Abstract
Purpose:
Previous studies report memory and functional connectivity of memory systems improve acutely after a single aerobic exercise session or with training, suggesting the acute effects of aerobic exercise may reflect initial changes that adapt over time. In this trial, for the first time, we test the proof-of-concept of whether the acute and training effects of aerobic exercise on working memory and brain network connectivity are related in the same participants.
Methods:
Cognitively normal older participants (N=34) were enrolled in a randomized clinical trial (NCT02453178). Participants completed fMRI resting state and a face working memory N-back task acutely after light and moderate intensity exercise and after a 12-week aerobic training intervention.
Results:
Functional connectivity did not change more after moderate compared with light intensity training. However, both training groups showed similar changes in cardiorespiratory fitness (maximal exercise oxygen uptake, VO2peak), limiting group-level comparisons. Acute effects of moderate intensity aerobic exercise on hippocampal-cortical connections in the default network predicted training enhancements in the same connections. Working memory also improved acutely, especially following moderate intensity, and greater acute improvements predicted greater working memory improvement with training. Exercise effects on functional connectivity of right lateralized fronto-parietal connections were related to both acute and training gains in working memory.
Conclusion:
Our data support the concept of acute aerobic exercise effects on functional brain systems and performance as an activity-evoked biomarker for exercise training benefits in the same outcomes. These findings may lead to new insights and methods for improving memory outcomes with aerobic exercise training.
Keywords: aerobic, cognitive aging, working memory, functional connectivity, default network
Introduction
Although aerobic exercise training promotes selective aspects of memory that decline with aging, benefits of exercise vary greatly across individuals (1). Identifying factors behind this variability could illustrate how to enhance exercise effects on memory for a wider aging population. Neuroplasticity and memory processes improve acutely (i.e., within 1 hour) following a single aerobic exercise session (1, 2), demonstrating overlap with training outcomes and suggesting exercise acutely evokes mechanisms involved in continued neural adaptation. However, it is unknown whether acute exercise predicts training outcomes in memory systems.
There is evidence for overlap in acute and training outcomes in both molecular and systems level mechanisms critical for memory. In rodent models, exercise upregulates hippocampal brain-derived neurotrophic factor (BDNF) expression both acutely (3) and after chronic training (4), and blocking hippocampal BDNF receptors during one week of exercise blocks exercise benefits on spatial memory (1). In humans, circulating BDNF increases acutely and chronically after aerobic exercise, and acute effects become stronger after aerobic exercise training (5). Moderate intensity aerobic exercise also enhances functional connectivity (fc) of hippocampal, parietal, and prefrontal regions in the Default Network (DN) in older adults both acutely (6) and after training for one year (7), and increases in resting serum BDNF after aerobic training relates to increased hippocampal fc (8).
Memory performance is also affected similarly acutely as with exercise training (2). One study found memory for relationships between faces and names improved acutely after moderate intensity cycling in young adults, coupled with increased serum BDNF (9). In the same study, face-name memory also improved after 5 weeks of aerobic training in some of the same participants, but the correlation between acute and training improvements was not reported. Additionally, a series of studies in young adults found that mnemonic discrimination, the ability to discriminate between similar memories, improved acutely after both light (10) and moderate intensity exercise (11), and performance was related to higher cardiorespiratory fitness (CRF) and greater physical activity (12). Finally, aerobic exercise acutely improves working memory (2, 13, 14), the capacity for maintaining and switching between multiple memory representations in current use. Together, these data support the predication that moderate intensity exercise has both acute and accumulated effects on memory processes critical for binding new relationships, precision, and flexibility of human memory.
Examining effects of exercise on the brain as organized by sub-systems carrying out distinct memory processes of binding, precision, and flexibility, would advance our understanding of how exercise could lead to specific memory improvements. The PMAT (posterior-medial anterior-temporal) framework (15–17), which overlaps with the DN, proposes such memory sub-systems (Figure 1A). The posterior-medial (PM) system includes the posterior hippocampus and DN cortical regions proposed to be involved in relational memory. On the other hand, the anterior-temporal (AT) system includes the anterior hippocampus and cortical regions involved in perception and discrimination between similar memories for objects. A third sub-system was identified as ventrolateral prefrontal cortex (VLPFC), including left and right inferior frontal regions, temporo-parietal junction, and pre- and post-central gyri (16). Although the PMAT framework makes no explicit predictions about the VLPFC, the region is involved in item and action selection and maintaining information in memory in the face of distraction (18, 19), in coordination with visual and perirhinal cortices (18, 20). Thus, the VLPFC sub-system may enable flexibility to bias relevant memory representations from either an active task state or long-term memory (21, 22).
Figure 1. Memory system networks.
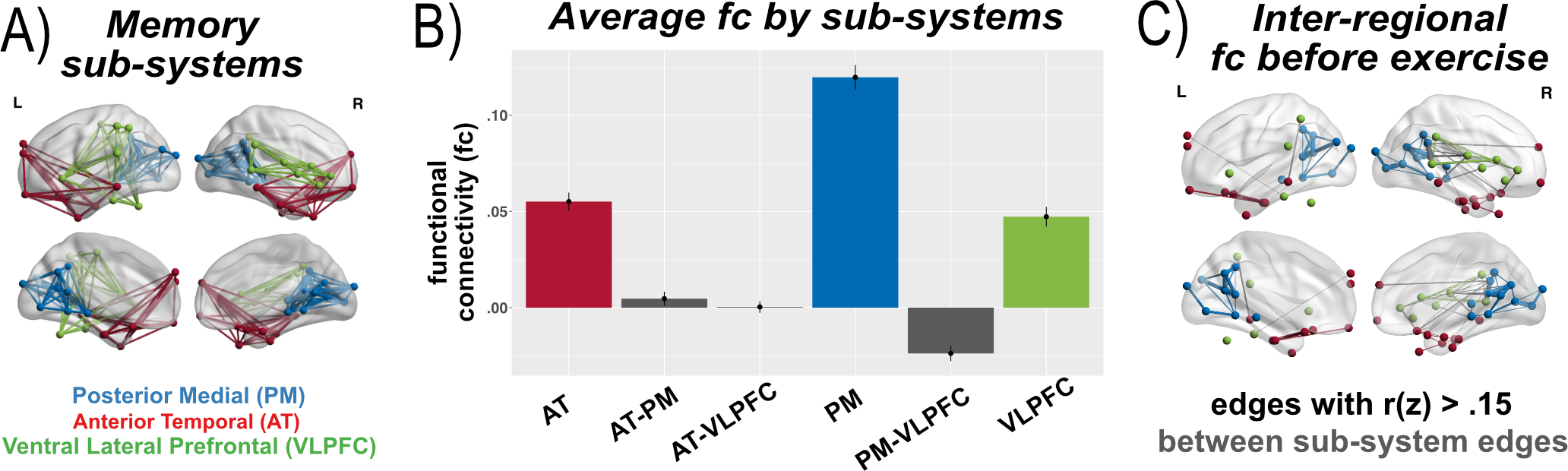
Panel (A) shows a priori memory sub-system regions of interest (ROIs) as defined in previous studies (see supplemental materials Table S1 for a full listing with MNI coordinate locations). (B) Average resting fc across both pre-scans from the acute phase. Average fc within and between sub-systems was significantly different based on sub-system groupings shown in the panel (F(5,396)=124.9, p<.05), and fc was stronger on average within (r(z)=.07) compared to between (r(z)=−.01), diff=.08 [95% CI .07–.09]. These results support modularity of the sub-systems in healthy older adults. (C) Inter-regional fc by node and edge demonstrates strongest within and between sub-system links. Note at the level of individual links (as compared to link averages in panel B), VLPFC connections are primarily right-lateralized, and strong between-system links are primarily between VLPFC and PM or AT, with very few strong links between AT and PM.
