Summary
We developed an adeno-associated virus (AAV) vector-based technique to label mouse neostriatal neurons comprising direct and indirect pathways with different fluorescent proteins and analyze their axonal projections. The AAV vector expresses GFP or RFP in the presence or absence of Cre recombinase and should be useful for labeling two cell populations exclusively dependent on its expression. Here, we describe the AAV vector design, stereotaxic injection of the AAV vector, and a highly sensitive immunoperoxidase method for axon visualization.
For complete details on the use and execution of this protocol, please refer to Okamoto et al. (2020).
Subject Areas: Gene Expression, Microscopy, Neuroscience
Graphical Abstract
Highlights
-
•
Exclusive labeling of two groups of neurons with different fluorescent proteins
-
•
Detailed protocols from stereotaxic virus injection to immunostaining methods
-
•
A signal enhancement method via peroxidase activity, BT-GO reaction
-
•
Significant improvement in a signal-to-noise ratio by the cost-effective reaction
We developed an adeno-associated virus (AAV) vector-based technique to label mouse neostriatal neurons comprising direct and indirect pathways with different fluorescent proteins and analyze their axonal projections. The AAV vector expresses GFP or RFP in the presence or absence of Cre recombinase and should be useful for labeling two cell populations exclusively dependent on its expression. Here, we describe the AAV vector design, stereotaxic injection of the AAV vector, and a highly sensitive immunoperoxidase method for axon visualization.
Before you begin
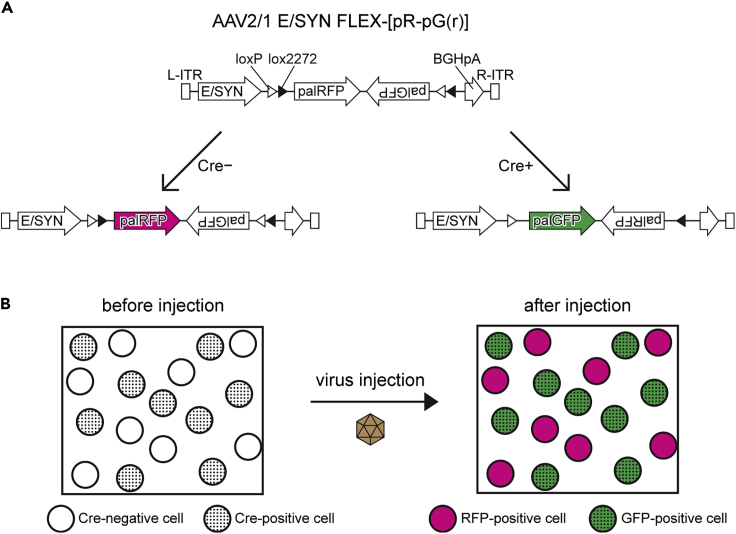
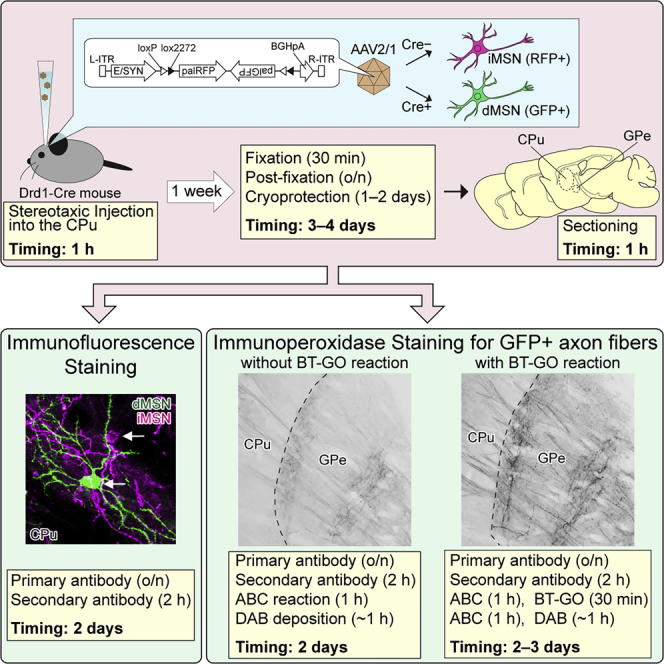
We developed a method using an adeno-associated virus (AAV) vector system to label two groups of neurons with different fluorescent proteins. The vector contains a flip-excision (FLEX) switch (Schnutgen et al., 2003), and expresses GFP or RFP based on the expression, or the lack, of Cre recombinase (Figures 1A and 1B). This labeling method offers a great advantage for the simultaneous labeling of mutually exclusive systems in the nervous system, such as excitatory and inhibitory neurons.
Figure 1.
Exclusive labeling of Cre-positive and -negative cells by an AAV vector
(A) Construct of the AAV vector. The vector expresses RFP or GFP in the absence or presence of Cre recombinase by a flip-excision (FLEX) switch. Both GFP and RFP are tagged with a membrane-targeting protein, palmitoylation signal (pal).
(B) Schematic diagram of the exclusive labeling method. Two cell populations are selectively labeled with different fluorescent proteins based on the expression of Cre recombinase.
BGHpA, a polyadenylation signal derived from bovine growth hormone gene; E/SYN, enhanced human synapsin I promoter (Hioki et al., 2007); ITR, inverted terminal repeat.
After preparation of the AAV vector, we injected the vector into the mouse neostriatum (caudate-putamen, CPu), and then analyzed the axonal projections of direct and indirect pathway medium-sized spiny neurons (dMSNs and iMSNs) (Okamoto et al., 2020). The production and purification methods of AAV vectors have been described in previous studies (Hamamoto et al., 2017; Ito et al., 2015; Iwano et al., 2018; Kataoka et al., 2014; Sohn et al., 2016; Sohn et al., 2017; Suzuki et al., 2015). We have also recently established a method to stably obtain a high-titer virus solution by recovering virus particles from cell pellets and the supernatant (medium) (Takahashi et al., 2020). For a detailed protocol of the AAV vector preparation, refer to the literature.
