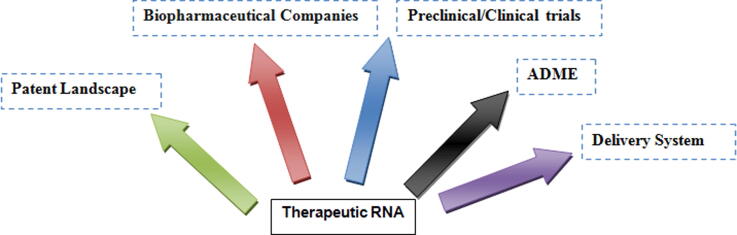
Graphical abstract
Keywords: miRNA, Next-generation medicine, Biopharmaceuticals
Abstract
miRNAs, a class of small endogenous RNAs, are one of the essential biopharmaceuticals which are in commercial spans as next-generation medicine in recent times. A snapshot of the current scenario regarding the miRNAs as biopharmaceuticals have been discussed. In this work, biopharmaceutical companies working with miRNAs and the current status of preclinical/clinical trials about miRNA therapeutics have been reviewed. Finally, recent updates on the absorption, distribution, metabolism, and excretion (ADME), as well as a delivery system of miRNAs, have been illustrated.
Introduction
With the landmark discovery of Victor Ambros's lab in 1993, microRNAs (miRNAs) were recognized as an essential molecule for gene regulation. In the Ambros work, the lin-4 was discovered as a gene from Caenorhabditis elegans, which was a miRNA gene, and the lin-4 gene contained complementary sequences to a repetitive sequence of the lin-14 mRNA (element in the 3ʹ UTR) [1]. At that time, this discovery was merely believed as a genetic discovery of worm. In anticipation of the subsequent invention, second miRNA-let-7, miRNA was not popularized. There are subsequent discoveries of miRNA where it was found that this class of gene is completely conserved among all species, as well as in humans [2], [3]. These two discoveries created interest in the research of various miRNAs in different animals, including human cells.
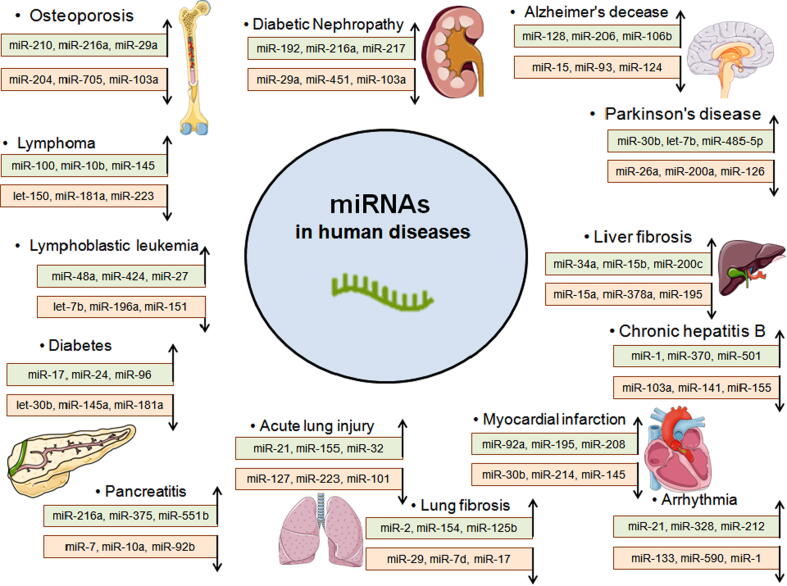
Principally miRNAs are a class of tiny nucleotide (nt) molecules. They are non-coding, as well as a single single-stranded group of RNAs. The length of the RNA is between 21 and 25 nts and acts as a gene regulator. miRNA biogenesis pathway is responsible for the processing of pre-miRNAs to mature miRNAs. The miRNA biogenesis pathway is well discussed by Bhattacharya et al. [4] and Chakraborty et al. [5]. Presently, it is already known that miRNA molecules regulate gene expression at the mRNA level [6]. Not only in gene regulation, but miRNAs are also influential regulators in a wide variety of diseases. A correlation has been observed between miRNAs and diseases such as immune disorders [7], Alzheimer's diseases [8], cardiovascular disease [9], rheumatoid arthritis[10], cancer [11], [12], etc. (Fig. 1). Recently, an association between various miRNAs and the regulation of some essential proteins in the insulin signaling pathway has been reported. A correlation of these proteins has also been observed with insulin resistance [13]. The functional role of miRNAs in an insulin signaling pathway has recently been illustrated, and their participation during the progression of pancreatic cancer has also been suggested [14]. miRNA deregulation is a usual characteristic of different diseases. Therefore, many types of research areas focus on the development of miRNA therapeutics for the treatment of an extensive range of human conditions. For the treatment of hepatitis C virus (HCV) infection, the first-ever miRNA therapeutic is in phase II clinical trials. A short locked nucleic acid (LNA) drug called miravirsen is for miR-122 and is rapidly moving from bench to clinic [15]. LNAs are novel nucleic acid analogues with some therapeutic value. Basically, they are changed or customized RNA nucleotides.
Fig. 1.
The potential of miRNAs in the treatment of human diseases.
In today's competitive world among pharma companies, search for a new therapeutic molecule that can act as a “new drug” is demanding. There are some qualifying criteria of a new molecule to be regarded as a “new drug” such as (i) a new drug must complete an unmet medical need, (ii) the compound should have a smart pharmacokinetic (PK) properties, and (iii) it should have a safety or efficacy over the current standard of care [16], [17]. Recent clinical trial data indicate that the anti-miR compounds, specific inhibitors for miRNAs, are the potential and a new class of drugs [18].
This review article aims to discuss the status of miRNA based therapeutics, which is currently in preclinical or clinical trials. Additionally, an illustration of the current patent landscape of miRNAs is provided. The article also discusses the current state of miRNA business and biopharmaceuticals companies. Finally, we have explained the fundamental views on ADME, recent delivery systems, and prospects for therapeutic miRNAs.
Recent patent updates for the therapeutic miRNAs
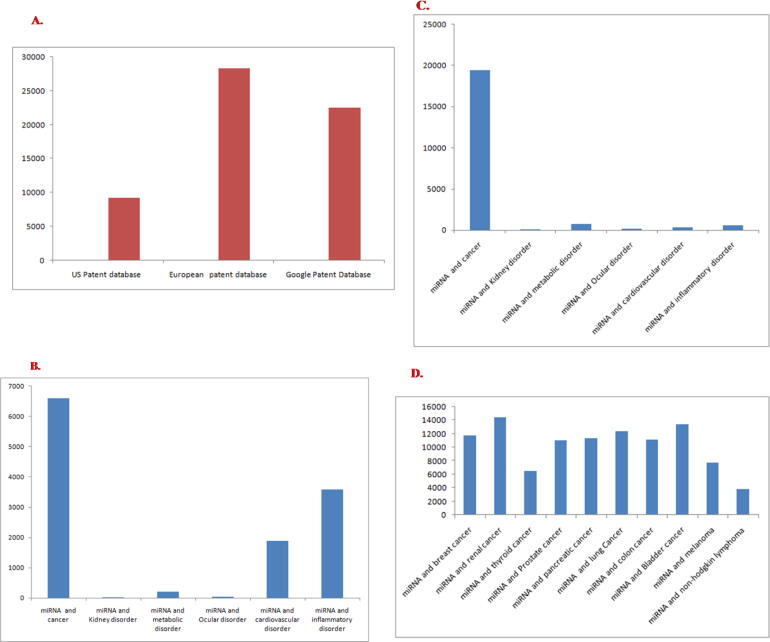
The intellectual property (IP) landscape is growing day by day in the area of innovation. Actually, patent protection is necessary for pharmaceutical companies. Pharma companies are pouring millions of dollars towards their research and development. Other than the developed countries, pharmaceutical companies are looking forward to the market of emerging industrial countries as their market is growing quicker. Therefore, there is a challenge for emerging economic states as well as developed countries to take the benefits of IP. Developing countries such as Brazil, Russia, India, and China are also aggressive in protecting their IP for innovations [19]. Nowadays, IP rights are not only restricted to pharmaceutical (chemically synthesized) products but are typical for other range of products also. For example, biological products such as monoclonal antibodies, recombinant vaccines are also patentable [20]. Furthermore, small-molecular drugs and biological drug candidates are also subject to patent rights. Focusing on commercial activity, recently, life science patents are growing in several United States of America (US) university campuses. Most of the universities are applying for patents for revenues from their innovations in the biomedical field [21]. Therefore, patents are playing a vital role in biopharmaceuticals and for the biopharmaceutical industry. It has been noted that biopharmaceutical companies are filing a number of patents in recent times. Currently, small RNA based therapeutics is a center of attraction for innovation and an important area to get patent rights. Tuschl et al. obtained a patent for their isolated nucleic acid (18–25 nucleotides), possessing at least 90% similarity to the complementary mature sequence of miRNA-122. This innovation is commonly termed as “Tuschl III” series [22]. Probably, this may be the first filed patent for miRNA. In Europe (2008), the first-ever miRNA-based patent was published. Van Rooij et al. performed a patent search related to miRNAs using the European patent database and the US patent database. They found 2136 published patent documents, of which 1661 were US publications, while 475 were European publications [17]. We have performed a smart search by utilizing the European patent database and the US patent database on miRNAs. The search revealed a collection of 7,055 US patents and 5,280 European patents from the database. We performed another search for miRNA related patents using “Google patent” and found 87,700 patents in total (Fig. 2A). Another quick search both from the US patent and European patent database showed that patents related to “miRNA and cancer” was too high compared to another disease (Fig. 2B, C).