Here, we utilized a randomized controlled design to test the concept that acute changes in working memory performance and memory system fc in response to moderate intensity exercise predict changes in the same outcomes after exercise training. We evaluated working memory with faces, using an n-back task we have previously shown acute performance improvements on after exercise in young adults (13). We evaluated memory sub-system function during the resting state, so that we could more broadly test our predictions about distinct memory sub-systems without a task biasing some processes over others. Indeed, the PMAT sub-systems are known to be intact and distinct at rest (6, 16), and in general, compared to task states, fMRI activation patterns during the resting state are less affected by expectancy and practice effects.
Our central prediction was that acute effects after a single session of moderate intensity aerobic exercise on memory sub-system fc and working memory would predict change in the same fc and memory outcomes after 12 weeks of exercise training. Based on prior studies showing aerobic exercise primarily affects posterior hippocampal-cortical and cognitive control systems critical for flexibility (e.g., 7, 23, 24), we hypothesized exercise would primarily strengthen fc within the PM and VLPFC sub-systems. Alternatively, if exercise effects are not organized according to PMAT sub-systems, acute fc changes predicting training effects may overlap with the DN regardless of PMAT sub-system. Therefore, the results could inform the functional principles by which aerobic exercise influences memory systems affected by aging.
Methods
The primary goal of the study was to test whether acute effects of aerobic exercise on the brain and cognition predict training-related changes within the same individuals. The study was pre-registered on clinicaltrials.gov (NCT02453178). Here, we focus on the primary outcome of brain network fc, as measured by the resting blood oxygenation level dependent (BOLD) signal in memory systems. We also report on secondary outcomes of working memory and CRF.
Participants.
Healthy older adults between the ages of 60 and 80 years were recruited from Iowa City, IA, USA, and the surrounding communities. Procedures were approved by the University of Iowa Institutional Review Board (IRB) and all participants read and signed an IRB-approved informed consent document. At the time of enrollment, participants were “currently inactive,” defined as not participating in regular moderate intensity physical activity (30 minutes accumulated per day) on any more than three days of the week. In addition, eligible participants must have had no history of neurological incident or disease, no chronic metabolic or psychiatric disease, corrected vision of 20/40, not currently taking medications that could interact with their heart rate during exercise such as beta-blockers, fluent in English, right-handed, able to complete an MRI, and score ≥ 25 on the Mini-Mental Status Exam (MMSE-2). Eligible participants further qualified as “low risk” for an acute cardiovascular event by published standards of the American College of Sports Medicine (25), and had no evidence of myocardial ischemia or cardiovascular disease on resting or maximal exercise test 12-lead electrocardiography (ECG) response supervised by a cardiologist.
The experimental design is shown in Figure 2A, and a full consort diagram describing our screening and enrollment is shown in the supplemental information (Figure S1). Overall, 34 participants completed the acute phase, and of those 33 participants were re-randomized to intervention training groups. Demographics for participants completing the acute and training phases are shown in Table 1.
Figure 2. Design of experiment and exercise manipulation data.
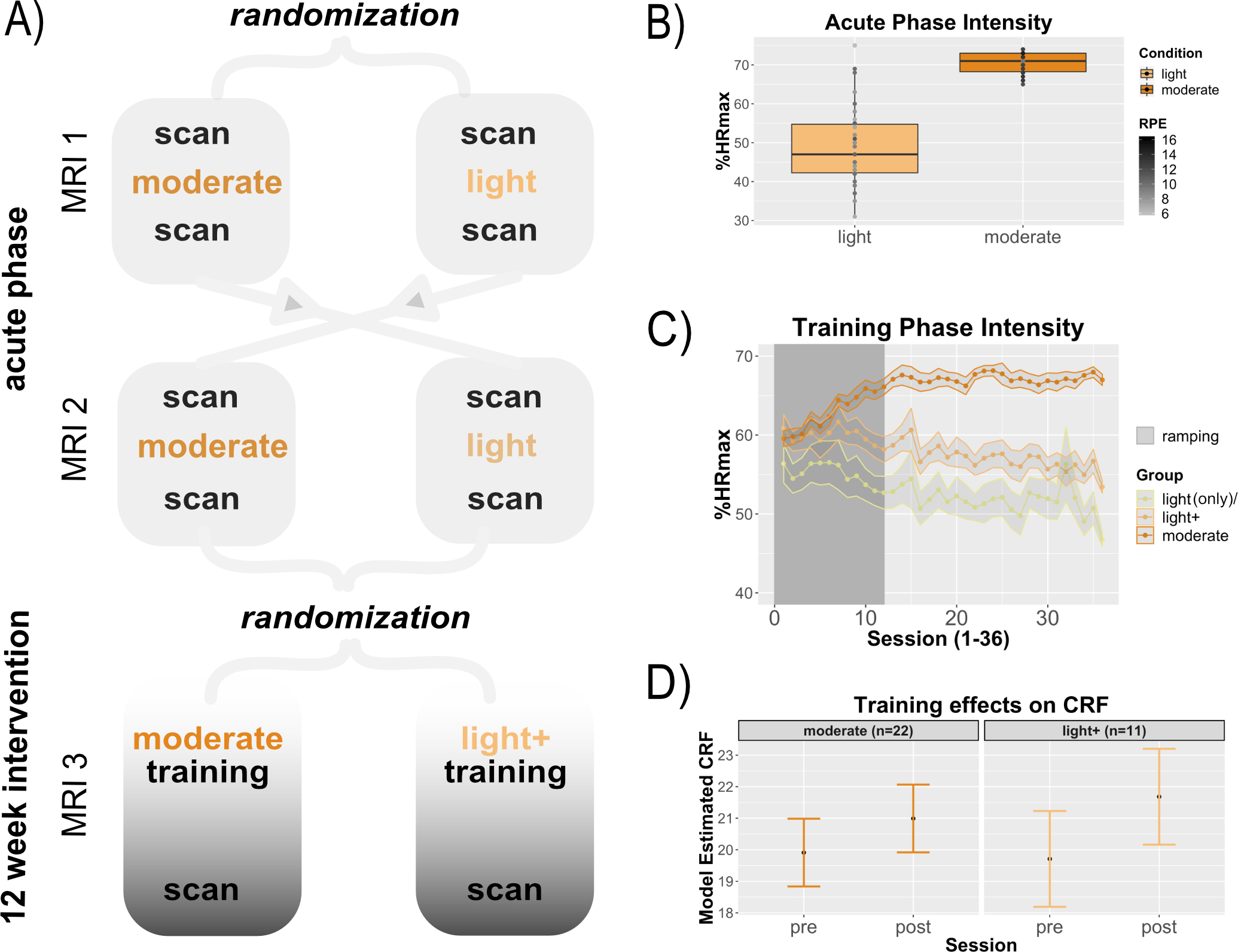
As shown in panel A, all participants completed both acute and intervention phases of the experiment. The acute phase was a within-subjects cross-over design, such that all participants completed sessions at light and moderate intensities (in a counter-balanced order) so that the effects of moderate intensity exercise could be examined within-subjects. The intervention training phase was a between-subjects design, such that all participants were randomized to either moderate or light training at a 2:1 ratio. (B) Recorded heart rate (HR) for the acute phase represented as percent of their maximum HR during their maximal exercise test, individual dots represent participants. Target zone 64–76% HRmax for the moderate intensity condition was achieved, and the target zone of <57% HRmax (“very light”) for light intensity was also achieved on average, albeit with more variability across participants. (C) Average HR data for the training sessions by group. The light intensity training group data are shown with four short moderate intervals (light+) as well as during passive cycling only (light(only)). (D) Training gains in cardiorespiratory fitness (CRF) were evident for both groups.