Here, we describe step-by-step procedures for stereotaxic injection of a virus solution into the mouse CPu, signal enhancement via peroxidase activity, and axon tracing. In particular, the signal enhancement method, namely the biotinylated tyramine-glucose oxidase (BT-GO) reaction, is cost-effective and straightforward (Furuta et al., 2009; Kuramoto et al., 2009) and should be useful for experiments that require specific staining with a high signal-to-noise ratio.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Biotinylated goat antibody anti-rabbit IgG | Vector Laboratories | Cat# BA-1000; RRID: AB_2313606 |
| Chicken polyclonal anti-GFP antibody | Aves Labs | Cat# GFP-1020; RRID: AB_10000240 |
| Goat anti-rabbit IgG (H+L) highly cross-adsorbed secondary antibody, Alexa Fluor 568 | Thermo Fisher Scientific | Cat# A-11036; RRID: AB_143157 |
| Goat anti-chicken IgY (H+L) secondary antibody, Alexa Fluor 488 | Thermo Fisher Scientific | Cat# A-11039; RRID: AB_2534096 |
| Affinity-purified rabbit antibody against GFP | Tamamaki et al., 2000; Nakamura et al., 2008 | N/A |
| Affinity-purified rabbit antibody against mRFP1 | Hioki et al., 2010 | N/A |
| Bacterial and virus strains | ||
| AAV2/1-E/SYN-FLEX-[pR-pG(r)] | Okamoto et al., 2020 | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| 1,4-Diazabicyclo [2.2.2] octane (DABCO) | Nacalai tesque | Cat# 34811-72 |
| Atipamezole hydrochloride (Antisedan) | Nihon Zenyaku Kogyo | N/A |
| Biotin–NHS | Calbiochem | Cat# 203112 |
| Bovine serum albumin (BSA) | Nacalai tesque | Cat# 01863-77 |
| Butorphanol tartrate (Vetorphale) | Meiji Seika Pharma | N/A |
| Diaminobenzidine-4HCl (DAB) | Dojindo | Cat# 347-00904 |
| Dimethyl sulfoxide (DMSO) | Nacalai tesque | Cat# 13407-45 |
| Di-sodium hydrogenphosphate (Na2HPO4) | Nacalai tesque | Cat# 31801-05 |
| Di-sodium hydrogenphosphate 12-water (Na2HPO4・12H2O) | Nacalai tesque | Cat# 31722-45 |
| Normal donkey serum | Sigma-Aldrich | Cat# S30-100ML |
| Formaldehyde solution | Nacalai tesque | Cat# 16223-55 |
| Glucose oxidase (GO) | Nacalai tesque | Cat# 16831-14 |
| Hydrochloric acid | Nacalai tesque | Cat# 18321-05 |
| Hydrogen peroxide (H2O2) (31% w/v) | Santoku | N/A |
| Medetomidine hydrochloride (Domitor) | Nihon Zenyaku Kogyo | N/A |
| Midazolam (Midazolam Sandoz) | Sandoz | N/A |
| Monoethanolamine (2-aminoethanol) | Nacalai tesque | Cat# 23405-55 |
| Sodium dihydrogenphosphate dihydrate (NaH2PO4・2H2O) | Nacalai tesque | Cat# 31718-15 |
| Sodium hydroxide solution (NaOH) (10 mol/L) | Nacalai tesque | Cat# 94611-45 |
| New MX | Matsunami Glass | Cat# FX00500 |
| OCT compound | Sakura Finetek | Cat# 4583 |
| Picric acid | Nacalai tesque | Cat# 27925-25 |
| Phosphate buffered saline (PBS) (10×) (pH 7.4) | Nacalai tesque | Cat# 27575-31 |
| Sodium azide | Nacalai tesque | Cat# 31233-55 |
| Sodium pentobarbital (Somnopentyl) | Kyoritsu Seiyaku | N/A |
| Tris(hydroxymethyl)aminomethane (Tris) | Nacalai tesque | Cat# 35434-21 |
| Triton X-100 | Nacalai tesque | Cat# 35501-15 |
| Tyramine hydrochloride | Sigma-Aldrich | Cat# T2879-1G |
| Xylene | Nacalai tesque | Cat# 36612-93 |
| β-D-Glucose | Nacalai tesque | Cat# 16804-32 |
| λ-Carrageenan | Wako Chemicals | Cat# 035-09693 |
| Critical commercial assays | ||
| VECTASTAIN Elite ABC Standard Kit | Vector Laboratories | Cat# PK-6100 |
| Experimental models: organisms/strains | ||
| Mouse: STOCK Tg (Drd1a-cre)150Gsat/Mmcd | MMRRC | RRID: MMRRC_029178-UCD |
| Software and algorithms | ||
| ADOBE ILLUSTRATOR CS3 | Adobe Systems | N/A |
| Other | ||
| Aminopropyltriethoxysilane (APS)-coated glass slides | Matsunami Glass | Cat# APS-01 |
| Auxiliary ear bar for mice | Narishige | EB-6 |
| Bamboo tablet | Wacom Corporation | CTL-470/K0 |
| Confocal laser scanning microscope TCS SP8 | Leica Microsystems | N/A |
| D-700 camera | Nikon | N/A |
| Electro freeze | Yamato Koki | MC-802A |
| Fine-tip tweezers | Fine Science Tools | Dumont #5 Forceps |
| Glass capillary (diameter 2 mm) | Narishige | G-2 |
| Hand drill | Minitor | Minimo |
| Low temperature circulator | EYELA | CCA-1112A |
| MICROPHOT-FXA | Nikon | N/A |
| Neo Sable round brush | Pentel | Cat# ZBNR-0 |
| NESCO DERMARK R (a surgical marker-pen) | Alfresa Pharma | Cat# ND-2 |
| Parafilm | Bemis Flexible Packaging | PM-996 |
| Picospritzer III | Parker Hannifin Corporation | N/A |
| Puller | Narishige | PC-100 |
| Sliding microtome | Leica Biosystems | SM2000R |
| Snow horn | Nippon Ekitan Corporation | N/A |
| Steel hole cutter (head diameter, 2.5 mm) | Minitor | BS1214 |
| Stereomicroscope | Leica Microsystems | MZ16 |
| Stereotaxic instruments for mice | Narishige | SR-5M-HT |
| Stereotaxic micromanipulator | Narishige | SM-15R |
| Digital slide scanner TOCO | CLARO | N/A |
Materials and equipment
Fixative solution (pH 7.4)
| Reagent | Final concentration | Amount |
|---|---|---|
| Saturated picric acid | 75% (v/v) | 750 mL |
| Na2HPO4 | 0.1 M | 14.2 g |
| Formaldehyde solution | 4% (v/v) | 108 mL |
| NaOH | N/A | N/A |
| ddH2O | N/A | up to 1,000 mL |
Adjust pH to 7.4 with NaOH.