Fig. 2.
Differentpatents of miRNAs (A) Total no of patents search from US patent database, the European patent office database and Google patent database (quick search through the keyword “miRNA”) (B) Number of patents in “miRNA” in case of the different diseases (from US patent database) (C) Number of patents in “miRNA” in case of the different diseases (from European patent office database) (D) Number of patents in “miRNA” in event of the different type of cancers (quick Search from Google patent database)(search was performed on 9th June 2020).
An analysis of diverse types of cancers from the US patents revealed that the maximum number of patents was granted for “miRNA and breast cancer” (2243 patents) while the lowest patent number was for “miRNA and renal cancer” (4 2 5) (Fig. 2D). Based on our search on patents related to miRNAs, a list of several important US, European and other countries have been prepared and summarized in Table 1. This table describes the significant information about the different US, European, and other country's patents such as patent number, county of origin, publication date, patent statement, inventors, and applicant.
Table 1.
Some recent patents issued for the discovery of miRNA molecules throughout the word.
| Patent No. | Patent Type (Country of Origin) | Publication Date | Patent Statement | Inventors | Applicant |
|---|---|---|---|---|---|
| US 20,170,240,898 A1 | US Patent | 10th March 2016 | Method for inhibiting Ebola virus via miRNA | Chenyu Zhang et al. | Jiangsu Micromedmark Biotech Co., Ltd (Taizhou, Jiangsu, CN) |
| US 20,180,171,334 A1 | US Patent | 21st June 2018 | Targeting microRNAs for metabolic disorders | BalkrishenBhat et al. | Regulus Therapeutics Inc. (San Diego, CA.USA) |
| US 20,170,121,711 A1 | US Patent | 4th May 2017 | miRNAs useful for identifying targets associated with cancer | Olorunseun O. Ogunwobi and Dibash K. Das | Research Foundation of the City University of New York (New York, NY,USA) |
| US 20,150,126,579 A1 | US Patent | 7th May 2015 | Micro-RNA inhibitors and their uses in disease | Pier Paolo Pandolfi et al. | Beth Israel Deaconess Medical Center (Boston, MA,USA) |
| CA3024953 A1 | European Patent | 11th October 2007 | Pharmaceutical composition comprising anti-miRNA antisense oligonucleotides | ElmenJoacim et al. | Roche Innovation Ct Copenhagen As |
| KR20180112180A | Korean Patent | 12th October 2018 | miRNA DNA DNA structure for validation of miRNA target | RyuSeong Ho and Choi Cheol Won | Soonchunhyang University Industry Academy Cooperation Foundation |
| WO2018181877 A1 | Japan Patent | 4th October 2018 | Cancer stem cell growth inhibitor using miRNA | Xin Wu et al. | Cancer Stem Tech Inc, Japan |
| US2018251858A1 | US Patent | 6th September 2018 | Method for identification of anti-HIV human miRNA mimics and miRNA inhibitors and anti-HIV pharmaceutical compounds | JerolenNaidoo et al. | CSIR [ZA] |
| WO2018157026A1 | US Patent | 30th August 2018 | Treatment of tumors with miRNA targeting CDK4/CDK6 | Amriti R Lulla and Wafik S EL-Deiry | Institute for Cancer Research and The Research Institute of Fox Chase Cancer Center, USA |
| CN108456692 A | China Patent | 28th August 2018 | Quadruple miRNA for resisting foot and mouth disease virus infection and building method | Jing Chen et al. | Institute of Animal Health, Guangdong Academy Agricultural Sciences,China |
| US2018223277 A1 | US Patent | 9th August 2018 | New Precursor miRNA and Applications in Tumor Therapy there of | Chenyu Zhang et al. | Jiangsu Micromedmark Biotech Co Ltd,China |
| CN108452307 A | China Patent | 28th August 2018 | Application of human miRNA-493-3p inhibitor in preparing medicine for treating renal fibrosis | Rui Du et al. | The Fourth Military Medical University, China |
| WO2018187673 A1 | US Patent | 11th October 2018 | miRNA signature expression in cancer | Marie Wood et al. | University of Vermont and State Agricultural College,USA |
| WO2018165929A1 | US Patent | 20th September 2018 | Dual miRNA inhibitory expression vector, construction method and application thereof | Jiyan Mao | Shenzhen Biocan Tech Co LTD, China |
| CA3012344A1 | US Patent | 17th August 2017 | Anti-AngiogenicmiRNA therapeutics for inhibiting corneal neovascularization | GuangpingGaoet al. | University Massachusetts, USA |
| CN108324946A | China Patent | 27th July 2018 | Application of miRNA708 and/or 301-cluster miRNA in the aspect of improving cardiac function | Zuoren Yu et al. | Shanghai East Hospital, China |
| CN108283646A | China Patent | 17th July 2018 | Application of hsa-miRNA-155-5p for preparing medicine for inhibiting human enterovirus 71 | Lingxiang Mao et al. | Zhenjiang First Peoples Hospital, China |
miRNA business and biopharmaceuticals companies
Currently, biopharmaceutical industries are a significant player for the biotechnology industry. The market for the biopharmaceutical industry is increasing, and the market was valued at $218,483.1 million in 2017. Between 2018 and 2023, the Compound Annual Growth Rate (CAGR) of about 8.59% has been proposed.
Among biopharmaceuticals, RNA therapeutics is a prominent business segment for the last 20 years.RNA based bio-pharmaceutical sare already entered into the market, and some are in the course of development. Clinical trials are being conducted for RNA-based drugs in various disease areas [23]. RNA-based biopharmaceuticals signify a new area for drug discovery and development. It has been estimated that RNA-based biopharmaceuticals will have a market value of about $1.2 billion by the end of 2020. Near about 65 academic institutes and 160 companies are presently involved in RNA-based therapies development [23]. However, the primary issue for RNA based drugs is to improve stability [24].
On the other hand, it is estimated that the typical cost to bring a new drug into the market is near about $1 billion [25], and the new compound may take 10–15 years to reach into the market. miRNA therapeutics is a significant area for RNA therapeutics and belong to the biopharmaceuticals. It is considered as “next-generation” therapeutics. The common miRNA market size was estimated at $ 160.5 million in 2017. It is estimated to rise at a CAGR of 18.6% over the tenure 2018–2020. Several biopharmaceutical companies are involved in RNA therapeutics development. Some are miRagen Therapeutics, Mirna Therapeutics, and Regulus Therapeutics, etc. These companies have made significant strides in the last 25 years. Major pharmaceutical companies are operating through a particular model whereby for R&D to clinical trials, they are utilizing little companies to develop new molecules. The major pharma giant companies have transformed themselves by following this vital trend over the past 20 years [26]. Now, RNA therapeutics is considered as trending investing area to enter into the profitable drug market. They are financing the development of new miRNA therapeutic molecules; so that they can come into the market quickly.
miRagen therapeutics Inc.
miRagen Therapeutics Inc.(formerly Signal Genetics, Inc.), is a biopharmaceutical company that is having clinical-platform for the discovery and development of RNA-based therapeutics. Their specific focus is to develop miRNAs therapeutics. It is located in Colorado, US. The company is focused on developing miRNA therapeutics for diseases with high unmet needs. They are using discoveries in miRNA biology to challenge conventional drug development paradigms. miRagen Therapeutics Inc. is producing an opening for novel therapeutic approaches and is performing a clinical trial of MRG-106 (inhibitor of miRNA-155), MRG-201 (a synthetic miRNA mimic to miRNA-29b) and MRG-110 (a synthetic miRNA inhibitor of miRNA-92).
Mirna therapeutics Inc.
