Abstract
Inflammatory myofibroblastic tumors (IMT) are rare soft tissue tumors of intermediate malignant potential with tendency for local recurrence. Although they can occur at all age groups, occurrence in infants is extremely unusual and their imaging characteristics are not well described. A 3-month-old female infant presented with gradually progressive abdominal distention without any fever or weight loss. She had a large ill-defined homogenous hypodense lesion of size 8.4 × 11.4 × 11.3 cm (APxTraxSag) in the abdomen showing mild delayed post contrast enhancement. She underwent exploratory laparotomy with gross total excision of mesenteric mass, histopathology of which was suggestive of IMT. She had recurrence within 6 months of complete resection with a well-defined heterogeneously enhancing lesion of size 1.8 × 1.8 × 2.3cm (APxTraxSag) in right paravesical region abutting the bladder without invasion with a similar lesion of size 4.4 × 2.1 × 3 cm (APxTraxSag) in left subdiaphragmatic region abutting superior surface of spleen (no invasion). Since, surgery in our patient would have entailed splenectomy and partial cystectomy, systemic therapy with ceritinib (anaplastic lymphoma kinase [ALK] inhibitor) was planned for her with which she had a near complete response after 2 months. A high index of suspicion is required to differentiate IMT from other common causes of mesenteric masses in children and role of radiologist is quintessential in this regard. Local recurrence with abutment but without invasion of surrounding structures points to the intermediate malignant pathology of IMT and may provide a clue to diagnosis. Systemic therapy is effective in patients who are ALK positive and destructive surgery should be avoided.
Keywords: Recurrent mesenteric inflammatory myofibroblastic tumor, Infant, Systemic therapy, ALK inhibitors, Case report
Abbreviations: IMT, inflammatory myofibroblastic tumor; IHC, immunohistochemistry; CT, computed tomography; US, ultrasound; ALK, anaplastic lymphoma kinase; DSRCT, desmoplastic small round cell tumor; RMS, rhabdomyosarcoma
Background
Inflammatory myofibroblastic tumor (IMT), originally a member of the Inflammatory pseudotumor family was first described in 1939 in the lung [1]. Since its first description, the understanding of biology and clinical features of IMT has gone a paradigm change. They are now considered potentially malignant with a propensity for local recurrence and rarely metastasis [2]. Most often presenting as a mass in the abdomino-pelvic region of children and young adults, they have been described in almost all anatomical locations [3,4]. Histologically, IMTs are characterized by a variably cellular spindle cell proliferation in a myxoid to collagenous stroma with a prominent inflammatory infiltrate composed primarily of plasma cells and lymphocytes, with occasional admixed eosinophils and neutrophils [3]. However, histological diagnosis is difficult and depends on evaluation by an expert sarcoma pathologist which are seldom available. Hence characterizing the imaging features of IMT becomes vital as it may give a clue to diagnosis and help in differentiation from other more common conditions like sarcomas, lymphomas or metastasis. Due to rarity of disease, literature on imaging features of IMT is scarce, more so for infantile IMT's [5], [6], [7].
Here we describe clinical and radiological findings of an 11-month-old female with mesenteric IMT who recurred within 6 months after complete resection and was treated with systemic therapy.