Filter the solution through filter paper to remove any debris.
The solution may be preserved at 20°C–25°C for up to 1 year.
Alternatives: 4% paraformaldehyde in 0.1 M PB can also be used with the fixative.
PBS-X
| Reagent | Final concentration | Amount |
|---|---|---|
| Triton X-100 | 0.3% (v/v) | 1.5 mL |
| 10× PBS | 1× | 50 mL |
| ddH2O | N/A | up to 500 mL |
The solution may be preserved at 20°C–25°C for up to 2 weeks.
20% sodium azide solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Sodium azide | 20% (w/v) | 2 g |
| ddH2O | N/A | up to 10 mL |
The solution may be preserved at 20°C–25°C for up to 1 year.
PBS-XCD
| Reagent | Final concentration | Amount |
|---|---|---|
| λ-carrageenan | 0.12% (w/v) | 120 mg |
| Normal donkey serum | 1% (v/v) | 1 mL |
| Triton X-100 | 0.3% (v/v) | 0.3 mL |
| 20% sodium azide solution | 0.02% (v/v) | 0.1 mL |
| 10× PBS | 1× | 10 mL |
| ddH2O | N/A | up to 100 mL |
The solution may be preserved at 4°C for up to 1 year.
Biotinylated tyramine (BT) solution
Prepare BT solution as follows (Figure 2):
-
-
Dissolve 3.5 mg of biotin–NHS in 36.5 μL of DMSO.
-
-
Dissolve 15 mg of tyramine hydrochloride in 300 μL of DMSO (in the next step, use only 36.5 μL out of 300 μL).
-
-
Mix well 36.5 μL of biotin–NHS solution and 36.5 μL of tyramine hydrochloride solution by inversion.
-
-
Incubate the mixture for 12–24 h at 20°C–25°C with rotation and protection from light.
-
-
Add 7.3 μL of monoethanolamine to the mixture.
-
-
Incubate it for 4 h at 20°C–25°C to inactivate the remaining free biotin–NHS with rotation and protection from light.
-
-
Keep the BT solution at 4°C for up to 2 months. For a more extended duration up to 3 years, store the solution at −80°C.
Note: When all biotin–NHS reacts with tyramine hydrochloride, 128 mM of BT solution will be obtained.
0.2 M phosphate buffer (PB) (pH 7.4)
| Reagent | Final concentration | Amount |
|---|---|---|
| NaH2PO4・2H2O | 0.04 M | 11.8 g |
| Na2HPO4・12H2O | 0.16 M | 116 g |
| ddH2O | N/A | up to 2 L |
The solution may be preserved at 4°C for up to 1 year.
Glucose oxidase (GO) solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Glucose oxidase | 1 mg/mL | 1 mg |
| 0.2 M PB | 0.1 M | 0.5 mL |
| ddH2O | N/A | 0.5 mL |
Dispense to 100 μL each and store at −80°C. The solution may be preserved at 4°C for up to 1 month.
ß-D-glucose solution
| Reagent | Final concentration | Amount |
|---|---|---|
| ß-D-glucose | 200 mg/mL | 200 mg |
| ddH2O | N/A | 1 mL |
Dispense to 100 μL each and store at −80°C. The solution may be preserved at 4°C for up to 1 month.
Mounting solution for fluorescence microscopy
| Reagent | Final concentration | Amount |
|---|---|---|
| Glycerol | 50% (v/v) | 10 mL |
| DABCO | 2.5% (w/v) | 0.5 g |
| 20% sodium azide solution | 0.2% (w/v) | 20 μL |
| 10× PBS | 1× | 2 mL |
| ddH2O | N/A | up to 20 mL |
The solution may be preserved at 4°C for up to 1 year.
0.5 M Tris-HCl (pH 7.6)
| Reagent | Final concentration | Amount |
|---|---|---|
| Tris | 0.5 M | 60.57 g |
| ddH2O | N/A | up to 1 L |
Adjust pH to 7.6 with HCl.
Before use, dilute the solution 10-fold with ddH2O (50 mM Tris-HCl).
The solution may be preserved at 20°C–25°C for up to 1 year.
Figure 2.
Chemical formula to produce biotin-tyramine
Step-by-step method details
Virus injection
Timing: ~1 h
Here, we describe the stereotaxic injection of an AAV vector solution (200 nL) into the mouse CPu.
-
1.
Disinfect the instruments for use in the injection experiment with 70% ethanol. Please refer to institutional guidelines for specific instructions on proper aseptic technique.
-
2.
Fabricate a glass micropipette using a PC-100 puller with a single pulling mode. Set the heating value to 73.5 and pulling force to 125 g (Figures 3A and 3B).
Note: The setting values will vary depending on the filament. Find the best conditions for your purpose.
-
3.
Fold the tip with fine-tip tweezers and check under a microscope that the tip is 50–70 μm in diameter (Figure 3C).
-
4.
Dilute AAV2/1-E/SYN-FLEX-[pR-pG(r)] vector solution with PBS containing 2% (w/v) BSA to 1 × 1011 infectious unit/mL.
-
5.
Place the diluted solution on ice.
Note: The solution may be preserved at 4°C for up to 1 month.
-
6.
Aspirate 500 nL of an AAV vector solution on a Parafilm sheet through the tip of a glass micropipette using capillary action, and mark the top surface of the solution with a permanent marker. Then, aspirate 200 nL of a virus solution again (700 nL in total).
CRITICAL: When injecting deep into the brain, the top surface of the solution becomes invisible, making it difficult to ascertain the injection volume. Aspirate the extra solution (e.g., 500 nL) and mark the level with a permanent marker so that you can inject the exact amount of the virus solution (Figure 3D).