Mirna Therapeutics, Inc. is a research and development company in the area of biotechnology. They are focused on miRNA based oncology therapies. This company is having world-class research capacity and facility, a robust understanding of miRNA and cancer biology, as well as an extensive IP portfolio. It is located in Austin, US. Mirna Therapeutics is a pioneer in capitalizing on the emerging field of miRNA-based therapeutics and their clinical trials. They are performing phase 1 clinical trial for MRX34 molecule (a miRNA molecule) on liver-associated cancers. Recently, Synlogic Inc. has acquired Mirna Therapeutics Inc.
Regulus therapeutics
Regulus was formed by the two companies which are Alnylam Pharmaceuticals and Isis Pharmaceuticals (presently, Ionis Pharmaceuticals), in September 2007. This company is a knowledge base company and has a focus on IP. It is located in California, USA. The company is paying attention to the discovery and development of miRNA based therapeutics. Currently, the company is performing a clinical trial on RG-012 (inhibits miR-21) and RGLS4326 (inhibit miR-17).
SantarisPharma
SantarisPharma is a biotechnological company explicitly working in the biopharmaceutical sector. It is situated in Copenhagen, Denmark, and was founded in 2003. The company has the IP rights for the therapeutic locked nucleic acid (LNA) technology for therapeutic applications. With the help of the LNA technology, the company is trying to develop the drugs for inhibiting miRNA and mRNA for a broad range of diseases. The company is currently performing clinical trials for miravirsen (SPC3649) (an inhibitor of miR-122). The company has received several awards for their continuous effort for developing RNA based drugs such as the Fierce 15 award by Fierce Biotechnology (2008), Red Herring Top 100 Europe Award (2010), Finalist Scrip Awards in the area of “Clinical Research Team of the Year”(2011), etc.
Current preclinical and clinical trials
There are some critical studies toward miRNAs as therapeutics, such as “extracellular vesicle-related crosstalk between the two components, immune system and melanoma [27]. The role of miRNAs in cancer is an emerging field [28]. We can understand this from our patent search about the increasing amount of work on miRNA based therapeutics in cancer. The therapeutic options for cancer treatment are increasing, along with the possibilities of the use of therapeutic miRNA. A recent review of the miRNA-based anticancer therapeutics has described the latest miRNA tools for cancer therapy, which is quite promising [29]. It has been noted in recent times HPV-related cancer cases are increasing. Casarotto et al. described the utility of non-coding RNAs, such as miRNAs in HPV-related cancer [30]. A colon or rectum related cancer is often termed as colorectal cancer which is associated with bowel movement and inflammation. A recent study of To et al. has described various miRNA based therapies for colorectal cancer [31]. On the other hand, it has been noted that exosomal miR-21 is responsible for colon adenocarcinoma. The study showed that in colon adenocarcinoma cells, miR-21 interacts with its target PDCD4. The exosomal miR-21 promotes invasion, proliferation, and extracellular formation for colon adenocarcinoma cells. Therefore, miR-21 may be a potential therapeutic approach for the treatment of colorectal cancer [32].
Similarly, miRNA is an interesting therapeutic tool for lung cancer, and is being studied by several scientists [33]. miRNA is also a treatment option for different drug resistance non-small cell lung cancer cells such as of gefitinib-resistant non-small cell lung cancer [34]. On the other hand, miRNA is an excellent therapeutic tool for acute myeloid leukemia (AML). It has been noted that two diverse loci of miR-15 and miR-16 create the diseases of AML. Therefore, miR-15 and miR-16 may be an outstanding therapeutic target to control the pathogenesis of AML [35]. Likewise, Chu-Tan et al. have described the miRNA based therapeutics in retinal disorders, which is very interesting for future studies [36]. However, there are significant challenges related to miRNA based cancer therapy, such as quick degradation and clearance in blood, limited penetration, the potential to activate the immune system, and unwanted side effects [37]. Some miRNA has been found to have an active role in toxicogenomics. miRNAs regulate genes that are affected by exposure to toxic substances and by the environmental changes [38].
To date, several miRNA molecules are in clinical trials. The first miRNA molecule that entered into the clinical trial is Miravirsen. The drug is under phase II clinical trials in several countries like the US, Slovakia, Poland, Romania, Netherlands, and Germany. For clinical trial approval, Investigational New Drug (IND) approval is necessary from the country's regulatory authority. The authority has to establish whether the new molecule would be safe initially to perform the clinical trial. It is also crucial to design a clinical trial protocol, and clinical trial investigators will observe the subject's safety. In the past few years, several miRNA-based therapeutics have been developed and are currently entered into different phases of clinical trials. Snapshots of these miRNA based therapeutics are listed in Table 2. These companies which are dealing with the clinical trials include mirage Therapeutics, SantarisPharma, Mirna Therapeutics, and Regulus Therapeutics. Clinical trials of numerous miRNAs have shown positive results in the initial phases. Some miRNAs molecules are in different stages of clinical trials that are described as following:
Table 2.
Major miRNA based therapeutics which are in the development phase.
| Therapeutic molecule | Disease | Target miRNA | Biotechnology or Biopharmaceutical Company | Stage of Development (Clinical trial/preclinical trial) |
|---|---|---|---|---|
| Miravirsen(SPC3649) | For the treatment of hepatitis C virus (HCV) infection | miR-122 | SantarisPharma | Phase II clinical trials |
| MRX34 | For the treatment of different types of cancers | miR-34a | miRNATherapeutics | Phase 1 clinical trial |
| RG-101 | For the treatment viral effect | miR-122 | Regulus Therapeutics | Phase 1B clinical trial |
| RG-012 | To prevent alport nephropathy | miRNA-21 | Regulus therapeutics (with the strategic alliance with Genzyme) | Preclinical stage |
| RGLS4326 | For the treatment of Polycystic kidney disease (PKD) | miR-17 | Regulus Therapeutics | Phase I clinical study |
| MGN-1374 | For the treatment of post-myocardial infarction | miRNA-15 and miR-195 | miRagen therapeutics | Preclinical stage |
| MGN-2677 | For the treatment of vascular disease | miR-143/145 | miRagen therapeutics | Preclinical stage |
| MGN-4220 | For the treatment of cardiac fibrosis | miR-29 | miRagen therapeutics | Preclinical stage |
| MGN-4893 | For the treatment of disorders like abnormal red blood cell production | miR-451 | miRagen therapeutics. | Preclinical stage |
| MGN-5804 | For the treatment of cardiometabolic disease | miR-378 | miRagen therapeutics | Preclinical stage |
| MGN-6114 | For the treatment of peripheral arterial disease | miR-92 | miRagen therapeutics | Preclinical stage |
| MGN-9103 | For the treatment of chronic heart failure | miR-208 | miRagen therapeutics | Preclinical stage |
| Cobomarsen (MRG-106) | For the treatment of cutaneous T-cell lymphoma (CTCL) | miR-155 | miRagen therapeutics | Phase-I clinical trial |
| MRG-107 | For the treatment of amyotrophic lateral sclerosis (ALS) | miR-155 | miRagen therapeutics | Completed preclinical trial and entering in clinical trial |
| MRG-110 | Target blood vessel growth and to control ischemia | miR-92a | miRagen therapeutics | Phase-I clinical trial |
| Remlarsen (MRG-201) | For the treatment of different type of fibrosis such as cutaneous fibrosis, idiopathic pulmonary fibrosis etc. | miR-29 | miRagen therapeutics | Phase-I clinical trial (for idiopathic pulmonary fibrosis and other fibrosis) |
Miravirsen(SPC3649)
Lately, for the treatment of hepatitis C virus (HCV) infection, a drug called Miravirsen (a locked nucleic acid (LNA) that inhibits miR-122)is under clinical trials [39], [40]. It is being given to the patient through subcutaneous injection during the clinical trials [41]. It is the first-ever drug that targets the miRNA. It entered into clinical trials and is now in phase II clinical trial undergoing assessment for its safety and effectiveness in the patients. It is a modified oligonucleotide that is made up of 15 nucleotides. The base sequence of Miravirsen binds to miR-122 and inhibits the miRNA. Santaris Pharma sponsors this molecule.
Mrx34
Several miRNAs can affect the suppression of tumors by repressing the tumor-suppressor genes [42]. miR-34a is known as a critical regulator of tumor suppression[43]. It has been noted that miR-34a down-regulates the expression of approximately 30 oncogenes through various oncogenic pathways [44]. It was given intravenously twice a week for three weeks in 4-week cycles. This drug targets miR-34a and can be used to treat a wide range of cancers, such as non-small-cell lung cancer (NSCLC), ovarian cancer, colon cancer, cervical cancer, hepatocellular carcinoma, etc. Currently, the molecule is in phase 1 clinical trial for the remedy of liver-based tumors. Mirna therapeutics is performing a phase 1 clinical trial for this molecule.