Case Presentation
Eleven-month-old female, born of full term normal vaginal delivery without any post-natal complications presented at three months of life with gradually progressive abdominal distention without any fever or weight loss. There was no significant past or family history. On examination, abdomen was distended with a large firm lump palpable centered around the umbilicus which was moving with respiration. An Ultrasound done at a private center showed a large ill-defined heterogeneously hypoechoic lesion in the right lumbar, umbilical and right iliac region abutting the liver superiorly displacing right kidney posteriorly and bowel loops to the left side. The lesion was showing vascularity on limited color Doppler scan. Contrast enhanced computed tomography (CT) scan of chest, abdomen and pelvis was done outside to characterize the lesion and stage the disease and reviewed at our center. In abdomen, there was evidence of a large ill-defined homogenous hypodense lesion of size 8.4 × 11.4 × 11.3 cm (APxTRAxSag) predominantly on the right side and in the midline. The lesion was showing mild heterogenous postcontrast enhancement on delayed images (at 5 minutes). Superiorly the mass was abutting the inferior surface of liver, gall bladder, and pancreas with focal loss of fat plane with pancreas and inferior surface of liver and inferiorly reaching up to the pelvis. It was displacing the small bowel loops to the left side and ascending colon and right kidney posterolateraly. However, there was no obvious infiltration of the above structures. These findings were suggestive of a mesenteric mass, likely malignant (Fig. 1a, b). She underwent exploratory laparotomy with gross total excision of mesenteric mass and resection anastomosis of involved small bowel. On histopathology, there was a spindle cell tumor will cells arranged in fascicular and haphazard pattern with abundant admixture of inflammatory cells rich in plasma cells, lymphocytes and few eosinophils. The tumor cells showed mild to moderate pleomorphism with finely dispersed chromatin and moderate to abundant eospinophilic cytoplasm. Variable mitosis was seen (4-5/10 per high power field) (Fig. 2a, b). Tumor cells showed nuclear immunorecativity for Anaplastic Lymphoma Kinase-1 (ALK-1) protein (Fig. 2c). In addition there was cytoplasmic positivity for smooth muscle actin (SMA) and desmin (Fig. 2d). Hence, a diagnosis of infantile IMT was offered. The child was kept on follow up after surgery. She developed abdominal pain after six months when a CECT abdomen was done which showed a well-defined heterogeneously enhancing lesion of size 1.8 × 1.8 × 2.3 cm (APxTraxSag) in right paravesical region indenting the right lateral wall of urinary bladder with loss of fat plane. Similar lesion of size 4.4 × 2.1 × 3 cm (APxTraxSag) was seen in left subdiaphragmatic region abutting superior surface of spleen with indentation and loss of fat plane but no obvious infiltration suggestive of recurrent disease (Fig. 3a-d). As resection would have entailed splenectomy and partial cystectomy, it was decided to offer the child systemic therapy with ceritinib 150 mg/day (Second generation ALK inhibitor) after discussion in multidisciplinary tumor board with which she had excellent response with no toxicity (Fig. 4a, b).
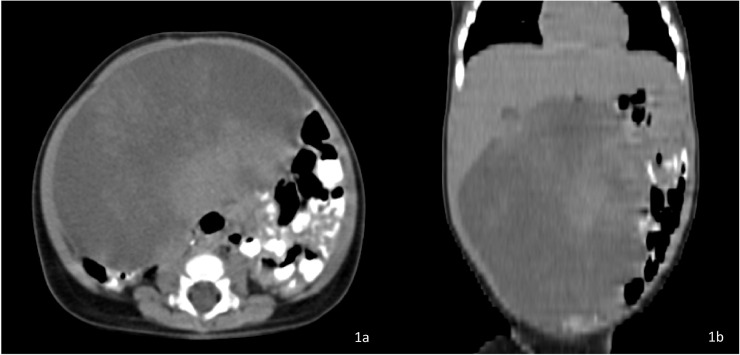
Fig. 1.
(a and b) Preoperative CECT Abdomen Axial + Coronal images showing large hypodense mesenteric lesion with mild heterogenous post contrast enhancement displacing small bowel loops to left side and ascending colon posteriorly and abutting inferior surface of liver with no obvious infiltration.