Table 1.
Summary statistics for demographics, cognitive status, and exercise variables at baseline before randomization for the acute phase
| Statistic | Female (proportion) | Age (yrs) | Education (yrs) | BMI | MMSE | MOCA | CRF (mL/kg/min) | CRF% |
|---|---|---|---|---|---|---|---|---|
| All participants in acute phase | ||||||||
| N | 34 | 34 | 34 | 34 | 34 | 31 | 34 | 34 |
| Mean | 0.6 | 67.1 | 17.4 | 29.1 | 29.1 | 27.3 | 20.1 | 6.5 |
| SD | 4.3 | 2.7 | 5.3 | 1.2 | 1.7 | 5.0 | 6.5 | |
| Min | 60 | 12 | 16.8 | 26 | 25.0 | 8.4 | 0.1 | |
| Pctl(25) | 65 | 16 | 25.6 | 29 | 26.0 | 16.9 | 1.6 | |
| Pctl(75) | 70 | 19 | 32.2 | 30 | 28.5 | 22.9 | 10.2 | |
| Max | 76 | 23 | 40.9 | 30 | 30.0 | 30.4 | 29.5 | |
| Light+ control training group | ||||||||
| N | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 11 |
| Mean | 0.6 | 66.7 | 17.4 | 29.0 | 29.2 | 26.8 | 19.7 | 5.7 |
| SD | 3.8 | 2.6 | 4.7 | 0.9 | 1.3 | 5.3 | 4.7 | |
| Min | 60 | 12 | 20.8 | 28 | 25 | 8.4 | 0.1 | |
| Pctl(25) | 65 | 16.5 | 25.4 | 28.5 | 26 | 17.2 | 2.0 | |
| Pctl(75) | 69 | 19.5 | 32.4 | 30 | 28 | 22.4 | 8.4 | |
| Max | 74 | 20 | 35.4 | 30 | 28 | 27.7 | 13.5 | |
| Moderate intensity training group | ||||||||
| N | 22 | 22 | 22 | 22 | 22 | 20 | 22 | 22 |
| Mean | 0.6 | 67.5 | 17.5 | 29.3 | 29.1 | 27.6 | 19.9 | 6.5 |
| SD | 4.6 | 2.8 | 5.7 | 1.3 | 1.9 | 4.8 | 7.1 | |
| Min | 60 | 12 | 16.8 | 26 | 25.0 | 12 | 0.4 | |
| Pctl(25) | 65.2 | 16 | 25.7 | 29 | 26.0 | 16.5 | 1.5 | |
| Pctl(75) | 71 | 19 | 31.6 | 30 | 29.0 | 22.9 | 10.2 | |
| Max | 76 | 23 | 40.9 | 30 | 30.0 | 30 | 29.5 | |
Cardiorespiratory Fitness (CRF) measurement.
CRF was measured during a maximal exercise test before the first acute session and following the intervention within 1 week of the last training session. VO2 peak (mL/kg/min) was measured with indirect calorimetry using a symptom-limited maximal exercise test on a cycle ergometer with workrate increasing in two-minute intervals. Oxygen consumption was calculated from expired air samples at 15-s intervals until peak VO2 was reached. VO2peak was determined when a) respiratory exchange rate (RER) exceeded 1.10, b) participant reached 90% of age-predicted HRmax, or c) HR and/or oxygen uptake plateaus despite an increase in workload. Otherwise, the test was terminated if the participant showed signs of distress or if physiological signals became abnormal (blood pressure, heart rate, ECG changes consistent with ischemia or dangerous arrhythmia).
Acute exercise paradigm.
Each participant completed two conditions of acute exercise in separate sessions (at least 1 week apart) in a randomized cross-over design (Figure 2A). Participants began acute sessions by sitting 30 minutes in a waiting room to establish a common baseline between acute phase visits. Participants then completed pre-exercise scans, followed by a 20-minute exercise session on a motor-driven stationary bicycle (Theracycle 200; Franklin, MA) located in a room directly next to the scanner. In the moderate intensity condition, participants increased their HR to 65% of their HRmax, as determined from the maximal exercise test, by pedaling against the cycle’s resistance. In order to isolate the effects of intensity, in the light intensity control condition, participants had their legs moved by the motorized pedals at the same rate as the moderate intensity session, which had a minimal effect on HR (Figure 2B). Thus, for both acute exercise conditions, participants used the same machine at the same pedal rate as determined during their orientation session, and we recorded HR continuously using a Polar RCX5. Salivary measures were collected before, during, and after the exercise protocol, but these were exploratory and beyond the scope of predictions tested in the current report.
Following exercise cessation, there was a 5-min cool-down before participants were escorted back to the scanner for post-exercise scanning. The elapsed time between exercise cessation and post-exercise scanning was 15.1±4.3 minutes for the moderate condition and 16.5±5.1 minutes for the light condition. The average number of days between acute visits was 9.18±5.94 (range: 6–35 days) with counterbalanced order. Borg Ratings of Perceived Exertion (RPE, 20-point scale 6 = no exertion, 20 = maximal exertion) were collected at 2-minute intervals during the acute exercise. Self-reported changes in affect were collected before, during, and immediately after completing each exercise bout (see supplementary materials).
Working memory outcome measure.
Working memory accuracy was assessed with an n-back task completed in the scanner directly following the resting state scans. Participants were presented a sequence of faces and instructed to detect whether each face matched the preceding face during the 1-back condition, or, for the 2-back condition, whether it matched the face presented two previously in the sequence. Each face was presented for 1000ms, participants had 1500ms to respond, and trials were separated by a 3000ms inter-stimulus interval. Face stimuli consisted of young adult male faces with neutral expressions chosen from the Center for Vital Longevity Face Database. Each set contained a total of six different faces, and importantly, a new set of faces for each experimental session. The task was composed of a total of 8 blocks with 14 trials in each block, alternating between blocks of 1-back and 2-back. Every block contained 4 target trials in which the face matched the preceding face during the 1-back or matched the face presented two previously for 2-back, yielding a total of 32 possible targets out of 112 trials throughout the task. Subjects completed two 12-minute n-back runs per session.
Intervention.
Following completion of their acute exercise sessions, participants were randomized to training groups (moderate vs. light intensity) based on a 2:1 ratio of moderate:light. A 2:1 ratio was used to enrich the number of participants with moderate intensity training, who we predicted would show the most similarity to acute effects after moderate intensity exercise. Randomization codes were generated by the PI, based on blocks of age (60–69,70–80) and sex. All participants attended a three-month supervised program that met 3 sessions/week. Both groups wore a Polar HR monitor (Model RCX5) during their training sessions. Target HR for each condition was based on the pre-intervention maximal exercise test, and in cases of discrepancy, RPE and HR were used to identify optimal training intensity.