Rg-101
For hepatitis C virus (HCV) replication miR-122 is a critical miRNA molecule. This miRNA amplifies HCV translation by determining the structure of the internal ribosomal entry site [45]. RG-101 is an N-acetylgalactosamine (GalNAc)-conjugated oligonucleotide. It has the ability to antagonizes miR-122 in hepatocytes. To understand the safety, tolerability as well as the antiviral effect of RG-101, it is currently undergoing phase 1B clinical trial [46]. RG-101molecule is developing by Regulus Therapeutics.
Rg-012
It has been observed that in fibrogenic diseases in numerous organs, including the kidneys, miR-21 is critical and plays a significant role in regulation. It works through the silencing of metabolic pathways for ROS production (critical for cellular ATP generation) and inflammatory signaling [47], [48]. Anti-miR-21oligonucleotides have an essential role in preventing Alport nephropathy [48]. Alport nephropathy is a fatal genetic kidney disorder that currently does not have an approved therapy. For the cure of Alport syndrome, RG-012 is being tried as a remedy as it has anti-miR capabilities against miR-21. This molecule is developed by Regulus therapeutics with the strategic alliance with Genzyme.
Rgls4326
Polycystic kidney disease (PKD) is a chronic genetic disorder. The existence of abundant fluid-filled cysts in the renal parenchyma is the usual characteristic of this disorder. miR-17 or miR-17~92(miRNA cluster) is responsible for the formation of kidney cysts. In mouse models of PKD, it has been reported that the expression level of this miRNA is often upregulated [49]. RGLS4326 is a new oligonucleotide that is designed to inhibit miR-17 [50]. At present, this molecule is in Phase I clinical trials to understand the safety, tolerability, pharmacodynamics, and pharmacokinetics. It is being developed by Regulus Therapeutics.
Mgn-1374
miRNA-15 has a crucial role in cardiac ischemic injury. A study has observed that cardiac ischemic injury can be prevented by inhibiting miR-15 [51]. On the other hand, miR-195 is expressed in cardiomyopathy or during heart failure [52]. Therefore, MGN-1374 is designed to inhibit the miRNA-15 and miR-195 for the treatment of post-myocardial infarction [53]. It is in the preclinical stage. This molecule is being developed by miRagen therapeutics.
Mgn-2677
The miRNA-143/-145 is a cluster and is required for the separation of vascular smooth muscle cells. Generally, heart and vascular smooth muscle cells express the miRNA-143/-145 gene [54]. MGN-2677 molecule targets miR-143/145 for the treatment of vascular disease [55].This molecule is in the pipeline and is being developed by miRagen therapeutics.
Mgn-4220
It has been noted that cardiac fibrosis can be prevented by inhibiting miR-29. miR-29 acts by activating the Wnt signaling pathway to support the pathological alteration of the heart [56]. The MGN-4220 molecule targets miR-29 for the treatment of cardiac fibrosis. This molecule is undergoing development at miRagen therapeutics.
Mgn-4893
miR-451 is responsible for the survival of the erythrocyte [57] and the regulation of erythroid maturation [58]. miR-451 can be targeted by the MGN-4893 molecule for the cure of ailments like abnormal red blood cell production. MGN-4893 is undergoing clinical developmental stages at miRagen therapeutics.
Mgn-5804
miR-378 has a role in cardiometabolic disease [59], [60]. It has been shown that miR-378 deficient mice are resistant to fat-diet induced obesity. The absence of miR-378 in insulin-target tissues often has high oxidative capabilities and enhanced mitochondrial fatty acid metabolism. The MGN-5804 molecule targets miR-378 for the treatment of cardiometabolic disease. MGN-5804 is being developed by miRagen therapeutics.
Mgn-6114
miR-92/MiR-92a has a significant role in Peripheral artery disease (PAD) and also regulates angiogenesis of endothelial cells [61], [62]. The MGN-6114 molecule targets miR-92 for the treatment of peripheral arterial disease. Currently, MGN-6114 is undergoing clinical developmental stages at miRagen therapeutics.
Mgn-9103
miR-208/miR-208a has a role in chronic heart failure. The cardiac function and heart failure can be recovered by the inhibition of miR-208a [63], [64]. For the treatment of chronic heart failure, the MGN-9103 molecule can be utilized to target miR-208. Moreover, a crucial role of MGN-9103 has been observed in diabetes/obesity mouse models [65]. This molecule is also in the pipeline now. Presently, miRagen therapeutics is developing MGN-9103 for its further efficacy.
Cobomarsen (MRG-106)
miR-155 has a role in lymphoma cells [66], [67]. To restore normal function, therapeutic inhibition of miR-155 in lymphoma cells is required. Patients with cutaneous T-cell lymphoma (CTCL) and be treated by inhibiting miR-155 byMRG-106 molecule. At present, the molecule is under Phase-I clinical trials for this indication. Besides, other phase-I clinical trials are also underway for some other indications such as adult T-cell lymphoma/leukemia, diffuse large-B cell lymphoma, and chronic lymphocytic leukemia. MRG-106 is also being developed by miRagen therapeutics.
Mrg-107
miR-155 has a role in neurodegenerative disorders like Amyotrophic Lateral Sclerosis (ALS)[68]. The inhibition of miR-155 has the potential to extend survival. MRG-107 has a potential role in neurodegeneration by targeting miR-155. For the treatment of ALS, the MRG-107 molecule has already completed a preclinical trial and is in further stages of development at miRagen therapeutics.
Mrg-110
miRNA-92a controls blood vessel growth and angiogenesis [69]. MRG-110 targets miR-92a to target blood vessel growth and to control ischemia. This molecule is in a phase-I clinical trial and is under development at miRagen therapeutics.
Remlarsen (MRG-201)
miR-29 is a controlling regulator of extracellular matrix synthesis and is a potential target for the therapy of pathological fibrosis [70]. Remlarsen targets miR-29 to control a different type of fibrosis, such as cutaneous fibrosis, idiopathic pulmonary fibrosis, etc. For cutaneous fibrosis, this molecule is in a phase-II clinical trial. While it is in a phase-I clinical trial for idiopathic pulmonary fibrosis and other fibrosis. Remlarsen is also being developed by miRagen therapeutics.
Setbacks to miRNA therapeutics development
During the therapeutics development process, often, setbacks have also been registered by the companies. One such example is from Mirna Therapeutics, Inc. MRX34 (microRNA liposomal injection) was evaluated for its efficacy against melanoma. However, in the Phase 1 clinical trial, the drug was withdrawn due to serious adverse effects (https://clinicaltrials.gov/ct2/show/NCT02862145). To date, most of the miRNAs are in their early phase of clinical trials; thus, it remains to be seen how other miRNAs that are undergoing clinical trial for human application fairs in terms of toxicity or side effects.
ADME for miRNA
Over the past two decades, methods for ADME (absorption, distribution, metabolism, elimination) have promptly advanced. For the drug discovery and development studies, one should perform the ADME studies. These studies are a must for recognizing a new chemical entity (NCE) [71], [72]. It creates an interdisciplinary interface between medicinal chemists, biologists, clinicians, formulators, toxicologists, and regulatory authorities along with drug development industries. All new pharmaceutical compounds (NCE molecule) have to undergo pharmacokinetics (PK), and pharmacodynamics (PD) studies before administration to the human body [73]. Pharmacokinetics is the assessment of ADME of the new drug molecule and is required for an in-depth understanding of the in vivo pharmacology. Recently, PK has been termed as biokinetics [74]. PK is a measure of how a particular drug may affect the body, once it is taken. It also indicates the kind of mechanism that is utilized by the drug molecule for absorption and distribution. Usually, during the process of ADME, drugs employ different drug-metabolizing enzymes and diverse kinds of drug transporters present in a variety of organs like kidney, small intestine, and liver [75]. Cytochrome P450 (CYP or P450) isoforms are usual drug-metabolizing enzymes and play a vital role during drug metabolism [76], [77]. Solute carrier (SLC) and ATP binding cassette (ABC) proteins are specific drug transporters that play vital roles in drug ADME [78], [79]. Preclinical toxicity testing is required to evaluate whether a drug is adequately safe enough before being applied to humans. In general, a safety analysis for the drug starts from the experimental animal models.
There are some efforts to develop miRNA-based drugs, which are (i) either in the form of miRNA mimics, and it augments the impact of a miRNA, or (ii) miRNA inhibitors / anti-miR[80]. Before clinical trials of an anti-miR/ miRNA mimics drug, all the nonclinical data on the drug substance, characterization and the safety data of animals are accumulated along with a clinical trial protocol (Phase I clinical trial) for humans to assess the safety and various doses of the new therapeutic [17], [81]. For an Investigational New Drug (IND) or Clinical Trials, these preclinical data are required and have to submit to the regulatory authorities or ethical committees to get permission. So, one has to prepare a new dossier to get new drug permission [82].