-
7.
Anesthetize a Drd1-Cre BAC transgenic mouse (8–16 weeks) by intraperitoneal injection of a mixture of medetomidine (Domitor; 0.3 mg/kg), midazolam (Midazolam Sandoz; 4 mg/kg), and butorphanol tartrate (Vetorphale; 5 mg/kg) in saline (0.9% NaCl). Please refer to institutional guidelines for proper anesthesia.
-
8.
Mount the anesthetized mouse into a stereotaxic instrument using an auxiliary ear bar. Adjust the position of the mouth and nose fixing clamp (incisor bar) until the lambda and bregma are equal in height (flat-skull position) (Figure 3E).
-
9.
Make an incision in the scalp with a scalpel along the midline to expose the skull.
-
10.
After marking the skull above the CPu with a surgical marker-pen (NESCO DERMARK R), thin the skull above the marked site using a hand drill with a steel hole cutter (Figure 3F). The injection coordinates for the CPu are as follows: 0.8 mm anterior to bregma, 2.0 mm lateral to the midline, and 2.5 mm ventral to the brain surface.
CRITICAL: While thinning the skull by a hand drill, wet with saline appropriately to avoid damage to the cortices by frictional heat.
-
11.
Carefully remove the remaining bone using fine-tip tweezers to expose the surface of the brain.
-
12.
Set the Picospritzer III to 40 psi injection pressure and 5 ms injection duration (Figures 3G and 3H).
CRITICAL: Eject a drop of virus solution from the tip of the glass micropipette to make sure it is not clogged, before inserting it into the brain. Troubleshooting 1
-
13.
Move the glass micropipette to the brain surface and slowly insert it into the target (Figure 3I). Pull the micropipette upward approximately 0.05 mm to make space for the virus solution to diffuse. Troubleshooting 2, 3, and 4
-
14.
Inject the virus solution into the mouse CPu by pressure pulses through a glass micropipette attached to Picospritzer III for 5 min. Troubleshooting 5
-
15.
Leave the micropipette in place for 5 min, and then remove it slowly.
-
16.
After closing and sterilizing the wound, administer antibiotics (e.g., gentamicin ointment) locally.
-
17.
Recover the mouse from anesthesia by intraperitoneal injection of atipamezole (Antisedan; 1.5 mg/kg) in saline after approximately 15 min. Please refer to institutional guidelines for anesthesia management.
-
18.
Maintain the mouse in specific pathogen-free conditions under a 12 h light/dark cycle with ad libitum access to food and water for 1 week after the AAV injection.
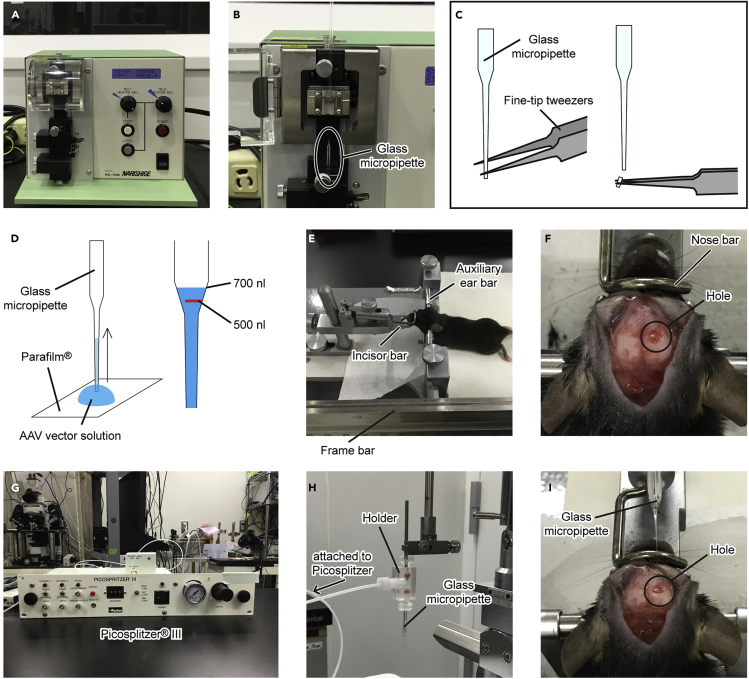
Figure 3.
Injection of the AAV vector solution
(A) PC-100 puller.
(B) Fabrication of a glass micropipette.
(C) Folding the tip of a glass micropipette
(D) Aspiration of the AAV vector solution into the glass micropipette. First, 500 nL of the solution is aspirated to mark the top surface with a permanent marker, and then another 200 nL is aspirated.
(E) Head fixation of an anesthetized mouse with a stereotaxic instrument.
(F) Perforation with a hand drill.
(G) Picospritzer III connected to a high-pressure N2 cylinder.
(H) Attaching the glass micropipette to the holder of Picospritzer III.
(I) Inserting the micropipette into the brain.
Tissue preparation
Timing: 2–3 days
Note: Filtrate PBS and the fixative solution through filter paper before perfusion.
Here, we introduce the preparation of sections for free-floating immunostaining.
-
19.
Anesthetize the mouse deeply by intraperitoneal injection of sodium pentobarbital (Somnopentyl; 200 mg/kg) in saline. Please refer to institutional guidelines for proper anesthesia.
-
20.
Open the thoracic cavity with surgical scissors, cut the right atrial appendage to bleed, and immediately insert a 22-gauge needle from the apex into the left ventricle of the heart.
-
21.
Perfuse with 20 mL of PBS using a syringe for 3 min to remove the blood from the circulatory system.
-
22.
Perfuse with 20 mL of a fixative solution using another syringe at the same speed.
CRITICAL: Be careful not to allow any small bubbles to enter during the perfusion. The inclusion of bubbles results in incomplete fixation.
-
23.
Remove the brain from the skull and place it in the same fixative solution for 12–24 h at 4°C.
-
24.
Replace the fixative solution with 30% (w/v) sucrose in 0.1 M PB and gently shake it for 1–2 days at 4°C to cryoprotect the brain tissue.
-
25.