Moderate intensity training.
Since participants were initially inactive, the moderate intensity group had a ramp-up period for duration spent in their moderate intensity zone. They started with a 5-minute warm-up, 20-minutes moderate intensity cycling and 20 minutes passive cycling, and 5-minute cool-down per session, for 3 sessions/week. In each additional week, we added 5 minutes of moderate intensity cycling per session, until the total time for moderate intensity was 40 minutes per session by the start of week 5 (with additional 5-minute warm-up and 5-minute cool-down). If participants could not attend an exercise session in the lab, they were given instructions on how to complete their session at home. Adherence was excellent, with 99.6% of sessions completed, and of these 4.5% of sessions were completed outside the lab. The target HR zone for moderate intensity training was 64–76% of HRmax. As expected, on average HR across training sessions was 65.7% (SD=3.8%) of HRmax.
Light intensity training.
This group completed primarily passive cycling whereby a motor in the stationary bicycle moved the pedals. To maintain participant expectancy for improvement and interest, we also included 4 × 1-minute bouts of moderate intensity cycling. The first 9 minutes after the warm-up were passive cycling and for the last 1-minute of the first 10-minute section, the participant applied force during pedaling to reach 64–76% HRmax. This was repeated 4 times throughout the 40-minute session, every 10 minutes. Self-report was offset from moderate intensity bouts so that RPE reflected passive cycling. The bouts of moderate intensity cycling accumulated to 12 minutes per week of moderate intensity exercise, and thus were intended to be ineffective for substantially increasing CRF over the course of the intervention. Adherence was excellent, with 97% of sessions completed, and of these 4.8% of sessions were completed outside the lab. As expected, average HR during passive cycling periods was 53.1% (SD=6.1%) of HRmax during training sessions, which was below the target of <57% HRmax. When considering the full session, average HR was slightly higher (58.2%, SD=4.4%) as a result of short moderate intensity bouts (Figure 2C).
Neuroimaging Methods.
All magnetic resonance imaging (MRI) was conducted at the Magnetic Resonance Research Facility (MRRF). During resting state fMRI scans, participants were instructed to attend their eyes on a fixation cross, relax, and remain awake for 10 minutes. To account for acute exercise-related changes in HR during scanning, HR was calculated from a photoplethysmography (PPG) signal collected on the big-toe during each scan by a BIOPAC MP150 system. The raw PPG signal was first normalized using z-scores, and the normalized timeseries was bandpass filtered (0.6 – 3.0 Hz; Butterworth filter). Peaks in the filtered PPG data (representing heart beats) were detected using MATLAB’s findpeaks algorithm, and HR was calculated as the median time difference between peaks and converted to beats per minute (bpm).
Acquisition Protocols.
Due to an unexpected change in scanner equipment during the project, imaging data were acquired using a 3.0T MRI Siemens TIM Trio and a 3.0T General Electric (GE) Discovery MR750w. However, all pre and post scans for a given participant were collected with the same scanner.
Siemens data were acquired with a 12-channel head coil. Anatomical 3D MPRAGE (Magnetization Prepared Rapid Gradient-Echo): inversion time=900 ms, echo time (TE)=3 ms, repetition time (TR)=2530 ms, flip angle=10°, matrix=256× 256×240mm, field of view (FOV)=256×256× 240mm. fMRI data were collected using T2*-weighted gradient-echo, echo-planar imaging (EPI) protocol sensitive to BOLD contrast: TR=2000 ms, TE=30 ms, FOV=220×220×124mm, image matrix=64×64, flip angle=80°, 3.4×3.4×4.0mm voxels, ascending acquisition of 31 contiguous axial slices of 4mm thickness. Dual-echo gradient field maps were collected with 4 mm-thick slices, 3.4 × 3.4 mm2 in-plane resolution, FOV = 64 × 64, TR = 388 ms, TE=4.89/7.35 ms, and flip angle=60°.
GE data were acquired with a 32-channel head coil. Anatomical 3D FSPGR (fast spoiled gradient echo) sequence with the following parameters: inversion time=450 ms, TE=3.376 ms, TR=8.588 ms, flip angle=12°, matrix=256× 256×240mm, FOV=256×256× 240mm. fMRI data were collected using a T2*-weighted gradient-echo, EPI protocol: TR=2000 ms, TE=30 ms, FOV=220× 220×124mm, image matrix=64×64, flip angle=80°, 3.4×3.4×4.0mm voxels, ascending acquisition of 31 contiguous axial slices of 4mm thickness. Gradient field maps were collected with 4 mm-thick slices, 3.4 × 3.4 mm2 in-plane resolution, FOV = 64 × 64, TR = 372.536 ms, TE=4.608 ms, and flip angle=60°. Prior to all GE scans, calibration-scan images were acquired (i.e., Phased array UnifoRmity Enhancement (PURE), which were used as reference images for post-processing that improved the uniformity of image signal intensity.
No imaging runs were discarded due to motion or artifact, as motion was highly reliable within-subjects (see supplementary materials). Head motion effects were accounted for with a data-driven variance decomposition tool described below (ICA-AROMA). Additionally, because scanner did not vary within-subject and did not interact with change over time in signal quality (see supplementary materials), all analyses collapse across scanner.
Image preprocessing and analysis.
Image processing and analyses were implemented with tools from FSL 5.0.10, AFNI, and MATLAB (https://github.com/HBClab/RestingState).
We first separated brain from non-brain tissue in the T1-weighted anatomical images, then utilized a multiple algorithm-based approach to create a brain mask (https://github.com/jdkent/MBA). Brain-masked anatomical images were segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) masks using FSL’s FAST. Anatomical images were registered to the MNI152 template using an affine boundary-based registration (FLIRT) in conjunction with a non-linear transform (FNIRT).
To process fMRI scans, we performed motion correction by using AFNI’s 3dvolreg to align the spatial orientation of all volumes to the middle time point. Motion-corrected images were spatially smoothed using a 6 mm kernel at full-width half-maximum (FSL’s SUSAN). After normalizing each global 4D dataset by the median intensity, we used an ICA-based method for further cleaning of motion-related artifacts (26). Denoised data were then temporally filtered using a bandpass range of 0.008 < f < 0.08 Hz, which reduces high frequency physiological signals and low frequency scanner drift. Finally, we regressed out tissue-based signals originating from subject-specific WM and CSF masks. After spatially transforming the partial volume estimates to EPI space, the WM and CSF probability masks were thresholded to 99% and eroded. The first 5 principal components from the WM and CSF timeseries were submitted to a multiple regression (using FSL’s FEAT tool) as independent variables predicting the observed fMRI data (i.e., nuisance regression). The residual time series data were then normalized using z standardization and re-centered to a mean of 1000 and utilized for subsequent analyses. For fMRI fc analyses, the average residual time series was extracted from ROIs transformed from MNI to native EPI space using transformations described above. ROIs were defined a priori from previous studies based on preferential connectivity with parahippocampal (PM) or perirhinal (AT) cortices (15, 16, see Table S1). Cross-correlation between ROIs was estimated with Pearson’s correlation coefficients and transformed to Fisher’s Z estimates Z(r) using Fisher’s r-to-z transformation.
Statistical analysis.