Due to the incredibly water-soluble character of polyanionic molecules, they are being used to develop anti-miRs. Polyanionic molecules belong to the group of polyelectrolytes, which are polymers. The usual molecular mass of these small polyanionic molecules is in the range of 2–6 kDa. Due to this small size range, polyanionic molecules are vital for developing anti-miRs. However, due to reduced absorption of miRNA-based NCEs through the intestinal membrane, there are regarded as a weak molecule for oral administration [83]. So, anti-miR oligonucleotides are presently administered through injection. There are two types of application of injectable routes that are currently in use. One is intravenous, and another is subcutaneous injections.
On the other hand, infusions are also used for the administration route of anti-miRs [17], [18], [84]. Because of the water solubility of anti-miR, it is simple to prepare its aqueous solution [85]. However, more studies are required to understand the ADME of intravenous and subcutaneous injections, along with infusions of anti-miR/ miRNA mimics. Moreover, to rule out frequent administration of anti-miRs it is also necessary to increase their biological half-lives.
Recent delivery systems
Anti-miR/ miRNA mimics drug delivery should include the effectiveness and the accurateness of anti-miR/ miRNA mimics drug delivery to the target cells. Some features that are a prerequisite for any model delivery systems are; (i) protecting the anti-miR/ miRNA mimics from early disintegration into the blood, (ii) bringing the anti-miR/ miRNA mimics close to the target cells, (iii) supporting cellular uptake of the anti-miR/ miRNA mimics, (iv) should not stimulate any immunogenic response, and (v) finally encompass the materials which are biocompatible and biodegradable [86], [87]. Some recent delivery systems in use are as follows:
Polymeric vectors
Polymeric vectors are a type of carrier that is recently being used as drug carriers specifically for RNA interference(RNAi) sequences. The polymeric vectors are usually made up of peptides and proteins and have low cytotoxicity and immunogenicity. Polymeric vector-like Polyethylenimines (PEIs) are one of the well-studied delivery vectors [88]. This type of vector is a cationic and amine-rich vector, having branched or linear structures. PEIs have been extensively studied for the delivery of miRNAs [89]. For example, a complex of miRs with PEIs was prepared for the deliveryof miR-145 and miR-33a in a colon cancer model and examined [90].
Atelocollagen (ATE)
Atelocollagen (ATE) is a type I collagen molecule that usually behaves like a positively charged polymer. Pepsin is the source of type I collagen. It is employed for the delivery of nucleic acid targeted therapy [91]. ATE-miR-34a complex was studied for its ability to suppress tumor growth [92]. Another ATE-miRNA complex was also used for anticancer therapy. In this study, ATE was utilized for carrying miR-15a and miR-16-1into bone metastasis of prostate cancer [93].
Poly Lactic-co-Glycolic acid (PLGA)
PLGA is a negatively charged nanocarrier delivery system having biocompatible and biodegradable characteristics. It is an FDA approved hydrophobic drug-delivery system. PLGA has been utilized to deliver miRNA to the cell; for example, miR-34a was delivered by PLGA and was effective in reducing the size of the tumor [94].
Polyamidoamine (PAMAM)
Polyamidoamine is a dendrimer-based polymeric vector. It is a cationic carrier that attaches to the negatively charged miRNAs. Fluorinated PAMAM dendrimers are being used for the delivery of miRNA to MRC-5 cells through the in vitro transfection process [95]. It has been observed that once negatively charged single-stranded oligonucleotides are attached with PAMAMs, it increases the cellular uptake of the miR-34a-containing plasmid. This conjugated particle was delivered to lung cancer cells in vitro [96].
Degradable dendrimers
Recently for the delivery of nanomedicines use of the degradable dendrimer-based system is growing. The advantage of using dendrimer is that it is possible to manipulate different parameters, such as chemical composition, surface functionality, and molecular weight [97]. The modification of chemical structure may help the dendrimer-based delivery of miRNAs. The adjustment of ester bonds to dendrimers can efficiently deliver the let-7 g miRNA mimics and increased the accumulation of the miRNA molecule in the liver tumor site in vivo. It reduced both tumor growth as well as dendrimer-related toxicity [98].
Inorganic nano-materials
There are several inorganic nano-materials, such as diamond, gold, ferric oxide, and silica, which comprise an efficient group of carriers. These inorganic nano-materials group of carriers can be utilized for the delivery of miRNAs efficiently to the cell [99]. Utilizing miR(1)-AuNP(10)-S-PEG(0.5), Ghosh et al. were successful for the delivery of miRNAs into the cancer cells. The study utilized gold nanoparticles as the delivery system for the delivery of miRNAs into the cancer cells. The delivery system demonstrated the lowest toxicity, efficient uptake, and highest payload [100]. For the delivery of pre-miR-145 into the breast and prostate cancer cells, Ekin et al. designed thiolated gold nanoparticles (AuNPs). The gold nanoparticle delivery system was efficient in delivering the pre-miR-145 into the cancer cells in vitro [101].
Lipid-based delivery systems
Lipids are well-studied components of various oily liquids. Lipid-based delivery systems are used to improve the bioavailability and the solubility of the drugs [102]. Usually, general organic structures constitute lipid-based delivery systems [103]. A study successfully demonstrated the delivery of miR-34a and let-7 using neutral lipid emulsion. The results were remarkable in the sense that a substantial decrease in lung tumor growth was observed [104]. Another study performed by Pramanik et al. utilized a liposomal delivery system for miRNAs. In this study, a delivery system composed of (i) cationic amphiphile (ii) co-lipids and (iii) miRNA in a 1:1:0.2 ratio was used to treat a particular type of pancreatic cancer (pancreatic ductal adenocarcinoma). Here, the researchers used a liposomal delivery system and miRNA (miR-34a or miR-143/145 cluster) to treat pancreatic cancer. They used this miRNA based liposomal molecule for systemic intravenous delivery [105].
Viral vectors
The viral vector can deliver nucleic acid into the target cells with high efficiency. In conjugation with viral vectors miRNAscan also be carried into tumor cells. Different types of viral vectors areused as a delivery system such as (i) adenoviruses, (ii) adeno-associated viruses, (iii) retroviruses, (iv) lentiviruses, and (v) hybrid vectors. In hepatoma cancer cells, miRNA replacement therapy was utilized to deliver miR-26a to the cells. A self-complementary adeno-associated vector was utilized to clone the nucleic acid of miR-26a, which was further conjugated with a green fluorescent protein. This conjugated molecule was administrated to a tumor-bearing animal [106]. Another research group used a lentivirus-encoded miRNA delivery system. The study utilized lentivirus-encoded miR-15–16 in the xenograft prostate cancer mice model. One week after treatment, the cancer cell growth was found arrested. While tumor treated with an empty viral vector showed no significant growth change [107].
Other delivery system
Recently several other novel miRNA deliver system has been described, such as RNA drug delivery using red blood cell extracellular vesicles. Specifically, O-RBCs has been proposed as the delivery system as they are devoid of DNA and universally accepted by all blood group types [108].
New possibilities of miRNA as bio-pharmaceuticals
Drug resistance is a major concern in the current drug treatment regime. In recent times several antibiotic resistance cases have been reported. Consequently, the alternatives are falling short for patients with resistance to available antibiotics. For the treatment of cancer, chemotherapy is a major therapy. However, nowadays, chemotherapy resistance is another issue that concerns the outcome of cancer treatment. In this direction, miRNA therapeutics is new hope for patients with therapeutic or drug resistance issues, and miRNAs are gaining interest day by day as new biopharmaceuticals. Currently, in the case of chemotherapy resistance, therapeutic miRNAs are being used along with chemotherapeutic agents for the treatment of cancer. Therapeutic miRNA combined with chemotherapeutic agents also reduces the drug doses for cancer treatment [109], [110]. It has been noted that miR-3622b-5p augment apoptosis and also sensitizes cells to cisplatin. Therefore, this miRNA can be used for the chemotherapy combined therapeutic approach for the treatment of cancer [111]. Thus, miRNA therapeutics might help counteract drug resistance issues in the near future.
Future prospects
Recently, one of the significant issues which are a major concern with miRNAs based therapeutics is the delivery system. The development of new in vivo delivery systems would be vital for the delivery of miRNAs. The delivery system should be target-specific, and it should be able to deliver the drugs to the targeted cells or tissues. The biotechnology or pharmaceutical organization should efficiently utilize these improved delivery systems to deliver commercial therapeutic miRNAs. On the other hand, according to the therapeutic need, the localized delivery of miRNA therapeutics is another challenge and requires efforts to resolve it.