Approximately 30 min before sectioning, turn on the low temperature circulator and set it to 4°C (Figure 4A).
-
26.
Mount the brain tissue onto the stage of a freezing microtome with OCT compound, and adjust the stage angle to keep the sagittal plane of the brain horizontal (Figure 4C).
-
27.
Turn on the electro-freezing component and set it to −25°C.
-
28.
Quickly freeze the brain tissue using dry ice powder and leave it in place for at least 5 min (Figure 4D).
Note: We recommend dry ice powder prepared by a snow horn for rapid tissue freezing (Figure 4B).
-
29.
Cut the brain tissue into 20-μm-thick parasagittal sections (Figure 4E), collect the sections with a paintbrush, and store them in 6 vials containing 0.02% sodium azide in 0.1 M PB (Figure 4F). Each vial will contain around 20 sections.
Note: For long-term storage (e.g., 5 years), increase the concentration of sodium azide to 0.2%.
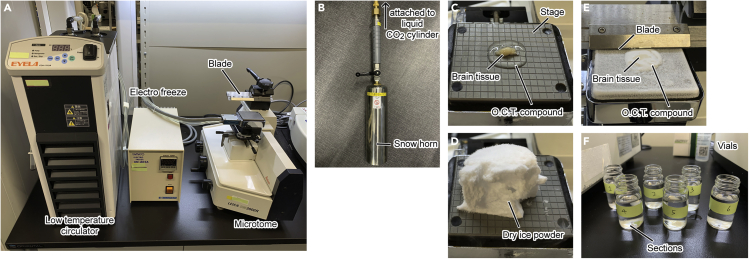
Figure 4.
Section preparation
(A) Sliding microtome equipped with a freezing device.
(B) Equipment to make dry ice powder (snow horn).
(C) Mounting brain tissue onto the stage with OCT compound.
(D) Freezing brain tissue with dry ice powder.
(E) Cutting brain tissue sections on the sliding microtome.
(F) Brain sections serially collected in 6 vials.
Immunofluorescence staining
Timing: 2 days
Here, we explain the procedure for double immunofluorescence staining for GFP and RFP. All the incubations are performed at 20°C–25°C with a gentle shake.
-
30.
Wash the sections with 8 mL of PBS for 10 min twice, and then with PBS-X for 30 min.
-
31.
Incubate the sections for 12–24 h with a mixture of 20 μg/mL of chicken polyclonal anti-GFP antibody and 1 μg/mL of affinity-purified rabbit antibody against mRFP1 in 500 μL of PBS-XCD.
-
32.
Wash the sections with 8 mL of PBS-X for 10 min twice.
-
33.
Incubate the sections for 2 h with a mixture of 5 μg/mL of AlexaFluor (AF) 488-conjugated antibody against chicken IgY and 5 μg/mL of AF568-conjugated antibody against rabbit IgG in 500 μL of PBS-XCD.
-
34.
Wash the sections with 8 mL of PBS-X for 20 min twice, and then with 8 mL of PBS for 20 min twice.
-
35.
Mount the sections onto APS-coated glass slides.
-
36.
Apply a coverslip with a mounting medium for fluorescence microscopy.
Note: The sections can be kept for about 2 years at −20°C.
-
37.
Observe the sections under a confocal laser scanning microscope (Figures 5A–5F). Troubleshooting 6 and 7
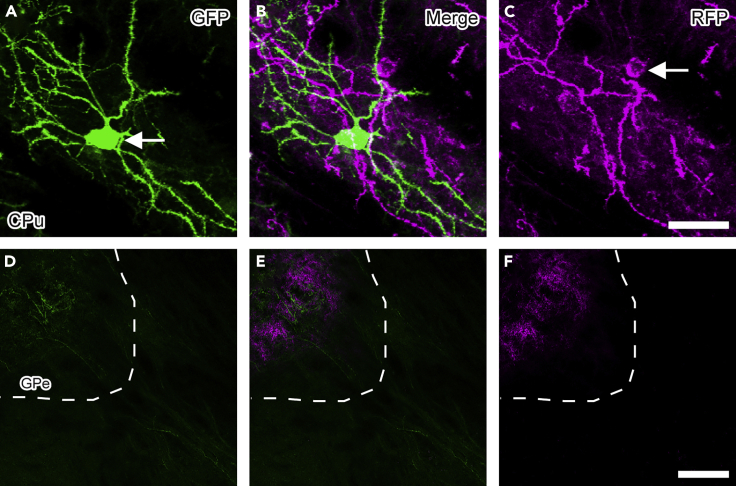
Figure 5.
Exclusive labeling of dMSNs and iMSNs
(A–C) The presence of spines on the dendrites indicates that GFP-positive and RFP-positive cells are dMSNs and iMSNs, respectively. Arrows indicate the cell bodies. Scale bar, 30 μm.
(D–F) In the GPe, GFP-positive fibers were sparsely distributed, whereas RFP-positive fibers were dense. GFP-positive, but not RFP-positive, fibers extend caudally beyond the GPe, forming the direct pathway to the output nuclei of the basal ganglia. Scale bar, 100 μm.
Immunoperoxidase staining
Timing: 4 days
CRITICAL: Before incubation with the sections, mix solutions A and B of ABC Elite kit at 1:100 dilution each in 500 μL of PBS-X and incubate the mixture for at least 30 min at 20°C–25°C with a gentle shake to form the avidin-biotinylated peroxidase complex (ABC).
We developed a cost-effective and straightforward signal amplification method called BT-GO reaction. Here, we describe the protocol in detail. All the incubations are performed at 20°C–25°C with a gentle shake.
-
38.
Wash the sections with 8 mL of PBS for 10 min twice.
-
39.
Incubate the sections for 30 min with 8 mL of 1% (v/v) H2O2 in PBS to inactivate endogenous peroxidase activity.
-
40.
Wash the sections with 8 mL of PBS-X for 10 min twice.
-
41.
Incubate the sections for 12–24 h with 0.1 μg/mL of affinity-purified rabbit antibody against GFP or mRFP1 in 500 μL of PBS-XCD.
-
42.
Wash the sections with 8 mL of PBS-X for 10 min twice.
-
43.