Linear mixed models (LMMs) were used to test whether acute changes in memory system fc and working memory performance predict changes in the same outcomes after training. Models adjusted for age and sex, and a standard t-test with estimated Kenward-Roger df were used to evaluate the null hypotheses. Detailed explanation of acute and training phase models is provided in supplementary materials. The p-values for brain network analyses were adjusted for multiple comparisons using an optimized False Discovery Rate (FDR) approach (27), yielding q-values.
A first model estimated acute effects. Age and sex were included as covariates, time was a categorical variable representing the pre- and post-exercise scans, and condition was a categorical variable representing moderate and light intensity. Time and the interaction between time and condition were entered as both fixed and random effects. Random effects were assumed to have a joint-normal distribution and independent of random error. Random errors were assumed uncorrelated over time, with correlation due to repeated outcome measures accounted for by the random effects structure. Random effects for the time x condition interaction provided participant-specific acute scores, defined for each person as: [(moderatepost −moderatepre)− (lightpost −lightpre)]. Higher acute scores indicated the participant responded acutely more to moderate than light intensity exercise. The LMM was estimated using maximum likelihood methods under the typical normality assumptions for random effects and random error. The acute phase LMM was estimated for each of the ROI-ROI connections (Figure 1A) and n-back accuracy.
A second model tested the relationship between acute and training changes, using the day 1 pre-test as a covariate, with additional covariates being those used in randomization (age, gender), along with exercise training group. Thus, in order to adjust for the fact that the day 1 pre-test was in both acute and training models, a residual change score was defined for both. The acute residual change model was defined as: (moderatepost − moderatepre) − (lightpost − lightpre) = β0 + β1PreM/L i + ri, where Pre/L is the day 1 pre-test score. r is the residualized change score for the acute model, defined as the difference of difference minus the intercept and slope of the pre-test scores. Similarly, the training residual change model was defined as post intervention minus pre-test score, and was modeled as a function of the pre-test score: Post intervention − PreM/L = β0 + β1PreM/Li + ei, where ei is the residualized change score for the training model, defined as the difference minus the intercept and slope of the pre-test scores.
Statistical power.
The sample size for our trial was based on the number of participants needed to detect an acute effect on cognition and training-related changes in CRF. We reasoned these effects were the premise for testing the unknown relationship between acute and training changes for outcomes theoretically enhanced by CRF improvements. We based anticipated effect sizes on a study with 13 control and 30 exercise participants in a between-subjects design, showing an effect size of d =.79 (9). Our within-subjects design increases statistical power, and our sample of N=34 in the acute phase is greater than the minimum sample size of N=18 proposed to satisfy 80% power for within-subjects designs detecting moderate-sized acute effects on cognition (d=.50) (28). Further, based on a meta-analytic effect size of g=.41 for CRF change with training in older adults (29), N=24 enrolled in moderate intensity training allowed 90% power to detect reliable change in CRF while allowing up to 6 drop-outs (30). Because only 1 participant dropped out during training (Figure S1), we are sufficiently powered for our manipulation check on CRF with N=22 randomized to moderate intensity training (Table 1).
Results
Participants and manipulation check.
Participant demographics, cognitive status, and baseline exercise variables are summarized in Table 1. As shown in Figure 2, participants achieved their target HR for each exercise condition in both the acute (2B) and training (2C) phase. Although we predicted moderate intensity training would selectively improve CRF, both groups improved CRF from training, shown by a statistically significant effect of time, F(1,33)=23.6, p<.05, with no training group x time interaction for CRF, F(1,33)=2.00, p=.17); Figure 2D). Supplementary analyses showed this was not due to the light group increasing their physical activity outside the lab (see supplementary materials). Thus, the 4 × 1-minute intermittent moderate intensity bouts per session in the light intensity group were sufficient to improve CRF over 12 weeks.
Do acute aerobic exercise effects predict exercise training-related changes in functional connectivity?
In the acute phase mixed-effects model, 66 connections showed a greater change for the moderate compared to light condition (p<.05), however none survived FDR correction with q <.05. Although this suggests the acute effects of moderate intensity exercise on resting fc were relatively weak at the group-level, our primary aim was to test the extent to which acute effects predicted training effects in the same individuals. Because intervention groups did not differ in CRF change, intervention group is not considered in further models. When testing the association between residualized change scores from the acute and training models, a total of 208 ROI connections showed effects with p<.05, and 12 of these connections survived FDR correction with q < .05 (see Figure 3A and 3B). In these 12 ROI pairs, heart rate acute scores during the resting state scan did not reliably relate to fc acute scores, with a range of correlations between r of −.57 and .47 and similar numbers of connections with negative, near zero, and positive correlations.
Figure 3. Effects of aerobic exercise on memory systems.
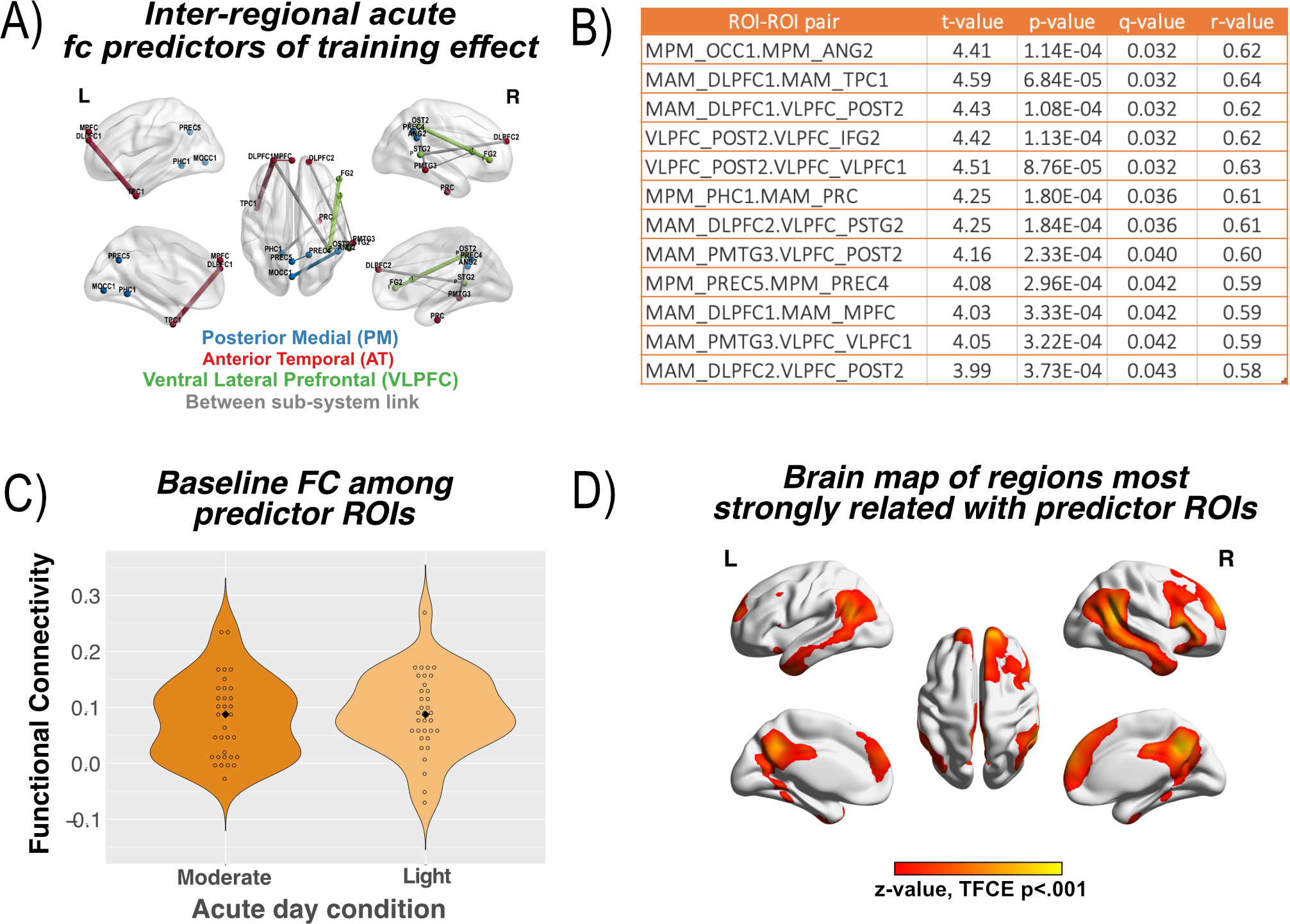
(A) Regional predictive ROI connections, where a preferential effect of moderate intensity exercise in the acute phase predicted a larger training effect in fc, FDR corrected at q<.05. ROI pair connections are colored by their PMAT sub-system membership (Figure 1), with inter-system links shown in gray. Width of the link corresponds to strength of the correlation between acute and training effects. (B) Table of the model statistics for each ROI pair. (C) Average fc for the set of predictive ROIs in panel A, with individual participants shown as open circles and the average as a closed circle. Note predictor ROIs show comparable strength of pre-exercise fc as PMAT sub-systems shown in Figure 1B. (D) A voxel-wise map of the regions that most strongly correlated with the average time-series of the acute predictor ROIs. The map has a strong spatial correlation with the DN (reported in main text), suggesting the DN is a hippocampal-cortical configuration sensitive to the acute effects of exercise which are also predictive of training effects.