Conclusions
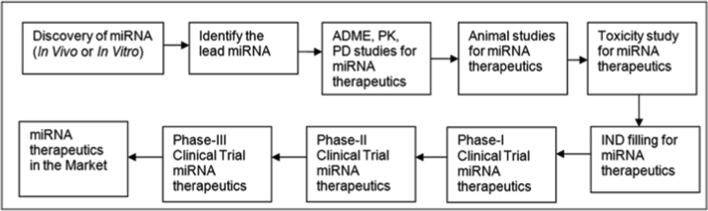
For pharmaceutical companies, the discovery of therapeutic miRNAs could be considered as one of the most exciting and significant therapeutic breakthroughs, and they have to cross different stages of the clinical trial to enter into the market (Fig. 3). miRNA-based therapeutics has confirmed that they might be the next generation drugs for the cure of various diseases. Presently, various Pharma companies are involved in the development of therapeutic miRNAs and novel delivery systems for the delivery of miRNAs to the targeted site. Therapeutic miRNAs being somewhat different from the NCE molecules and requires novel approaches for their development. In the next two decades, miRNA-based therapeutics will comprise several new drugs that will be introduced into the market. At last, it may be expected that currently developed therapeutic miRNAs will make to the clinics as next-generation therapeutics. Being able to regulate, specifically, therapeutic miRNAs, are the perfect choice for future medication.
Fig. 3.
The different stages of the therapeutic miRNA development procedure. A snapshot of all the stages, beginning from “miRNA discovery” to “miRNA therapeutics in the market”.
Compliance with Ethical Standards
Research involving Human Participants and/or Animals.
Not any human Participants and/or Animals are used for the research.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported by Hallym University Research Fund and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF2020R1C1C1008694).
Biographies

Dr. Chiranjib Chakraborty is a Professor, Department of Biotechnology at Adamas University Kolkata. He was former Professor, Galgotias University, India and former Associate Professor, VIT University, Vellore, India. Dr. Chakraborty is also a visiting Professor at Institute for Skeletal Aging & Orthopedic Surgery, Hallym University, South Korea. He is Academic Editor of iScience, Associate Editor of ‘Frontiers in Pharmacology’ and Editorial Board member of ‘Nature Scientific Reports’, ‘Interdisciplinary Sciences: Computational Life Sciences’, etc. His research interest is structural and functional bioinformatics of medically important proteins, diabetes, miRNA, etc. He published more than 140 SCI papers and five books and two edited books.

Ashish Ranjan Sharma is working as Research Professor at the Institute of Skeletal Ageing & Orthopedic Surgery, Hallym University. He obtained his Ph.D. degree in Medical Sciences (Pharmacology) from Hallym University, South Korea in Feb 2013. Currently, his research area is focused on studying various aspects of bone biology, cancer cell biology, nanotechnology, computational biology, and drug discovery. He has already co-authored some seventy peer-reviewed scientific publications.

Garima Sharma has obtained her Ph.D. degree in 2012 in microbiology from Singhania University, India and is currently working as a post-doctoral fellow at Neuropsychopharmacology and Toxicology Program, College of Pharmacy, Kangwon National University, South Korea. Her research interest is on neuropharmacology and cell signaling pathways. In addition, she is also working on nanoparticles based drug development for cancer and bone-related diseases. She has co-authored some Fifty peer-reviewed scientific publications.

Sang–Soo Lee is a Professor, Department of Orthopedic Surgery at Hallym University-hospital and director of Institute of Skeletal Ageing & Orthopedic Surgery. He received his medical degree from the college of medicine, Hallym University. He received a Ph.D. degree in the field of basic orthopedic research from Hallym University-Graduate School. He had completed his residency at the Hallym University Medical Center, Korea, and fellowship at the Hospital for Special Surgery, NY, USA. He has coauthored about Sixty peer-reviewed scientific publications in the field of orthopedic research.
Footnotes
Peer review under responsibility of Cairo University.
Contributor Information
Chiranjib Chakraborty, Email: drchiranjib@yahoo.com, chiranjib.chakravartty@adamasuniversity.ac.in.
Sang-Soo Lee, Email: 123sslee@gmail.com, totalhip@hallym.ac.kr.
References
- 1.Lee R.C., Feinbaum R.L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 2.Pasquinelli A.E. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000;408(6808):86. doi: 10.1038/35040556. [DOI] [PubMed] [Google Scholar]
- 3.Reinhart B.J. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403(6772):901. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharya M. The crucial role and regulations of miRNAs in zebrafish development. Protoplasma. 2017;254(1):17–31. doi: 10.1007/s00709-015-0931-1. [DOI] [PubMed] [Google Scholar]
- 5.Chakraborty C. miRNAs in insulin resistance and diabetes-associated pancreatic cancer: the 'minute and miracle' molecule moving as a monitor in the 'genomic galaxy'. Curr Drug Targets. 2013;14(10):1110–1117. doi: 10.2174/13894501113149990182. [DOI] [PubMed] [Google Scholar]
- 6.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 7.Taganov K.D., Boldin M.P., Baltimore D. MicroRNAs and immunity: tiny players in a big field. Immunity. 2007;26(2):133–137. doi: 10.1016/j.immuni.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Gupta P. miRNAs in Alzheimer disease–a therapeutic perspective. Curr Alzheimer Res. 2017;14(11):1198–1206. doi: 10.2174/1567205014666170829101016. [DOI] [PubMed] [Google Scholar]
- 9.Small E.M., Olson E.N. Pervasive roles of microRNAs in cardiovascular biology. Nature. 2011;469(7330):336. doi: 10.1038/nature09783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma A.R. miRNA-Regulated Key Components of Cytokine Signaling Pathways and Inflammation in Rheumatoid Arthritis. Med Res Rev. 2016;36(3):425–439. doi: 10.1002/med.21384. [DOI] [PubMed] [Google Scholar]
- 11.Chakraborty C., Chin K.-Y., Das S. miRNA-regulated cancer stem cells: understanding the property and the role of miRNA in carcinogenesis. Tumor Biol. 2016;37(10):13039–13048. doi: 10.1007/s13277-016-5156-1. [DOI] [PubMed] [Google Scholar]
- 12.Chakraborty C. MicroRNAs mediated regulation of MAPK signaling pathways in chronic myeloid leukemia. Oncotarget. 2016;7(27):42683. doi: 10.18632/oncotarget.7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakraborty C. Influence of miRNA in insulin signaling pathway and insulin resistance: micro-molecules with a major role in type-2 diabetes. Wiley Interdisc Reviews: RNA. 2014;5(5):697–712. doi: 10.1002/wrna.1240. [DOI] [PubMed] [Google Scholar]
- 14.Chakraborty C., Priya D., Bandyopadhyay S. miRNAs in insulin resistance and diabetes-associated pancreatic cancer: the 'minute and miracle' molecule moving as a monitor in the 'genomic galaxy'. Curr Drug Targets. 2013;14(10):1110–1117. doi: 10.2174/13894501113149990182. [DOI] [PubMed] [Google Scholar]
- 15.Lindow M., Kauppinen S. Rockefeller University Press; 2012. Discovering the first microRNA-targeted drug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoelder S., Clarke P.A., Workman P. Discovery of small molecule cancer drugs: successes, challenges and opportunities. Mol Oncol. 2012;6(2):155–176. doi: 10.1016/j.molonc.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Rooij E., Purcell A.L., Levin A.A. Developing microRNA therapeutics. Circ Res. 2012;110(3):496–507. doi: 10.1161/CIRCRESAHA.111.247916. [DOI] [PubMed] [Google Scholar]
- 18.Chakraborty C. Therapeutic miRNA and siRNA: moving from bench to clinic as next generation medicine. Mol Therapy-Nucleic Acids. 2017;8:132–143. doi: 10.1016/j.omtn.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reichman J.H. Intellectual property in the twenty-first century: will the developing countries lead or follow? Houston law review/University of Houston. 2009;46(4):1115. [PMC free article] [PubMed] [Google Scholar]
- 20.Rai A.K., Sherkow J.S. The changing life science patent landscape. Nat Biotechnol. 2016;34(3):292. doi: 10.1038/nbt.3504. [DOI] [PubMed] [Google Scholar]
- 21.Owen-Smith J., Powell W.W. The expanding role of university patenting in the life sciences: assessing the importance of experience and connectivity. Res Policy. 2003;32(9):1695–1711. [Google Scholar]
- 22.McLeod B.W. The'real world'utility of miRNA patents: lessons learned from expressed sequence tags. Nat Biotechnol. 2011;29(2):129. doi: 10.1038/nbt.1765. [DOI] [PubMed] [Google Scholar]