Incubate the sections for 2 h with 10 μg/mL biotinylated goat antibody against rabbit IgG in 500 μL of PBS-XCD.
-
44.
Wash the sections with 8 mL of PBS-X for 10 min twice.
-
45.
Incubate the sections for 1 h with 1:100-diluted avidin-biotinylated peroxidase complex (ABC) in 500 μL of PBS-X.
-
46.
Wash the sections with 8 mL of PBS-X for 10 min twice.
-
47.
Wash the sections with 8 mL of 0.1 M PB for 10 min twice.
-
48.
Incubate the sections for 5 min with a BT-GO reaction mixture containing 1:50,000-diluted BT solution, 3 μg/mL of GO, and 1% BSA in 1 mL of 0.1 M PB.
Note: The optimal concentration of the BT solution used in the reaction should be determined by the end user. We recommend a dilution of the solution from 1:5,000 to 1:500,000 for the trial.
-
49.
Add 10 μL of β-D-glucose solution to a final concentration of 2 mg/mL and mix the solution and sections well.
-
50.
Incubate the sections for 30 min (Figure 6A).
-
51.
Wash the sections with 8 mL of 0.1 M PB for 10 min.
-
52.
Wash the sections with 8 mL of PBS-X for 10 min twice.
-
53.
Incubate the sections for 1 h with 1/100-diluted ABC in 500 μL of PBS-X.
-
54.
Wash the sections with 8 mL of PBS-X for 10 min twice.
-
55.
Wash the sections with 8 mL of PBS for 10 min twice.
-
56.
Incubate the sections for 30–60 min with 0.02% (w/v) DAB and 0.0001% (v/v) H2O2 in 8 mL of 50 mM Tris-HCl (pH 7.6).
CRITICAL: Check the progress of DAB deposition under the microscope and stop the reaction at a high signal-to-noise ratio. If DAB deposition is weak and slow, additional H2O2 can be added directly to the reaction solution.
-
57.
Wash the sections with 8 mL of PBS for 10 min three times.
-
58.
Mount all the stained sections serially onto APS-coated glass slides.
-
59.
Dry up the sections.
-
60.
Soak the glass slides in ethanol-water mixtures of 30, 50, and 70% (v/v) for 30 min each, and dip the slides into 95% (v/v) ethanol in water.
-
61.
Soak the glass slides in 100% ethanol for 12–24 h.
-
62.
Dip the glass slides into a xylene-ethanol mixture of 50% (v/v) and soak the slides in 100% xylene for 12–24 h.
-
63.
Coverslip the sections with a mounting medium for bright-field microscopy, NEW MX.
-
64.
Observe the sections under a bright-field microscope, MICROPHOT-FXA, (Figures 6B and 6C) and capture images using a Nikon D-700 camera. Troubleshooting 8
Note: Specimens can be stored for at least 10 years at 20°C–25°C with protection from light.
Figure 6.
Signal enhancement with the BT-GO reaction
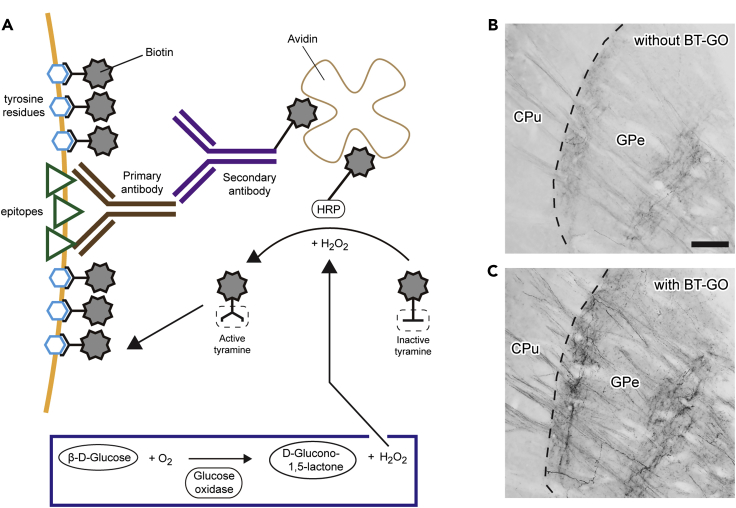
(A) Schematic diagram of the signal amplification method with the BT-GO reaction. BT is deposited on tyrosine residues in the tissue via the peroxidase activity of ABC. This deposition requires H2O2, and the chemical reaction between glucose and GO supplies H2O2 stably.
(B and C) Visualization of GFP-labeled dMSN axon fibers in the GPe without (B) or with (C) the BT-GO reaction. While DAB deposition through the peroxidase activity of ABC gave a weak signal (B), the BT-GO reaction markedly amplified the signals of the axon fibers. (C) is the adjacent section of (B). Scale bar, 100 μm.
Axon tracing
Timing: ~4 days
By using the virtual slide system, the efficiency of the two-dimensional reconstruction is greatly increased. We briefly describe the reconstruction method using a TOCO virtual slide scanner.
-
65.
Acquire a color image of the entire section with a spatial resolution of 1.038 μm/pixel using the digital slide scanner TOCO with a 10× objective lens.
-
66.
Trace and digitize the axons of DAB-stained neurons on the images with a digital pen tablet and a graphic software ADOBE ILLUSTRATOR CS3.
Alternatives: Virtual slide systems from other manufacturers are also available.
Note: Axons are sometimes out of focus, where multiple axon fibers cross one another. Additional observations with a bright field microscope may also be required to reconstruct the axons accurately.
Expected outcomes
The stereotaxic injection method allows us to deliver the transgene to the restricted area of the mouse CPu (Figure 3). The range of the infection area will be isotropic, with a radius of 0.5 to 0.8 mm. If the infection area is further confined, it may be more effective to use iontophoresis than pressure injection (Oh et al., 2014; Wang et al., 2014). Moreover, section preparation with a freezing microtome not only completes the process in a short time but may also lead to the improvement of antibody penetration and a signal-to-noise ratio due to freeze-thaw treatment (Figure 4).