Figure 3A shows that ROI connections where acute effects predicted training effects (i.e., predictor ROIs) are spread across the PMAT sub-systems and appear to show a DN-like configuration, supporting our alternative hypothesis. This observation is supported by the fact that the average fc between the predictor ROIs is comparable or stronger than the PMAT sub-systems (see Figures 1B and 3C). To further evaluate whether predictor ROIs reflect the DN, we computed a voxel-wise map of the correlation between the average time-series across all predictor ROIs (i.e., a multi-ROI seed) and every voxel. Voxel-wise statistics were determined by a 1-sample t-test computed with the FSL program randomise (31), and multiple comparisons controlled with threshold-free cluster enhancement (32). As shown in Figure 3D, the spatial configuration indeed resembles the DN, and was spatially correlated with separate DN maps derived from primarily young adults (r = .47) (33) and older adults (r = .46) (34).
Do acute aerobic exercise effects on working memory performance predict exercise training-related changes in performance?
In the acute phase mixed-effects model, there was a time x exercise-condition interaction (b = .05 (SE = .02), t(182) = 2.34, p = .02), and a time x task-condition interaction (b = .04 (SE =.02), t(181) = 2.10, p=.04). Thus, similar to our earlier report with young adults (13), physical activity acutely improved working memory performance, and accuracy improvements were stronger for moderate compared to light intensity and for the 2-back compared to the 1-back condition (Figure 4A). The 3-way interaction of time x exercise-condition x task-condition was not statistically significant (b =.02 (SE=.04), t(181)= .42, p=.67), reflecting the trend for acute improvements in both 1- and 2-back conditions. Overlap of acute and training post-test accuracy for all participants is shown in Figure 4A. Finally, again using the residualized change scores, stronger acute effects for moderate compared to light intensity predicted accuracy improvements after 12-weeks of training (b =1.14 (SE=.06), t=2.58, p=.02).
Figure 4. Effects of aerobic exercise on working memory.
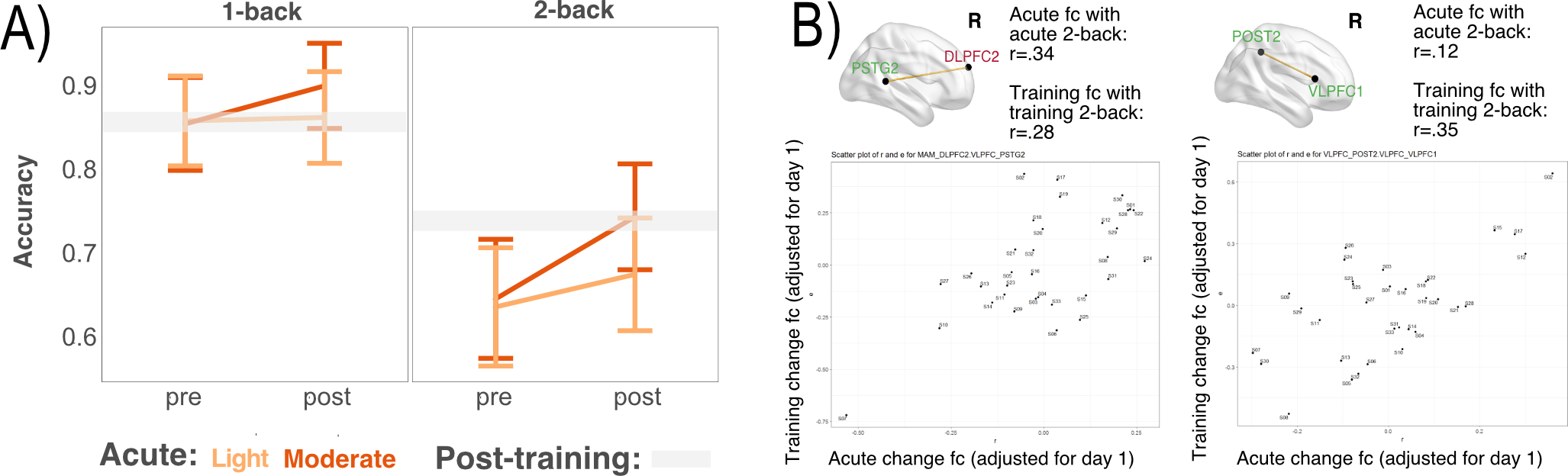
(A) Working memory (n-back) performance was improved following the moderate compared to light intensity condition, as both the task-condition x time and exercise-condition x time effects were statistically significant. The light gray bar marks average post-training accuracy for each condition, for all participants, demonstrating the accuracy boost after 20 minutes of moderate intensity exercise matches the boost after 12 weeks of training that improved CRF. (B) The strongest effect sizes linking changes in fc with changes in 2-back performance across acute and training phases. Nodes are color-coded according to networks in Figure 1A, and to the right are effect sizes for the correspondence between change in fc and performance. Scatter plots show correspondence of acute and training changes in fc in these ROI connections for individual subjects, who are labeled by subject number in the plot.
Are changes in network fc related to improved performance?
Using exploratory correlational analyses, we examined whether changes in fc were linked to improved performance on the 2-back condition, for both acute and training residual change scores. Among the 12 ROI connections passing FDR correction above, the same two ROI connections were the two strongest effect sizes for acute and training associations, including (1) a link between the right post-central/parietal cortex (VLPFC) and the right ventral lateral prefrontal cortex (VLPFC), and (2) between the right posterior superior temporal gyrus (VLPFC) and the right dorsolateral prefrontal cortex (AT) (Figure 4B). Thus, both connections were right lateralized and included aspects of the lateral prefrontal cortex, further supporting a role for the VLPFC sub-system in working memory and its potential facilitation through physical exercise.