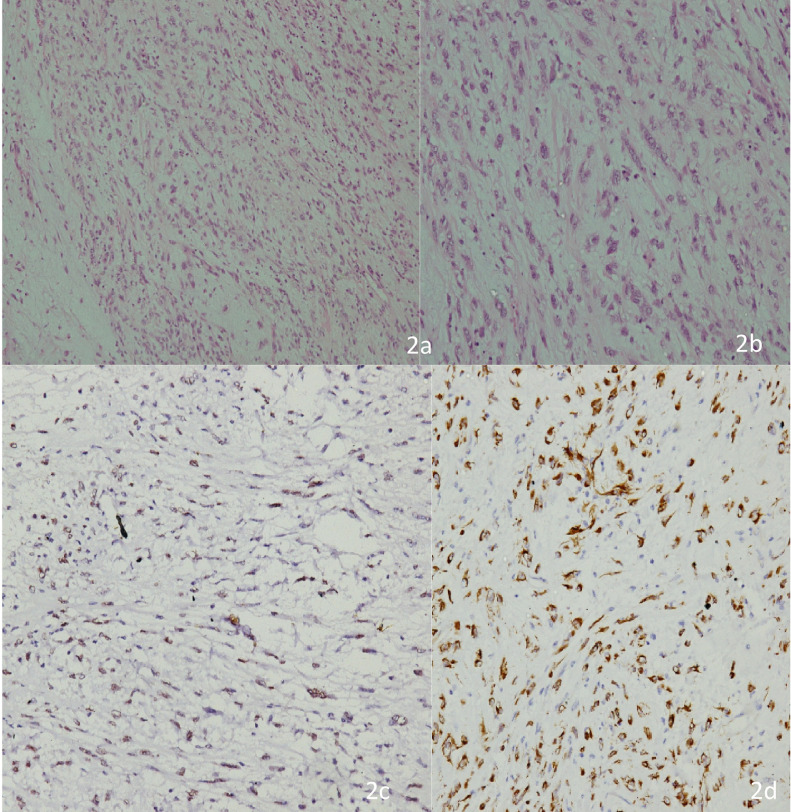
Fig. 2.
(a) Low power photomicrograph of the tumor showing cells arranged in fascicles as well as haphazard pattern with edematous background and admixed inflammatory cells. (b) High power picture showing spindle cell population exhibiting myofibroblastic differentiation with mild to moderate nuclear pleomorphism, finely dispersed chromatin and moderate to abundant cytoplasm. The inflammatory cells are rich in plasma cells with lymphocytes and few eosinophils (H&E 200x). (c) Immunostain for ALK-1 showing nuclear reactivity in cells with myofibroblastic differentiation. (d) Immunostain for SMA showing cytoplasmic reactivity in cells with myofibroblastic differentiation.
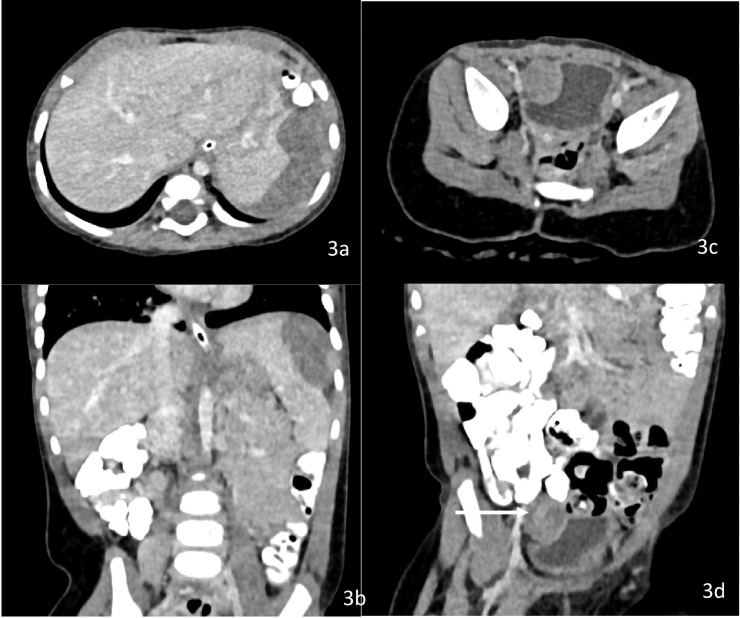
Fig. 3.
(a and b) CECT abdomen at recurrence Axial + Coronal images showing heterogeneously enhancing lesion in left subdiaphragmatic region abutting superior surface of spleen with indentation and loss of fat plane. (c, d) CECT abdomen Axial + Coronal images showing heterogeneously enhancing lesion in right paravesical region indenting the right lateral wall of urinary bladder with loss of fat plane.