By injecting an AAV vector solution into the CPu of a Drd1-Cre mouse, we successfully visualized dMSNs and iMSNs with different fluorescent proteins, GFP and RFP. While the projection of RFP-positive axon fibers was restricted to the external segment of the globus pallidus (GPe), GFP-positive axon fibers extended caudally beyond the region (Figure 5). We then amplified the signals of GFP-positive and RFP-positive axon fibers in the GPe using the BT-GO reaction (Figure 6). The reaction remarkably enhanced the signals while leaving the noise unaltered, thus contributing to the increase in the signal-to-noise ratio.
Limitations
The present exclusive labeling method allows us to visualize two cell populations with different fluorescent proteins based on the expression of Cre recombinase. However, the Cre-negative population may contain multiple cell types. Indeed, in addition to iMSNs, interneurons in the CPu not expressing Cre recombinase were labeled with RFP in the present study. Nonetheless, they can be distinguished by their morphology, such as the cell body size and the presence of spines on the dendrites (Okamoto et al., 2020). Given that interneurons do not send axons outside of the CPu, the distributions of dMSN and iMSN axon fibers, which are the focus of our study, can be quantitatively analyzed within the GPe. When using this exclusive labeling method, careful attention should be paid to the characteristics of the Cre-negative cell population.
Direct delivery of the virus vectors with stereotaxic injections has the advantage of being suitable for any specific region of the animal brain at any time. On the other hand, a disadvantage is that the infection area is limited. When a wide-area gene transfer is required, an AAV serotype that efficiently crosses the blood-brain barrier, such as AAV-PHP.eB (Chan et al., 2017), should be administrated intravenously.
The BT-GO reaction efficiently increases the signal-to-noise ratio; we (Furuta et al., 2009; Kuramoto et al., 2009) and others (Igarashi et al., 2012; Sun et al., 2020) have applied this method to axonal projection analysis. However, bright-field staining by DAB deposition via the BT-GO reaction has the drawback that the GFP and RFP signals cannot be observed simultaneously in the same section. The development of signal amplification methods that enable fluorescent multi-color labeling is expected in future studies.
Troubleshooting
Problem 1
No virus solution is released from the glass micropipette (step 12).
Potential solution
Set the injection duration of Picospritzer III to a longer time. If the solution is still not ejected, check the shape of the glass micropipette tip under a stereomicroscope.
Problem 2
The glass micropipette cannot be inserted into the brain (step 13).
Potential solution
Perform a small incision in the dura mater with fine-tip tweezers or a needle.
Problem 3
The infection site is out of the target position (step 13).
Potential solution
In stereotactic injection, it is critical to mount animals into a stereotaxic apparatus properly. In particular, ensure that the ear bars are inserted properly.
Problem 4
Infected cells are frequently observed in the area of passage of the glass micropipette (step 13).
Potential solution
The following points should be verified: 1) the glass micropipette is thin, 2) there is no bubble inside the glass micropipette, 3) slow injection of the virus solution, and 4) slow removal of the glass micropipette.
Problem 5
Excessive numbers of cells are labeled with the virus vectors (step 14).
Potential solution
The concentration of the solution should be determined according to the purpose of the experiment. Lower concentrations label fewer neurons.
Problem 6
The expression of fluorescent proteins is low (step 37).
Potential solution
The survival time of animals after virus injection should be determined based on the purpose of the experiments. In general, longer survival times result in higher expression.
Problem 7
The fluorescent signal rapidly fades (step 37).
Potential solution
It is recommended to evaporate as much water as possible in the mounting medium for fluorescence microscopy.
Problem 8
High background immunostaining (step 64).
Potential solution
Higher concentrations of primary antibodies may result in higher background immunostaining, especially in immunoperoxidase staining. First, the concentration of the primary antibody and then the concentration of the BT solution should be reduced (primary antibodies, a factor of 5; BT solution, a factor of 10–100).
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Hiroyuki Hioki (h-hioki@juntendo.ac.jp).
Materials availability
All plasmids used in this study are available from the Lead Contact upon request.
Data and code availability
This study did not generate any new data or code.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing. This study was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and the Japan Society for the Promotion of Science (JSPS) for Early Career Scientists (JP18K14844 to J.S.), Scientific Research (JP20K07231 to K.Y; JP17K08522 to M.K.; JP20H03549 to F.F.; JP16H04663 to H.H.), Exploratory Research (JP20K20671 to F.F.; JP17K19451 to H.H.), and Scientific Research on Innovative Areas, “Adaptive Circuit Shift” (JP26112001 to F.F.; JP15H01430 to H.H.), ‘‘Hyper-Adaptability’’ (JP20H05484 to F.F.), and “Resonance Bio” (JP18H04743 to H.H.). This study was also supported by the Japan Agency for Medical Research and Development (AMED) (JP20dm0207063 to T.F.; JP20dm0207064 to H.H.), Grants-in-Aid from the Research Institute for Diseases of Old Age at the Juntendo University School of Medicine (X1915 to K.Y.; X1904 to H.H.), and MEXT Private University Research Branding Project (Juntendo University).
Author contributions
Conceptualization, S.O. and H.H.; methodology, S.O., K.Y., J.S., M.T., Y.I., T.F., and H.H.; investigation, S.O., K.Y., J.S., and H.H.; writing – original draft, S.O., K.Y., and H.H.; writing – review & editing, S.O., K.Y., J.S., T.F., M.K., F.F., and H.H.; funding acquisition, J.S., K.Y., T.F., M.K., F.F., and H.H.
Declaration of interests
The authors declare no competing interests.