Discussion
The goal of our study was to test whether acute effects of moderate intensity exercise on memory systems and performance predicted exercise training-related improvements in the same outcomes. Although both training groups showed similar changes in CRF, limiting group-level comparisons in training intensity-related changes, we show support for the relation of acute and training outcomes in working memory and brain network fc within the same individuals. The predictive acute fc effects were strongest in temporal-parietal-prefrontal connections from PMAT and VLPFC system regions overlapping with the DN, and when working memory demands were greatest. Results support the potential for acute exercise effects on the brain and cognition to characterize who may benefit most from training for these outcomes.
In the current study, predictive acute effects of moderate intensity exercise on fc fit a DN node configuration similar to regions we have previously found related to CRF independent of light or moderate intensity physical activity levels (34). We propose that these results fit together when considering initial physiological effects in the brain as an activity-evoked biomarker of the capacity for a continued, adaptive response when repeatedly exposed to moderate intensity physical activity. It is worth emphasizing that we designed our study to isolate acute effects of moderate intensity exercise over and above movement alone, so that we could examine individual differences in response to an exercise intensity that would improve CRF if repeated. The passive movement in the acute light condition is theoretically informative because it is plausible that movement alone could intensify synchronized neural activity in the hippocampus, sparking increased neural activity-dependent BDNF expression and enhancing memory (1). However, here we show that intensity mattered more than movement for eliciting a response in brain and cognitive outcomes predicting training-related change coupled with improved CRF. In future studies, further support for dose-response intensity effects will ideally come from ≥3 intensity levels, particularly with intensities straddling relevant physiological thresholds such as the lactate threshold (35) and high cortisol responses (3).
We focused on working memory outcomes in the acute and training phase, which was based on data from our lab and others on acute effects of exercise on working memory (2, 6, 36). Here we replicated the acute effects of aerobic exercise on working memory in older adults, and we showed participants with the biggest acute benefit also showed the biggest training benefit on performance. This could not be solely due to practice-effects because the order of intensity conditions was counter-balanced, thus half of the participants completed their light exercise condition a week after the moderate intensity condition. Given the robust effect of aging on non-verbal working memory as assessed with the n-back task (37), the result that a single session of aerobic exercise mimics the effects of 12-weeks of training on performance has important implications both practically and theoretically.
Practically, our results suggest acute benefits of aerobic exercise on working memory generalize to physically inactive older adults. Although the acute benefit is expected to be transient, future studies describing the duration of benefits and whether they interact with time-of-day will help further inform potential applications. Theoretically, results show that a single aerobic exercise session could unmask performance levels achieved after training 12 weeks, suggesting a constraint on working memory was affected. One candidate is the neurotransmitter dopamine, whose striatal and cortical reductions with aging have been associated with age-related decline in working memory (38), and rodent studies have shown extracellular dopamine in the striatum (39) and hippocampus (40) increase during and after exercise.
As with any study, limitations should be noted. We had a relatively small sample size with a homogeneous population that were screened as cognitively normal and inactive but without common chronic health concerns such as heart disease, diabetes, or recent cancer. Because of our interest in the physiological response to exercise in this small sample, we also excluded participants taking beta-blockers. Therefore, to test our central prediction across a broader population at risk for age-related cognitive decline, it will be critical to extend this design to a larger sample of older adults with more variability in cognitive status, medication usage, health history, and physical activity profiles. Given our interest in acute effects as an evoked biomarker of potential for cognitive protection from exercise, it will also be important to test the specificity of predictive effects for different types of hippocampal-dependent memory negatively affected by aging but positively by CRF, such as relational memory and mnemonic discrimination (1). Regarding the relatively weak acute effects for fc on average, we note participants in this study had lower CRF (6.5th percentile) than our previous report (6) investigating acute effects on brain functional connectivity (41.5th percentile). It is unknown how CRF affects the acute brain response in older adults, but our results suggest this question warrants future study. Finally, we focused on functional brain systems during the resting state, for methodological reasons and because this is the state we have previously shown both acute and long-term effects of exercise on DN fc (6, 7, 34). Functional MRI collected during working memory performance will be the focus of a separate report.
In summary, here we show acute effects of moderate intensity aerobic exercise on memory systems affected by aging may serve as an evoked biomarker of adaptive long-term changes with continued training. Results support further development of the acute exercise paradigm as a tool to understand how initial effects on brain physiology and performance predict successive neurobiological and behavioral outcomes associated with aerobic exercise training.
Supplementary Material
Footnotes
Conflicts of interest, acknowledgements, and source of funding: None of the authors declare a conflict of interest. We thank Dr. Phillip G. Schmid for his efforts in overseeing maximal exercise tests, and we thank James Kent and Matthew Sodoma for contributions to data management and collection. We also thank the Data Safety and Monitoring Board (DSMB) for their oversight of the trial. The DSMB included Dr. Yaakov Stern, Dr. Michael Marsiske, and Dr. Kurt Kroenke. Research support provided by 5R21AG048170 from the National Institutes of Health/National Institute on Aging. The results of the present study do not constitute endorsement by ACSM. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
References
- 1.Voss MW, Soto C, Yoo S, Sodoma M, Vivar C, van Praag H. Exercise and hippocampal memory systems. Trends Cogn Sci. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roig M, Nordbrandt S, Geertsen S, Nielsen J. The effects of cardiovascular exercise on human memory: a review with meta-analysis. Neurosci Biobehav Rev. 2013;37(8):1645–66. doi: 10.1016/j.neubiorev.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Soya H, Nakamura T, Deocaris C, Kimpara A, Iimura M, Fujikawa T, et al. BDNF induction with mild exercise in the rat hippocampus. Biochem Biophys Res Commun. 2007;358(4):961–7. doi: 10.1016/j.bbrc.2007.04.173. [DOI] [PubMed] [Google Scholar]