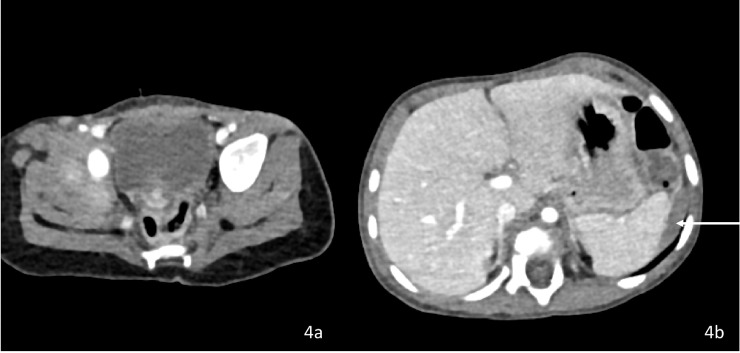
Fig. 4.
(a and b) Two moths post Ceritinib CECT Abdomen axial images showing complete resolution of right paravesical lesion and near complete resolution of left subdiaphragmatic lesion.
Discussion
Although IMT's have been described in all age groups ranging from infants to elderly, median age is around ten years and presentation in infancy is extremely rare [3]. Primary site of origin is equally distributed between lung and abdomen, with abdomen being more common in pediatric age groups. In the abdomen, mesentery usually accounts for 40%-50% of cases although this estimate seems slightly higher in pediatric context [8]. In a recent large case series of 32 pediatric IMT, median age was 9.5 years with abdomen as the most common site (28%) followed by head and neck (22%) and intrathoracic (22%). Only two patients had infantile IMT with one arising in the abdomen highlighting the rarity of the presentation [9]. Another study elaborating imaging findings of 9 cases of abdominal IMT in children described liver as the most common site (5 cases) with 2 cases each from mesentery and stomach/intestine [5]. There was no case of IMT in infants in this case series. In the largest case series of infantile IMT described in literature (12 cases), 6 were abdominal (50%), though exact number of mesenteric cases was not detailed and imaging findings were not described [10]. The majority of patients with abdominal IMT present with abdominal mass without other symptoms similar to our case, though rarely presentation with acute abdomen or systemic inflammatory response syndrome with fever and weight loss has been described [11].
Majority of abdominal cysts/masses in infants are of renal origin (55%) followed by gastrointestinal (15%, 5%-6% mesenteric origin) and extension from pelvic lesions (15%); majority are benign [12]. Important differential diagnosis of a solid mesenteric mass include mesenteric fibromatosis, desmoplastic small round cell tumor, high grade sarcoma (usually rhabdomyosarcoma or extraskeletal Ewing sarcoma), high grade lymphoma (usually Burkitt lymphoma), IMT and castleman disease.
Although pathology is the gold standard for diagnosis, radiological differential diagnosis is essential and may be a basis for starting treatment when patients condition warrants urgent therapy (as in rhabdomyosarcoma and burkitt lymphoma) [13]. Since IMT's can mimic other common malignant diseases and there is scarcity of literature on their imaging findings, a high index of suspicion is necessary on part of the radiologist to recognize this entity.
Ultrasound (US) is usually the most common first investigation done to evaluate an abdominal mass in infants. At US, IMT typically appears as an ill- or well-defined, heterogeneous, solid, hypo- or hyperechoic mass. A recent case series of US findings of mesenteric IMT described them as irregular hypoechoic masses in all cases with vascularity similar to our patient [14]. The margins can be well defined or ill defined, but usually infiltration to surrounding structures is lacking due to borderline malignant nature of these lesions. Frankly malignant IMT can have infiltrative features, though they are uncommon [14]. All the cases described in above case series were solitary similar to our case and is consistent with previous literature [5,14].