- 23.Lundstrom K. Latest development on RNA-based drugs and vaccines. Future Sci OA. 2018;4(5):p. FSO300. doi: 10.4155/fsoa-2017-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burgess D.J. RNA stability: remember your driver. Nat Rev Genet. 2012;13(2):72. doi: 10.1038/nrg3159. [DOI] [PubMed] [Google Scholar]
- 25.DiMasi J.A., Hansen R.W., Grabowski H.G. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22(2):151–185. doi: 10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- 26.Gautam A., Pan X. The changing model of big pharma: impact of key trends. Drug Discovery Today. 2016;21(3):379–384. doi: 10.1016/j.drudis.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Pretti M.A. Extracellular vesicle-mediated crosstalk between melanoma and the immune system: Impact on tumor progression and therapy response. J Leukoc Biol. 2020 doi: 10.1002/JLB.3MR0320-644R. [DOI] [PubMed] [Google Scholar]
- 28.Jin, J.O., et al., Nucleic acid nanotechnology for cancer treatment. Biochimica et Biophysica Acta (BBA)-Reviews on cancer, 2020.1847(1):188377. [DOI] [PubMed]
- 29.To K.K. Advances in the discovery of microRNA-based anticancer therapeutics: latest tools and developments. Expert Opin Drug Discov. 2020;15(1):p.63-83 doi: 10.1080/17460441.2020.1690449. [DOI] [PubMed] [Google Scholar]
- 30.Casarotto M. Beyond MicroRNAs: Emerging Role of Other Non-Coding RNAs in HPV-Driven Cancers. Cancers. 2020;12(5):1246. doi: 10.3390/cancers12051246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.To K.K. MicroRNAs in the prognosis and therapy of colorectal cancer: From bench to bedside. World J Gastroenterol. 2018;24(27):2949. doi: 10.3748/wjg.v24.i27.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun L.H. Exosomal miR-21 promotes proliferation, invasion and therapy resistance of colon adenocarcinoma cells through its target PDCD4. Sci Rep. 2020;10(1):p.1-8 doi: 10.1038/s41598-020-65207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xue J. MicroRNA-targeted therapeutics for lung cancer treatment. Expert Opin Drug Discov. 2017;12(2):141–157. doi: 10.1080/17460441.2017.1263298. [DOI] [PubMed] [Google Scholar]
- 34.Sin T.K. Implications of MicroRNAs in the Treatment of Gefitinib-Resistant Non-Small Cell Lung Cancer. Int J Mol Sci. 2016;17(2):237. doi: 10.3390/ijms17020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lovat, F., et al., Combined loss of function of two different loci of miR-15/16 drives the pathogenesis of acute myeloid leukemia. Proceedings of the National Academy of Sciences, 2020.117(22):p. 12332–40. [DOI] [PMC free article] [PubMed]
- 36.Chu-Tan J.A., Natoli R. The potential for microRNA-based therapeutics in retinal disorders. Ann Transl Med. 2020;8(7):419. doi: 10.21037/atm.2020.03.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Segal M., Slack F.J. Challenges identifying efficacious miRNA therapeutics for cancer. Expert Opin Drug Discov. 2020.18:p.1-5. doi: 10.1080/17460441.2020.1765770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong W.Y., Cho W.C. The role of microRNAs in toxicology. Arch Toxicol. 2015;89(3):319–325. doi: 10.1007/s00204-014-1440-2. [DOI] [PubMed] [Google Scholar]
- 39.Gebert L.F. Miravirsen (SPC3649) can inhibit the biogenesis of miR-122. Nucleic Acids Res. 2013;42(1):609–621. doi: 10.1093/nar/gkt852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janssen H.L. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368(18):1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 41.Titze-de-Almeida R., David C., Titze-de-Almeida S.S. The race of 10 synthetic RNAi-based drugs to the pharmaceutical market. Pharm Res. 2017;34(7):1339–1363. doi: 10.1007/s11095-017-2134-2. [DOI] [PubMed] [Google Scholar]
- 42.Okada N. A positive feedback between p53 and miR-34 miRNAs mediates tumor suppression. Genes Dev. 2014;28(5):438–450. doi: 10.1101/gad.233585.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slabáková E. Alternative mechanisms of miR-34a regulation in cancer. Cell Death Dis. 2017;8(10) doi: 10.1038/cddis.2017.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beg M.S. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Invest New Drugs. 2017;35(2):180–188. doi: 10.1007/s10637-016-0407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schult P. microRNA-122 amplifies hepatitis C virus translation by shaping the structure of the internal ribosomal entry site. Nat Commun. 2018;9(1):2613. doi: 10.1038/s41467-018-05053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Ree M.H. Safety, tolerability, and antiviral effect of RG-101 in patients with chronic hepatitis C: a phase 1B, double-blind, randomised controlled trial. The Lancet. 2017;389(10070):709–717. doi: 10.1016/S0140-6736(16)31715-9. [DOI] [PubMed] [Google Scholar]
- 47.Kölling M. Therapeutic miR-21 silencing ameliorates diabetic kidney disease in mice. Mol Ther. 2017;25(1):165–180. doi: 10.1016/j.ymthe.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gomez I.G. Anti–microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. J Clin Investig. 2015;125(1):141–156. doi: 10.1172/JCI75852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel V. miR-17~ 92 miRNA cluster promotes kidney cyst growth in polycystic kidney disease. Proc Natl Acad Sci. 2013;110(26):10765–10770. doi: 10.1073/pnas.1301693110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valencia, T., et al. Preclinical evaluation of RGLS4326 for the treatment of autosomal dominant polycystic kidney diseases. in nephrology dialysis transplantation. 2018. Oxford univ press great clarendon st, Oxford ox2 6dp, England.
- 51.Hullinger T.G. inhibition of miR-15 protects against cardiac ischemic injury. Circ Res. 2012;110(1):71–81. doi: 10.1161/CIRCRESAHA.111.244442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Divakaran V., Mann D.L. The emerging role of microRNAs in cardiac remodeling and heart failure. Circ Res. 2008;103(10):1072–1083. doi: 10.1161/CIRCRESAHA.108.183087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hydbring P., Badalian-Very G. Clinical applications of microRNAs. F1000Research. 2013;2 doi: 10.12688/f1000research.2-136.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao W., Zhao S.-P., Zhao Y.-H. MicroRNA-143/-145 in cardiovascular diseases. Biomed Res Int. 2015. 2015. doi: 10.1155/2015/531740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leimena C., Qiu H. Non-Coding RNA in the Pathogenesis, Progression and Treatment of Hypertension. Int J Mol Sci. 2018;19(4):927. doi: 10.3390/ijms19040927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sassi Y. Cardiac myocyte miR-29 promotes pathological remodeling of the heart by activating Wnt signaling. Nat Commun. 2017;8(1):1614. doi: 10.1038/s41467-017-01737-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rivkin N. Erythrocyte survival is controlled by microRNA-142. Haematologica. 2017;102(4):676–685. doi: 10.3324/haematol.2016.156109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pase L. miR-451 regulates zebrafish erythroid maturation in vivo via its target gata2. Blood. 2009;113(8):1794–1804. doi: 10.1182/blood-2008-05-155812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Urbich C., Kuehbacher A., Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res. 2008;79(4):581–588. doi: 10.1093/cvr/cvn156. [DOI] [PubMed] [Google Scholar]
- 60.Carrer M. et al., Control of mitochondrial metabolism and systemic energy homeostasis by microRNAs 378 and 378. Proceedings of the National Academy of Sciences, 2012. 109(38): p. 15330–5. [DOI] [PMC free article] [PubMed]
- 61.Hamburg M., Leeper N.J. Therapeutic potential of modulating microRNA in peripheral artery disease. Curr Vasc Pharmacol. 2015;13(3):316–323. [PubMed] [Google Scholar]
- 62.Zhang L. MiR-92a regulates viability and angiogenesis of endothelial cells under oxidative stress. Biochem Biophys Res Commun. 2014;446(4):952–958. doi: 10.1016/j.bbrc.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Montgomery R.L. Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation. 2011;124(14):1537–1547. doi: 10.1161/CIRCULATIONAHA.111.030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wong L. MicroRNA and heart failure. Int J Mol Sci. 2016;17(4):502. doi: 10.3390/ijms17040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grueter C.E. A cardiac microRNA governs systemic energy homeostasis by regulation of MED13. Cell. 2012;149(3):671–683. doi: 10.1016/j.cell.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kluiver, J., et al., Mir-155 Enhances B-Cell Lymphoma Growth By Targeting TBRG1, 2015, Am Soc Hematology.