- 4.Hatchard T, Ting JJ, Messier C. Translating the impact of exercise on cognition: methodological issues in animal research. Behav Brain Res. 2014;273:177–88. Epub 2014/07/16. doi: 10.1016/j.bbr.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 5.Szuhany KL, Bugatti M, Otto MW. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J Psychiatr Res. 2015;60:56–64. Epub 2014/12/03. doi: 10.1016/j.jpsychires.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weng TB, Pierce GL, Darling WG, Falk D, Magnotta VA, Voss MW. The Acute Effects of Aerobic Exercise on the Functional Connectivity of Human Brain Networks. Brain Plasticity. 2017;2(2):171–90. Epub 2017/03/28. doi: 10.3233/bpl-160039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voss M, Prakash R, Erickson K, Basak C, Chaddock L, Kim J, et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2010;2. doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voss MW, Erickson KI, Prakash RS, Chaddock L, Kim JS, Alves H, et al. Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav Immun. 2013;28:90–9. Epub 2012/11/06. doi: 10.1016/j.bbi.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffin É, Mullally S, Foley C, Warmington S, O’Mara S, Kelly A. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol Behav. 2011;104(5):934–41. doi: 10.1016/j.physbeh.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Suwabe K, Byun K, Hyodo K, Reagh ZM, Roberts JM, Matsushita A, et al. Rapid stimulation of human dentate gyrus function with acute mild exercise. Proc Natl Acad Sci U S A. 2018;115(41):10487–92. Epub 2018/09/27. doi: 10.1073/pnas.1805668115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suwabe K, Hyodo K, Byun K, Ochi G, Yassa MA, Soya H. Acute moderate exercise improves mnemonic discrimination in young adults. Hippocampus. 2017;27(3):229–34. Epub 2016/12/21. doi: 10.1002/hipo.22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suwabe K, Hyodo K, Byun K, Ochi G, Fukuie T, Shimizu T, et al. Aerobic fitness associates with mnemonic discrimination as a mediator of physical activity effects: evidence for memory flexibility in young adults. Sci Rep. 2017;7(1):5140 Epub 2017/07/13. doi: 10.1038/s41598-017-04850-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weng TB, Pierce GL, Darling WG, Voss MW. Differential Effects of Acute Exercise on Distinct Aspects of Executive Function. Med Sci Sports Exerc. 2015;47(7):1460–9. Epub 2014/10/12. doi: 10.1249/MSS.0000000000000542. [DOI] [PubMed] [Google Scholar]
- 14.Pontifex MB, Hillman CH, Fernhall B, Thompson KM, Valentini TA. The effect of acute aerobic and resistance exercise on working memory. Med Sci Sports Exerc. 2009;41(4):927–34. Epub 2009/03/12. doi: 10.1249/MSS.0b013e3181907d69. [DOI] [PubMed] [Google Scholar]
- 15.Libby LA, Ekstrom AD, Ragland JD, Ranganath C. Differential connectivity of perirhinal and parahippocampal cortices within human hippocampal subregions revealed by high-resolution functional imaging. J Neurosci. 2012;32(19):6550–60. Epub 2012/05/11. doi: 10.1523/JNEUROSCI.3711-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ritchey M, Yonelinas AP, Ranganath C. Functional connectivity relationships predict similarities in task activation and pattern information during associative memory encoding. J Cogn Neurosci. 2014;26(5):1085–99. Epub 2013/11/29. doi: 10.1162/jocn_a_00533. [DOI] [PubMed] [Google Scholar]
- 17.Kim S, Voss JL. Selective and coherent activity increases due to stimulation indicate functional distinctions between episodic memory networks. Scientific Advances. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ranganath C Working memory for visual objects: complementary roles of inferior temporal, medial temporal, and prefrontal cortex. Neuroscience. 2006;139(1):277–89. Epub 2005/12/14. doi: 10.1016/j.neuroscience.2005.06.092. [DOI] [PubMed] [Google Scholar]
- 19.Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45(13):2883–901. Epub 2007/08/07. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 20.Rissman J, Gazzaley A, D’Esposito M. Dynamic adjustments in prefrontal, hippocampal, and inferior temporal interactions with increasing visual working memory load. Cereb Cortex. 2008;18(7):1618–29. Epub 2007/11/15. doi: 10.1093/cercor/bhm195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranganath C, DeGutis J, D’Esposito M. Category-specific modulation of inferior temporal activity during working memory encoding and maintenance. Brain Res Cogn Brain Res. 2004;20(1):37–45. Epub 2004/05/08. doi: 10.1016/j.cogbrainres.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 22.Spreng RN, DuPre E, Selarka D, Garcia J, Gojkovic S, Mildner J, et al. Goal-congruent default network activity facilitates cognitive control. J Neurosci. 2014;34(42):14108–14. doi: 10.1523/JNEUROSCI.2815-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voss M, Erickson K, Prakash R, Chaddock L, Malkowski E, Alves H, et al. Functional connectivity: a source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia. 2010;48(5):1394–406. doi: 10.1016/j.neuropsychologia.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boraxbekk CJ, Salami A, Wahlin A, Nyberg L. Physical activity over a decade modifies age-related decline in perfusion, gray matter volume, and functional connectivity of the posterior default-mode network-A multimodal approach. Neuroimage. 2016;131:133–41. Epub 2015/12/26. doi: 10.1016/j.neuroimage.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 25.ACSM. ACSM’s Guidelines for Exercise Testing and Prescription 9th Edition ed. Medicine ACoS, editor: Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 26.Pruim R, Mennes M, van Rooij D, Llera A, Buitelaar J, Beckmann C. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015;112:267–77. doi: 10.1016/j.neuroimage.2015.02.064. [DOI] [PubMed] [Google Scholar]
- 27.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the royal statistical society Series B (Methodological). 1995:289–300. [Google Scholar]
- 28.Pontifex MB, McGowan AL, Chandler MC, Gwizdala KL, Parks AC, Fenn K, et al. A primer on investigating the after effects of acute bouts of physical activity on cognition. Psychology of Sport and Exercise. 2019;40:1–22. doi: 10.1016/j.psychsport.2018.08.015. [DOI] [Google Scholar]
- 29.RAND. Exercise programs for older adults: a systematic review and meta-analysis. Santa Monica CA: Southern California Evidence-Based Practice Center: 2007. [Google Scholar]
- 30.Portney LG, Watkins MP. Foundations of Clinical Research. 3rd Edition ed. Upper Saddle River, New Jersey: Pearson Prentice Hall; 2009. [Google Scholar]
- 31.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92:381–97. Epub 2014/02/18. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. Epub 2008/05/27. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 33.Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo XN, Holmes AJ, et al. Local-Global Parcellation of the Human Cerebral Cortex from Intrinsic Functional Connectivity MRI. Cereb Cortex. 2017:1–20. Epub 2017/10/06. doi: 10.1093/cercor/bhx179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voss M, Weng T, Burzynska A, Wong C, Cooke G, Clark R, et al. Fitness, but not physical activity, is related to functional integrity of brain networks associated with aging. NeuroImage. 2016;131:113–25. doi: 10.1016/j.neuroimage.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferris LT, Williams JS, Shen CL. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med Sci Sports Exerc. 2007;39(4):728–34. Epub 2007/04/07. doi: 10.1249/mss.0b013e31802f04c7. [DOI] [PubMed] [Google Scholar]
- 36.Pontifex M, Hillman C, Fernhall B, Thompson K, Valentini T. The effect of acute aerobic and resistance exercise on working memory. Med Sci Sports Exerc. 2009;41(4):927–34. doi: 10.1249/MSS.0b013e3181907d69. [DOI] [PubMed] [Google Scholar]
- 37.Bopp KL, Verhaeghen P. Aging and n-Back Performance: A Meta-Analysis. The Journals of Gerontology: Series B. 2018. doi: 10.1093/geronb/gby024. [DOI] [PubMed] [Google Scholar]
- 38.Bäckman L, Karlsson S, Fischer H, Karlsson P, Brehmer Y, Rieckmann A, et al. Dopamine D1 receptors and age differences in brain activation during working memory. Neurobiology of aging. 2011;32(10):1849–56. [DOI] [PubMed] [Google Scholar]
- 39.Hattori S, Naoi M, Nishino H. Striatal dopamine turnover during treadmill running in the rat: relation to the speed of running. Brain research bulletin. 1994;35(1):41–9. [DOI] [PubMed] [Google Scholar]
- 40.Goekint M, Bos I, Heyman E, Meeusen R, Michotte Y, Sarre S. Acute running stimulates hippocampal dopaminergic neurotransmission in rats, but has no influence on brain-derived neurotrophic factor. J Appl Physiol (1985). 2012;112(4):535–41. doi: 10.1152/japplphysiol.00306.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