Contrast CT remains the investigation of choice in evaluating abdominal masses in children due to ease of performance and excellent sensitivity and specificity. IMT's are generally visualized as well demarcated homogenous hypodense masses which are usually bulky. Contrast enhancement is usually variable due to mixed pathology of the lesion consisting of myxoid, fibrous, and inflammatory components and can be early, delayed or even absent. Calcification are rarely seen, although when present they may provide a clue to diagnosis [5,13,15]. IMT's rarely show aggressive growth patterns such as circumferential growth around vessels and extension into the bowel with mural infiltration and ulceration. When such a pattern is present, other differentials like sarcoma and mesenteric fibromatosis should be higher on the differential. Concomitant diffuse peritoneal involvement suggests DSCRT, intense post contrast enhancement suggests castleman disease and infiltrative growth pattern may indicate mesenteric fibromatosis although basic imaging features are same as IMT [15]. A history of colonic polyps may suggest familial adenomatous polyps and suggest a diagnosis of mesenteric fibromatosis in this setting. An important differential is sporadic Burkitt Lymphoma; a more diffuse involvement, predilection for right lower quadrant of the abdomen, central necrosis due to rapid turnover and surrounding lymphadenopathy may give clues to diagnosis. Our patient had similar features as described in literature and imaging at recurrence showed similar imaging features though it was multifocal. In our patient, imaging findings at baseline were not suggestive of aggressive disease biology as there were no invasion or infiltration of surrounding structures; still our an early recurrence suggests that imaging can only be complementary and cannot predict clinical course in an individual patient.
Surgery remains the primary modality of treatment of IMT and complete resection in curative in a large majority of patients. Local recurrence occurs in around one-fourth of cases and only <5% develop distant metastasis [16]. Although re-excision is generally preferred for recurrent tumors, radical organ sacrificing resections should be avoided as even recurrent tumors have good long term outcomes above 80% [16]. Rearrangements in ALK can be seen in 50% of tumors and Introduction of tyrosine kinase inhibitors targeting ALK (mainly crizotinib and ceritinib) has changed the treatment paradigm for ALK positive IMT [17] Majority of data for ALK inhibitors exist in adults with few case reports of their successful use in pediatric population [18,19]. Since surgery in our patient would have entailed splenectomy and partial cystectomy, we started our patient on ceritinib 150 mg once a day with which she had an excellent response.
Conclusions
This is the first reported case of mesenteric IMT in an infant from India and describes similar radiological findings as seen in adults. CT remains the investigation of choice for diagnosing abdominal masses in children and is more practical than an MR in this scenario. Although pathology is the gold standard for diagnosis, a good radiological differential diagnosis is essential for the clinician. Characteristic CT appearance with findings of abutment and indentation of vital organs without frank invasion may provide a clue to diagnosis. This case also highlights the enigmatic problem of IMT where in imaging features seldom predict clinical behavior. Systemic therapy is effective in patients who express ALK on IHC and extensive surgery should be avoided in this scenario.
Consent for publication
Written informed consent was obtained from the patient's father (as patient is 11 month old girl) for publication of this case report and accompanying images.
Authors' contributions
AG and SS reported the radiological findings of the case. AB reviewed the pathology. AM and SR treated the patient. AG, SS and AM reviewed the literature and wrote the manuscript. All authors read and approved the final manuscript.
Consent Statement
Written informed consent was obtained from the patient's father for publication of this manuscript.
Footnotes
Competing interests: None.
Funding: None.
Acknowledgments: None.
References
- 1.Brunn H. Two interesting benign lung tumors of contradictory histopathology. J Thorac Surg. 1939;9:119–131. [Google Scholar]
