Highlights
-
•
Lemierre’s syndrome is a rare and potentially fatal entity.
-
•
Lemierre syndrome (Ls) is a rare septic thromboembolic complication of an infection of the oropharyngeal.
-
•
The incidence of Lemierre’s disease appears to be increasing.
-
•
A multidisciplinary approach is major point to treat patients with Lemierre’s syndrome.
Keywords: Lemierre’s syndrome, Oropharyngeal-infection, Jugular thrombophlebitis
Abstract
Introduction
Lemierre's syndrome is a rare and potentially fatal entity characterized by the spread of an oropharyngeal infection, with secondary suppurative thrombophlebitis of the internal jugular vein and septic emboli.
Presentation of case
We discuss the case of a 52-year-old male who developed Lemierre’s syndrome following peritonsillar abscess. He presented with submandibular and submental swelling extending into the neck. His management included; incision and drainage of the abscesses; and prolonged anticoagulant therapy.
Conclusion
The incidence of Lemierre's disease appears to be increasing, perhaps due to ignorance of the disease by many clinicians, and diagnosis is often delayed with potentially fatal consequences.
1. Introduction
Lemierre syndrome (Ls) is a rare septic thromboembolic complication of an infection of the oropharyngeal by anaerobes living in the mucosa. It is a life-threatening syndrome described by Andre in 1936 which associates post-anginal septicemia due typically to Fusobacterium necrophorum and complicated by internal jugular vein (IJV) thrombophlebitis and distant septic emboli, it affects mainly young adults from 15 to 30 years old, without medical history [1] the progression can be rapid and beyond control hence the need for urgent care [2]. Mortality rate up to 40%, particularly in the presence of mediastinal extension; however, this rate has significantly diminished with the rise of modern antibiotics [3].
The purpose of this study; from our case and literature review is to increase awareness about this condition among medical professionals. This work is reported by following the surgical case report (SCARE) guidelines [4].
2. Case presentation
A 52-year-old male presented to the emergency department with the main complaints of sore throat and a right laterocervical swelling for 10 days (Fig. 1).
Fig. 1.
Anterior view of the neck shows submandibular and submental swelling extending into the neck.
The medical history found diabetes, no pharmacological allergies, no psychosocial problems including drug, smoking, and no family genetic disease.
Clinical examination revealed inflammatory, indurated painful right laterocervical swelling. Examination of the oropharynx revealed marked trismus, a raised tongue, right peritonsillar swelling, and very bad dentition. General review showed early signs of sepsis, with a low-grade temperature of 38.5 °C and mild tachycardia.
Laboratory examinations revealed the following results: hemoglobin 13,2 g/dl, WBC: 13120/mm3, Absolute neutrophils 22530/mm3, C-reactive protein, 441.9 mg/l; creatinine 76.5 mg/L, urea: 0.69 g/L, ASAT: 585 UI/l; ALAT 607 UI/l; PT: 48%, fasting blood glucose: 3.05 g/L. Bacteriological examination of the pus after endobucal adenophlegmon enhancement showed a multidrug-resistant streptococcus intermedius; resistant to all betalactamine groupe, tetracyclin, ciprofloxacin, erythromycin, lincomycin, and trimethoprim/Sulfamethoxasol and sensitive only to levofloxacin, moxifloxacin, and vancomycin.
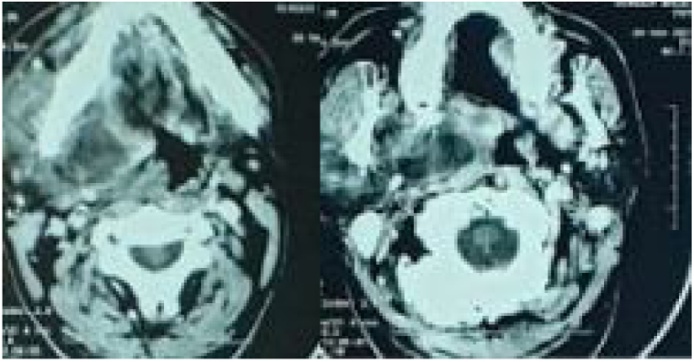
A computed tomography (CT) scan with intravenous contrast demonstrated a thrombosed right IJV, right tonsil abscess, associated with several abscessed bilateral submandibular lymph nodes more on the right side, narrowing the adjacent pharyngeal lumen (Fig. 2, Fig. 3).
Fig. 2.
CT scan of the neck revealing right tonsil abscess, associated with several abscessed bilateral submandibular lymph nodes more on the right side, narrowing the adjacent pharyngeal lumen.