- 67.Bedewy A.M. Prognostic value of miRNA-155 expression in B-cell non-Hodgkin lymphoma. Turkish J Hematol. 2017;34(3):207. doi: 10.4274/tjh.2016.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parisi C. Dysregulated microRNAs in amyotrophic lateral sclerosis microglia modulate genes linked to neuroinflammation. Cell Death Dis. 2013;4(12) doi: 10.1038/cddis.2013.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bonauer A. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324(5935):1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 70.Cushing L. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;45(2):287–294. doi: 10.1165/rcmb.2010-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Caldwell G.W. Compound optimization in early-and late-phase drug discovery: acceptable pharmacokinetic properties utilizing combined physicochemical, in vitro and in vivo screens. Curr Opin Drug Discov Devel. 2000;3(1):30–41. [PubMed] [Google Scholar]
- 72.Ruiz-Garcia A. pharmacokinetics in drug discovery. J Pharm Sci. 2008;97(2):654–690. doi: 10.1002/jps.21009. [DOI] [PubMed] [Google Scholar]
- 73.Pandey S. Bioanalysis in drug discovery and development. Pharm Methods. 2010;1(1):14–24. doi: 10.4103/2229-4708.72223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bonate, P.L. and D.R. Howard, Pharmacokinetics in Drug Development: Problems and Challenges in Oncology2016: Springer International Publishing.
- 75.Roberts S. Drug metabolism and pharmacokinetics in drug discovery. Curr Opin Drug Discov Devel. 2003;6(1):66–80. [PubMed] [Google Scholar]
- 76.Lin J.H., Lu A.Y. Inhibition and induction of cytochrome P450 and the clinical implications. Clin Pharmacokinet. 1998;35(5):361–390. doi: 10.2165/00003088-199835050-00003. [DOI] [PubMed] [Google Scholar]
- 77.Guengerich F.P. Cytochrome P450s and other enzymes in drug metabolism and toxicity. AAPS J. 2006;8(1):E101–E111. doi: 10.1208/aapsj080112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nigam S.K. What do drug transporters really do? Nat Rev Drug Discovery. 2015;14(1):29. doi: 10.1038/nrd4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rukov J.L. Pharmaco-miR: linking microRNAs and drug effects. Briefings Bioinf. 2013;15(4):648–659. doi: 10.1093/bib/bbs082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Husain S. Gene therapy for cancer: regulatory considerations for approval. Cancer Gene Ther. 2015;22(12):554. doi: 10.1038/cgt.2015.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vashisth S., Singh G., Nanda A. A comparative study of regulatory trends of pharmaceuticals in Brazil, Russia, India and China (BRIC) countries. J Generic Med. 2012;9(3):128–143. [Google Scholar]
- 82.Khatsenko O. absorption of antisense oligonucleotides in rat intestine: effect of chemistry and length. Antisense Nucleic Acid Drug Dev. 2000;10(1):35–44. doi: 10.1089/oli.1.2000.10.35. [DOI] [PubMed] [Google Scholar]
- 83.Stenvang J. inhibition of microRNA function by antimiR oligonucleotides. Silence. 2012;3(1):1. doi: 10.1186/1758-907X-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang L. Cytosolic co-delivery of miRNA-34a and docetaxel with core-shell nanocarriers via caveolae-mediated pathway for the treatment of metastatic breast cancer. Sci Rep. 2017;7:46186. doi: 10.1038/srep46186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang X. Enhanced hepatic delivery of siRNA and microRNA using oleic acid based lipid nanoparticle formulations. J Control Release. 2013;172(3):690–698. doi: 10.1016/j.jconrel.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang H.-W. Gadolinium-functionalized nanographene oxide for combined drug and microRNA delivery and magnetic resonance imaging. Biomaterials. 2014;35(24):6534–6542. doi: 10.1016/j.biomaterials.2014.04.057. [DOI] [PubMed] [Google Scholar]
- 87.Tamboli V., Mishra G.P., Mitra A.K. Polymeric vectors for ocular gene delivery. Therapeutic Delivery. 2011;2(4):523–536. doi: 10.4155/tde.11.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Höbel S., Aigner A. Polyethylenimines for siRNA and miRNA delivery in vivo. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013;5(5):484–501. doi: 10.1002/wnan.1228. [DOI] [PubMed] [Google Scholar]
- 89.Ibrahim A.F. MicroRNA replacement therapy for miR-145 and miR-33a is efficacious in a model of colon carcinoma. Cancer Res. 2011;71(15):5214–5224. doi: 10.1158/0008-5472.CAN-10-4645. [DOI] [PubMed] [Google Scholar]
- 90.Koenig O. An atelocollagen coating for efficient local gene silencing by using small interfering RNA. Mol Therapy-Nucleic Acids. 2017;6:290–301. doi: 10.1016/j.omtn.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tazawa H. Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells. Proc Natl Acad Sci. 2007;104(39):15472–15477. doi: 10.1073/pnas.0707351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hao Z. Efficient delivery of micro RNA to bone-metastatic prostate tumors by using aptamer-conjugated atelocollagen in vitro and in vivo. Drug Delivery. 2016;23(3):864–871. doi: 10.3109/10717544.2014.920059. [DOI] [PubMed] [Google Scholar]
- 93.Cosco D. delivery of miR-34a by chitosan/PLGA nanoplexes for the anticancer treatment of multiple myeloma. Sci Rep. 2015;5:17579. doi: 10.1038/srep17579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Öztuna A., Nazır H. In vitro transfection potential of fluorinated G5 PAMAM dendrimers for miRNA delivery to MRC-5 cells. Eur Res J. 2017;4(2):92–100. [Google Scholar]
- 95.Wang H. Aptamer-dendrimer bioconjugates for targeted delivery of miR-34a expressing plasmid and antitumor effects in non-small cell lung cancer cells. PLoS ONE. 2015;10(9) doi: 10.1371/journal.pone.0139136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Leiro V. The present and the future of degradable dendrimers and derivatives in theranostics. Bioconjug Chem. 2015;26(7):1182–1197. doi: 10.1021/bc5006224. [DOI] [PubMed] [Google Scholar]
- 97.Zhou K. Modular degradable dendrimers enable small RNAs to extend survival in an aggressive liver cancer model. Proc Natl Acad Sci. 2016;113(3):520–525. doi: 10.1073/pnas.1520756113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chakraborty C. Therapeutic microRNA delivery strategies with special emphasis on cancer therapy and tumorigenesis: current trends and future challenges. Curr Drug Metab. 2016;17(5):469–477. doi: 10.2174/1389200217666160126142408. [DOI] [PubMed] [Google Scholar]
- 99.Ghosh R. A gold nanoparticle platform for the delivery of functional microRNAs into cancer cells. Biomaterials. 2013;34(3):807–816. doi: 10.1016/j.biomaterials.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 100.Ekin A. Designing a gold nanoparticle-based nanocarrier for microRNA transfection into the prostate and breast cancer cells. J Gene Med. 2014;16(11–12):331–335. doi: 10.1002/jgm.2810. [DOI] [PubMed] [Google Scholar]
- 101.Alexander A. A review on novel therapeutic strategies for the enhancement of solubility for hydrophobic drugs through lipid and surfactant based self micro emulsifying drug delivery system: a novel approach. Am. J. Drug Disc. Develop. 2012;2(4):143–183. [Google Scholar]
- 102.Lv H. toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release. 2006;114(1):100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 103.Trang P. Systemic delivery of tumor suppressor microRNA mimics using a neutral lipid emulsion inhibits lung tumors in mice. Mol Ther. 2011;19(6):1116–1122. doi: 10.1038/mt.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pramanik D. Restitution of tumor suppressor microRNAs using a systemic nanovector inhibits pancreatic cancer growth in mice. Mol Cancer Ther. 2011;10(8):1470–1480. doi: 10.1158/1535-7163.MCT-11-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kota J. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137(6):1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bonci D. The miR-15a–miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat Med. 2008;14(11):1271. doi: 10.1038/nm.1880. [DOI] [PubMed] [Google Scholar]
- 107.Ramaiah, MJ. Functions and epigenetic aspects of miR-15/16: Possible future cancer therapeutics.Gene Reports, 2018: 1(12):p.149–64.
- 108.Usman W.M. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nat Commun. 2018;9(1):p.1-5 doi: 10.1038/s41467-018-04791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chakraborty C. The novel strategies for next-generation cancer treatment: miRNA combined with chemotherapeutic agents for the treatment of cancer. Oncotarget. 2018;9(11):10164–10174. doi: 10.18632/oncotarget.24309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yu H.W., Cho W.C. The emerging role of miRNAs in combined cancer therapy. Expert Opin Biol Ther. 2015;15(7):923–925. doi: 10.1517/14712598.2015.1030390. [DOI] [PubMed] [Google Scholar]
- 111.Vernon M. Functional miRNA screening identifies wide-ranging antitumor properties of miR-3622b-5p and reveals a new therapeutic combination strategy in ovarian tumor organoids. Mol Cancer Ther. 2020 doi: 10.1158/1535-7163. [DOI] [PubMed] [Google Scholar]