- 2.Meis JM, Enzinger FM. Inflammatory fibrosarcoma of the mesentery and retroperitoneum. A tumor closely simulating inflammatory pseudotumor. Am J Surg Pathol. 1991;15:1146–1156. doi: 10.1097/00000478-199112000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Coffin CM, Watterson J, Priest JR, Dehner LP Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859–872. doi: 10.1097/00000478-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Cook JR, Dehner LP, Collins MH, Ma Z, Morris SW, Coffin CM. Anaplastic lymphoma kinase (ALK) expression in the inflammatory myofibroblastic tumor: a comparative immunohistochemical study. Am J Surg Pathol. 2001;25:1364–1371. doi: 10.1097/00000478-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Kim SJ, Kim WS, Cheon JE, Shin SM, Youn BJ, Kim IO. Inflammatory myofibroblastic tumors of the abdomen as mimickers of malignancy: imaging features in nine children. AJR Am J Roentgenol. 2009;193(5):1419–1424. doi: 10.2214/AJR.09.2433. [DOI] [PubMed] [Google Scholar]
- 6.Taratuta E, Krinsky G, Genega E, Roche K, Geneisier N. Pediatric inflammatory pseudotumor of the stomach: contrast-enhanced CT and MR imaging findings. AJR. 1996;167:919–920. doi: 10.2214/ajr.167.4.8819383. [DOI] [PubMed] [Google Scholar]
- 7.Day DL, Sane S, Dehner LP. Inflammatory pseudotumor of the mesentery and small intestine. Pediatr Radiol. 1986;16:210–215. doi: 10.1007/BF02456289. [DOI] [PubMed] [Google Scholar]
- 8.Coffin CM, Fletcher JA. Inflammatory myofibroblastic tumor. In: Fletcher CDM, Bridge JA, Hogendoorn PWC, Mertens F, editors. WHO Classification of Tumours of Soft Tissue and Bone. IARC Press; Lyon, France: 2013. pp. 83–84. [Google Scholar]
- 9.Dalton BG, Thomas PG, Sharp NE, Manalang MA, Fisher JE, Moir CR. Inflammatory myofibroblastic tumors in children. J Pediatr Surg. 2016;51(4):541–544. doi: 10.1016/j.jpedsurg.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Nunez O, John I, Panasiti RN, Ranganathan S, Santoro L, Grelaud D. Infantile inflammatory myofibroblastic tumors: clinicopathological and molecular characterization of 12 cases. Mod Pathol. 2020;33(4):576–590. doi: 10.1038/s41379-019-0406-6. [DOI] [PubMed] [Google Scholar]
- 11.Stringer MD, Ramani P, Yeung CK, Capps SN, Kiely EM, Spitz L. Abdominal inflammatory myofibroblastic tumours in children. Br J Surg. 1992;79:1357–1360. doi: 10.1002/bjs.1800791239. [DOI] [PubMed] [Google Scholar]
- 12.Hartman GE, Shochat SJ. Abdominal mass lesions in the newborn: diagnosis and treatment. Clin Perinatol. 1989;16(1):123–135. [PubMed] [Google Scholar]
- 13.Chung EM, Biko DM, Arzamendi AM, Meldrum JT, Stocker JT. Solid tumors of the peritoneum, omentum, and mesentery in children: radiologic-pathologic correlation: from the radiologic pathology archives. Radiographics. 2015;35(2):521–546. doi: 10.1148/rg.352140273. [DOI] [PubMed] [Google Scholar]
- 14.Qian J, Zhu K, Ye J. Ultrasonic manifestations of mesenteric inflammatory myofibroblastic tumors in children. Front Pediatr. 2019;7:39. doi: 10.3389/fped.2019.00039. Published 2019 Mar 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patnana M, Sevrukov AB, Elsayes KM, Viswanathan C, Lubner M, Menias CO. Inflammatory pseudotumor: the great mimicker. AJR Am J Roentgenol. 2012;198(3):W217–W227. doi: 10.2214/AJR.11.7288. [DOI] [PubMed] [Google Scholar]
- 16.Singer S, Neilsen T, Antonescu CR. In: 9th ed. DeVita VT, Lawrence TS, Rosenberg SA, editors. Lippincott Williams and Wilkins; Philadelphia, PA: 2012. p. 1540. International edition. [Google Scholar]
- 17.Coffin CM, Patel A, Perkins S, Elenitoba-Johnson KS, Perlman E, Griffin CA. ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod Pathol. 2001;14:569–576. doi: 10.1038/modpathol.3880352. [DOI] [PubMed] [Google Scholar]
- 18.Schöffski P, Sufliarsky J, Gelderblom H, Blay JY, Strauss SJ, Stacchiotti S. Crizotinib in patients with advanced, inoperable inflammatory myofibroblastic tumours with and without anaplastic lymphoma kinase gene alterations (European Organisation for Research and Treatment of Cancer 90101 CREATE): a multicentre, single-drug, prospective, non-randomised phase 2 trial. Lancet Respir Med. 2018;6(6):431–441. doi: 10.1016/S2213-2600(18)30116-4. [DOI] [PubMed] [Google Scholar]
- 19.Brivio E, Zwaan CM. ALK inhibition in two emblematic cases of pediatric inflammatory myofibroblastic tumor: Efficacy and side effects. Pediatr Blood Cancer. 2019;66(5):e27645. doi: 10.1002/pbc.27645. [DOI] [PubMed] [Google Scholar]