Fig. 3.
CT scan of the neck demonstrating right internaljugular vein thrombosis and aspect of emphisematous cervical cellulitis.
The patient was immediately hospitalized and started multidisciplinary management with the endocrinologist, the cardiologist and the nephrologist. No bacteremia or metastatic infectious lesions were identified, but the patient was previously under broad-spectrum antibiotic therapy, and the first blood cultures were collected after the first administration of intravenous antibiotics.
He received as treatment:
-
•
Intravenous injectable treatment based on (amoxicillin/clavulanic acid (1 g every 8 h) + metronidazole (500 mg every 8 h) + gentamycin (160 mg per day on a single injection) during 4 days, after receiving the results of the antibiogram; the antibiotic therapy was switched to moxifloxacin (400 mg per day) + metronidazole (500 mg every 8 h) for 3 weeks.
-
•
Daily puncture of the right tonsil abscess,
-
•
Anticoagulant therapy based on Enoxaparin (60 mg/0.6 mL every 12 h) for 4 days switched by Acenocoumarol.
-
•
Endobucal adenophlegmon incision under local anesthesia in order to drain the pus and improve his trismus.
The evolution was marked by the increase in lateral cervical tumefaction. The control CT scan showed an aspect of emphysematous cervical facial cellulitis (Fig. 4, Fig. 5).
Fig. 4.
Sagittal scan CT of the head and neck demonstrated several collections at the level of the oral floor, some of which contain air bubbles.
Fig. 5.
CT scan coronal view of emphisematous cervical cellulitis.
Because of these results, the decision was to drain the collections under general anesthesia performed by a resident with 5 years of specialized training, a sebileau incision was made and we discovered several cervical facial collections, one of which communicates with the oral cavity and necrosis of the submandibular gland. A delbet drain was placed and the communications were closed in multiple layers (Fig. 6).
Fig. 6.
Lateral view of the patient after incision and drainage of the abscesses.
The patient adhered well to the treatment received with a good tolerance to the surgery and post-operative care including antibiotics and local care.
Postoperatively, the patient presented no complications. He was anticoagulated with heparin. An echocardiogram was negative for endocarditis. He was discharged on hospital day 12.
3. Discussion
Lemierre syndrome is a rare and serious septic complication that can quickly be life-threatening if an urgent charge is not taken due to the very high mortality [5]. It is characterized by anaerobic bacteremia and thrombophlebitis of the internal jugular vein (IJV) [6]. According to a recent Danish retrospective study, the incidence from 2010 to 2014 was 2.8 cases / million / year in the general population and 9.4 cases / million / year in the population of young people aged between 15 to 24 years old [7]. Lemierre’s syndrome occurs more commonly in men, with a 2:1 male: female ratio described in some studies [8]. Although this incidence seems to be underestimated, in the last two decades which can be explained by the misuse of antibiotics responsible for the emergence of resistant forms, an overconsumption of non-steroidal anti-inflammatory drugs during ENT infections [9].
Risk factors that can guide the diagnosis such as dental procedures or persistent fevers or history of sinusitis or include recurrent pharyngitis/tonsillitis [10] or diabetes mellitus and history of ENT malignancies [11]. The early symptoms of acute pharyngitis might not orient to recognize septicemia and other complications but persistent high fever and neck pain with tenderness can be signs for suspecting LS in its typical presentation. The delay between oropharyngeal infection and septicemia is usually one week in most infections [6]. Our patient was a diabetic man and had a bad oral condition with overconsumption of non-steroidal anti-inflammatory. The pathogen most commonly implicated is F. necrophorum (FN), followed by Fusobacterium nucleatum [11,12]. Other germs can be responsible for LS either in association with the FN with a rate of 10–30%, or exclusively. several species have been isolated such as Streptococcus (group A, B, C, oralis, constellatus, intermedium), Staphylococcus (methicillin-sensitive / resistant aureus, epidermidis), Enterococcus, Proteus mirabillis, Klebsiella pneumoniae, Pseudomonas aeruginosa, Bacteroides, Ekinella corrodens, Leptotricha buccalis, Porphyromonas (asaccherolityca, endodontalis), Arcanobacterium haemolyticum or Prevotella bivia [13]. These clinical symptoms are objectified by biological examinations revealing : inflammatory syndrome, leukopenia or hyperleukocytosis, thrombocytopenia, elevated liver enzymes or creatininaemia, hydro electrolytic disorders, hyperbilirubinemia, hypoxemia, or an increase in lactates [13]. Our patient presented most of these clinical and biological examinations.