References
- Chan K.Y., Jang M.J., Yoo B.B., Greenbaum A., Ravi N., Wu W.L., Sanchez-Guardado L., Lois C., Mazmanian S.K., Deverman B.E. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat. Neurosci. 2017;20:1172–1179. doi: 10.1038/nn.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta T., Kaneko T., Deschenes M. Septal neurons in barrel cortex derive their receptive field input from the lemniscal pathway. J. Neurosci. 2009;29:4089–4095. doi: 10.1523/JNEUROSCI.5393-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamoto M., Kiyokage E., Sohn J., Hioki H., Harada T., Toida K. Structural basis for cholinergic regulation of neural circuits in the mouse olfactory bulb. J. Comp. Neurol. 2017;525:574–591. doi: 10.1002/cne.24088. [DOI] [PubMed] [Google Scholar]
- Hioki H., Kameda H., Nakamura H., Okunomiya T., Ohira K., Nakamura K., Kuroda M., Furuta T., Kaneko T. Efficient gene transduction of neurons by lentivirus with enhanced neuron-specific promoters. Gene Ther. 2007;14:872–882. doi: 10.1038/sj.gt.3302924. [DOI] [PubMed] [Google Scholar]
- Hioki H., Nakamura H., Ma Y.F., Konno M., Hayakawa T., Nakamura K.C., Fujiyama F., Kaneko T. Vesicular glutamate transporter 3-expressing nonserotonergic projection neurons constitute a subregion in the rat midbrain raphe nuclei. J. Comp. Neurol. 2010;518:668–686. doi: 10.1002/cne.22237. [DOI] [PubMed] [Google Scholar]
- Igarashi K.M., Ieki N., An M., Yamaguchi Y., Nagayama S., Kobayakawa K., Kobayakawa R., Tanifuji M., Sakano H., Chen W.R. Parallel mitral and tufted cell pathways route distinct odor information to different targets in the olfactory cortex. J. Neurosci. 2012;32:7970. doi: 10.1523/JNEUROSCI.0154-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Hioki H., Sohn J., Okamoto S., Kaneko T., Iino S., Oliver D.L. Convergence of lemniscal and local excitatory inputs on large GABAergic tectothalamic neurons. J. Comp. Neurol. 2015;523:2277–2296. doi: 10.1002/cne.23789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwano S., Sugiyama M., Hama H., Watakabe A., Hasegawa N., Kuchimaru T., Tanaka K.Z., Takahashi M., Ishida Y., Hata J. Single-cell bioluminescence imaging of deep tissue in freely moving animals. Science. 2018;359:935–939. doi: 10.1126/science.aaq1067. [DOI] [PubMed] [Google Scholar]
- Kataoka N., Hioki H., Kaneko T., Nakamura K. Psychological stress activates a dorsomedial hypothalamus-medullary raphe circuit driving brown adipose tissue thermogenesis and hyperthermia. Cell Metab. 2014;20:346–358. doi: 10.1016/j.cmet.2014.05.018. [DOI] [PubMed] [Google Scholar]
- Kuramoto E., Furuta T., Nakamura K.C., Unzai T., Hioki H., Kaneko T. Two types of thalamocortical projections from the motor thalamic nuclei of the rat: a single neuron-tracing study using viral vectors. Cereb. Cortex. 2009;19:2065–2077. doi: 10.1093/cercor/bhn231. [DOI] [PubMed] [Google Scholar]
- Nakamura K.C., Kameda H., Koshimizu Y., Yanagawa Y., Kaneko T. Production and histological application of affinity-purified antibodies to heat-denatured green fluorescent protein. J. Histochem. Cytochem. 2008;56:647–657. doi: 10.1369/jhc.2008.950915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S.W., Harris J.A., Ng L., Winslow B., Cain N., Mihalas S., Wang Q., Lau C., Kuan L., Henry A.M. A mesoscale connectome of the mouse brain. Nature. 2014;508:207–214. doi: 10.1038/nature13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S., Sohn J., Tanaka T., Takahashi M., Ishida Y., Yamauchi K., Koike M., Fujiyama F., Hioki H. Overlapping projections of neighboring direct and indirect pathway neostriatal neurons to globus pallidus external segment. iScience. 2020;23:101409. doi: 10.1016/j.isci.2020.101409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnutgen F., Doerflinger N., Calleja C., Wendling O., Chambon P., Ghyselinck N.B. A directional strategy for monitoring Cre-mediated recombination at the cellular level in the mouse. Nat. Biotechnol. 2003;21:562–565. doi: 10.1038/nbt811. [DOI] [PubMed] [Google Scholar]
- Sohn J., Okamoto S., Kataoka N., Kaneko T., Nakamura K., Hioki H. Differential inputs to the perisomatic and distal-dendritic compartments of vip-positive neurons in layer 2/3 of the mouse barrel cortex. Front. Neuroanat. 2016;10:124. doi: 10.3389/fnana.2016.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn J., Takahashi M., Okamoto S., Ishida Y., Furuta T., Hioki H. A single vector platform for high-level gene transduction of central neurons: adeno-associated virus vector equipped with the tet-off system. PLoS One. 2017;12:e0169611. doi: 10.1371/journal.pone.0169611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Wang J., Liang S.-H., Ge J., Lu Y.-C., Li J.-N., Chen Y.-B., Luo D.-S., Li H., Li Y.-Q. Involvement of the ventrolateral periaqueductal gray matter-central medial thalamic nucleus- basolateral amygdala pathway in neuropathic pain regulation of rats. Front. Neuroanat. 2020;14 doi: 10.3389/fnana.2020.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Kiyokage E., Sohn J., Hioki H., Toida K. Structural basis for serotonergic regulation of neural circuits in the mouse olfactory bulb. J. Comp. Neurol. 2015;523:262–280. doi: 10.1002/cne.23680. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Ishida Y., Kataoka N., Nakamura K., Hioki H. Efficient labeling of neurons and identification of postsynaptic sites using adeno-associated virus vector. In: Luján R., Ciruela F., editors. Receptor and ion channel detection in the brain: methods and protocols. Springer; 2020. [Google Scholar]
- Tamamaki N., Nakamura K., Furuta T., Asamoto K., Kaneko T. Neurons in Golgi-stain-like images revealed by GFP-adenovirus infection in vivo. Neurosci. Res. 2000;38:231–236. doi: 10.1016/s0168-0102(00)00176-0. [DOI] [PubMed] [Google Scholar]
- Wang Q., Henry A.M., Harris J.A., Oh S.W., Joines K.M., Nyhus J., Hirokawa K.E., Dee N., Mortrud M., Parry S. Systematic comparison of adeno-associated virus and biotinylated dextran amine reveals equivalent sensitivity between tracers and novel projection targets in the mouse brain. J. Comp. Neurol. 2014;522:1989–2012. doi: 10.1002/cne.23567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate any new data or code.