasis in Haiti. Am J Trop Med Hyg. 2004;71(5):598–601. [PubMed] [Google Scholar]
- 37. Oscar R, Lemoine JF, Direny AN et al. . Haiti National Program for the Elimination of Lymphatic Filariasis – a model of success in the face of adversity. PLoS Negl Trop Dis. 2014;8(7):e2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lemoine JF, Desormeaux AM, Monestime F et al. . Controlling neglected tropical diseases (NTDs) in Haiti: implementation strategies and evidence of their success. PLoS Negl Trop Dis. 2016;10(10):e0004954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Addiss DG, Louis-Charles J, Roberts J et al. . Feasibility and effectiveness of basic lymphedema management in Leogane, Haiti, an area endemic for Bancroftian filariasis. PLoS Negl Trop Dis. 2010;4(4):e668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. World Health Organization Lymphatic filariasis. Key facts. https://www.who.int/news-room/fact-sheets/detail/lymphatic-filariasis [accessed 11 July 2020]. [Google Scholar]