Identification of the germs specially FN by blood culture, bacteriological examination is possible in 80–90% of cases. It can also be highlighted from the cerebrospinal fluid, pleural fluid, or by removal of abscessed collections [14].
Contrast-enhanced computed tomography (CT) of the neck still the best way to diagnose vascular thrombosis of the IJV showed as intraluminal filling defects and enhancement of the IJV along with soft tissue swelling. The CT can also diagnose other complications such as neck’s cellulitis, pulmonary emboli, osteomyelitis, as well as brain or epidural abscess [15]. Doppler ultrasonography can also highlight LS; the IJV thrombosis will be objectified as an echogenic region within a dilated vein or a complex mass of cystic and solid components [15].
A multidisciplinary approach is a major point to treat patients with Lemierre’s syndrome. Collaboration with infectious disease experts, pharmacologists, radiologists, otolaryngologists, and thoracic surgeons is important to reach a prompt diagnosis and with the good therapeutic outcome [2].
A recent systematic review [16] showed that most patients were treated with antibiotics. Amoxicillin/clavulanic acid (AMC) and ceftriaxone (CRO) were the preferred drugs, and metronidazole (MTZ) came in second (37.5%). Other authors reported that antimicrobial therapy should be prescribed for 3–6 weeks. Therapy can be completed orally once the infection is controlled. Despite providing appropriate antimicrobial treatment, the observed clinical response might be slow [15].
Currently, the use of anticoagulant therapy in LS management is controversial, due to subsequent lack of controlled studies [17]. But some authors strongly recommend anticoagulation for this case: a) failure of 48–72 h of adequate antimicrobial therapy, b) persistent bacteremia, c) underlying thrombophilia and/or d) progression to intracranial thrombosis [18].
Finally, drainage of infected cervicofacial tissue and abscessed collections may be necessary in around 50% of cases. On the other hand, the ligature of the internal jugular vein is exceptional, only reserved for the persistence of embolisms septic patients under optimal antibiotic treatment, uncontrolled severe sepsis, or even extensive septic thrombosis [13,14].
Sinave et al. reported that the lungs are the most common site for septic emboli with a percentage ranging from 85% to 97% [15]. These lung lesions are commonly appeared as necrotic cavitary lesions but can also be present as infiltrates, pleural effusions, empyema, lung abscesses, and necrotizing mediastinitis [15]. Our patient did not present metastatic infectious. A systematic review noted that 58% of patients having LS syndrome required intensive care [17].
Lemierre syndrome is a septic thromboembolic complication, which has become increasingly rare but which should not be forgotten, because the course is unpredictable. We recommend to start immediately a large spectre antibiotic therapy without delaying surgical drainage. The antibiotic treatment must be adapted according to the results of the antibiogram and the evolution.
4. Conclusion
Lemierre syndrome is an increasingly common and potentially fatal complication of pharyngitis. A high clinical suspicion in the emergency department must quickly evoke the diagnosis of Lemierre syndrome so that prompt treatment can be instituted since early treatment reduces its morbidity and mortality rates.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Funding
This research did not receive any specific grant(s) from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
I certify that this kind of manuscript does not require ethical approval by the Ethical Committee of our institution.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
K. Chaker: conception and design of the study.
O. Berrada: conception and design of the study.
M. Lyoubi: acquisition of data.
Y. Oukessou: drafting the article.
S. Rouadi: drafting the article.
R. Abada: revising the article.
M. Roubal: revising the article.
M. Mahtar: final approval of the version to be submitted.
Registration of research studies
Not applicable.
Guarantor
Kaoutar Chaker.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Contributor Information
Kaoutar Chaker, Email: kaoutar1990ckr@gmail.com.
Omar Berrada, Email: omarberrada7o@gmail.com.
Mouna Lyoubi, Email: mounalyoubi03@gmail.com.
Youssef Oukessou, Email: oukessouyoussef87@gmail.com.
Reda Allah Abada, Email: larbiabada75@gmail.com.
Sami Rouadi, Email: sami.rouadii80@gmail.com.
Mohammed Roubal, Email: roubal.mohmmed@gmail.com.
Mohammed Mahtar, Email: mohamedmahtar17@gmail.com.
References
- 1.Lemierre A. On certain septicaemias due to anaerobic organisms. Lancet. 1936;227(5874):701–703. [Google Scholar]
- 2.Lee W.-S., Jean S.-S., Chen F.-L., Hsieh S.-M., Hsueh P.-R. Lemierre’s syndrome: a forgotten and re-emerging infection. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.03.027. [DOI] [PubMed] [Google Scholar]
- 3.Wu M.-Y., Hou Y.-T., Ke J.-Y., Yiang G.-T. Case of internal jugular vein thrombosis and fever: Lemierre’s syndrome or Trousseau’s syndrome? Tzu-Chi Med. J. 2020;32(1):91. doi: 10.4103/tcmj.tcmj_34_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agha R., Franchi T., Sohrabi C., Mathew G., Kerwan A., Thoma A. The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020 doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 5.Mesrar H., Mesrar J., Maillier B., Kraoua S., Chapoutot L., Delclaux B. Lemierre’s syndrome: diagnosis, exploration, treatment. Rev. Med. Interne. 2018;39(5):339. doi: 10.1016/j.revmed.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Surapaneni B.K., Omar H., Iguina M.M., Suarez M. Fusobacterium necrophorum septicemia leading to Lemierre’s syndrome in an immunocompetent individual: a case report. Cureus. 2020;12(3) doi: 10.7759/cureus.7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bank S., Jensen A., Nielsen H.M., Kristensen L.H., Voldstedlund M., Prag J. Fusobacterium necrophorum findings in Denmark from 2010 to 2014 using data from the Danish microbiology database. Apmis. 2016;124(12):1087–1092. doi: 10.1111/apm.12606. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong, Spooner, Sanders Lemierre’s syndrome. Curr. Infect. Dis. Rep. 2000;2(2):168–173. doi: 10.1007/s11908-000-0030-z. [DOI] [PubMed] [Google Scholar]
- 9.Kristensen L.H., Prag J. Lemierre’s syndrome and other disseminated Fusobacterium necrophorum infections in Denmark: a prospective epidemiological and clinical survey. Eur. J. Clin. Microbiol. Infect. Dis. 2008;27(9):779–789. doi: 10.1007/s10096-008-0496-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riordan T. Human infection with Fusobacterium necrophorum (Necrobacillosis), with a focus on Lemierre’s syndrome. Clin. Microbiol. Rev. 2007;20(4):622–659. doi: 10.1128/CMR.00011-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai Y.-J., Lin Y.-C., Harnnd D.-J., Chiang R.P.-Y., Wu H.-M. A Lemierre syndrome variant caused by Klebsiella pneumoniae. J. Formos. Med. Assoc. 2012;111(7):403–405. doi: 10.1016/j.jfma.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Noh H.J., de Freitas C.A., Simões J.C., Kosugi E.M. Lemierre syndrome: a rare complication of pharyngotonsillitis. Braz. J. Otorhinolaryngol. 2015;81(5):568–570. doi: 10.1016/j.bjorl.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jariwala R.H., Srialluri S., Huang M.Z., Boppana S.B. Methicillin-resistant Staphylococcus aureus as a cause of Lemierre’s syndrome. Pediatr. Infect. Dis. J. 2017;36(4):429–431. doi: 10.1097/INF.0000000000001460. [DOI] [PubMed] [Google Scholar]
- 14.Kuppalli K., Livorsi D., Talati N.J., Osborn M. Lemierre’s syndrome due to Fusobacterium necrophorum. Lancet Infect. Dis. 2012;12(10):808–815. doi: 10.1016/S1473-3099(12)70089-0. [DOI] [PubMed] [Google Scholar]
- 15.Li Y.-H., Wang C.-H., Jean S.-S., Chen F.-L., Lee W.-S., Chang J.-H. Lemierre syndrome complicating deep neck infection and descending necrotizing mediastinits caused by odontogenic infections. J. Microbiol. Immunol. Infect. 2020;53(2):357–359. doi: 10.1016/j.jmii.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Moretti M., De Geyter D., Goethal L., Allard S.D. Lemierre’s syndrome in adulthood, a case report and systematic review. Acta Clin. Belg. 2020:1–11. doi: 10.1080/17843286.2020.1731661. [DOI] [PubMed] [Google Scholar]
- 17.Karkos P.D., Asrani S., Karkos C.D., Leong S.C., Theochari E.G., Alexopoulou T.D. Lemierre’s syndrome: a systematic review. Laryngoscope. 2009;119(8):1552–1559. doi: 10.1002/lary.20542. [DOI] [PubMed] [Google Scholar]
- 18.Cupit-Link M.C., Rao A.N., Warad D.M., Rodriguez V. Lemierre syndrome: a retrospective study of the role of anticoagulation and thrombosis outcomes. Acta Haematol. 2017;137(2):59–65. doi: 10.1159/000452855. [DOI] [PubMed] [Google Scholar]